Supplemental Silicon and Boron Alleviates Aluminum-Induced Oxidative Damage in Soybean Roots
Abstract
1. Introduction
2. Results
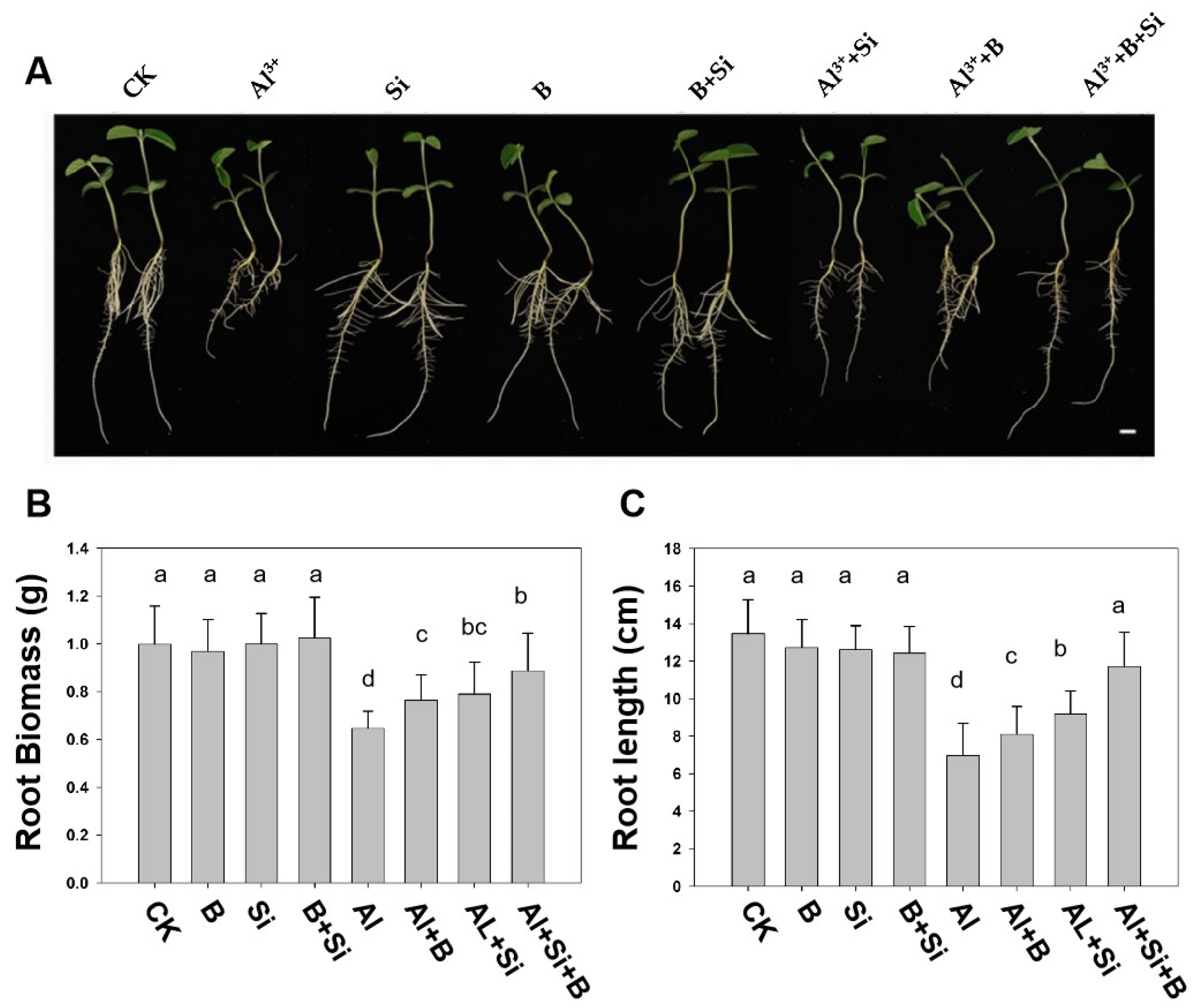
2.1. Effects of B, Si, and Their Combination on Soybean Root Growth Parameters under Al Stress
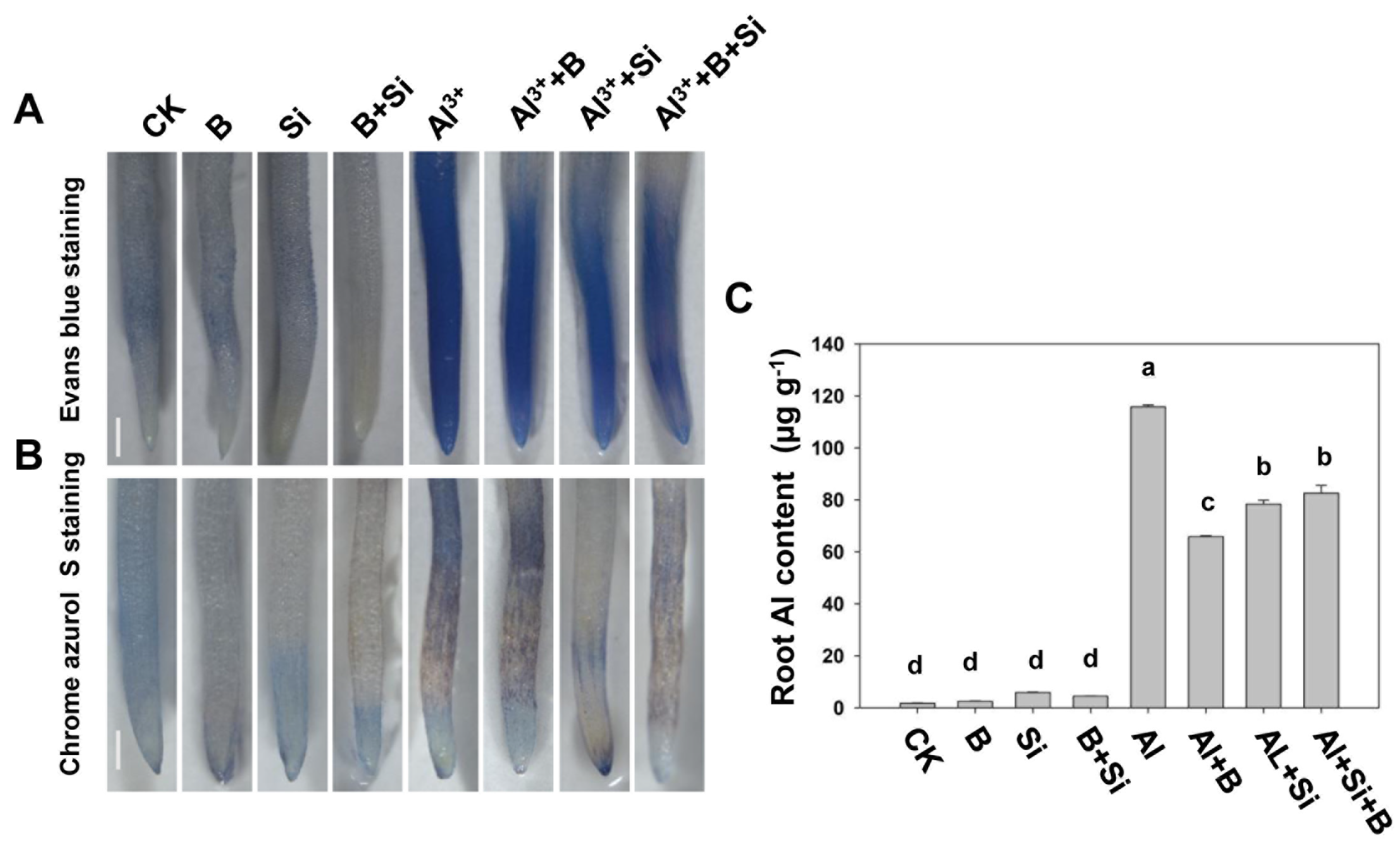
2.2. Effect of B, Si, and Their Combination on Al Toxicity-Induced PCD and Al Accumulation in Soybean Roots
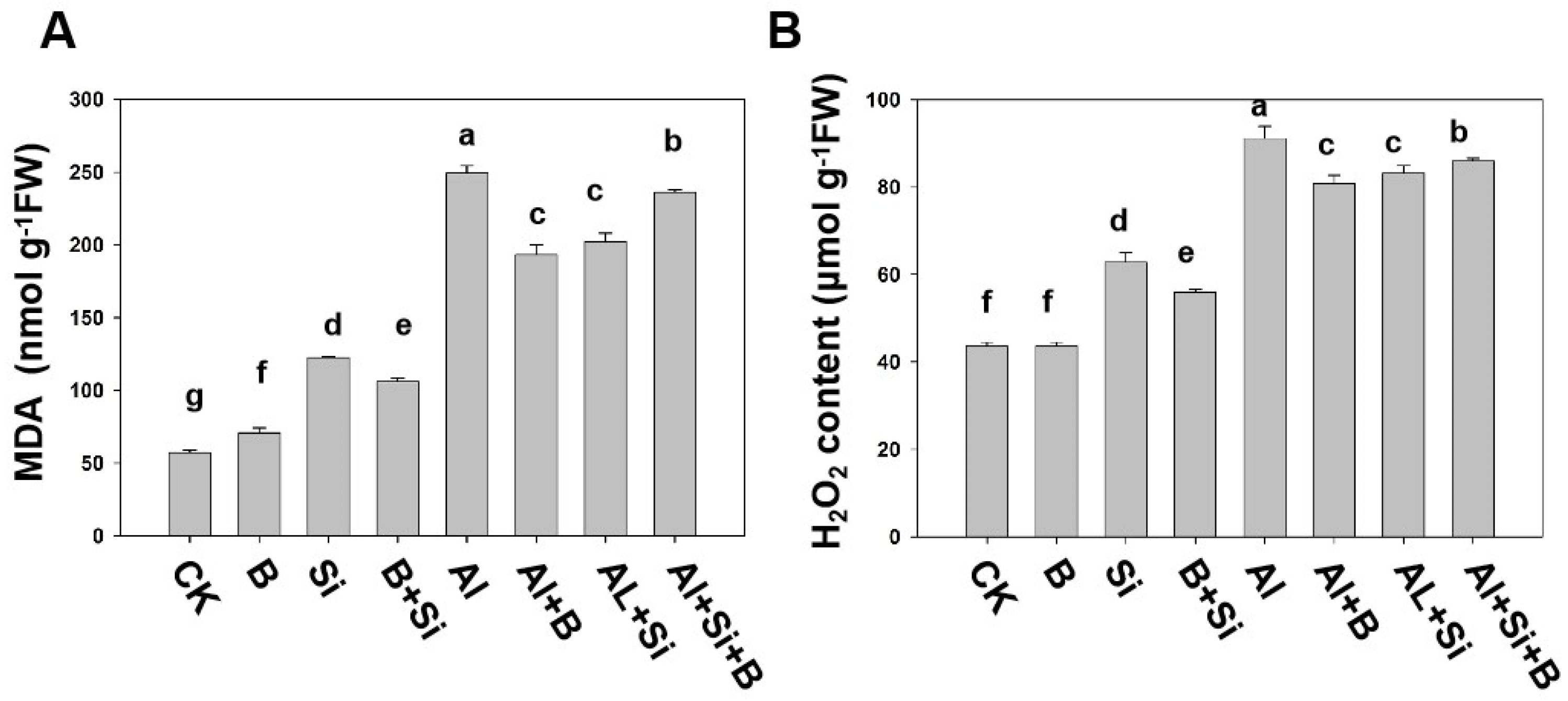
2.3. Effects of B, Si, and Their Combination on Lipid Peroxidation and H2O2 Content in Soybean Roots under Al Stress
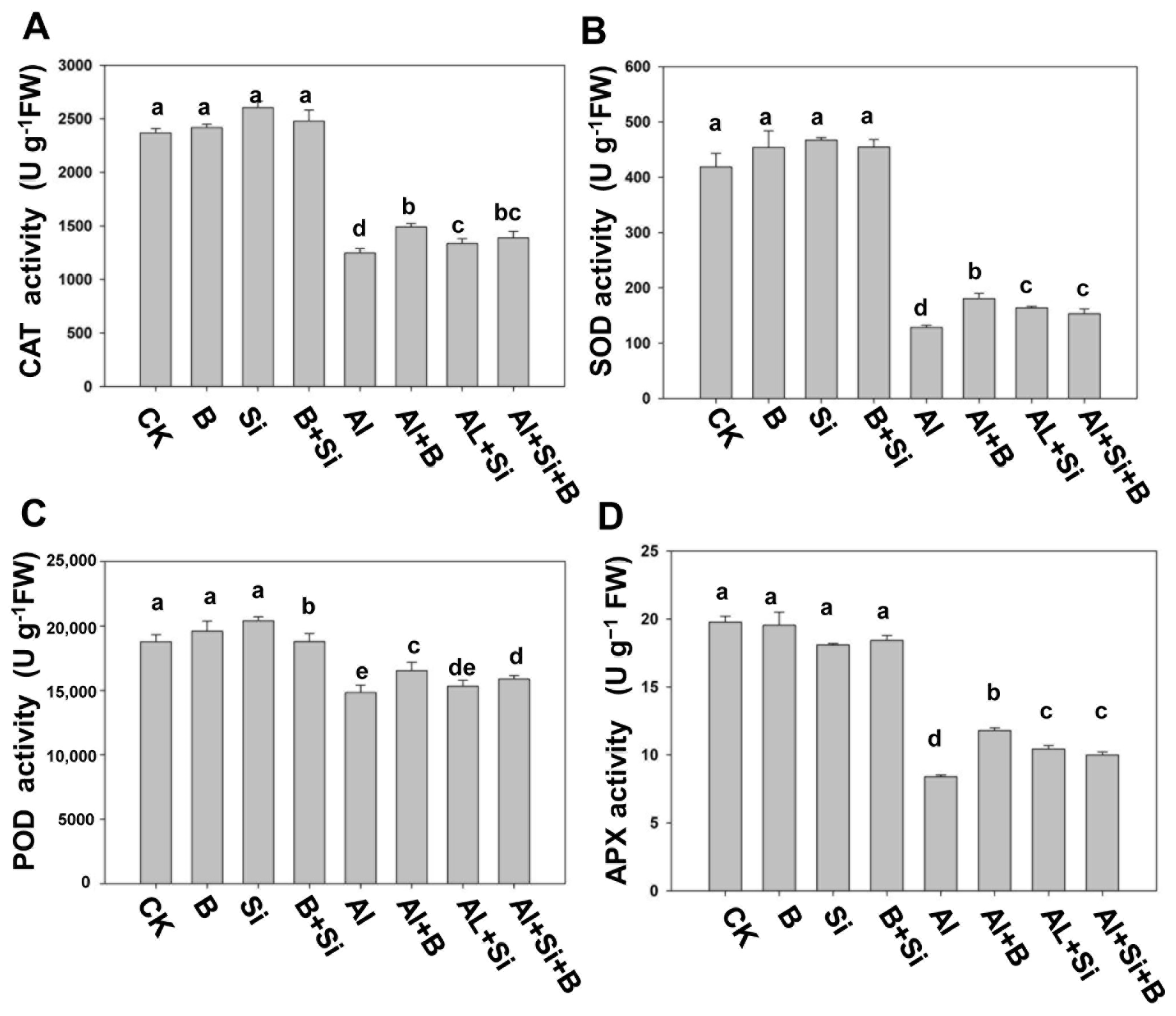
2.4. Effects of B, Si, and Their Combination on Antioxidant Activities in Soybean Roots under Al Stress
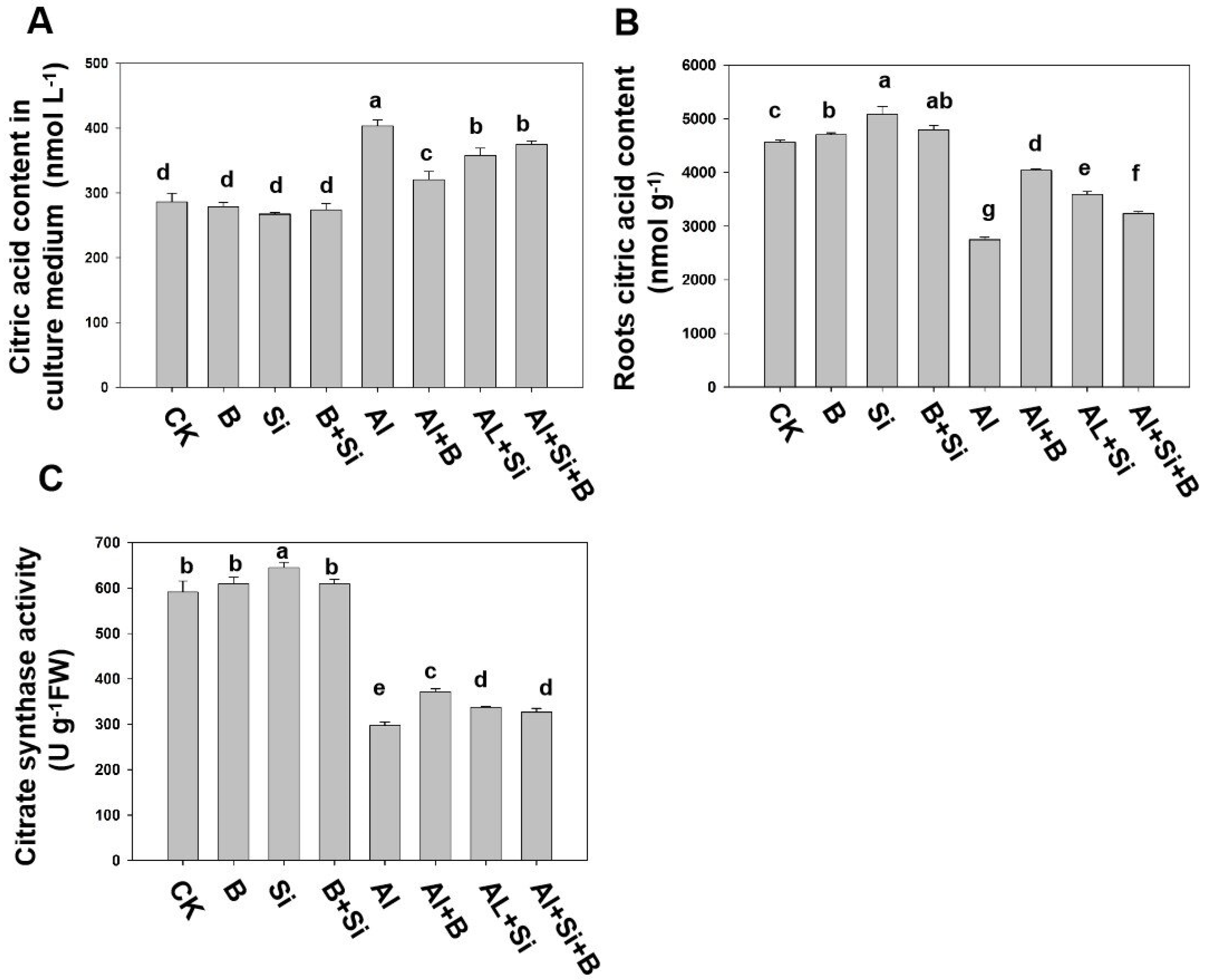
2.5. Effects of B, Si, and Their Combination on the Secretion of Citric Acid in Soybean Roots under Al Stress
3. Discussion
4. Materials and Methods
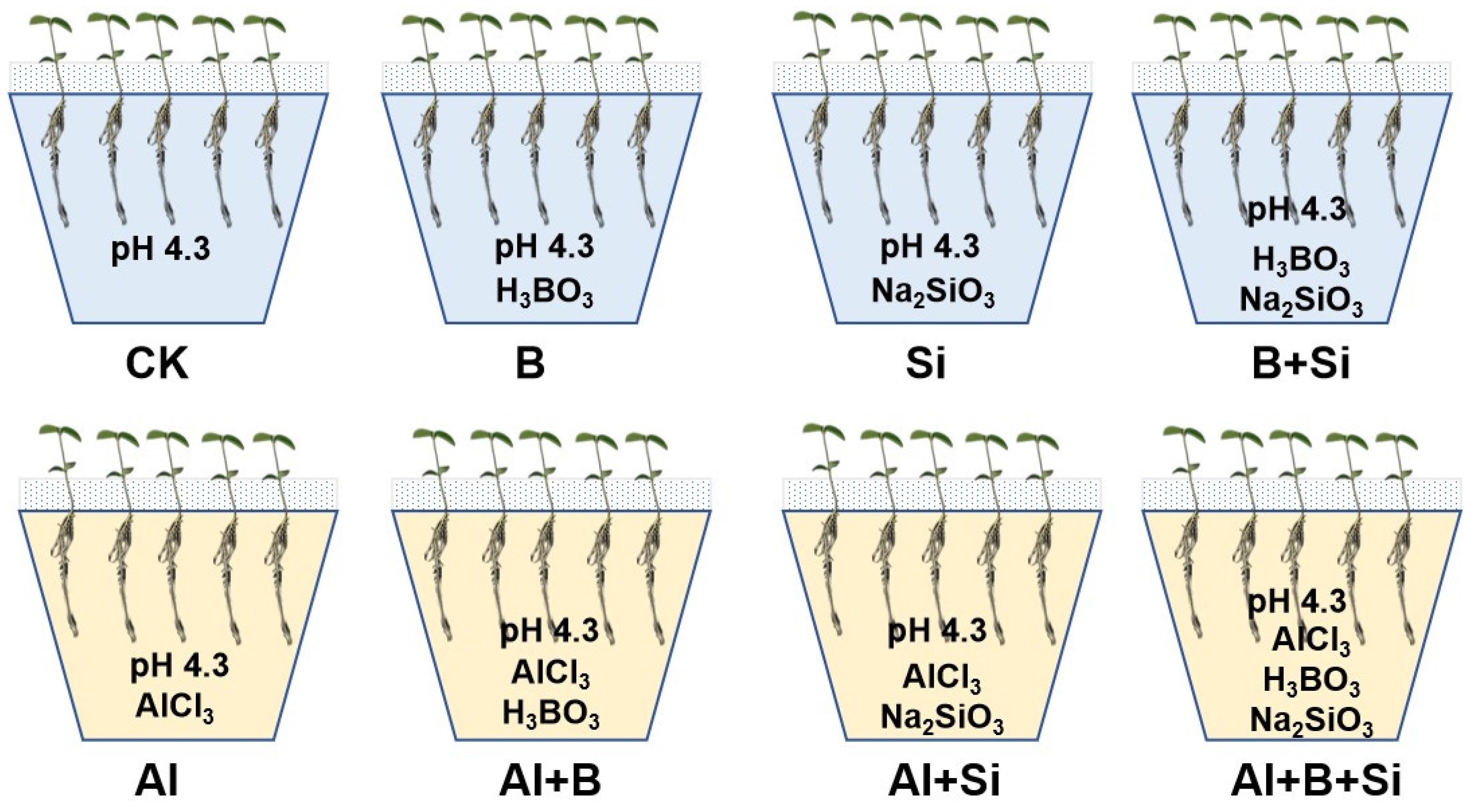
4.1. Plant Growth and Experimental Design
4.2. Root Biomass and Length Measurement
4.3. Determination of Al Content in Root
4.4. Root Cell Death Detection
4.5. Lipid Peroxidation (MDA) and Hydrogen Peroxide (H2O2) Content in Soybean Roots
4.6. Determination of Antioxidant Enzyme Activities in Soybean Roots
4.7. Determination of Citrate Synthase Activity and Citric Acid Content in Soybean Roots and Citric Acid Content in Treatment Solution
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kochian, L.V.; Piñeros, M.A.; Liu, J.; Magalhaes, J.V. Plant adaptation to acid soils: The molecular basis for crop aluminum resistance. Annu. Rev. Plant Biol. 2015, 66, 571–598. [Google Scholar] [CrossRef]
