Genome-Wide Identification and Expression Analysis of the PUB Gene Family in Zoysia japonica under Salt Stress
Abstract
1. Introduction
2. Results
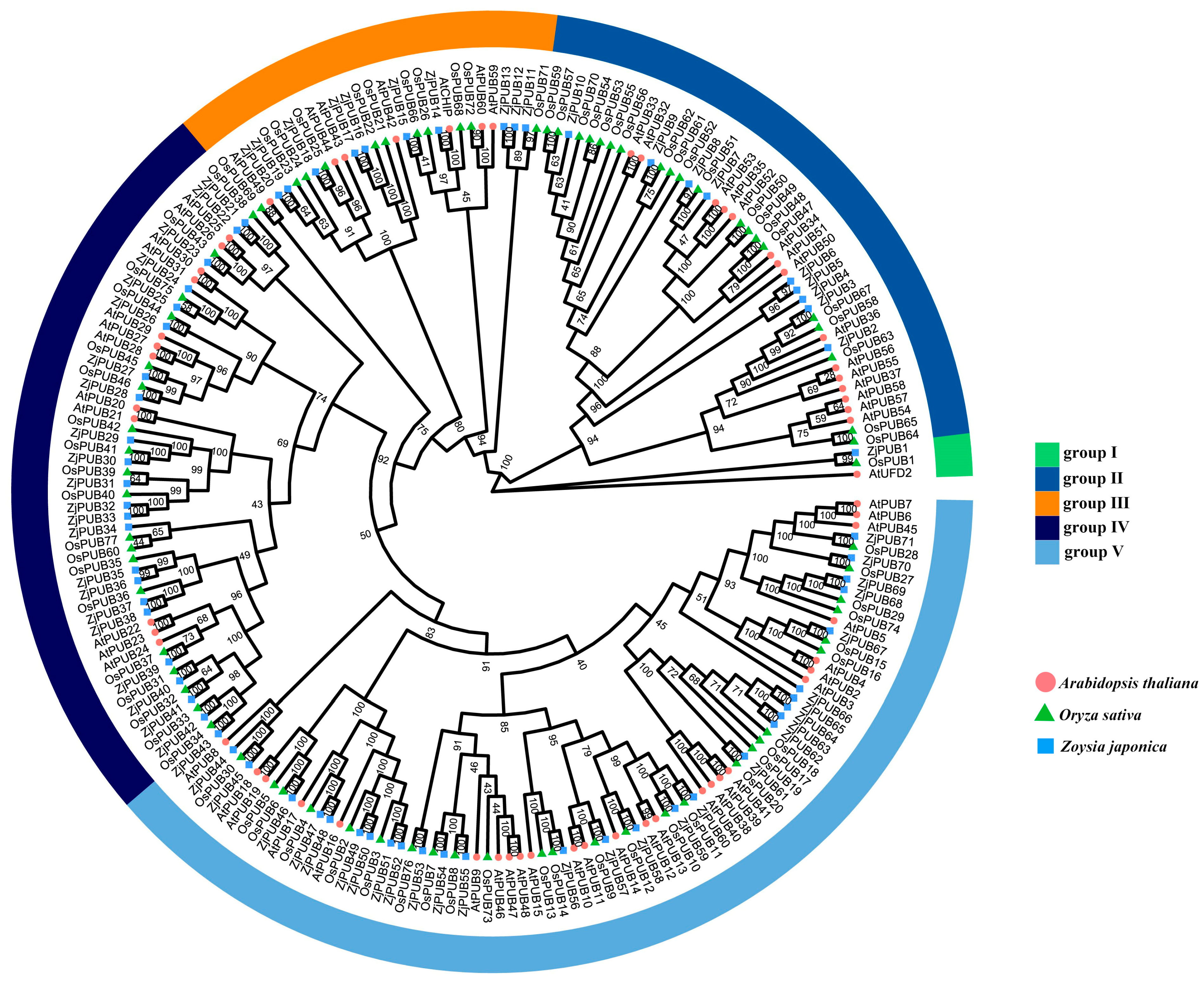
2.1. Identification and Protein Tree Construction of the PUB Gene Family in Z. japonica
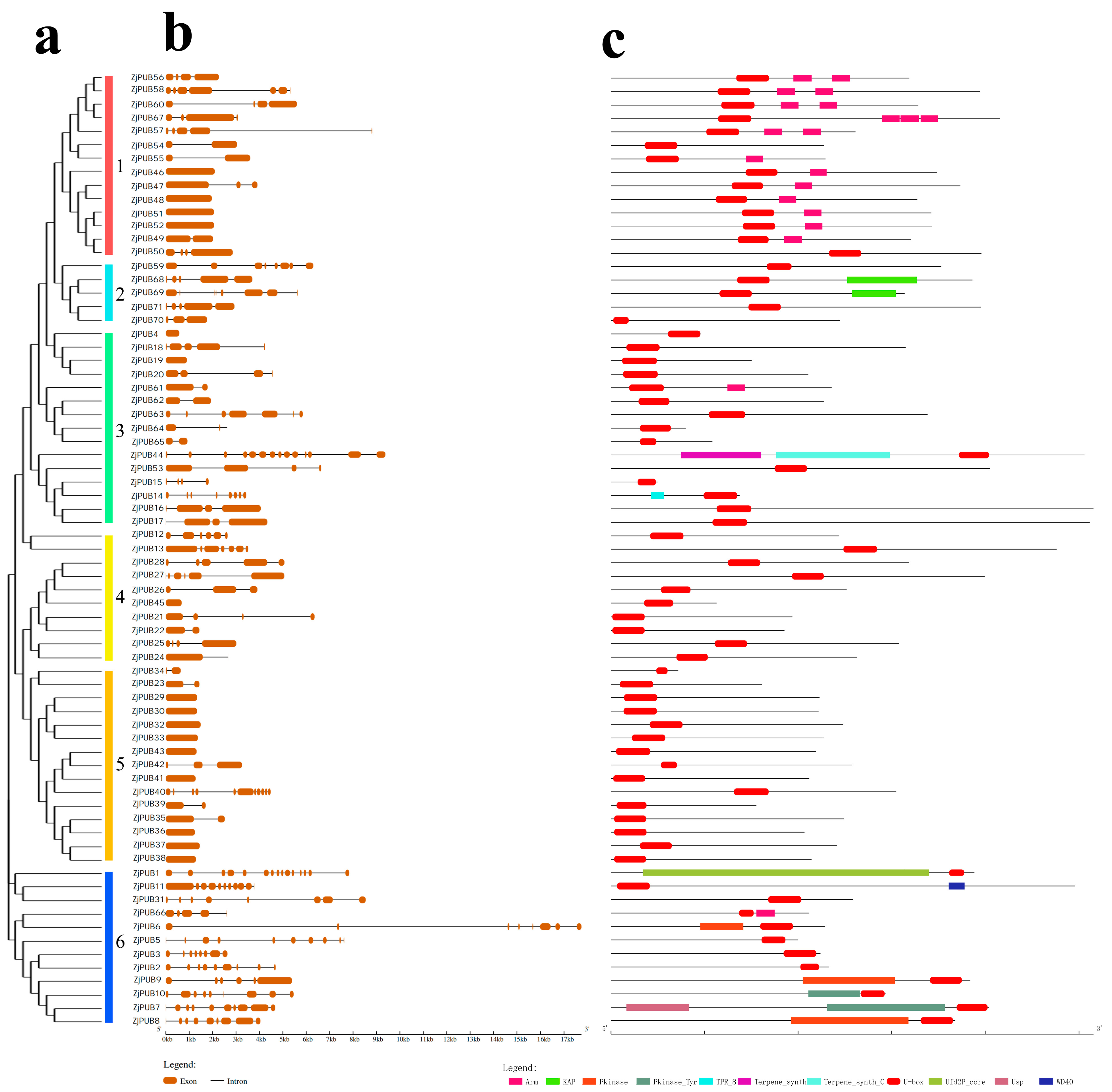
2.2. Characteristics, Gene Structure and Domain Analysis of PUB Genes in Z. japonica
2.3. Cis-Acting Regulatory Element Prediction in Promoter Regions of ZjPUB Family Members
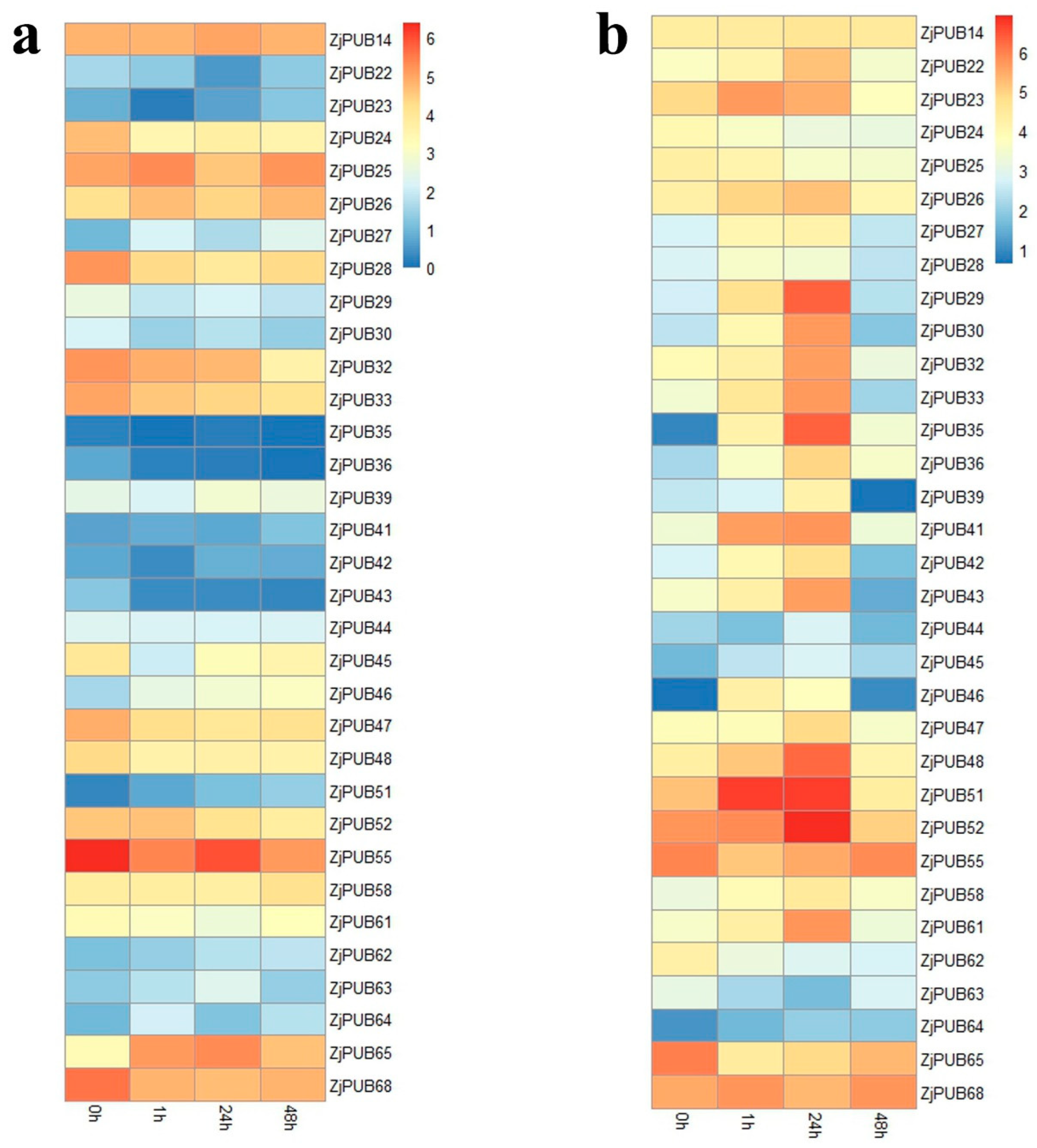
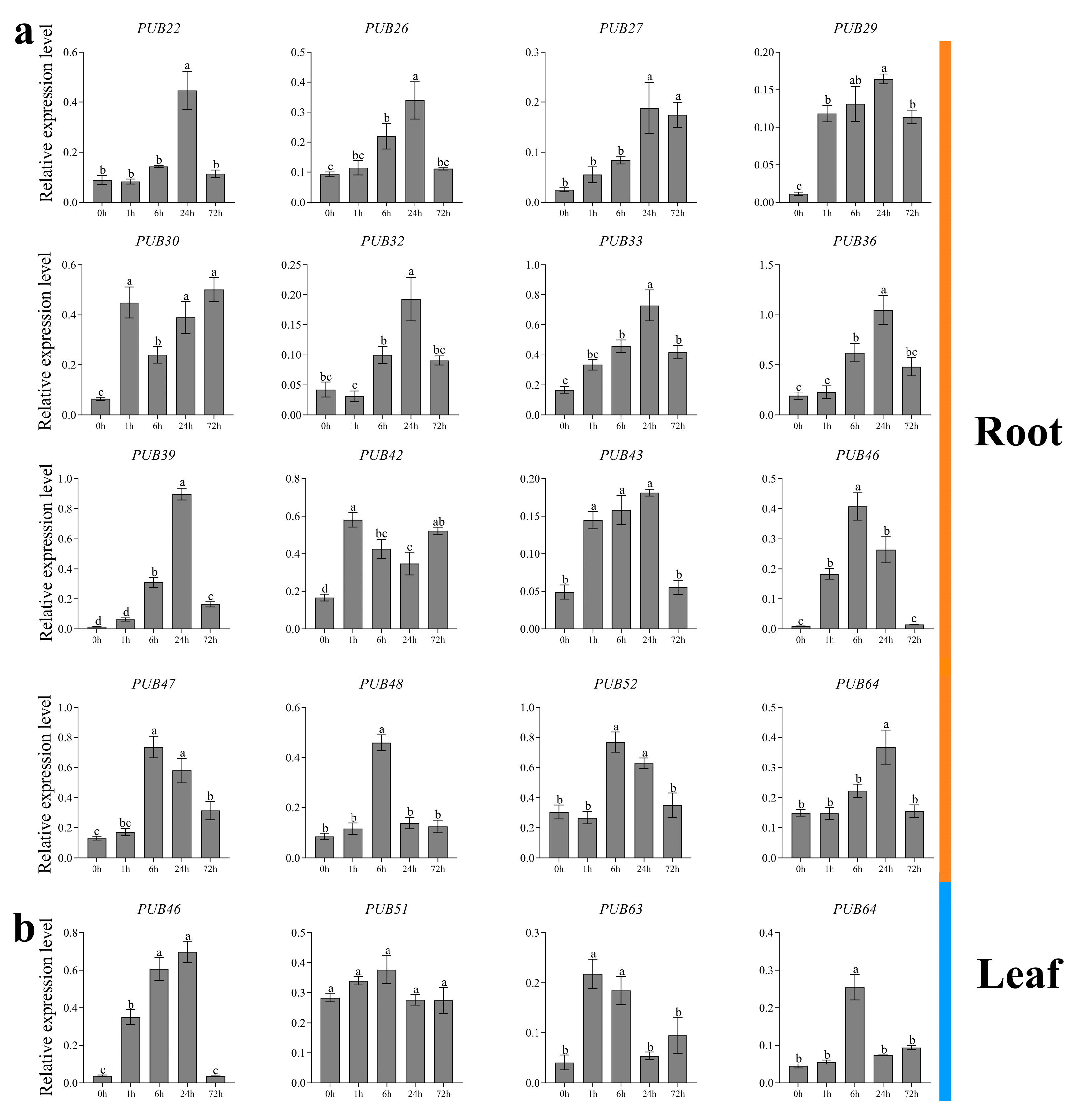
2.4. Expression Analysis of ZjPUB Genes under Salt Stress
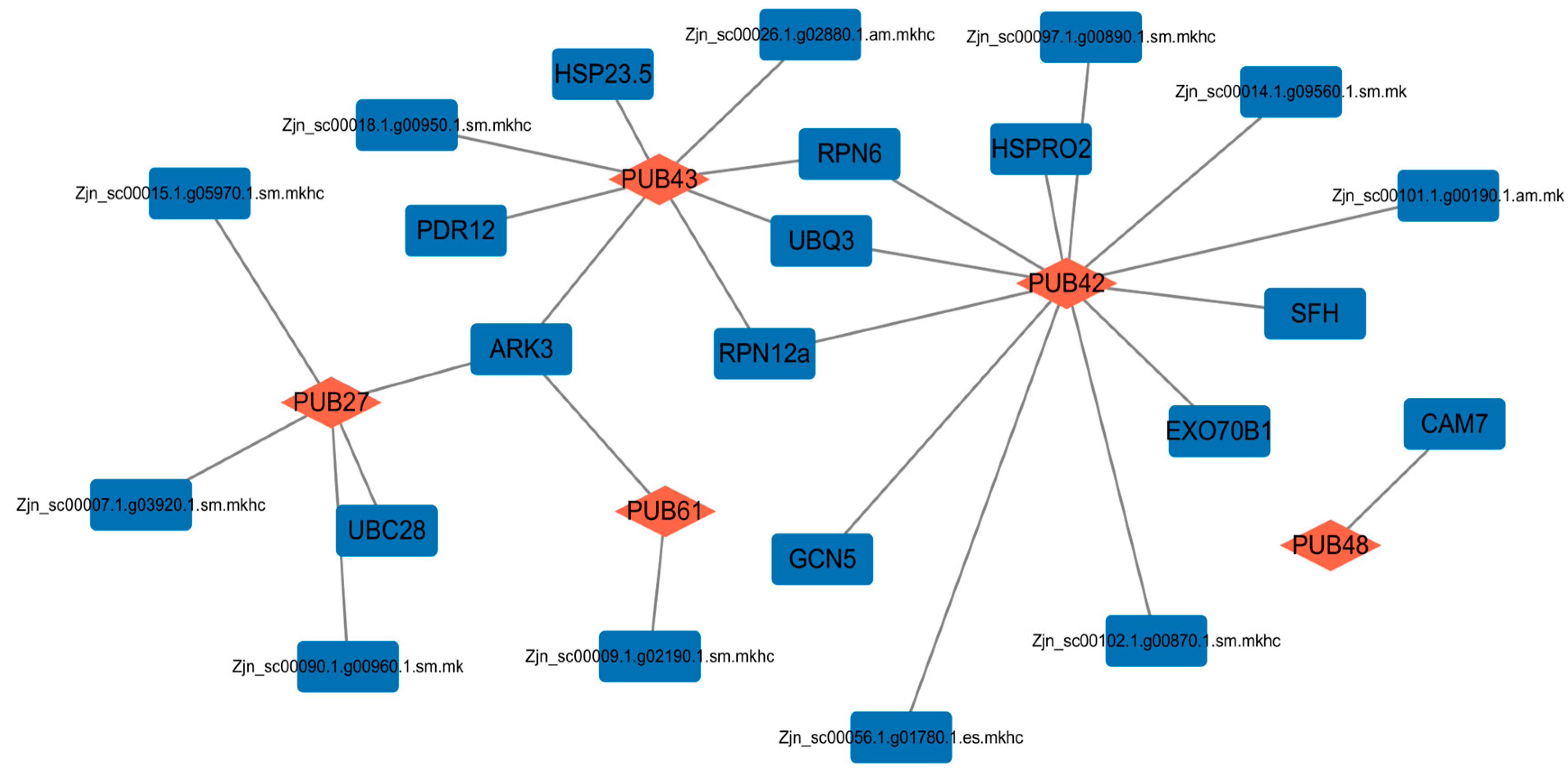
2.5. Protein–Protein Interaction (PPI) Network Analysis of Differentially Expressed ZjPUB Members under Salt Stress
3. Discussion
4. Materials and Methods
4.1. Genome and Transcriptome Data Sources
4.2. Identification of ZjPUBs and Construction of the Protein Tree
4.3. Characteristics, Gene Structure and Domain Analysis of PUB Genes in Z. japonica
4.4. Cis-Acting Elements within the Promoter Region of ZjPUB Genes
4.5. Gene Expression Analysis of ZjPUBs under Salt Stress
4.6. PPI Network Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhou, J.; Hu, Y.P.; Li, J.J.; Yu, Z.Y.; Guo, Q. Genome-wide identification and expression analysis of the plant U-box protein gene family in Phyllostachys edulis. Front. Genet. 2021, 12, 710113. [Google Scholar] [CrossRef]
- Sharma, B.; Joshi, D.; Yadav, P.K.; Gupta, A.K.; Bhatt, T.K. Role of ubiquitin-mediated degradation system in plant biology. Front. Plant Sci. 2016, 7, 806. [Google Scholar] [CrossRef]
- Smalle, J.; Vierstra, R.D. The ubiquitin 26S proteasome proteolytic pathway. Annu. Rev. Plant Biol. 2004, 55, 555–590. [Google Scholar] [CrossRef]
- Stone, S.L. Role of the ubiquitin proteasome system in plant response to abiotic stress. Int. Rev. Cell Mol. Biol. 2019, 343, 65–110. [Google Scholar]
- Wang, D.R.; Zhang, X.W.; Xu, R.R.; Wang, G.L.; You, C.X.; An, J.P. Apple U-box-type E3 ubiquitin ligase MdPUB23 reduces cold-stress tolerance by degrading the cold-stress regulatory protein MdICE1. Hortic. Res. 2022, 9, uhac171. [Google Scholar] [CrossRef]
- Serrano, I.; Campos, L.; Rivas, S. Roles of E3 ubiquitin-ligases in nuclear protein homeostasis during plant stress responses. Front. Plant Sci. 2018, 9, 139. [Google Scholar] [CrossRef]
- Lu, X.K.; Shu, N.; Wang, D.L.; Wang, J.J.; Chen, X.G.; Zhang, B.L.; Wang, S.; Guo, L.X.; Chen, C.; Ye, W.W. Genome-wide identification and expression analysis of PUB genes in cotton. BMC Genom. 2020, 21, 213. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Zhao, J.; Chen, D.; Wang, Y. E3 ubiquitin ligases: Styles, structures and functions. Mol. Biomed. 2021, 2, 23. [Google Scholar] [CrossRef] [PubMed]
- Buetow, L.; Huang, D.T. Structural insights into the catalysis and regulation of E3 ubiquitin ligases. Nat. Rev. Mol. Cell Biol. 2016, 17, 626–642. [Google Scholar] [CrossRef]
