Improving Soybean Germination and Nodule Development with Nitric Oxide-Releasing Polymeric Nanoparticles
Abstract
1. Introduction
2. Results
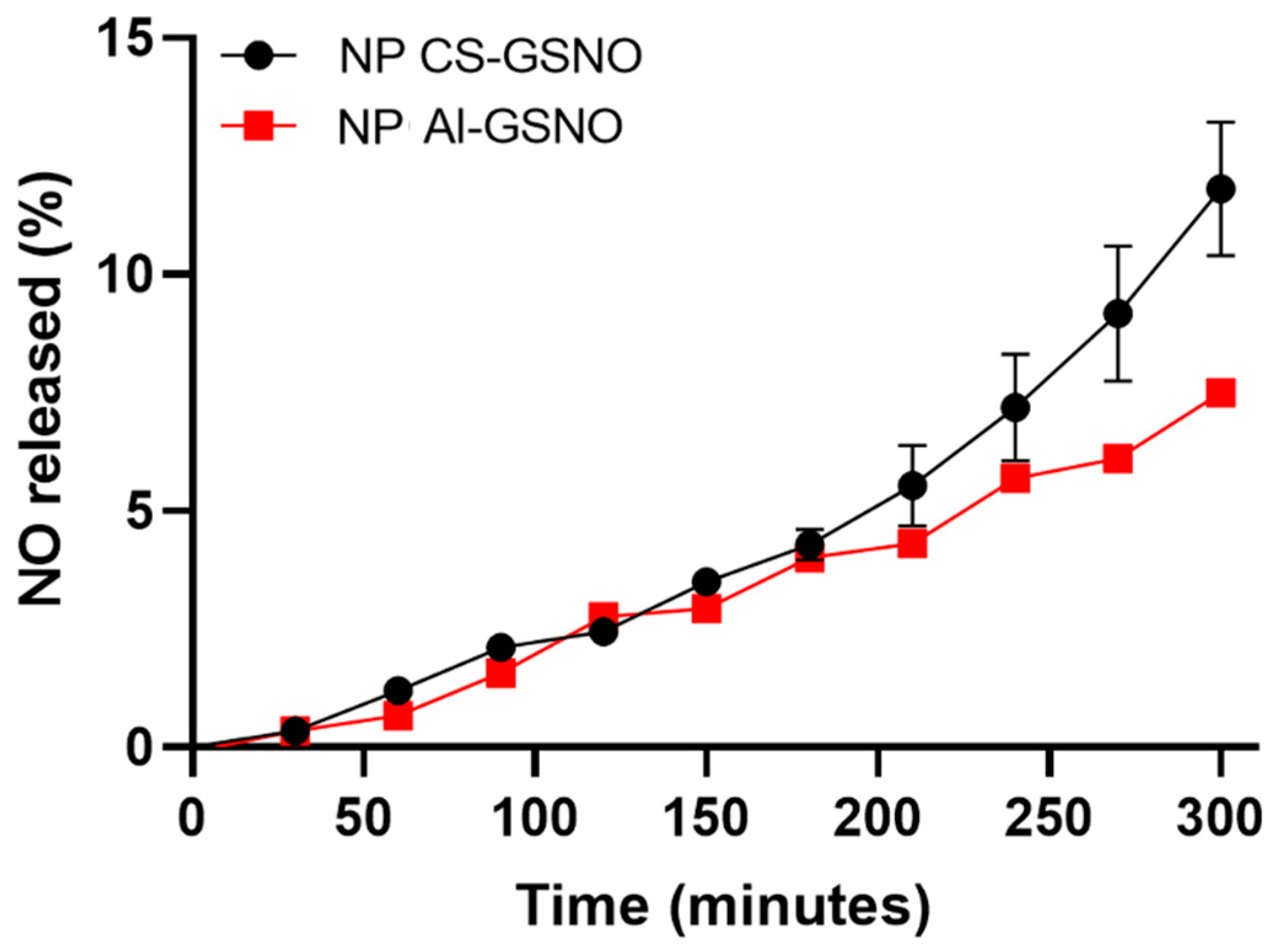
2.1. Characterization of Nanoparticles and Kinetics of NO Release
2.2. In Vitro Experiment with Soybean
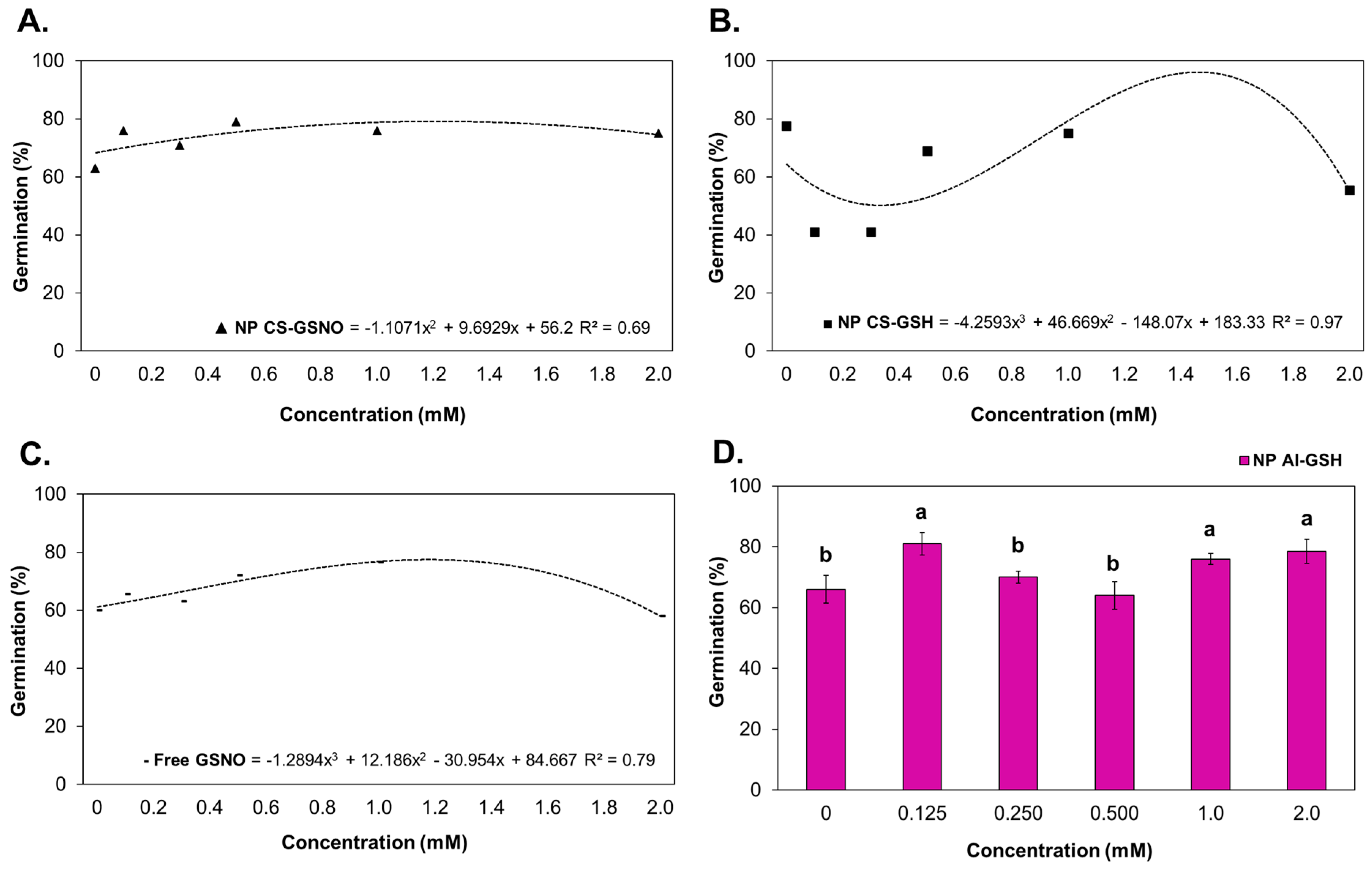
2.2.1. Germination Parameters
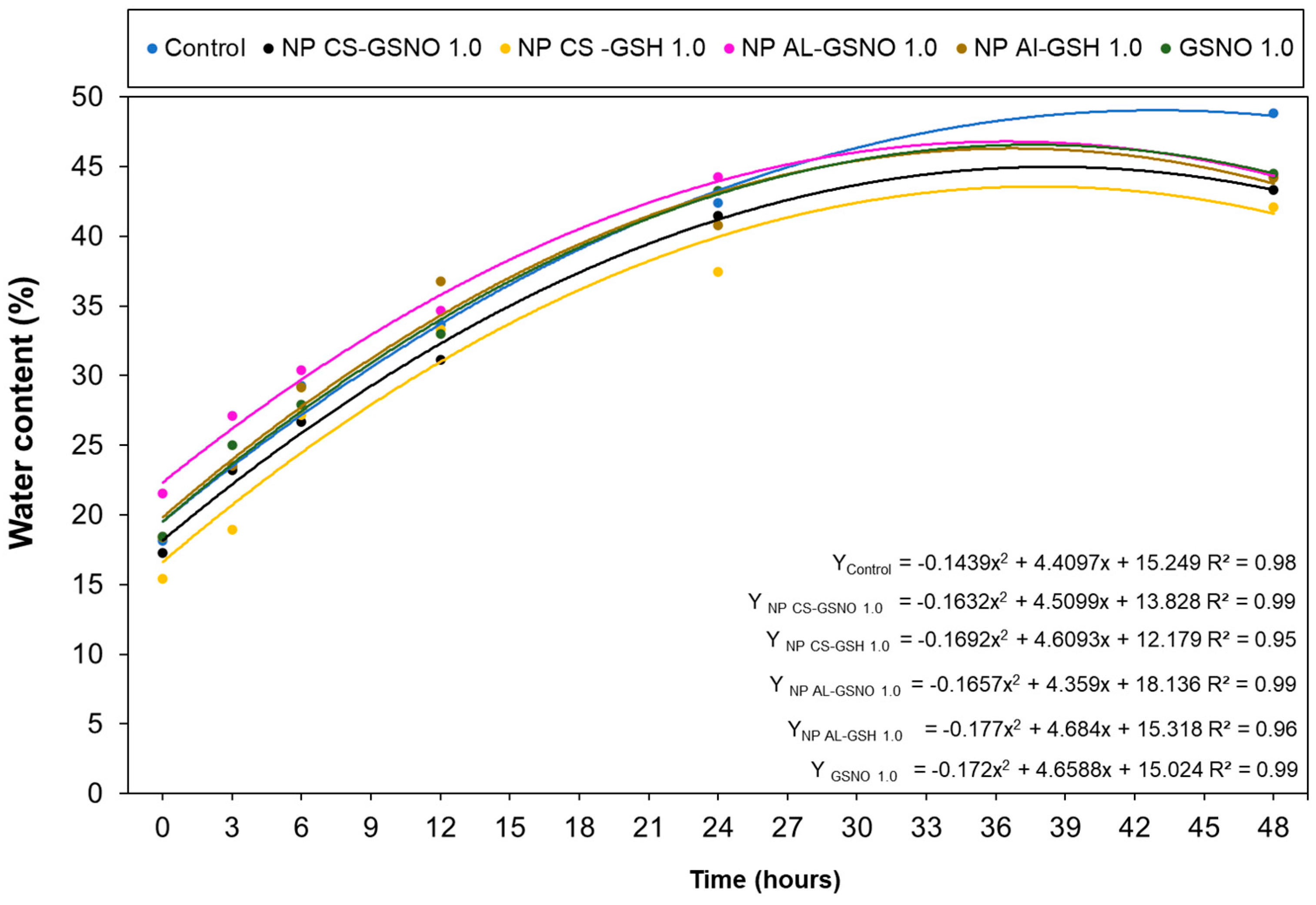
2.2.2. Imbibition Curve
2.2.3. Biochemical Analyses
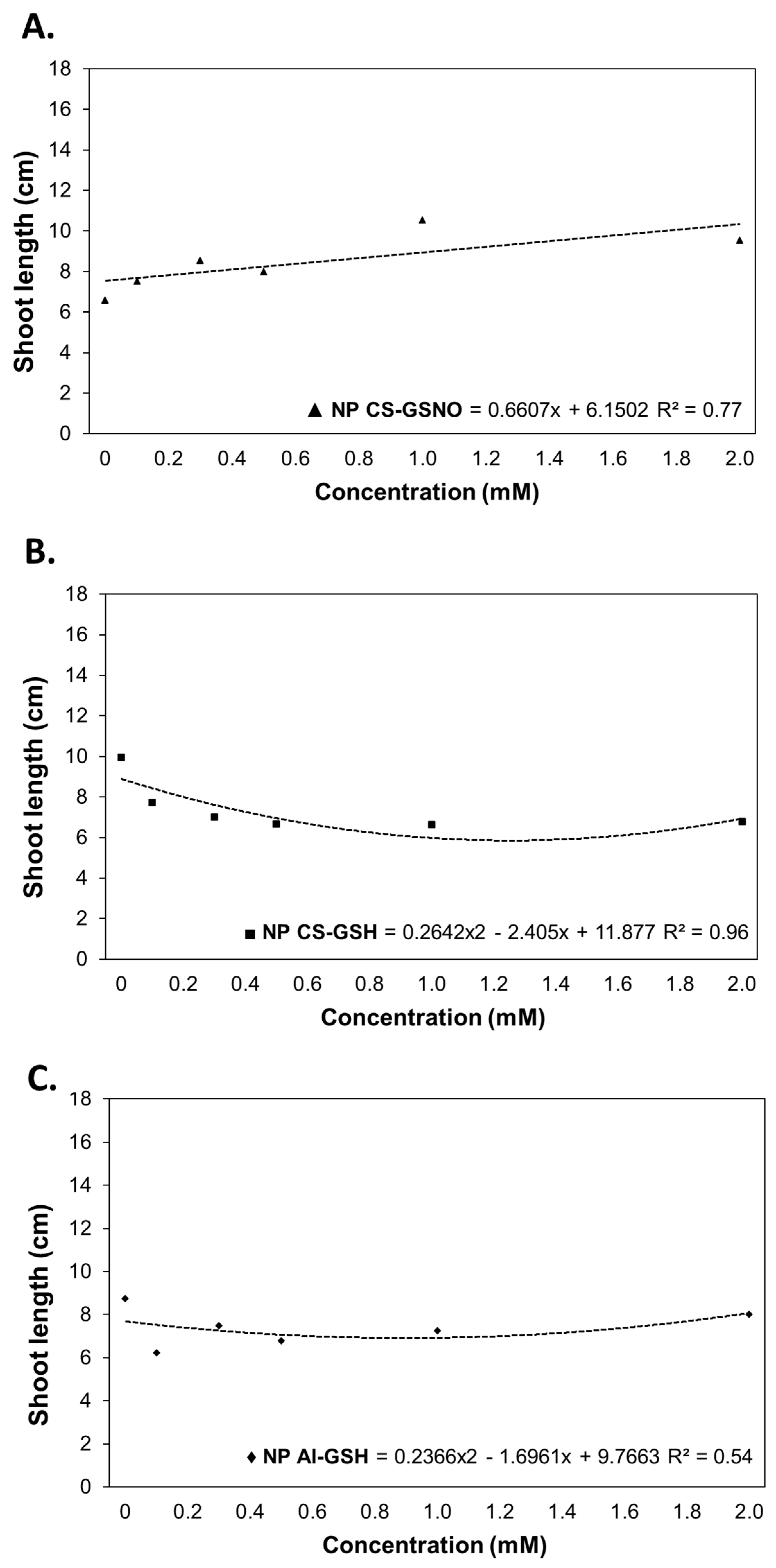
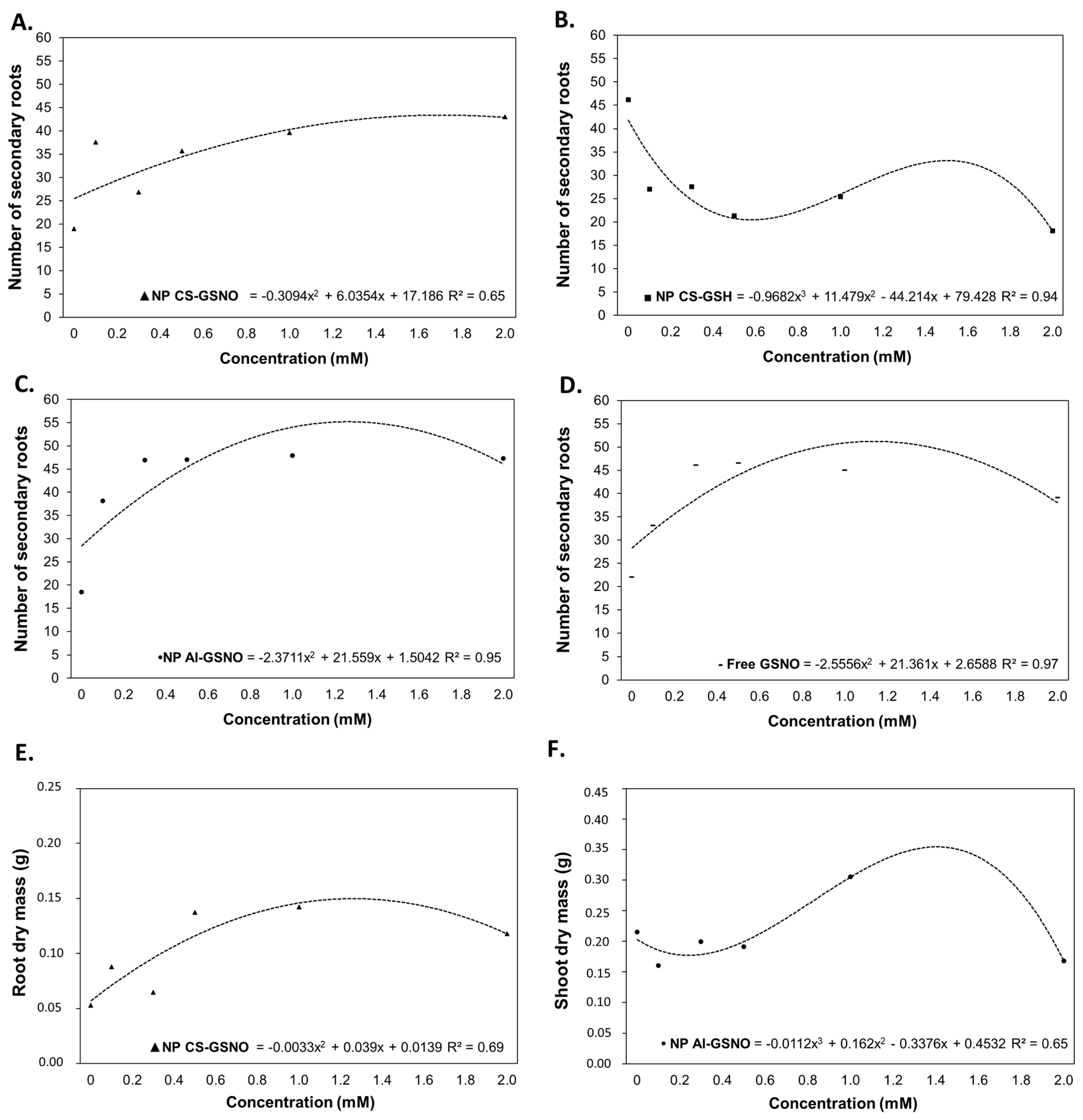
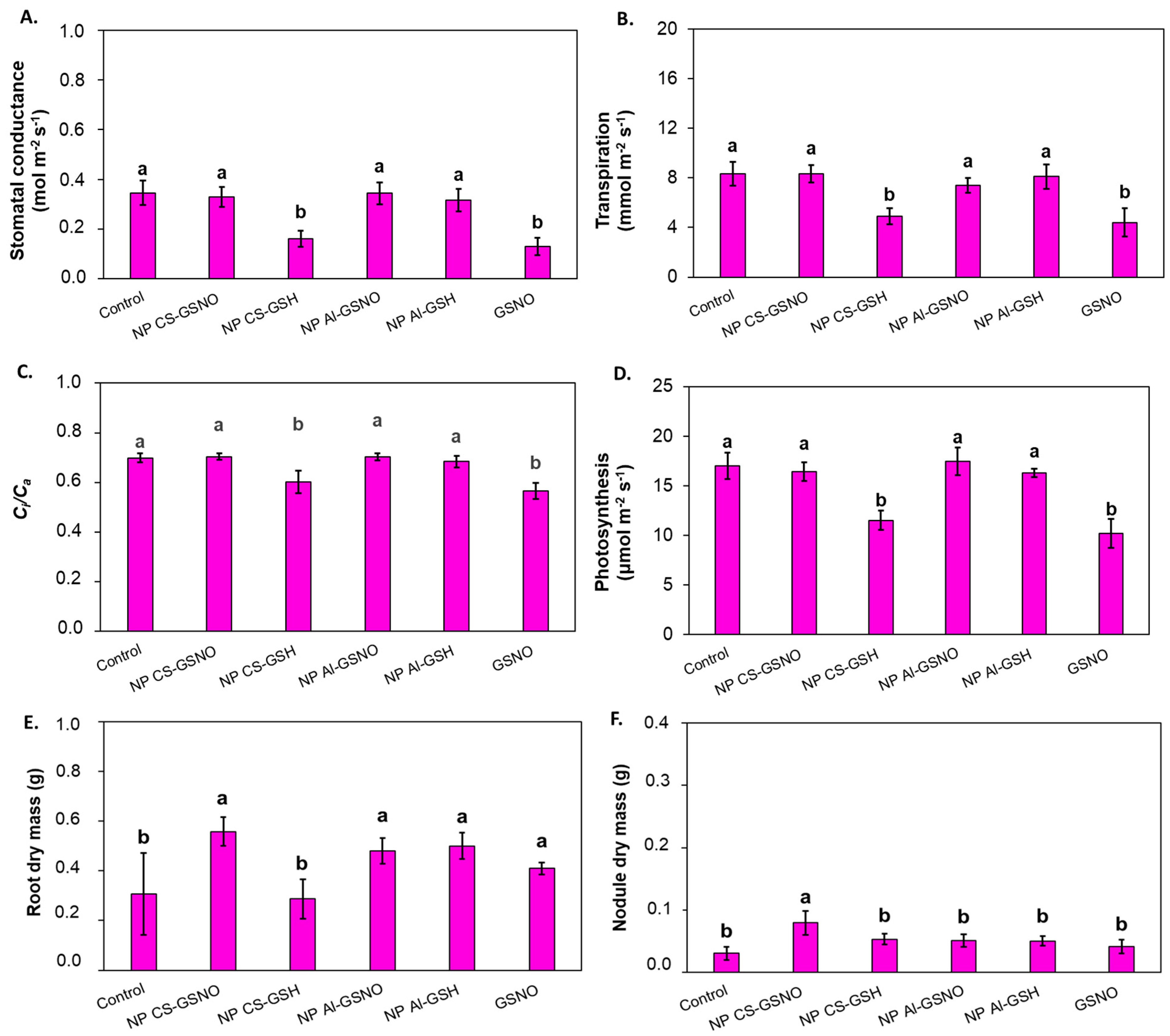
2.3. Greenhouse Experiment
3. Discussion
4. Conclusions
5. Material and Methods
5.1. Preparation of Formulations
5.1.1. Chitosan Nanoparticles
5.1.2. Alginate Nanoparticles
5.1.3. Free S-Nitrosoglutathione
5.2. Characterization of Nanoparticles and NO Release
5.3. Plant Material and Treatments
5.3.1. In Vitro Experiment
Germination Analyses
Water Absorption Assay
Biochemical Analysis
5.3.2. Greenhouse Experiment
5.4. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Reed, R.C.; Bradford, K.J.; Khanday, I. Seed germination and vigor: Ensuring crop sustainability in a changing climate. Heredity 2022, 128, 450–459. [Google Scholar] [CrossRef] [PubMed]
- Finch-Savage, W.E.; Bassel, G.W. Seed vigour and crop establishment: Extending performance beyond adaptation. J. Exp. Bot. 2016, 67, 567–591. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, N.; Yang, R.; Wang, L.; Sun, Q.; Li, D.; Cao, Y.; Weeda, S.; Zhao, B.; Ren, S.; et al. Melatonin promotes seed germination under high salinity by regulating antioxidant systems, ABA and GA 4 interaction in cu-cumber (Cucumis sativus L.). J. Pineal Res. 2014, 57, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Lavres, J.; Castro Franco, G.; De Sousa Câmara, G.M. Soybean seed treatment with nickel improves biological nitrogen fixation and urease activity. Front. Environ. Sci. 2016, 4, 37. [Google Scholar] [CrossRef]
- Rossaman, D.R.; Byrne, A.M.; Chilvers, M.I. Profitability and efficacy of soybean seed treatment in Michigan. Crop Prot. 2018, 114, 44–52. [Google Scholar] [CrossRef]
- Arif, A.B.; Yuliani, S.; Hernani, Q.; Agustinisar, I.; Winarti, C. Effects of chitosan nanoparticles coating on delaying of seed soybean (Gycine max) deterioration. Emir. J. Food Agric. 2023, 3, 232–241. [Google Scholar] [CrossRef]