- Bojórquez-Quintal, E.; Escalante-Magaña, C.; Echevarría-Machado, I.; Martínez-Estévez, M. Aluminum, a friend or foe of higher plants in acid soils. Front. Plant Sci. 2017, 8, 1767. [Google Scholar] [CrossRef]
- Huang, W.; Yang, X.; Yao, S.; LwinOo, T.; He, H.; Wang, A.; Li, C.; He, L. Reactive oxygen species burst induced by aluminum stress triggers mitochondria-dependent programmed cell death in peanut root tip cells. Plant Physiol. Biochem. 2014, 82, 76–84. [Google Scholar] [CrossRef]
- Ofoe, R.; Gunupuru, L.R.; Wang-Pruski, G.; Fofana, B.; Thomas, R.H. Abbey, Lord Seed priming with pyroligneous acid mitigates aluminum stress, and promotes tomato seed germination and seedling growth. Plant Stress 2022, 4, 100083. [Google Scholar] [CrossRef]
- Guo, P.; Qi, Y.P.; Cai, Y.T.; Yang, T.Y.; Yang, L.T.; Huang, Z.R.; Chen, L.S. Aluminum effects on photosynthesis, reactive oxygen species and methylglyoxal detoxification in two Citrus species differing in aluminum tolerance. Tree Physiol. 2018, 38, 1548–1565. [Google Scholar] [CrossRef]
- Ofoe, R.; Thomas, R.H.; Asiedu, S.K.; Wang-Pruski, G.; Fofana, B.; Abbey, L. Aluminum in plant: Benefits, toxicity and tolerance mechanisms. Front. Plant Sci. 2023, 13, 1085998. [Google Scholar] [CrossRef]
- Yamamoto, Y. Aluminum toxicity in plant cells: Mechanisms of cell death and inhibition of cell elongation. Soil Sci. Plant Nutr. 2019, 65, 41–55. [Google Scholar] [CrossRef]
- Chen, D.; Chen, D.; Xue, R.; Long, J.; Lin, X.; Lin, Y.; Jia, L.; Zeng, R.; Song, Y. Effects of boron, silicon and their interactions on cadmium accumulation and toxicity in rice plants. J. Hazard. Mater. 2019, 367, 447–455. [Google Scholar] [CrossRef]