- Jiang, L.Y.; Lin, Y.X.; Wang, L.X.; Peng, Y.T.; Yang, M.; Jiang, Y.Y.; Hou, G.Y.; Liu, X.Y.; Li, M.Y.; Zhang, Y.T. Genome-wide identification and expression profiling reveal the regulatory role of U-box E3 ubiquitin ligase genes in strawberry fruit ripening and abiotic stresses resistance. Front. Plant Sci. 2023, 14, 1171056. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.L.; Wang, W.Q.; Wu, Y.Z.; Li, Q.X.; Zhang, G.Q.; Shi, R.R.; Yang, J.J.; Wang, Y.; Wang, W. The involvement of wheat U-box E3 ubiquitin ligase TaPUB1 in salt stress tolerance. J. Integr. Plant Biol. 2020, 62, 631–651. [Google Scholar] [CrossRef]
- Azevedo, C.; Santos-Rosa, M.J.; Shirasu, K. The U-box protein family in plants. Trends Plant Sci. 2001, 6, 354–358. [Google Scholar] [CrossRef]
- Zeng, L.R.; Park, C.H.; Venu, R.; Gough, J.; Wang, G.L. Classification, expression pattern, and E3 ligase activity assay of rice U-box-containing proteins. Mol. Plant 2008, 1, 800–815. [Google Scholar] [CrossRef]
- Cui, J.H.; Ren, G.Z.; Bai, Y.Z.; Gao, Y.K.; Yang, P.Y.; Chang, J.H. Genome-wide identification and expression analysis of the U-box E3 ubiquitin ligase gene family related to salt tolerance in sorghum (Sorghum bicolor L.). BMC Genom. 2023, 14, 1141617. [Google Scholar] [CrossRef]
- Wang, C.M.; Song, B.B.; Dai, Y.Q.; Zhang, S.L.; Huang, X.S. Genome-wide identification and functional analysis of U-box E3 ubiquitin ligases gene family related to drought stress response in Chinese white pear (Pyrus bretschneideri). BMC Plant Biol. 2021, 21, 235. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.G.; Yu, C.M.; Li, L.; Wang, M.; Yang, L.L.; Zhang, Y.N.; Zhang, Y.F.; Wang, J.Y.; Li, C.N.; Reynolds, M.P. How many faces does the plant U-Box E3 ligase have? Int. J. Mol. Sci. 2022, 23, 2285. [Google Scholar] [CrossRef] [PubMed]
- Song, J.B.; Mo, X.W.; Yang, H.Q.; Yue, L.M.; Song, J.; Mo, B.X. The U-box family genes in Medicago truncatula: Key elements in response to salt, cold, and drought stresses. PLoS ONE 2017, 12, e0182402. [Google Scholar] [CrossRef] [PubMed]
- Bergler, J.; Hoth, S. Plant U-box armadillo repeat proteins AtPUB18 and AtPUB19 are involved in salt inhibition of germination in Arabidopsis. Plant Biol. 2011, 13, 725–730. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.Z.; Wang, W.L.; Li, Q.X.; Zhang, G.Q.; Zhao, X.Y.; Li, G.Y.; Li, Y.L.; Wang, Y.; Wang, W. The wheat E3 ligase TaPUB26 is a negative regulator in response to salt stress in transgenic Brachypodium distachyon. Plant Sci. 2020, 294, 110441. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Kim, M.S.; Seo, Y.W. Overexpression of a plant U-box gene TaPUB4 confers drought stress tolerance in Arabidopsis thaliana. Plant Physiol. Biochem. 2023, 196, 596–607. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.L.; Yang, Q.Y.; Lanhuang, B.; Lin, H.T.; Shi, Y.; Dhanasekaran, S.; Godana, E.A.; Zhang, H.Y. Genome-wide investigation and analysis of U-box Ubiquitin–Protein ligase gene family in apple: Expression profiles during Penicillium expansum infection process. Physiol. Mol. Plant Pathol. 2020, 111, 101487. [Google Scholar] [CrossRef]