- Soumare, A.; Diedhiou, A.G.; Thuita, M.; Hafidi, M.; Ouhdouch, Y.; Gopalakrishnan, S.; Kouisni, L. Exploiting biological nitrogen fixation: A route towards a sustainable agriculture. Plants 2020, 9, 1011. [Google Scholar] [CrossRef]
- Berger, A.; Boscari, A.; Horta Araújo, N.; Maucourt, M.; Hanchi, M.; Bernillon, S.; Brouquisse, R. Plant nitrate reductases regulate nitric oxide production and nitrogen-fixing metabolism during the Medicago truncatula–Sinorhizobium meliloti symbiosis. Front. Plant Sci. 2020, 11, 1313. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.W.; Murray, J.D. The role of flavonoids in nodulation host-range specificity: An update. Plants 2016, 5, 33. [Google Scholar] [CrossRef]
- Calabrese, E.J.; Agathokleous, E. Nitric oxide, hormesis and plant biology. Sci. Total Environ. 2023, 866, 161299. [Google Scholar] [CrossRef]
- Lombardo, M.C.; Lamattina, L. Nitric oxide is essential for vesicle formation and trafficking in Arabidopsis root hair growth. J. Exp. Bot. 2012, 63, 4875–4885. [Google Scholar] [CrossRef] [PubMed]
- Kolbert, Z.; Barroso, J.; Brouquisse, R.; Corpas, F.; Gupta, K.; Lindermayr, C.; Loake, G.; Palma, J.; Petřivalský, M.; Wendehenne, D.; et al. A forty year journey: The generation and roles of NO in plants. Nitric Oxide 2019, 93, 53–70. [Google Scholar] [CrossRef]
- Pande, A.; Mun, B.G.; Lee, D.S.; Khan, M.; Lee, G.M.; Hussain, A.; Yun, B.W. Network for plant-Microbe communication underground: A review. Front. Plant Sci. 2021, 12, 431. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Ali, S.; Al Azzawi, T.N.I.; Yun, B.W. Nitric oxide acts as a key signaling molecule in plant development under stressful conditions. Int. J. Mol. Sci. 2023, 24, 4782. [Google Scholar] [CrossRef]
- Cam, Y.; Pierre, O.; Boncompagni, E.; Hérouart, D.; Meilhoc, E.; Bruand, C. Nitric oxide (NO): A key player in the senescence of Medicago truncatula root nodules. New Phytol. 2012, 196, 548–560. [Google Scholar] [CrossRef] [PubMed]
- Seabra, A.B.; Silveira, N.M.; Ribeiro, R.V.; Pieretti, J.C.; Barroso, J.B.; Corpas, F.J.; Palma, J.M.; Hancock, J.T.; Petřivalský, M.; Gupta, K.J.; et al. Nitric oxide-releasing nanomaterials: From basic research to potential biotechnological applications in agriculture. New Phytol. 2022, 234, 1119–1125. [Google Scholar] [CrossRef] [PubMed]
