Biochemical Characterization of the Seed Quality of a Collection of White Lupin Landraces from Southern Italy
Abstract
1. Introduction
2. Results and Discussion
2.1. Samples Description
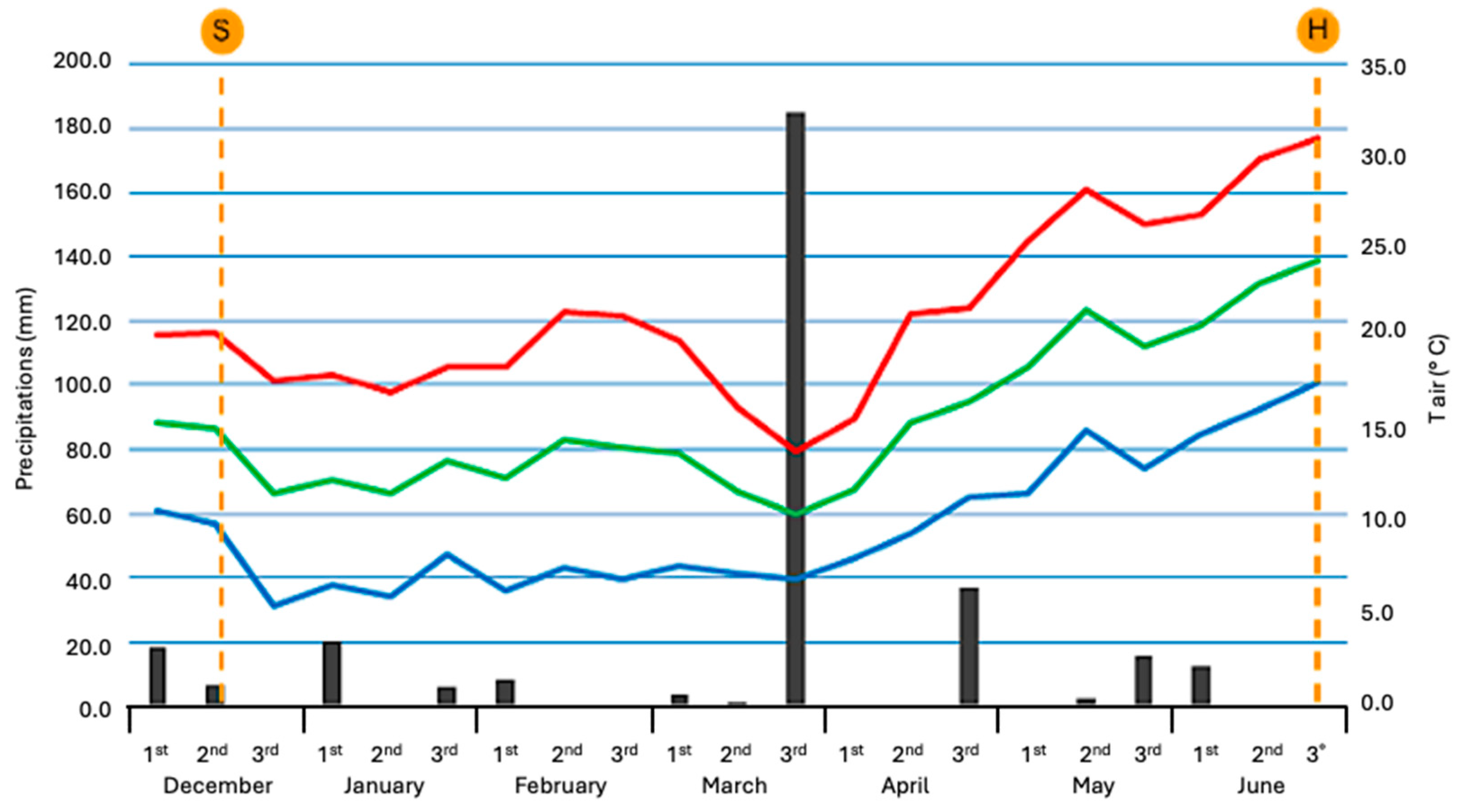
2.2. Description of the Main Climatic Data
2.3. Plants Phenological Features
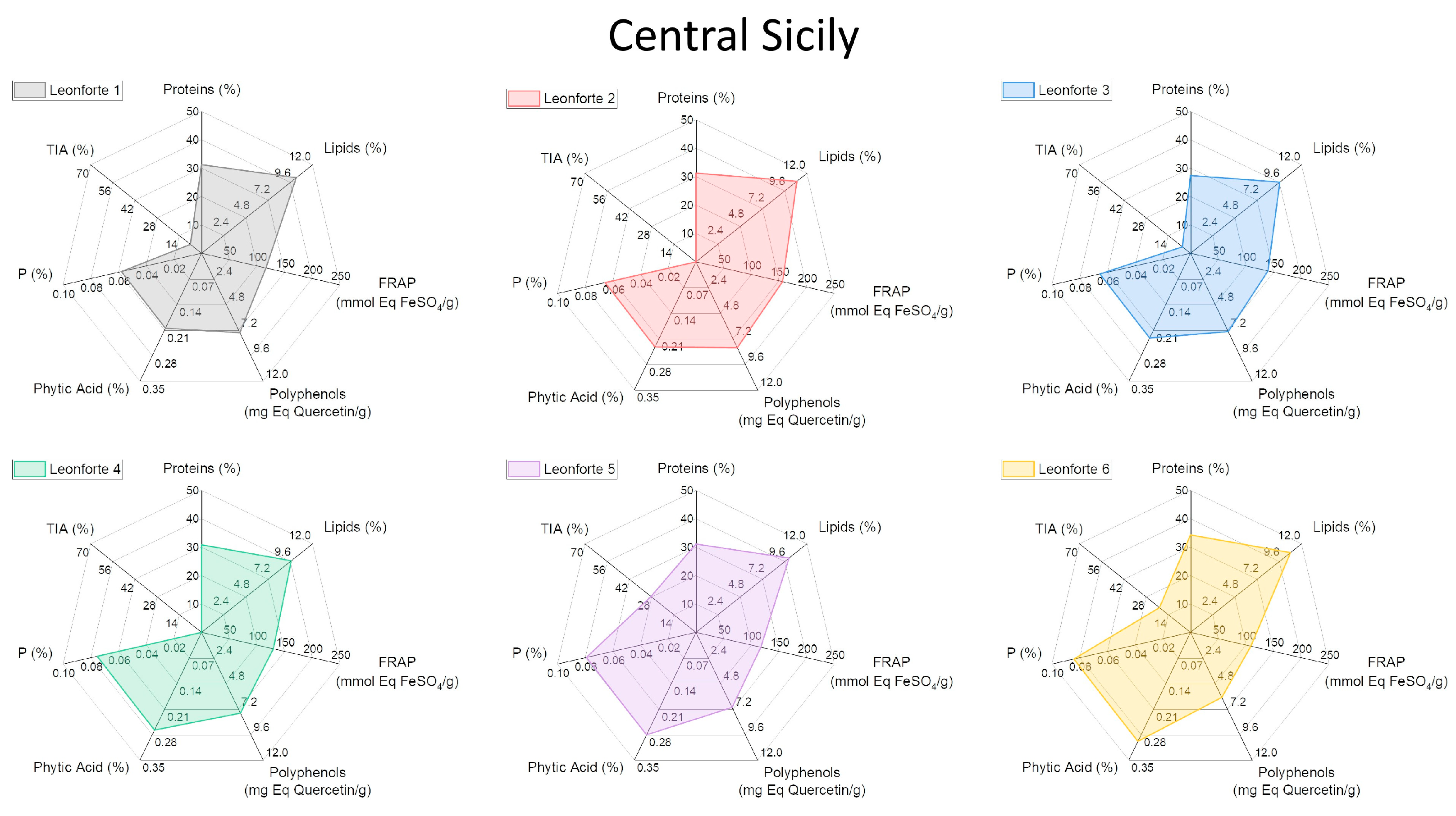
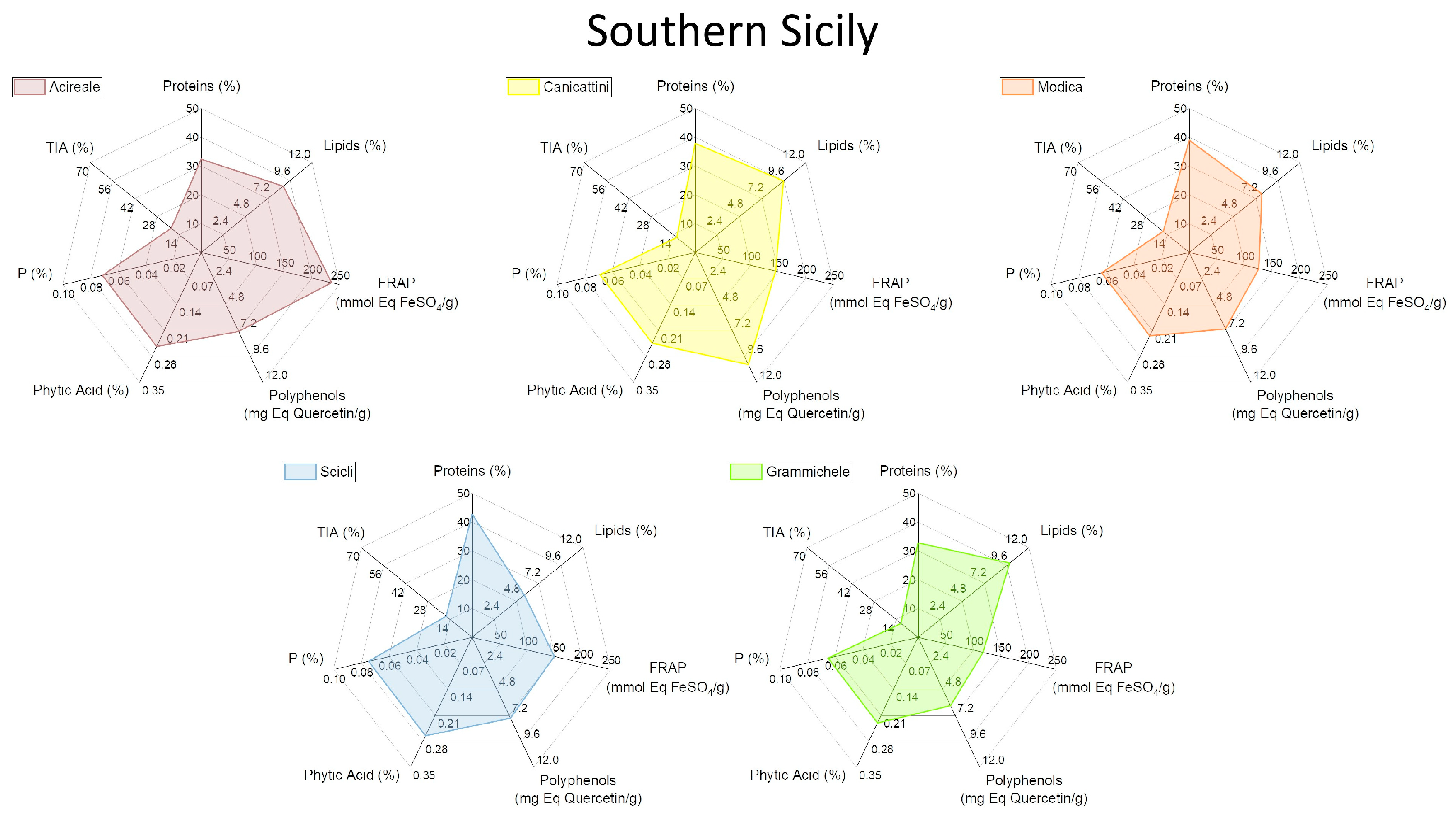
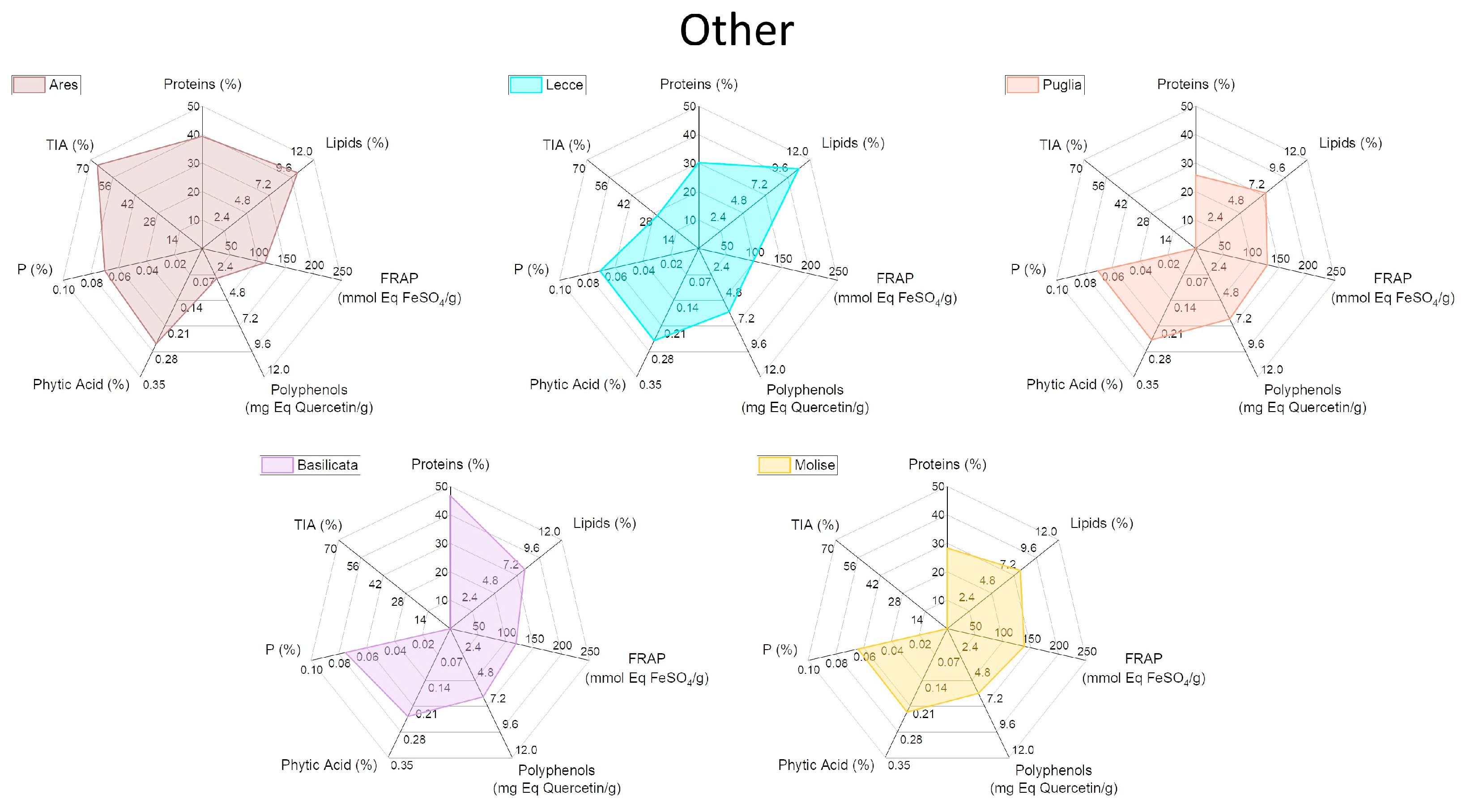
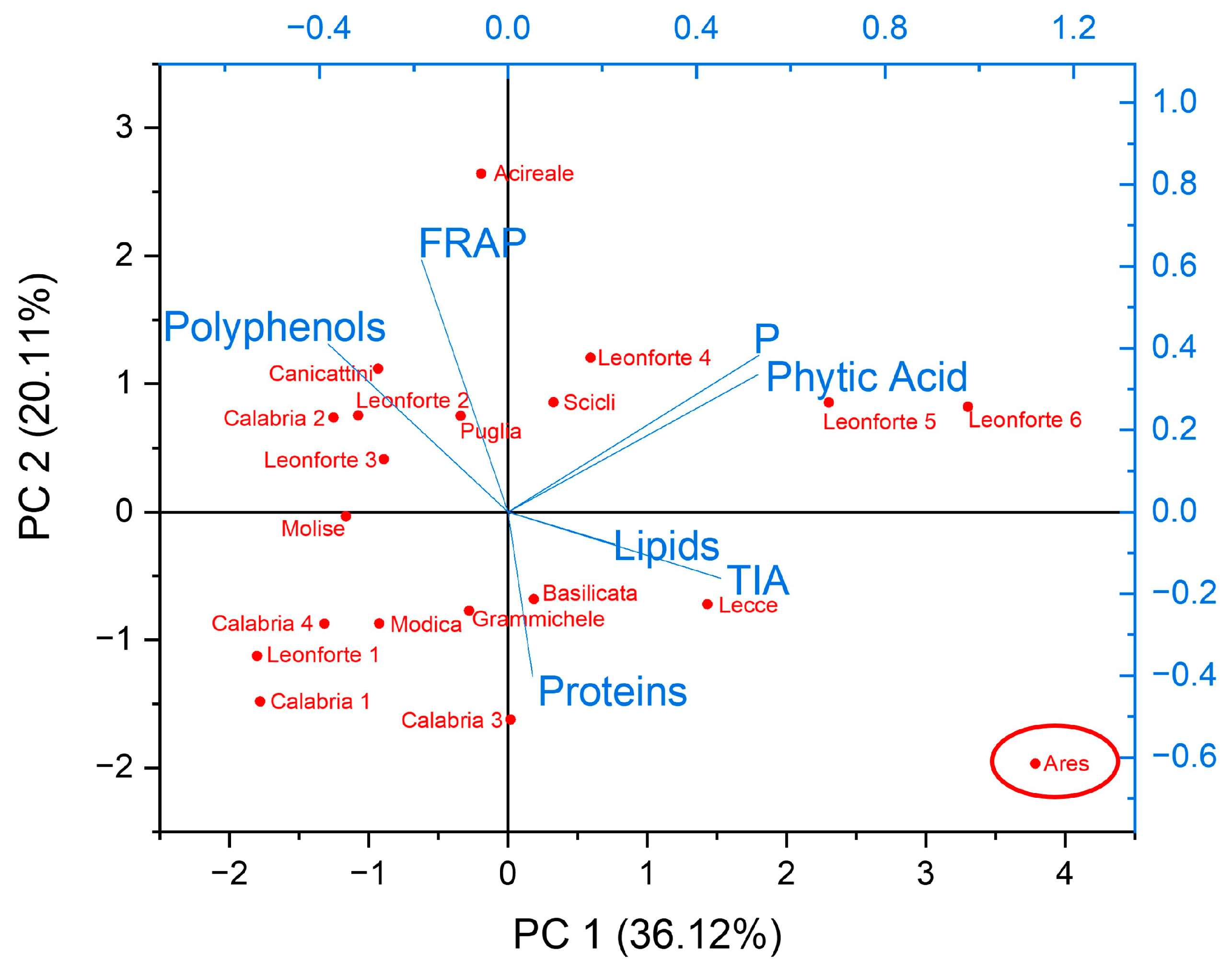
2.4. Characterization of Biochemical and Nutritional Features
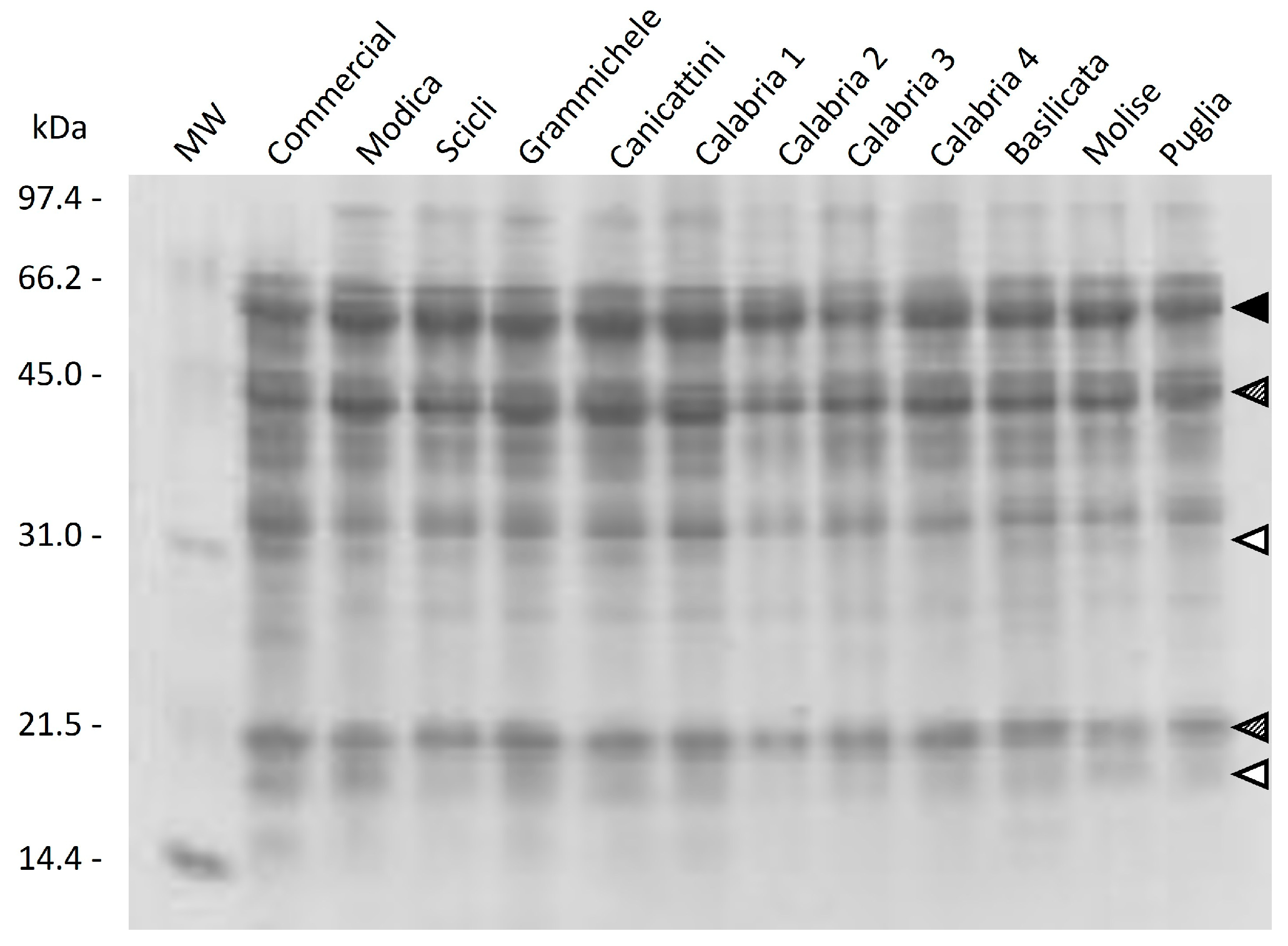
2.5. Qualitative Protein Characterization
3. Materials and Methods
3.1. Plant Material and Sampling
3.2. Experimental Site and Climatic Details
3.3. Plants Phenological Measurements
3.4. Protein Extraction and Quantification
3.5. SDS-PAGE
3.6. Lipids Quantification
3.7. Trypsin Inhibitor Activity
3.8. Reducing Activity
3.9. Phytic Acid and Phosphorus Content Assay
3.10. Total Phenolic Extraction and Quantification
3.11. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Considine, M.J.; Siddique, K.H.M.; Foyer, C.H. Nature’s Pulse Power: Legumes, Food Security and Climate Change. J. Exp. Bot. 2017, 68, 1815. [Google Scholar] [CrossRef] [PubMed]
- Parry, M.L.; Rosenzweig, C.; Iglesias, A.; Livermore, M.; Fischer, G. Effects of Climate Change on Global Food Production under SRES Emissions and Socio-Economic Scenarios. Glob. Environ. Chang. 2004, 14, 53–67. [Google Scholar] [CrossRef]
- Kemp, J.; Lotter, D.; Meyer, A.; Kleinert, A.; Pérez-Fernández, M.; Valentine, A. Variation in Rhizosphere Nutrient Cycling Affects the Source of Nitrogen Acquisition in Wild and Cultivated Aspalathus Linearis (N.L. Burm.) R. Dahlgren Plants. Appl. Soil Ecol. 2018, 130, 26–33. [Google Scholar] [CrossRef]
- Rabara, R.C.; Tripathi, P.; Rushton, P.J. Comparative Metabolome Profile between Tobacco and Soybean Grown under Water-Stressed Conditions. BioMed Res. Int. 2017, 2017, 3065251. [Google Scholar] [CrossRef] [PubMed]