- Guo, H.L.; Xuan, J.P.; Liu, J.X.; Zhang, Y.M.; Zheng, Y.Q. Association of molecular markers with cold tolerance and green period in zoysiagrass (Zoysia Willd.). Breed. Sci. 2012, 62, 320–327. [Google Scholar] [CrossRef]
- Wang, W.; Shao, A.; Xu, X.; Fan, S.G.; Fu, J.M. Comparative genomics reveals the molecular mechanism of salt adaptation for zoysiagrasses. BMC Plant Biol. 2022, 22, 355. [Google Scholar] [CrossRef]
- Guo, H.L.; Ding, W.W.; Chen, J.B.; Chen, X.; Zheng, Y.Q.; Wang, Z.Y.; Liu, J.X. Genetic linkage map construction and QTL mapping of salt tolerance traits in Zoysiagrass (Zoysia japonica). PLoS ONE 2014, 9, e107249. [Google Scholar] [CrossRef]
- Van Zelm, E.; Zhang, Y.X.; Testerink, C. Salt tolerance mechanisms of plants. Annu. Rev. Plant Biol. 2020, 71, 403–433. [Google Scholar] [CrossRef]
- Yuan, F.; Wang, X.; Zhao, B.Q.; Xu, X.J.; Shi, M.; Leng, B.Y.; Dong, X.X.; Lu, C.X.; Feng, Z.T.; Guo, J.R. The genome of the recretohalophyte Limonium bicolor provides insights into salt gland development and salinity adaptation during terrestrial evolution. Mol. Plant 2022, 15, 1024–1044. [Google Scholar] [CrossRef]
- Teng, K.; Tan, P.H.; Guo, W.E.; Yue, Y.S.; Fan, X.F.; Wu, J.Y. Heterologous expression of a novel Zoysia japonica C2H2 zinc finger gene, ZjZFN1, improved salt tolerance in Arabidopsis. Front. Plant Sci. 2018, 9, 1159. [Google Scholar] [CrossRef] [PubMed]
- Teng, K.; Tan, P.H.; Xiao, G.Z.; Han, L.B.; Chang, Z.H.; Chao, Y.H. Heterologous expression of a novel Zoysia japonica salt-induced glycine-rich RNA-binding protein gene, ZjGRP, caused salt sensitivity in Arabidopsis. Plant Cell Rep. 2017, 36, 179–191. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.H.; Hong, M.J.; Kim, D.Y.; Kim, J.Y.; Jung, J.H.; Seo, Y.W. Molecular characterisation of the Cu/Zn superoxide dismutase gene (ZjSOD1) induced by salt stress in Zoysia japonica. J. Hortic. Sci. Biotechnol. 2012, 87, 640–646. [Google Scholar] [CrossRef]
- Du, Y.H.; Hei, Q.; Liu, Y.X.; Zhang, H.; Xu, K.; Xia, T. Isolation and characterization of a putative vacuolar Na+/H+ antiporter gene from Zoysia japonica L. J. Plant Biol. 2010, 53, 251–258. [Google Scholar] [CrossRef]
- Byun, M.Y.; Cui, L.H.; Oh, T.K.; Jung, Y.J.; Lee, A.; Park, K.Y.; Kang, B.G.; Kim, W.T. Homologous U-box E3 ubiquitin ligases OsPUB2 and OsPUB3 are involved in the positive regulation of low temperature stress response in rice (Oryza sativa L.). Front. Plant Sci. 2017, 8, 16. [Google Scholar] [CrossRef] [PubMed]
- Qin, Q.; Wang, Y.X.; Huang, L.Y.; Du, F.P.; Zhao, X.Q.; Li, Z.K.; Wang, W.S.; Fu, B.Y. A U-box E3 ubiquitin ligase OsPUB67 is positively involved in drought tolerance in rice. Plant Mol. Biol. 2020, 102, 89–107. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.H.; Seo, D.H.; Kang, B.G.; Kwak, J.M.; Kim, W.T. Suppression of Arabidopsis AtPUB30 resulted in increased tolerance to salt stress during germination. Plant Cell Rep. 2015, 34, 277–289. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.J.; An, C.; Guo, H.L.; Yang, X.Y.; Chen, J.B.; Zong, J.Q.; Li, J.J.; Liu, J.X. Physiological and transcriptomic analyses reveal the mechanisms underlying the salt tolerance of Zoysia japonica Steud. BMC Plant Biol. 2020, 20, 114. [Google Scholar] [CrossRef]