- Mgadi, K.; Ndaba, B.; Roopnarain, A.; Rama, H.; Adeleke, R. Nanoparticle applications in agriculture: Overview and response of plant-associated microorganisms. Front. Microbiol. 2024, 15, 1354440. [Google Scholar] [CrossRef]
- Fukamachi, K.; Konishi, Y.; Nomura, T. Disease control of Phytophthora infestans using cyazofamid encapsulated in poly lactic-co-glycolic acid (PLGA) nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2019, 577, 315–322. [Google Scholar] [CrossRef]
- Li, R.; He, J.; Xie, H.; Wang, W.; Bose, S.K.; Sun, Y.; Yin, H. Effects of chitosan nanoparticles on seed germination and seed-ling growth of wheat (Triticum aestivum L.). Int. J. Biol. Macromol. 2019, 126, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Schnoor, B.; Elhendawy, A.; Joseph, S.; Putman, M.; Chacón-Cerdas, R.; Flores-Mora, D.; Bravo-Moraga, F.; Gonzalez-Nilo, F.; Salvador-Morales, C. Engineering atrazine loaded poly (lactic-co-glycolic acid) nanoparticles to ameliorate environmental challenges. J. Agric. Food Chem. 2018, 66, 7889–7898. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.M.; Tiwari, A.; Maity, D.; Saha, S. Recent progress of nanomaterials in sustainable agricultural applications. J. Mater. Sci. 2022, 57, 10836–10862. [Google Scholar] [CrossRef]
- Steven, S.; Islam, M.S.; Ghimire, A.; Methela, N.J.; Kwon, E.H.; Yun, B.W.; Lee, I.J.; Kim, S.H.; Kim, Y. Chitosan-GSNO Nanoparticles and Silicon Priming Enhance the Germination and Seedling Growth of Soybean (Glycine max L.). Plants 2024, 13, 1290. [Google Scholar] [CrossRef]
- Spielman-Sun, E.; Avellan, A.; Bland, G.D.; Tappero, R.V.; Acerbo, A.S.; Unrine, J.M.; Giraldo, J.P.; Lowry, G.V. Nano-particle surface charge influences translocation and leaf distribution in vascular plants with contrasting anatomy. Environ. Sci. Nano 2019, 6, 2508–2519. [Google Scholar] [CrossRef]
- Bai, T.; Zhang, P.; Guo, Z.; Chetwynd, A.J.; Zhang, M.; Adeel, M.; Rui, Y. Different physiological responses of C3 and C4 plants to nanomaterials. Environ. Sci. Pollut. Res. 2021, 28, 25542–25551. [Google Scholar] [CrossRef] [PubMed]