- Chauhan, D.K.; Yadav, V.; Vaculík, M.; Gassmann, W.; Pike, S.; Arif, N.; Singh, V.P.; Deshmukh, R.; Sahi, S.; Tripathi, D.K. Aluminum toxicity and aluminum stress-induced physiological tolerance responses in higher plants. Crit. Rev. Biotechnol. 2021, 41, 715–730. [Google Scholar] [CrossRef]
- Liu, H.; Zhu, R.; Shu, K.; Lv, W.; Wang, S.; Wang, C. Aluminum stress signaling, response, and adaptive mechanisms in plants. Plant Signal. Behav. 2022, 17, 2057060. [Google Scholar] [CrossRef]
- Sachdev, S.; Ansari, S.A.; Ansari, M.I.; Fujita, M.; Hasanuzzaman, M. Abiotic stress and reactive oxygen species: Generation, signaling, and defense mechanisms. Antioxidants 2021, 10, 277. [Google Scholar] [CrossRef]
- Du, H.; Ryan, P.R.; Liu, C.; Li, H.; Hu, W.; Yan, W.; Huang, Y.; He, W.; Luo, B.; Zhang, X.; et al. ZmMATE6 from maize encodes a citrate transporter that enhances aluminum tolerance in transgenic Arabidopsis thaliana. Plant Sci. 2021, 311, 111016. [Google Scholar] [CrossRef]
- Melo, J.O.; Martins, L.G.C.; Barros, B.A.; Pimenta, M.R.; Lana, U.G.P.; Duarte, C.E.M.; Pastina, M.M.; Guimaraes, C.T.; Schaffert, R.E.; Kochian, L.V.; et al. Repeat variants for the SbMATE transporter protect sorghum roots from aluminum toxicity by transcriptional interplay in cis and trans. Proc. Natl. Acad. Sci. USA 2019, 116, 313–318. [Google Scholar] [CrossRef]
- Ligaba, A.; Katsuhara, M.; Ryan, P.R.; Shibasaka, M.; Matsumoto, H. The BnALMT1 and BnALMT2 genes from rape encode aluminum-activated malate transporters that enhance the aluminum resistance of plant cells. Plant Physiol. 2006, 142, 1294–1303. [Google Scholar] [CrossRef]