- Classen, A.T.; Sundqvist, M.K.; Henning, J.A.; Newman, G.S.; Moore, J.A.M.; Cregger, M.A.; Moorhead, L.C.; Patterson, C.M. Direct and Indirect Effects of Climate Change on Soil Microbial and Soil Microbial-Plant Interactions: What Lies Ahead? Ecosphere 2015, 6, 1–21. [Google Scholar] [CrossRef]
- Ravasi, R.A.; Paleari, L.; Vesely, F.M.; Movedi, E.; Thoelke, W.; Confalonieri, R. Ideotype Definition to Adapt Legumes to Climate Change: A Case Study for Field Pea in Northern Italy. Agric. For. Meteorol. 2020, 291, 108081. [Google Scholar] [CrossRef]
- Singh, M.; Kumar, S.; Basandrai, A.K.; Basandrai, D.; Malhotra, N.; Saxena, D.R.; Gupta, D.; Sarker, A.; Singh, K. Evaluation and Identification of Wild Lentil Accessions for Enhancing Genetic Gains of Cultivated Varieties. PLoS ONE 2020, 15, e0229554. [Google Scholar] [CrossRef]
- Wang, X.; Guo, R.; Li, M.; Liu, Y.; Zhao, M.; Fu, H.; Liu, X.; Wang, S.; Shi, L. Metabolomics Reveals the Drought-Tolerance Mechanism in Wild Soybean (Glycine soja). Acta Physiol. Plant. 2019, 41, 161. [Google Scholar] [CrossRef]
- Karamać, M.; Orak, H.H.; Amarowicz, R.; Orak, A.; Piekoszewski, W. Phenolic Contents and Antioxidant Capacities of Wild and Cultivated White Lupin (Lupinus albus L.) Seeds. Food. Chem. 2018, 258, 1–7. [Google Scholar] [CrossRef]
- Yang, G.; Roy, J.; Veresoglou, S.D.; Rillig, M.C. Soil Biodiversity Enhances the Persistence of Legumes under Climate Change. New Phytol. 2021, 229, 2945–2956. [Google Scholar] [CrossRef]
- Okagu, I.U.; Ndefo, J.C.; Aham, E.C.; Obeme-Nmom, J.I.; Agboinghale, P.E.; Aguchem, R.N.; Nechi, R.N.; Lammi, C. Lupin-Derived Bioactive Peptides: Intestinal Transport, Bioavailability and Health Benefits. Nutrients 2021, 13, 3266. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-López, M.A.; Barrientos-Ramírez, L.; García-López, P.M.; Valdés-Miramontes, E.H.; Zamora-Natera, J.F.; Rodríguez-Macias, R.; Salcedo-Pérez, E.; Bañuelos-Pineda, J.; Vargas-Radillo, J.J. Nutritional and Bioactive Compounds in Mexican Lupin Beans Species: A Mini-Review. Nutrients 2019, 11, 1785. [Google Scholar] [CrossRef] [PubMed]
- Magni, C.; Scarafoni, A.; Herndl, A.; Sessa, F.; Prinsi, B.; Espen, L.; Duranti, M. Combined 2D Electrophoretic Approaches for the Study of White Lupin Mature Seed Storage Proteome. Phytochemistry 2007, 68, 997–1007. [Google Scholar] [CrossRef] [PubMed]
- Foley, R.C.; Gao, L.L.; Spriggs, A.; Soo, L.Y.C.; Goggin, D.E.; Smith, P.M.C.; Atkins, C.A.; Singh, K.B. Identification and Characterisation of Seed Storage Protein Transcripts from Lupinus angustifolius. BMC Plant Biol. 2011, 11, 59. [Google Scholar] [CrossRef] [PubMed]
- Restani, P.; Duranti, M.; Cerletti, P.; Simonetti, P. Subunit Composition of the Seed Globulins of Lupinus albus. Phytochemistry 1981, 20, 2077–2083. [Google Scholar] [CrossRef]
- Jimenez-Lopez, J.C. Narrow-Leafed Lupin (Lupinus angustifolius L.) β-Conglutin: A Multifunctional Family of Proteins with Roles in Plant Defence, Human Health Benefits, and Potential Uses as Functional Food. Legum. Sci. 2020, 2, e33. [Google Scholar] [CrossRef]
- Jimenez-Lopez, J.C.; Melser, S.; DeBoer, K.; Thatcher, L.F.; Kamphuis, L.G.; Foley, R.C.; Singh, K.B. Narrow-Leafed Lupin (Lupinus angustifolius) Β1- and Β6-Conglutin Proteins Exhibit Antifungal Activity, Protecting Plants against Necrotrophic Pathogen Induced Damage from Sclerotinia sclerotiorum and Phytophthora nicotianae. Front. Plant Sci. 2016, 7, 1856. [Google Scholar] [CrossRef]
- Heinzl, G.C.; Tretola, M.; De Benedetti, S.; Silacci, P.; Scarafoni, A. Lupinus albus γ-Conglutin: New Findings about Its Action at the Intestinal Barrier and a Critical Analysis of the State of the Art on Its Postprandial Glycaemic Regulating Activity. Nutrients 2022, 14, 3666. [Google Scholar] [CrossRef]