- Preisler, A.C.; Guariz, H.R.; Carvalho, L.B.; Santo Pereira, A.D.E.; de Oliveira, J.L.; Fraceto, L.F.; Dalazen, G.; Oliveira, H.C. Phytotoxicity evaluation of poly (ε-caprolactone) nanocapsules prepared using different methods and compositions in Brassica juncea seeds. Plant Nano Biol. 2022, 1, 100003. [Google Scholar] [CrossRef]
- Oliveira, H.C.; Gomes, B.C.; Pelegrino, M.T.; Seabra, A.B. Nitric oxide-releasing chitosan nanoparticles alleviate the effects of salt stress in maize plants. Nitric Oxide 2016, 61, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Fu, L.; Li, S.; Yan, J.; Sun, M.; Giraldo, J.P.; Matyjaszewski, K.; Tilton, R.D.; Lowry, G.V. Star polymer size, charge content, and hydrophobicity affect their leaf uptake and translocation in plants. Environ. Sci. Technol. 2021, 55, 10758–10768. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Chen, D.; Yu, D.; Regenstein, J.M.; Jiang, Q.; Dong, J.; Chen, W.; Xia, W. Modulating physicochemical, anti-microbial and release properties of chitosan/zein bilayer films with curcumin/nisin-loaded pectin nanoparticles. Food Hydrocoll. 2022, 133, 107955. [Google Scholar] [CrossRef]
- Parkinson, S.J.; Tungsirisurp, S.; Joshi, C.; Richmond, B.L.; Gifford, M.L.; Sikder, A.; Lynch, I.; O’Reilly, R.K.; Napier, R.M. Polymer nanoparticles pass the plant interface. Nat. Commun. 2022, 13, 7385. [Google Scholar] [CrossRef]
- Preisler, A.C.; Carvalho, L.B.; Saraiva-Santos, T.; Verri, W.A., Jr.; Mayer, J.L.S.; Fraceto, L.F.; Dalazen, G.; Oliveira, H.C. Interaction of nanoatrazine and target organism: Evaluation of fate and photosystem II inhibition in hydroponically grown mustard (Brassica juncea) plants. J. Agric. Food Chem. 2022, 70, 7644–7652. [Google Scholar] [CrossRef]
- Zhang, Y.; Martinez, M.R.; Sun, H.; Sun, M.; Yin, R.; Yan, J.; Marelli, B.; Giraldo, J.P.; Matyjaszewski, K.; Tilton, R.D.; et al. Charge, aspect ratio, and plant species affect uptake efficiency and translocation of polymeric agrochemical nanocarriers. Environ. Sci. Technol. 2023, 57, 8269–8279. [Google Scholar] [CrossRef] [PubMed]
- Methela, N.J.; Islam, M.S.; Lee, D.S.; Yun, B.W.; Mun, B.G. S-nitrosoglutathione (GSNO)-mediated lead detoxification in soybean through the regulation of ROS and metal-related transcripts. Int. J. Mol. Sci. 2023, 24, 9901. [Google Scholar] [CrossRef] [PubMed]