- Zhu, X.; Wang, P.; Bai, Z.; Herde, M.; Ma, Y.; Li, N.; Liu, S.; Huang, C.F.; Cui, R.; Ma, H.; et al. Calmodulin-like protein CML24 interacts with CAMTA2 and WRKY46 to regulate ALMT1-dependent Al resistance in Arabidopsis thaliana. New Phytol. 2022, 233, 2471–2487. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, Y.; Cui, W.; Gong, L.; He, Y.; Zhang, Q.; Meng, X.; Yang, Z.; You, J. Characterization of GmMATE13 in its contribution of citrate efflux and aluminum resistance in soybeans. Front. Plant Sci. 2022, 13, 1027560. [Google Scholar] [CrossRef]
- Wen, Z.; Yang, M.; Han, H.; Fazal, A.; Liao, Y.; Ren, R.; Yin, T.; Qi, J.; Sun, S.; Lu, G.; et al. Mycorrhizae Enhance Soybean Plant Growth and Aluminum Stress Tolerance by Shaping the Microbiome Assembly in an Acidic Soil. Microbiol. Spectr. 2023, 11, e0331022. [Google Scholar] [CrossRef]
- Zhang, W.; Xie, Z.; Lang, D.; Cui, J.; Zhang, X. Beneficial effects of silicon on abiotic stress tolerance in legumes. J. Plant Nutr. 2017, 40, 2224–2236. [Google Scholar] [CrossRef]
- Zhu, Y.X.; Gong, H.J.; Yin, J.L. Role of silicon in mediating salt tolerance in plants: A Review. Plants 2019, 8, 147. [Google Scholar] [CrossRef]
- Bhat, J.A.; Shivaraj, S.M.; Singh, P.; Navadagi, D.B.; Tripathi, D.K.; Dash, P.K.; Solanke, A.U.; Sonah, H.; Deshmukh, R. Role of silicon in mitigation of heavy metal stresses in crop plants. Plants 2019, 8, 71. [Google Scholar] [CrossRef]
- Dorneles, A.O.S.; Pereira, A.S.; Sasso, V.M.; Possebom, G.; Tarouco, C.P.; Schorr, M.R.W.; Rossato, L.; Ferreira, P.A.A.; Tabaldi, L.A. Aluminum stress tolerance in potato genotypes grown with silicon. Bragantia 2019, 78, 12–25. [Google Scholar] [CrossRef]
- Souri, Z.; Khanna, K.; Karimi, N.; Ahmad, P. Silicon and Plants: Current Knowledge and Future Prospects. J. Plant Growth Regul. 2021, 40, 906–925. [Google Scholar] [CrossRef]