- Magni, C.; Sessa, F.; Accardo, E.; Vanoni, M.; Morazzoni, P.; Scarafoni, A.; Duranti, M. Conglutin γ, a Lupin Seed Protein, Binds Insulin In Vitro and Reduces Plasma Glucose Levels of Hyperglycemic Rats. J. Nutr. Biochem. 2004, 15, 646–650. [Google Scholar] [CrossRef]
- Lima-Cabello, E.; Alche, V.; Foley, R.C.; Andrikopoulos, S.; Morahan, G.; Singh, K.B.; Alche, J.D.; Jimenez-Lopez, J.C. Narrow-Leafed Lupin (Lupinus angustifolius L.) β-Conglutin Proteins Modulate the Insulin Signaling Pathway as Potential Type 2 Diabetes Treatment and Inflammatory-Related Disease Amelioration. Mol. Nutr. Food Res. 2017, 61, 1600819. [Google Scholar] [CrossRef]
- Souza Cândido, E.; Pinto, M.F.S.; Pelegrini, P.B.; Lima, T.B.; Silva, O.N.; Pogue, R.; Grossi-de-Sá, M.F.; Franco, O.L. Plant Storage Proteins with Antimicrobial Activity: Novel Insights into Plant Defense Mechanisms. FASEB J. 2011, 25, 3290–3305. [Google Scholar] [CrossRef] [PubMed]
- Zafeiriou, I.; Polidoros, A.N.; Baira, E.; Kasiotis, K.M.; Machera, K.; Mylona, P.V. Mediterranean White Lupin Landraces as a Valuable Genetic Reserve for Breeding. Plants 2021, 10, 2403. [Google Scholar] [CrossRef] [PubMed]
- Harouna, D.V.; Venkataramana, P.B.; Ndakidemi, P.A.; Matemu, A.O. Under-Exploited Wild Vigna Species Potentials in Human and Animal Nutrition: A Review. Glob. Food Secur. 2018, 18, 1–11. [Google Scholar] [CrossRef]
- Romeo, F.V.; Fabroni, S.; Ballistreri, G.; Muccilli, S.; Spina, A.; Rapisarda, P. Characterization and Antimicrobial Activity of Alkaloid Extracts from Seeds of Different Genotypes of Lupinus spp. Sustainability 2018, 10, 788. [Google Scholar] [CrossRef]
- Duranti, M.; Consonni, A.; Magni, C.; Sessa, F.; Scarafoni, A. The Major Proteins of Lupin Seed: Characterisation and Molecular Properties for Use as Functional and Nutraceutical Ingredients. Trends Food Sci. Technol. 2008, 19, 624–633. [Google Scholar] [CrossRef]