- do Carmo, G.C.; Iastrenski, L.F.; Debiasi, T.V.; da Silva, R.C.; Gomes, D.G.; Pelegrino, M.T.; Oliveira, H.C. Nanoencapsulation improves the protective effects of a nitric oxide donor on drought-stressed Heliocarpus popayanensis seedlings. Ecotoxicol. Environ. Saf. 2021, 225, 112713. [Google Scholar] [CrossRef] [PubMed]
- Silveira, N.M.; Seabra, A.B.; Marcos, F.C.; Pelegrino, M.T.; Machado, E.C.; Ribeiro, R.V. Encapsulation of S-nitrosoglutathione into chitosan nanoparticles improves drought tolerance of sugarcane plants. Nitric Oxide 2019, 84, 38–44. [Google Scholar] [CrossRef]
- Seligman, K.; Saviani, E.E.; Oliveira, H.C.; Pinto-Maglio, C.A.F.; Salgado, I. Floral transition and nitric oxide emission during flower development in Arabidopsis thaliana is affected in nitrate reductase-deficient plants. Plant Cell Physiol. 2008, 49, 1112–1121. [Google Scholar] [CrossRef]
- Farnese, F.S.; Menezes-Silva, P.E.; Gusman, G.S.; Oliveira, J.A. When bad guys become good ones: The key role of reactive oxygen species and nitric oxide in the plant responses to abiotic stress. Front. Plant Sci. 2016, 7, 471. [Google Scholar] [CrossRef] [PubMed]
- León, J.; Costa-Broseta, Á. Present knowledge and controversies, deficiencies, and misconceptions on nitric oxide synthesis, sensing, and signaling in plants. Plant Cell Environ. 2020, 43, 1–15. [Google Scholar] [CrossRef]
- Yan, A.; Chen, Z. The control of seed dormancy and germination by temperature, light and nitrate. Bot. Rev. 2020, 86, 39–75. [Google Scholar] [CrossRef]
- Khator, K.; Parihar, S.; Jasik, J.; Shekhawat, G.S. Nitric oxide in plants: An insight on redox activity and responses toward abiotic stress signaling. Plant Signal. Behav. 2024, 19, 2298053. [Google Scholar] [CrossRef]
- Sun, H.; Feng, F.; Liu, J.; Zhao, Q. The interaction between auxin and nitric oxide regulates root growth in response to iron deficiency in rice. Front. Plant Sci. 2017, 8, 2169. [Google Scholar] [CrossRef]
- Lombardo, M.C.; Lamattina, L. Abscisic acid and nitric oxide modulate cytoskeleton organization, root hair growth and ectopic hair formation in Arabidopsis. Nitric Oxide 2018, 80, 89–97. [Google Scholar] [CrossRef]