- Trenner, J.; Monaghan, J.; Saeed, B.; Quint, M.; Shabek, N.; Trujillo, M. Evolution and functions of plant U-box proteins: From protein quality control to signaling. Annu. Rev. Plant Biol. 2022, 73, 93–121. [Google Scholar] [CrossRef]
- Kim, M.S.; Kang, K.K.; Cho, Y.G. Molecular and functional analysis of U-box E3 ubiquitin ligase gene family in rice (Oryza sativa). Int. J. Mol. Sci. 2021, 22, 12088. [Google Scholar] [CrossRef]
- Samuel, M.A.; Salt, J.N.; Shiu, S.H.; Goring, D.R. Multifunctional arm repeat domains in plants. Int. Rev. Cytol. 2006, 253, 1–26. [Google Scholar]
- Yang, C.W.; González-Lamothe, R.; Ewan, R.A.; Rowland, O.; Yoshioka, H.; Shenton, M.; Ye, H.; O’Donnell, E.; Jones, J.D.; Sadanandom, A. The E3 ubiquitin ligase activity of Arabidopsis PLANT U-BOX17 and its functional tobacco homolog ACRE276 are required for cell death and defense. Plant Cell 2006, 18, 1084–1098. [Google Scholar] [CrossRef]
- Libault, M.; Wan, J.; Czechowski, T.; Udvardi, M.; Stacey, G. Identification of 118 Arabidopsis transcription factor and 30 ubiquitin-ligase genes responding to chitin, a plant-defense elicitor. Mol. Plant-Microbe Interact. 2007, 20, 900–911. [Google Scholar] [CrossRef]
- Lescot, M.; Déhais, P.; Thijs, G.; Marchal, K.; Moreau, Y.; Van de Peer, Y.; Rouzé, P.; Rombauts, S. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002, 30, 325–327. [Google Scholar] [CrossRef]
- Liu, Y.C.; Wu, Y.R.; Huang, X.H.; Sun, J.; Xie, Q. AtPUB19, a U-box E3 ubiquitin ligase, negatively regulates abscisic acid and drought responses in Arabidopsis thaliana. Mol. Plant 2011, 4, 938–946. [Google Scholar] [CrossRef]
- Wang, N.; Liu, Y.P.; Cong, Y.H.; Wang, T.T.; Zhong, X.T.; Yang, S.P.; Li, Y.; Gai, J.Y. Genome-wide identification of soybean U-box E3 ubiquitin ligases and roles of GmPUB8 in negative regulation of drought stress response in Arabidopsis. Plant Cell Physiol. 2016, 57, 1189–1209. [Google Scholar] [CrossRef]
- Wei, Y.; Jin, J.T.; Xu, Y.X.; Liu, W.T.; Yang, G.X.; Bu, H.D.; Li, T.; Wang, A.D. Ethylene-activated MdPUB24 mediates ubiquitination of MdBEL7 to promote chlorophyll degradation in apple fruit. Plant J. 2021, 108, 169–182. [Google Scholar] [CrossRef] [PubMed]
- Seo, P.J.; Xiang, F.N.; Qiao, M.; Park, J.Y.; Lee, Y.N.; Kim, S.G.; Lee, Y.-H.; Park, W.J.; Park, C.M. The MYB96 transcription factor mediates abscisic acid signaling during drought stress response in Arabidopsis. Plant Physiol. 2009, 151, 275–289. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Wang, R.L.; Yang, X.P.; Ju, Q.; Li, W.Q.; Lü, S.Y.; Tran, L.S.P.; Xu, J. The R2R3-MYB transcription factor AtMYB49 modulates salt tolerance in Arabidopsis by modulating the cuticle formation and antioxidant defence. Plant Cell Environ. 2020, 43, 1925–1943. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.Y.; Shi, Y.T.; Yang, S.H. Insights into the regulation of C-repeat binding factors in plant cold signaling. J. Integr. Plant Biol. 2018, 60, 780–795. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.X.; Feng, Y.; Yu, S.H.; Fan, Z.Q.; Li, X.L.; Li, J.Y.; Yin, H.F. The flavonoid biosynthesis network in plants. Int. J. Mol. Sci. 2021, 22, 12824. [Google Scholar] [CrossRef] [PubMed]