- Siger, A.; Grygier, A.; Czubinski, J. Comprehensive Characteristic of Lipid Fraction as a Distinguishing Factor of Three Lupin Seed Species. J. Food Compos. 2023, 115, 104945. [Google Scholar] [CrossRef]
- Estivi, L.; Brandolini, A.; Gasparini, A.; Hidalgo, A. Lupin as a Source of Bioactive Antioxidant Compounds for Food Products. Molecules 2023, 28, 7529. [Google Scholar] [CrossRef] [PubMed]
- Erbas, M.; Certel, M.; Uslu, M.K. Some Chemical Properties of White Lupin Seeds (Lupinus albus L.). Food Chem. 2004, 89, 341–345. [Google Scholar] [CrossRef]
- Egana, J.I.; Uauy, R.; Cassorla, X.; Barrera, G.; Yanez, E. Sweet Lupin Protein Quality in Young Men. J. Nutr. 1992, 122, 2341–2347. [Google Scholar] [CrossRef]
- Medic, J.; Atkinson, C.; Hurburgh, C.R. Current Knowledge in Soybean Composition. J. Am. Oil Chem. Soc. 2014, 91, 363–384. [Google Scholar] [CrossRef]
- Rybiński, W.; Święcicki, W.; Bocianowski, J.; Börner, A.; Starzycka-Korbas, E.; Starzycki, M. Variability of Fat Content and Fatty Acids Profiles in Seeds of a Polish White Lupin (Lupinus albus L.) Collection. Genet. Resour. Crop. Evol. 2018, 65, 417–431. [Google Scholar] [CrossRef]
- Lampart-Szczapa, E.; Korczak, J.; Nogala-Kalucka, M.; Zawirska-Wojtasiak, R. Antioxidant Properties of Lupin Seed Products. Food Chem. 2003, 83, 279–285. [Google Scholar] [CrossRef]
- Pastor-Cavada, E.; Juan, R.; Pastor, J.E.; Alaiz, M.; Vioque, J. Antioxidant Activity in the Seeds of Four Wild Lupinus Species from Southern Spain. J. Food Biochem. 2010, 34, 149–160. [Google Scholar] [CrossRef]
- Król, A.; Amarowicz, R.; Weidner, S. Content of Phenolic Compounds and Antioxidant Properties in Seeds of Sweet and Bitter Cultivars of Lupine (Lupinus angustifolius). Nat. Prod. Commun. 2018, 13, 1934578X1801301027. [Google Scholar] [CrossRef]