- Berger, A.; Boscari, A.; Frendo, P.; Brouquisse, R. Nitric oxide signaling, metabolism and toxicity in nitrogen-fixing symbiosis. J. Exp. Bot. 2019, 70, 4505–4520. [Google Scholar] [CrossRef]
- Altamura, M.M.; Piacentini, D.; Della Rovere, F.; Fattorini, L.; Falasca, G.; Betti, C. New paradigms in brassinosteroids, strigolactones, sphingolipids, and nitric oxide interaction in the control of lateral and adventitious root formation. Plants 2023, 12, 413. [Google Scholar] [CrossRef] [PubMed]
- Signorelli, S.; Sainz, M.; Tabares-da Rosa, S.; Monza, J. The role of nitric oxide in nitrogen fixation by legumes. Front. Plant Sci. 2020, 11, 521. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; Shah, F.; Ali, F.; Yun, B.W. Role of nitric oxide in plant senescence. Front. Plant Sci. 2022, 13, 851631. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Huang, J.; Li, Y.; Li, C.; Hou, J.; Liang, W. Involvement of nitric oxide-mediated alternative pathway in tolerance of wheat to drought stress by optimizing photosynthesis. Plant Cell Rep. 2016, 35, 2033–2044. [Google Scholar] [CrossRef] [PubMed]
- Silveira, N.M.; Frungillo, L.; Marcos, F.C.; Pelegrino, M.T.; Miranda, M.T.; Seabra, A.B.; Salgado, I.; Machado, E.C.; Ribeiro, R.V. Exogenous nitric oxide improves sugarcane growth and photosynthesis under water deficit. Planta 2016, 244, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Ahammed, G.J.; Li, X.; Mao, Q.; Wan, H.; Zhou, G.; Cheng, Y. The SlWRKY81 transcription factor inhibits stomatal closure by attenuating nitric oxide accumulation in the guard cells of tomato under drought. Physiol. Plant. 2021, 172, 885–895. [Google Scholar] [CrossRef]
- Desikan, R.; Griffiths, R.; Hancock, J.; Neill, S. A new role for an old enzyme: Nitrate reductase-mediated nitric oxide generation is required for abscisic acid-induced stomatal closure in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2002, 99, 16314–16318. [Google Scholar] [CrossRef] [PubMed]