- Yoon, Y.; Seo, D.H.; Shin, H.; Kim, H.J.; Kim, C.M.; Jang, G. The role of stress-responsive transcription factors in modulating abiotic stress tolerance in plants. Agronomy 2020, 10, 788. [Google Scholar] [CrossRef]
- Seo, D.H.; Ahn, M.Y.; Park, K.Y.; Kim, E.Y.; Kim, W.T. The N-terminal UND motif of the Arabidopsis U-box E3 ligase PUB18 is critical for the negative regulation of ABA-mediated stomatal movement and determines its ubiquitination specificity for exocyst subunit Exo70B1. Plant Cell 2016, 28, 2952–2973. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, X.; Tang, Y.J.; Zhao, T.; Huang, C.B.; Li, Y.; Zhang, C.H. Exocyst subunit VviExo70B is degraded by ubiquitin ligase VviPUB19 and they regulate drought and salt tolerance in grapevine. Environ. Exp. Bot. 2023, 206, 105175. [Google Scholar] [CrossRef]
- Kang, J.; Hwang, J.U.; Lee, M.; Kim, Y.Y.; Assmann, S.M.; Martinoia, E.; Lee, Y. PDR-type ABC transporter mediates cellular uptake of the phytohormone abscisic acid. Proc. Natl. Acad. Sci. USA 2010, 107, 2355–2360. [Google Scholar] [CrossRef] [PubMed]
- Trujillo, M. News from the PUB: Plant U-box type E3 ubiquitin ligases. J. Exp. Bot. 2018, 69, 371–384. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.Y.; Cheng, J.K.; Zhu, Y.J.; Ding, Y.L.; Meng, J.J.; Chen, Z.Z.; Xie, Q.; Guo, Y.; Li, J.G.; Yang, S.H. Degradation of the ABA co-receptor ABI1 by PUB12/13 U-box E3 ligases. Nat. Commun. 2015, 6, 8630. [Google Scholar] [CrossRef] [PubMed]
- Park, J.J.; Yi, J.; Yoon, J.; Cho, L.H.; Ping, J.; Jeong, H.J.; Cho, S.K.; Kim, W.T.; An, G. OsPUB15, an E3 ubiquitin ligase, functions to reduce cellular oxidative stress during seedling establishment. Plant J. 2011, 65, 194–205. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.R.; Li, B.; Wang, J.Y.; Chang, X.P.; Mao, X.G.; Jing, R.L. TaPUB15, a U-Box E3 ubiquitin ligase gene from wheat, enhances salt tolerance in rice. Food Energy Secur. 2021, 10, e250. [Google Scholar] [CrossRef]
- Tanaka, H.; Hirakawa, H.; Kosugi, S.; Nakayama, S.; Ono, A.; Watanabe, A.; Hashiguchi, M.; Gondo, T.; Ishigaki, G.; Muguerza, M. Sequencing and comparative analyses of the genomes of zoysiagrasses. DNA Res. 2016, 23, 171–180. [Google Scholar] [CrossRef]
- Mistry, J.; Chuguransky, S.; Williams, L.; Qureshi, M.; Salazar, G.A.; Sonnhammer, E.L.; Tosatto, S.C.; Paladin, L.; Raj, S.; Richardson, L.J. Pfam: The protein families database in 2021. Nucleic Acids Res. 2021, 49, D412–D419. [Google Scholar] [CrossRef]
- Kawahara, Y.; de la Bastide, M.; Hamilton, J.P.; Kanamori, H.; McCombie, W.R.; Ouyang, S.; Schwartz, D.C.; Tanaka, T.; Wu, J.; Zhou, S. Improvement of the Oryza sativa Nipponbare reference genome using next generation sequence and optical map data. Rice 2013, 6, 4. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547. [Google Scholar] [CrossRef]