- Etesami, H.; Jeong, B.R. Silicon (Si): Review and future prospects on the action mechanisms in alleviating biotic and abiotic stresses in plants. Ecotoxicol. Environ. Saf. 2018, 147, 881–896. [Google Scholar] [CrossRef]
- Pavlovic, J.; Kostic, L.; Bosnic, P.; Kirkby, E.A.; Nikolic, M. Interactions of Silicon with Essential and Beneficial Elements in Plants. Front. Plant Sci. 2021, 12, 697592. [Google Scholar] [CrossRef]
- Cheraghi, M.; Motesharezadeh, B.; Mousavi, S.M.; Ma, Q.; Ahmadabadi, Z. Silicon (Si): A Regulator Nutrient for Optimum Growth of Wheat Under Salinity and Drought Stresses—A Review. J. Plant Growth Regul. 2023, 42, 5354–5378. [Google Scholar] [CrossRef]
- Xiao, Z.; Ye, M.; Gao, Z.; Jiang, Y.; Zhang, X.; Nikolic, N.; Liang, Y. Silicon Reduces Aluminum-Induced Suberization by Inhibiting the Uptake and Transport of Aluminum in Rice Roots and Consequently Promotes Root Growth. Plant Cell Physiol. 2022, 63, 340–352. [Google Scholar] [CrossRef]
- Xiao, Z.; Yan, G.; Ye, M.; Liang, Y. Silicon relieves aluminum-induced inhibition of cell elongation in rice root apex by reducing the deposition of aluminum in the cell wall. Plant Soil 2021, 462, 189–205. [Google Scholar] [CrossRef]
- Vega, I.; Nikolic, M.; Pontigo, S.; Godoy, K.; de La Luz Mora, M.; Cartes, P. Silicon improves the production of high antioxidant or structural phenolic compounds in barley cultivars under aluminum stress. Agronomy 2019, 9, 388. [Google Scholar] [CrossRef]
- Kopittke, P.M.; Gianoncelli, A.; Kourousias, G.; Green, K.; McKenna, B.A. Alleviation of Al toxicity by Si Is associated with the formation of Al–Si complexes in root tissues of sorghum. Front. Plant Sci. 2017, 8, 2189. [Google Scholar] [CrossRef]