- Bright, J.; Desikan, R.; Hancock, J.T.; Weir, I.S.; Neill, S.J. ABA-induced NO generation and stomatal closure in Arabidopsis are dependent on H2O2 synthesis. Plant J. 2006, 45, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Calvo-Begueria, L.; Rubio, M.C.; Martínez, J.I.; Pérez-Rontomé, C.; Delgado, M.J.; Bedmar, E.J.; Becana, M. Redefining nitric oxide production in legume nodules through complementary insights from electron paramagnetic resonance spectroscopy and specific fluorescent probes. J. Exp. Bot. 2018, 69, 3703–3714. [Google Scholar] [CrossRef]
- Seabra, A.B.; Fabbri, G.K.; Pelegrino, M.T.; Silva, L.C.; Rodrigues, T. Synthesis, characterization and cytotoxicity of S-nitroso-mercaptosuccinic acid-containing alginate/chitosan nanoparticles. J. Phys. Conf. Ser. 2017, 838, 012032. [Google Scholar] [CrossRef]
- Urzedo, A.L.; Goncalves, M.C.; Nascimento, M.H.; Lombello, C.B.; Nakazato, G.; Seabra, A.B. Multifunctional alginate nanoparticles containing nitric oxide donor and silver nanoparticles for biomedical applications. Mater. Sci. Eng. C 2020, 112, 110933. [Google Scholar] [CrossRef] [PubMed]
- Pelegrino, M.T.; Pieretti, J.C.; Lange, C.N.; Kohatsu, M.Y.; Freire, B.M.; Batista, B.L.; Fincheira, P.; Tortella, G.R.; Rubilar, O.; Seabra, A.B. Foliar spray application of CuO nanoparticles (NPs) and S-nitrosoglutathione enhances productivity, physiological and biochemical parameters of lettuce plants. J. Chem. Technol. Biotechnol. 2021, 96, 2185–2196. [Google Scholar] [CrossRef]
- Lopes-Oliveira, P.J.; Gomes, D.G.; Pelegrino, M.T.; Bianchini, E.; Pimenta, J.A.; Stolf-Moreira, R.; Oliveira, H.C. Effects of nitric oxide-releasing nanoparticles on neotropical tree seedlings submitted to acclimation under full sun in the nursery. Sci. Rep. 2019, 9, 17371. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.E.S.; Oliveira, H.C.; Fraceto, L.F.; Santaella, C. Nanotechnology potential in seed priming for sustainable agriculture. Nanomaterials 2021, 11, 267. [Google Scholar] [CrossRef]
- Pelegrino, M.T.; Weller, R.B.; Chen, X.; Bernardes, J.S.; Seabra, A.B. Chitosan nanoparticles for nitric oxide delivery in human skin. MedChemComm 2017, 8, 713–719. [Google Scholar] [CrossRef]
- Giaretta, D.; de Lima, V.A.; Schmidt, C.A.P.; Carpes, S.T. Chromatographic characterization of isoflavones in soy flour variety BRS 257, and recognition of their patterns by chemometrics. LWT-Food Sci. Technol. 2015, 64, 1209–1216. [Google Scholar] [CrossRef][Green Version]