- Nguyen, L.T.; Schmidt, H.A.; Von Haeseler, A.; Minh, B.Q. IQ-TREE: A fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Smith, D.K.; Zhu, H.; Guan, Y.; Lam, T.T.Y. ggtree: An R package for visualization and annotation of phylogenetic trees with their covariates and other associated data. Methods Ecol. Evol. 2017, 8, 28–36. [Google Scholar] [CrossRef]
- Duvaud, S.; Gabella, C.; Lisacek, F.; Stockinger, H.; Ioannidis, V.; Durinx, C. Expasy, the Swiss Bioinformatics Resource Portal, as designed by its users. Nucleic Acids Res. 2021, 49, W216–W227. [Google Scholar] [CrossRef]
- Hu, B.; Jin, J.P.; Guo, A.Y.; Zhang, H.; Luo, J.C.; Gao, G. GSDS 2.0: An upgraded gene feature visualization server. Bioinformatics 2015, 31, 1296–1297. [Google Scholar] [CrossRef]
- Savojardo, C.; Martelli, P.L.; Fariselli, P.; Profiti, G.; Casadio, R. BUSCA: An integrative web server to predict subcellular localization of proteins. Nucleic Acids Res. 2018, 46, W459–W466. [Google Scholar] [CrossRef]
- Chen, C.J.; Wu, Y.; Li, J.W.; Wang, X.; Zeng, Z.H.; Xu, J.; Liu, Y.L.; Feng, J.T.; Chen, H.; He, Y.H. TBtools-II: A “one for all, all for one” bioinformatics platform for biological big-data mining. Mol. Plant 2023, 16, 1733–1742. [Google Scholar] [CrossRef]
- Kolde, R.; Kolde, M.R. Package ‘pheatmap’. R Package 2015, 1, 790. [Google Scholar]
- Li, X.H.; Ye, G.; Shen, Z.Y.; Li, J.J.; Hao, D.L.; Kong, W.Y.; Wang, H.R.; Zhang, L.; Chen, J.B.; Guo, H.L. Na+ and K+ homeostasis in different organs of contrasting Zoysia japonica accessions under salt stress. Environ. Exp. Bot. 2023, 214, 105455. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Snel, B.; Lehmann, G.; Bork, P.; Huynen, M.A. STRING: A web-server to retrieve and display the repeatedly occurring neighbourhood of a gene. Nucleic Acids Res. 2000, 28, 3442–3444. [Google Scholar] [CrossRef] [PubMed]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef] [PubMed]

| Gene Name | Transcript ID | CDS Length (bp) | Amino Acids (aa) | MW (Da) | pI | Subcellular Localization |
|---|---|---|---|---|---|---|
| ZjPUB1 | Zjn_sc00010.1.g02110.1.am.mkhc | 2331 | 777 | 88,914 | 5.35 | endomembrane system |
| ZjPUB2 | Zjn_sc00182.1.g00060.1.sm.mkhc | 1398 | 466 | 52,315.6 | 6.02 | nucleus |
| ZjPUB3 | Zjn_sc00102.1.g00610.1.sm.mkhc | 1344 | 448 | 49,809.3 | 5.03 | nucleus |
| ZjPUB4 | Zjn_sc00022.1.g06260.1.sm.mk | 576 | 192 | 21,098.8 | 4.86 | nucleus |
| ZjPUB5 | Zjn_sc00041.1.g00960.1.am.mk | 1200 | 400 | 44,426.1 | 7.1 | chloroplast |
| ZjPUB6 | Zjn_sc00049.1.g01240.1.sm.mk | 1374 | 458 | 51,715.7 | 5.5 | nucleus |
| ZjPUB7 | Zjn_sc00049.1.g00160.1.sm.mkhc | 2424 | 808 | 90,586 | 7.46 | nucleus |
| ZjPUB8 | Zjn_sc00067.1.g02790.1.am.mkhc | 2208 | 736 | 82,274.4 | 7.26 | nucleus |
| ZjPUB9 | Zjn_sc00066.1.g00450.1.am.mk | 2304 | 768 | 85,501.4 | 6.09 | nucleus |
| ZjPUB10 | Zjn_sc00102.1.g00620.1.am.mk | 1764 | 588 | 65,486.1 | 8.06 | nucleus |
| ZjPUB11 | Zjn_sc00026.1.g00430.1.sm.mkhc | 2976 | 992 | 108,074.2 | 6.65 | chloroplast |
| ZjPUB12 | Zjn_sc00004.1.g08300.1.am.mkhc | 1464 | 488 | 53,820.9 | 4.55 | nucleus |
| ZjPUB13 | Zjn_sc00023.1.g01280.1.sm.mkhc | 2859 | 953 | 104,355 | 6.38 | chloroplast |