- Siger, A.; Czubinski, J.; Kachlicki, P.; Dwiecki, K.; Lampart-Szczapa, E.; Nogala-Kalucka, M. Antioxidant Activity and Phenolic Content in Three Lupin Species. J. Food Compos. Anal. 2012, 25, 190–197. [Google Scholar] [CrossRef]
- Reddy, N.R.; Sathe, S.K.; Salunkhe, D.K. Phytates in Legumes and Cereals. Adv. Food Res. 1982, 28, 1–92. [Google Scholar] [CrossRef]
- Sinkovič, L.; Pipan, B.; Šibul, F.; Nemeš, I.; Tepić Horecki, A.; Meglič, V. Nutrients, Phytic Acid and Bioactive Compounds in Marketable Pulses. Plants 2023, 12, 170. [Google Scholar] [CrossRef]
- Avilés-Gaxiola, S.; Chuck-Hernández, C.; Serna Saldívar, S.O. Inactivation Methods of Trypsin Inhibitor in Legumes: A Review. J. Food Sci. 2018, 83, 17–29. [Google Scholar] [CrossRef]
- Duranti, M.; Gius, C. Field Crops Research Legume Seeds: Protein Content and Nutritional Value. Field Crops Res. 1997, 53, 31–45. [Google Scholar] [CrossRef]
- Spina, A.; Saletti, R.; Fabroni, S.; Natalello, A.; Cunsolo, V.; Scarangella, M.; Rapisarda, P.; Canale, M.; Muccilli, V. Multielemental, Nutritional, and Proteomic Characterization of Different Lupinus spp. Genotypes: A Source of Nutrients for Dietary Use. Molecules 2022, 27, 8771. [Google Scholar] [CrossRef]
- Błażejczyk, K.; Baranowski, J.; Jendritzky, J.; Błażejczyk, A.; Bröde, P.; Fiala, D. Regional features of the bioclimate of central and southern Europe against the background of the Köppen Geiger climate classification. Geogr. Pol. 2015, 88, 439–453. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Laemmli, U.K. Cleavage of Structural Proteins during the Assembly of the Head of Bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- ISO 14902:2001; Animal Feeding Stuffs—Determination of Trypsin Inhibitor Activity of Soya Products. International Organization for Standardization: Geneva, Switzerland, 2001.