- de Sousa, A.; Saleh, A.M.; Habeeb, T.H.; Hassan, Y.M.; Zrieq, R.; Wadaan, M.A.M.; Hozzein, W.N.; Selim, S.; Matos, M.; AbdElgawad, H. Silicon dioxide nanoparticles ameliorate the phytotoxic hazards of aluminum in maize grown on acidic soil. Sci. Total Environ. 2019, 693, 133636. [Google Scholar] [CrossRef]
- Kohli, S.K.; Kaur, H.; Khanna, K.; Handa, N.; Bhardwaj, R.; Rinklebe, J.; Ahmad, P. Boron in plants: Uptake, deficiency and biological potential. Plant Growth Regul. 2023, 100, 267–282. [Google Scholar] [CrossRef]
- Yan, L.; Li, S.; Cheng, J.; Zhang, Y.; Jiang, C. Boron-mediated lignin metabolism in response to aluminum toxicity in citrus (Poncirus trifoliata (L.) Raf.) root. Plant Physiol. Biochem. 2022, 185, 1–12. [Google Scholar] [CrossRef]
- Riaz, M.; Yan, L.; Wu, X.; Hussain, S.; Aziz, O.; Jiang, C. Mechanisms of organic acids and boron induced tolerance of aluminum toxicity: A review. Ecotoxicol. Environ. Saf. 2018, 165, 25–35. [Google Scholar] [CrossRef]
- Yan, L.; Riaz, M.; Wu, X.; Du, C.; Liu, Y.; Jiang, C. Ameliorative effects of boron on aluminum induced variations of cell wall cellulose and pectin components in trifoliate orange (Poncirus trifoliate (L.) Raf.) rootstock. Environ. Pollut. 2018, 240, 764–774. [Google Scholar] [CrossRef]
- Yan, L.; Riaz, M.; Du, C.; Liu, Y.; Zeng, Y.; Jiang, C. Ameliorative role of boron to toxicity of aluminum in trifoliate orange roots. Ecotoxicol. Environ. Saf. 2019, 179, 212–221. [Google Scholar] [CrossRef]
- Yan, L.; Riaz, M.; Liu, J.; Liu, Y.; Zeng, Y.; Jiang, C. Boron reduces aluminum deposition in alkali-soluble pectin and cytoplasm to release aluminum toxicity. J. Hazard. Mater. 2021, 401, 123388. [Google Scholar] [CrossRef]