| ZjPUB14 | Zjn_sc00150.1.g00150.1.sm.mkhc | 825 | 275 | 31,037 | 7.17 | nucleus |
| ZjPUB15 | Zjn_sc00016.1.g06570.1.sm.mkhc | 303 | 101 | 11,515.1 | 5.7 | nucleus |
| ZjPUB16 | Zjn_sc00093.1.g01230.1.sm.mkhc | 3093 | 1031 | 112,713.5 | 6.61 | nucleus |
| ZjPUB17 | Zjn_sc00131.1.g00970.1.sm.mkhc | 3069 | 1023 | 111,844.9 | 6.15 | nucleus |
| ZjPUB18 | Zjn_sc00165.1.g00170.1.sm.mkhc | 1890 | 630 | 69,830.4 | 5.31 | plasma membrane |
| ZjPUB19 | Zjn_sc00007.1.g00090.1.cf.mkhc | 903 | 301 | 34,248.6 | 4.84 | nucleus |
| ZjPUB20 | Zjn_sc00010.1.g03830.1.sm.mkhc | 1266 | 422 | 46,902.2 | 5.08 | nucleus |
| ZjPUB21 | Zjn_sc00006.1.g02940.1.sm.mk | 1164 | 388 | 42,146.4 | 5.02 | nucleus |
| ZjPUB22 | Zjn_sc00058.1.g02220.1.sm.mk | 1113 | 371 | 39,634.4 | 8.65 | nucleus |
| ZjPUB23 | Zjn_sc00020.1.g01280.1.sm.mk | 969 | 323 | 33,963.6 | 5.69 | nucleus |
| ZjPUB24 | Zjn_sc00003.1.g07570.1.am.mk | 1578 | 526 | 56,312.6 | 8.25 | nucleus |
| ZjPUB25 | Zjn_sc00002.1.g09550.1.am.mk | 1848 | 616 | 66,461.4 | 8.93 | chloroplast |
| ZjPUB26 | Zjn_sc00012.1.g05350.1.am.mk | 1512 | 504 | 51,890.3 | 9.76 | chloroplast |
| ZjPUB27 | Zjn_sc00020.1.g01450.1.am.mk | 2397 | 799 | 85,768.3 | 9.95 | nucleus |
| ZjPUB28 | Zjn_sc00006.1.g03530.1.am.mk | 1911 | 637 | 67,282.2 | 9.7 | nucleus |
| ZjPUB29 | Zjn_sc00002.1.g08810.1.am.mk | 1338 | 446 | 47,119.9 | 7.7 | mitochondrion |
| ZjPUB30 | Zjn_sc00003.1.g06760.1.am.mk | 1332 | 444 | 47,189 | 7.88 | nucleus |
| ZjPUB31 | Zjn_sc00093.1.g00320.1.am.mk | 1554 | 518 | 55,204.8 | 10.25 | nucleus |
| ZjPUB32 | Zjn_sc00007.1.g05420.1.am.mk | 1488 | 496 | 53,359.2 | 8.73 | chloroplast |
| ZjPUB33 | Zjn_sc00022.1.g01940.1.sm.mk | 1368 | 456 | 48,826.9 | 8.17 | nucleus |
| ZjPUB34 | Zjn_sc00006.1.g06155.1.br | 432 | 144 | 14,928.8 | 6.79 | nucleus |
| ZjPUB35 | Zjn_sc00004.1.g08630.1.am.mk | 1494 | 498 | 54,381.3 | 8.31 | nucleus |
| ZjPUB36 | Zjn_sc00023.1.g01610.1.sm.mk | 1242 | 414 | 45,167.9 | 7.34 | nucleus |
| ZjPUB37 | Zjn_sc00007.1.g08400.1.am.mk | 1449 | 483 | 52,598.4 | 5.33 | nucleus |
| ZjPUB38 | Zjn_sc00022.1.g04900.1.sm.mk | 1287 | 429 | 46,311 | 4.85 | nucleus |
| ZjPUB39 | Zjn_sc00028.1.g01890.1.am.mk | 933 | 311 | 33,132.5 | 6.7 | nucleus |
| ZjPUB40 | Zjn_sc00034.1.g05980.1.am.mk | 1830 | 610 | 66,145.2 | 10.03 | nucleus |
| ZjPUB41 | Zjn_sc00004.1.g14050.1.sm.mk | 1272 | 424 | 45,043.1 | 7.06 | nucleus |
| ZjPUB42 | Zjn_sc00020.1.g01520.1.am.mk | 1545 | 515 | 55,689.2 | 9.83 | nucleus |
| ZjPUB43 | Zjn_sc00006.1.g03610.1.am.mk | 1314 | 438 | 46,999.6 | 7.94 | nucleus |
| ZjPUB44 | Zjn_sc00071.1.g01640.1.am.mk | 3036 | 1012 | 111,234.8 | 7.37 | chloroplast thylakoid membrane |
| ZjPUB45 | Zjn_sc04324.1.g00010.1.am.mk | 678 | 226 | 23,553.7 | 7.64 | nucleus |
| ZjPUB46 | Zjn_sc00018.1.g06300.1.sm.mk | 2091 | 697 | 73,597.5 | 7.31 | chloroplast |
| ZjPUB47 | Zjn_sc00047.1.g02090.1.am.mk | 2241 | 747 | 81,591.7 | 5.39 | endomembrane system |
| ZjPUB48 | Zjn_sc00174.1.g00010.1.sm.mk | 1965 | 655 | 70,775.8 | 6.66 | nucleus |
| ZjPUB49 | Zjn_sc00012.1.g07180.1.sm.mkhc | 1923 | 641 | 68,015.4 | 7.07 | nucleus |