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Rossi, J.A.J.; Singleton, V.L. Colorimetry of Total Phenolics with Phosphomolybdic Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
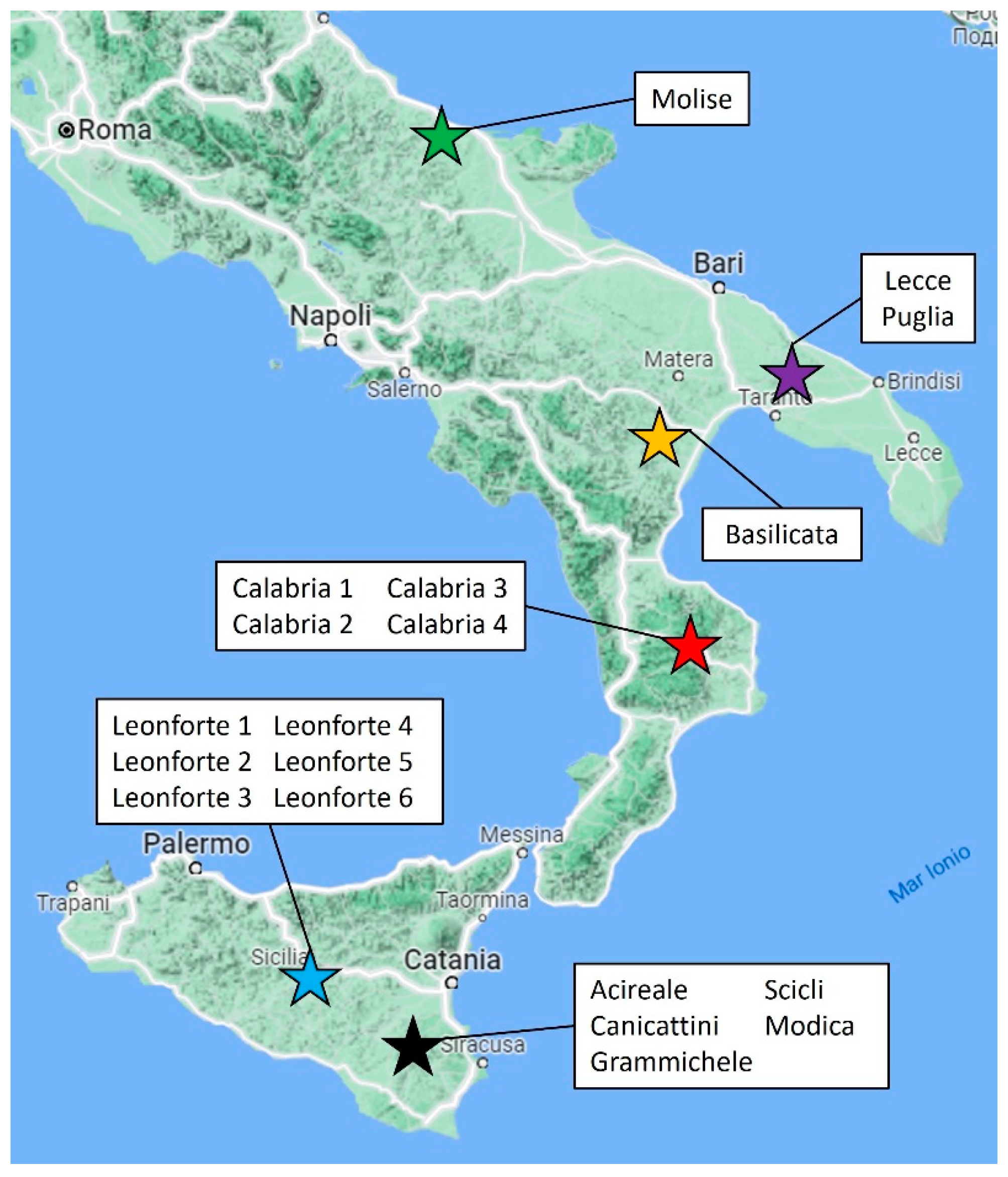

| Name | Type | Region/Province of Collection |
|---|---|---|
| Leonforte 1 | Landrace | Enna (Sicily, Italy) |
| Leonforte 2 | Landrace | Enna (Sicily, Italy) |
| Leonforte 3 | Landrace | Enna (Sicily, Italy) |
| Leonforte 4 | Landrace | Enna (Sicily, Italy) |
| Leonforte 5 | Landrace | Enna (Sicily, Italy) |
| Leonforte 6 | Ecotype | Enna (Sicily, Italy) |
| Acireale | Landrace | Catania (Italy) |
| Canicattini | Landrace | Siracusa (Sicily, Italy) |
| Modica | Landrace | Ragusa (Sicily, Italy) |
| Scicli | Landrace | Ragusa (Sicily, Italy) |
| Grammichele | Landrace | Catania (Sicily, Italy) |
| Calabria 1 | Landrace | Calabria (Italy) |
| Calabria 2 | Landrace | Calabria (Italy) |
| Calabria 3 | Landrace | Calabria (Italy) |
| Calabria 4 | Landrace | Calabria (Italy) |
| Puglia | Landrace | Apulia (Italy) |
| Lecce | Landrace | Apulia (Italy) |
| Basilicata | Landrace | Basilicata (Italy) |
| Molise | Landrace | Molise (Italy) |
| Ares | Commercial | Nd |
| Sample | End Germination–Beginning Plant Emergence | Main Stem Flowering | Main Pods Growth | Pod and Seed Maturity |
|---|---|---|---|---|
| Leonforte 1 | 12.0 ± 0.00 bc | 93.0 ± 0.00 gh | 106.5 ± 0.71 ghi | 175.0 ± 0.00 def |
| Leonforte 2 | 11.5 ± 0.71 bc | 89.5 ± 0.71 hi | 103.0 ± 1.41 hi | 174.5 ± 0.71 ef |
| Leonforte 3 | 13.0 ± 0.00 abc | 103.5 ± 0.71 e | 118.5 ± 0.71 cdef | 176.0 ± 0.00 cdef |
| Leonforte 4 | 12.5 ± 0.71 abc | 96.0 ± 0.00 g | 111.0 ± 0.00 g | 175.0 ± 0.00 def |
| Leonforte 5 | 12.0 ± 0.00 bc | 91.5 ± 0.71 hi | 107.5 ± 0.71 gh | 175.0 ± 0.00 def |
| Leonforte 6 | 11.0 ± 0.00 c | 80.5 ± 0.71 jk | 95.5 ± 0.71 j | 174.0 ± 0.00 f |
| Acireale | 11.0 ± 0.00 c | 81.5 ± 0.71 j | 97.5 ± 0.71 j | 174.0 ± 0.00 f |
| Canicattini | 11.0 ± 0.00 c | 80.0 ± 0.00 jk | 95.0 ± 0.00 j | 174.0 ± 0.00 f |
| Modica | 12.0 ± 0.00 bc | 93.5 ± 0.71 gh | 106.5 ± 0.71 ghi | 175.0 ± 0.00 def |
| Scicli | 13.0 ± 0.00 abc | 104.5 ± 0.71 de | 117.5 ± 0.71 ef | 176.5 ± 0.71 bcde |