- Zhu, C.Q.; Cao, X.C.; Zhu, L.F.; Hu, W.J.; Hu, A.Y.; Abliz, B.; Bai, Z.G.; Huang, J.; Liang, Q.D.; Sajid, H.; et al. Boron reduces cell wall aluminum content in rice (Oryza sativa) roots by decreasing H 2 O 2 accumulation. Plant Physiol. Biochem. 2019, 138, 80–90. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, M.; Feng, F.; Tian, Z. Toward a “Green Revolution” for Soybean. Mol. Plant 2020, 13, 688–697. [Google Scholar] [CrossRef]
- Wang, H.; Yin, X.; Du, D.; Liang, Z.; Han, Z.; Nian, H.; Ma, Q. GsMYB7 encoding a R2R3-type MYB transcription factor enhances the tolerance to aluminum stress in soybean (Glycine max L.). BMC Genom. 2022, 23, 529. [Google Scholar] [CrossRef]
- Sade, H.; Meriga, B.; Surapu, V.; Gadi, J.; Sunita, M.S.L.; Suravajhala, P.; Kavi Kishor, P.B. Toxicity and tolerance of aluminum in plants: Tailoring plants to suit to acid soils. Biometals 2016, 29, 187–210. [Google Scholar] [CrossRef]
- He, H.; Li, Y.; He, L.F. Aluminum toxicity and tolerance in Solanaceae plants. S. Afr. J. Bot. 2019, 123, 23–29. [Google Scholar] [CrossRef]
- Bilal, S.; Khan, A.; Imran, M.; Khan, A.L.; Asaf, S.; Al-Rawahi, A.; Al-Azri, M.S.A.; Al-Harrasi, A.; Lee, I.J. Silicon-and Boron-Induced Physio-Biochemical Alteration and Organic Acid Regulation Mitigates Aluminum Phytotoxicity in Date Palm Seedlings. Antioxidants 2022, 11, 1063. [Google Scholar] [CrossRef]
- He, H.; Huang, W.; Oo, T.L.; Gu, M.; He, L.F. Nitric oxide inhibits aluminum-induced programmed cell death in peanut (Arachis hypoganea L.) root tips. J. Hazard. Mater. 2017, 333, 285–292. [Google Scholar] [CrossRef]
- Huang, J.; Han, R.; Ji, F.; Yu, Y.; Wang, R.; Hai, Z.; Liang, W.; Wang, H. Glucose-6-phosphate dehydrogenase and abscisic acid mediate programmed cell death induced by aluminum toxicity in soybean root tips. J. Hazard. Mater. 2022, 425, 127964. [Google Scholar] [CrossRef]