| ZjPUB50 | Zjn_sc00025.1.g05030.1.am.mk | 2376 | 792 | 84,931.1 | 10.13 | endomembrane system |
| ZjPUB51 | Zjn_sc00009.1.g08070.1.sm.mk | 2055 | 685 | 73,004.8 | 6.71 | chloroplast |
| ZjPUB52 | Zjn_sc00040.1.g03750.1.am.mk | 2061 | 687 | 73,448.3 | 6.24 | nucleus |
| ZjPUB53 | Zjn_sc00090.1.g01510.1.am.mk | 2430 | 810 | 87,041.7 | 6.28 | nucleus |
| ZjPUB54 | Zjn_sc00006.1.g06160.1.sm.mk | 1368 | 456 | 49,690 | 5.01 | nucleus |
| ZjPUB55 | Zjn_sc00020.1.g03330.1.sm.mkhc | 1377 | 459 | 49,914.2 | 5.94 | nucleus |
| ZjPUB56 | Zjn_sc00015.1.g04170.1.am.mk | 1914 | 638 | 70,795.3 | 7.92 | nucleus |
| ZjPUB57 | Zjn_sc00022.1.g02290.1.sm.mkhc | 1569 | 523 | 57,066.2 | 5.52 | nucleus |
| ZjPUB58 | Zjn_sc00184.1.g00310.1.sm.mkhc | 2367 | 789 | 85,448.1 | 7.41 | nucleus |
| ZjPUB59 | Zjn_sc00002.1.g06310.1.sm.mkhc | 2118 | 706 | 76,646.2 | 8.2 | nucleus |
| ZjPUB60 | Zjn_sc00038.1.g02650.1.sm.mkhc | 1971 | 657 | 71,535.9 | 5.22 | nucleus |
| ZjPUB61 | Zjn_sc00093.1.g00050.1.sm.mk | 1416 | 472 | 49,182.2 | 6.32 | chloroplast outer membrane |
| ZjPUB62 | Zjn_sc00018.1.g06490.1.sm.mk | 1365 | 455 | 47,388.5 | 7.28 | chloroplast |
| ZjPUB63 | Zjn_sc00091.1.g00910.1.am.mk | 2031 | 677 | 70,993.7 | 8.76 | nucleus |
| ZjPUB64 | Zjn_sc00039.1.g04330.1.am.mk | 480 | 160 | 16,782.6 | 7.09 | chloroplast |
| ZjPUB65 | Zjn_sc00039.1.g04340.1.am.mk | 651 | 217 | 23,010.2 | 5.02 | chloroplast |
| ZjPUB66 | Zjn_sc00008.1.g00320.1.sm.mk | 1272 | 424 | 46,803.7 | 6.76 | nucleus |
| ZjPUB67 | Zjn_sc00071.1.g00460.1.am.mkhc | 2496 | 832 | 90,311.8 | 5.76 | nucleus |
| ZjPUB68 | Zjn_sc00007.1.g07460.1.sm.mkhc | 2319 | 773 | 86,679.4 | 5.64 | nucleus |
| ZjPUB69 | Zjn_sc00022.1.g03900.1.sm.mkhc | 1884 | 628 | 69,200 | 6.42 | nucleus |
| ZjPUB70 | Zjn_sc00056.1.g00870.1.am.mkhc | 1470 | 490 | 54,562.6 | 4.82 | nucleus |
| ZjPUB71 | Zjn_sc00009.1.g03620.1.am.mkhc | 2373 | 791 | 86,729.6 | 6.14 | nucleus |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, D.; Xu, J.; Wang, H.; Guo, H.; Chen, Y.; Zhang, L.; Li, J.; Hao, D.; Yao, X.; Li, X. Genome-Wide Identification and Expression Analysis of the PUB Gene Family in Zoysia japonica under Salt Stress. Plants 2024, 13, 788. https://doi.org/10.3390/plants13060788
Sun D, Xu J, Wang H, Guo H, Chen Y, Zhang L, Li J, Hao D, Yao X, Li X. Genome-Wide Identification and Expression Analysis of the PUB Gene Family in Zoysia japonica under Salt Stress. Plants. 2024; 13(6):788. https://doi.org/10.3390/plants13060788
Chicago/Turabian StyleSun, Daojin, Jingya Xu, Haoran Wang, Hailin Guo, Yu Chen, Ling Zhang, Jianjian Li, Dongli Hao, Xiang Yao, and Xiaohui Li. 2024. "Genome-Wide Identification and Expression Analysis of the PUB Gene Family in Zoysia japonica under Salt Stress" Plants 13, no. 6: 788. https://doi.org/10.3390/plants13060788
APA StyleSun, D., Xu, J., Wang, H., Guo, H., Chen, Y., Zhang, L., Li, J., Hao, D., Yao, X., & Li, X. (2024). Genome-Wide Identification and Expression Analysis of the PUB Gene Family in Zoysia japonica under Salt Stress. Plants, 13(6), 788. https://doi.org/10.3390/plants13060788