- Ministério da Agricultura e Reforma Agrária (MAPA). Secretaria Nacional de Defesa Agropecuária. Regras para Análise de Sementes. Brasília. 2009. Available online: https://www.gov.br/agricultura/pt-br/assuntos/insumos-agropecuarios/arquivos-publicacoes-insumos/2946_regras_analise__sementes.pdf (accessed on 30 April 2024).
- Singkaew, J.; Miyagawa, S.; Wongs-Aree, C.; Vichitsoonthonkul, T.; Sokaokha, S.; Photchanachai, S. Season, fruit maturity, and storage affect on the physiological quality of F1 hybrid ‘VTM580’ tomato seeds and seedlings. Hortic. J. 2017, 86, 121–131. [Google Scholar] [CrossRef]
- Oliveira, A.B.D.; Bosco, M.R.D.O. Biometria, determinação da curva de absorção de água em sementes e emergência inicial de plântulas de Copernicia hospita Martius. Rev. Bras. Agroecol. 2013, 8, 66–74. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Preisler, A.C.; do Carmo, G.C.; da Silva, R.C.; Simões, A.L.d.O.; Izidoro, J.d.C.; Pieretti, J.C.; dos Reis, R.A.; Jacob, A.L.F.; Seabra, A.B.; Oliveira, H.C. Improving Soybean Germination and Nodule Development with Nitric Oxide-Releasing Polymeric Nanoparticles. Plants 2025, 14, 17. https://doi.org/10.3390/plants14010017
Preisler AC, do Carmo GC, da Silva RC, Simões ALdO, Izidoro JdC, Pieretti JC, dos Reis RA, Jacob ALF, Seabra AB, Oliveira HC. Improving Soybean Germination and Nodule Development with Nitric Oxide-Releasing Polymeric Nanoparticles. Plants. 2025; 14(1):17. https://doi.org/10.3390/plants14010017
Chicago/Turabian StylePreisler, Ana Cristina, Giovanna Camargo do Carmo, Rafael Caetano da Silva, Ana Luisa de Oliveira Simões, Juliana de Carvalho Izidoro, Joana Claudio Pieretti, Roberta Albino dos Reis, André Luiz Floriano Jacob, Amedea Barozzi Seabra, and Halley Caixeta Oliveira. 2025. "Improving Soybean Germination and Nodule Development with Nitric Oxide-Releasing Polymeric Nanoparticles" Plants 14, no. 1: 17. https://doi.org/10.3390/plants14010017
APA StylePreisler, A. C., do Carmo, G. C., da Silva, R. C., Simões, A. L. d. O., Izidoro, J. d. C., Pieretti, J. C., dos Reis, R. A., Jacob, A. L. F., Seabra, A. B., & Oliveira, H. C. (2025). Improving Soybean Germination and Nodule Development with Nitric Oxide-Releasing Polymeric Nanoparticles. Plants, 14(1), 17. https://doi.org/10.3390/plants14010017