- Pontigo, S.; Godoy, K.; Jiménez, H.; Gutiérrez-Moraga, A.; Mora, M.D.L.L.; Cartes, P. Silicon-mediated alleviation of aluminum toxicity by modulation of al/si uptake and antioxidant performance in ryegrass plants. Front. Plant Sci. 2017, 8, 642. [Google Scholar] [CrossRef]
- Yan, L.; Li, S.; Cheng, J.; Liu, Y.; Liu, J.; Jiang, C. Boron contributes to excessive aluminum tolerance in trifoliate orange (Poncirus trifoliata (L.) Raf.) by inhibiting cell wall deposition and promoting vacuole compartmentation. J. Hazard. Mater. 2022, 437, 129275. [Google Scholar] [CrossRef]
- Liu, C.; Cheng, H.; Wang, S.; Yu, D.; Wei, Y. Physiological and Transcriptomic Analysis Reveals That Melatonin Alleviates Aluminum Toxicity in Alfalfa (Medicago sativa L.). Int. J. Mol. Sci. 2023, 24, 17221. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Cheng, H.; Wei, Y. Supplemental Silicon and Boron Alleviates Aluminum-Induced Oxidative Damage in Soybean Roots. Plants 2024, 13, 821. https://doi.org/10.3390/plants13060821
Wang S, Cheng H, Wei Y. Supplemental Silicon and Boron Alleviates Aluminum-Induced Oxidative Damage in Soybean Roots. Plants. 2024; 13(6):821. https://doi.org/10.3390/plants13060821
Chicago/Turabian StyleWang, Shuwei, Haijing Cheng, and Yunmin Wei. 2024. "Supplemental Silicon and Boron Alleviates Aluminum-Induced Oxidative Damage in Soybean Roots" Plants 13, no. 6: 821. https://doi.org/10.3390/plants13060821
APA StyleWang, S., Cheng, H., & Wei, Y. (2024). Supplemental Silicon and Boron Alleviates Aluminum-Induced Oxidative Damage in Soybean Roots. Plants, 13(6), 821. https://doi.org/10.3390/plants13060821