| Grammichele | 11.0 ± 0.00 c | 77.5 ± 0.71 k | 90.5 ± 0.71 k | 174.0 ± 0.00 f |
| Calabria 1 | 13.0 ± 0.00 abc | 106.5 ± 0.71 cde | 119.5 ± 0.71 bcde | 176.5 ± 0.71 bcde |
| Calabria 2 | 13.0 ± 0.00 abc | 104.0 ± 0.71 e | 118.0 ± 0.71 def | 175.5 ± 0.71 def |
| Calabria 3 | 15.0 ± 0.00 a | 111.5 ± 0.71 a | 121.5 ± 0.71 abcd | 180.0 ± 0.00 a |
| Calabria 4 | 13.0 ± 0.00 abc | 107.5 ± 0.71 cd | 122.5 ± 0.71 ab | 177.0 ± 0.00 bcd |
| Puglia | 14.0 ± 1.41 ab | 108.0 ± 0.00 b | 123.0 ± 0.00 ab | 178.0 ± 0.00 abc |
| Lecce | 13.0 ± 0.00 abc | 106.0 ± 0.00 cde | 122.0 ± 0.00 abc | 176.0 ± 0.00 cdef |
| Basilicata | 12.5 ± 0.71 abc | 99.5 ± 0.71 f | 115.5 ± 0.71 f | 175.0 ± 0.00 def |
| Molise | 15.0 ± 0.00 a | 109.0 ± 0.00 ab | 124.0 ± 0.00 a | 178.5 ± 0.71 ab |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spina, A.; De Benedetti, S.; Heinzl, G.C.; Ceravolo, G.; Magni, C.; Emide, D.; Castorina, G.; Consonni, G.; Canale, M.; Scarafoni, A. Biochemical Characterization of the Seed Quality of a Collection of White Lupin Landraces from Southern Italy. Plants 2024, 13, 785. https://doi.org/10.3390/plants13060785
Spina A, De Benedetti S, Heinzl GC, Ceravolo G, Magni C, Emide D, Castorina G, Consonni G, Canale M, Scarafoni A. Biochemical Characterization of the Seed Quality of a Collection of White Lupin Landraces from Southern Italy. Plants. 2024; 13(6):785. https://doi.org/10.3390/plants13060785
Chicago/Turabian StyleSpina, Alfio, Stefano De Benedetti, Giuditta Carlotta Heinzl, Giulia Ceravolo, Chiara Magni, Davide Emide, Giulia Castorina, Gabriella Consonni, Michele Canale, and Alessio Scarafoni. 2024. "Biochemical Characterization of the Seed Quality of a Collection of White Lupin Landraces from Southern Italy" Plants 13, no. 6: 785. https://doi.org/10.3390/plants13060785
APA StyleSpina, A., De Benedetti, S., Heinzl, G. C., Ceravolo, G., Magni, C., Emide, D., Castorina, G., Consonni, G., Canale, M., & Scarafoni, A. (2024). Biochemical Characterization of the Seed Quality of a Collection of White Lupin Landraces from Southern Italy. Plants, 13(6), 785. https://doi.org/10.3390/plants13060785









