Genetic Analyses of Flower Main Traits from Two Pitayas and Their Progenies: A Cactus Plant
Abstract
1. Introduction
2. Results
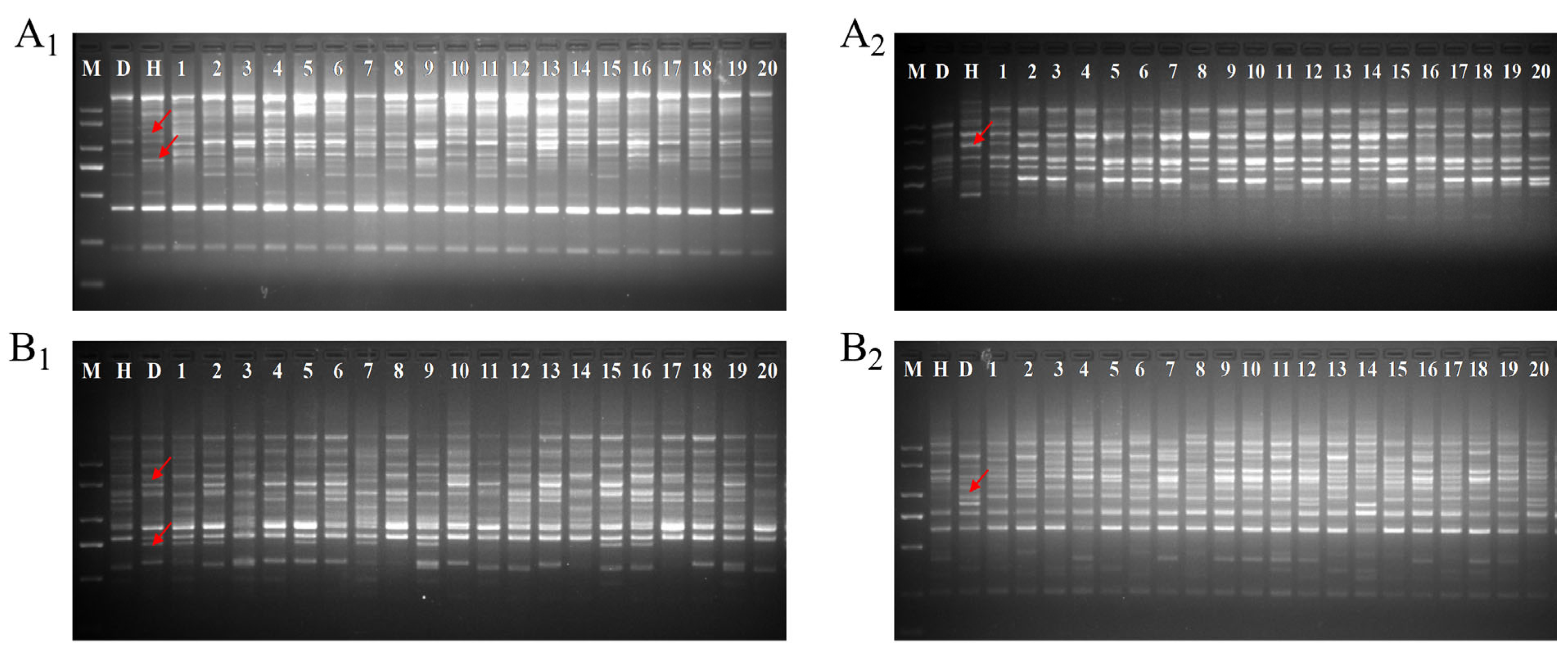
2.1. Hybrid Authenticity Verification
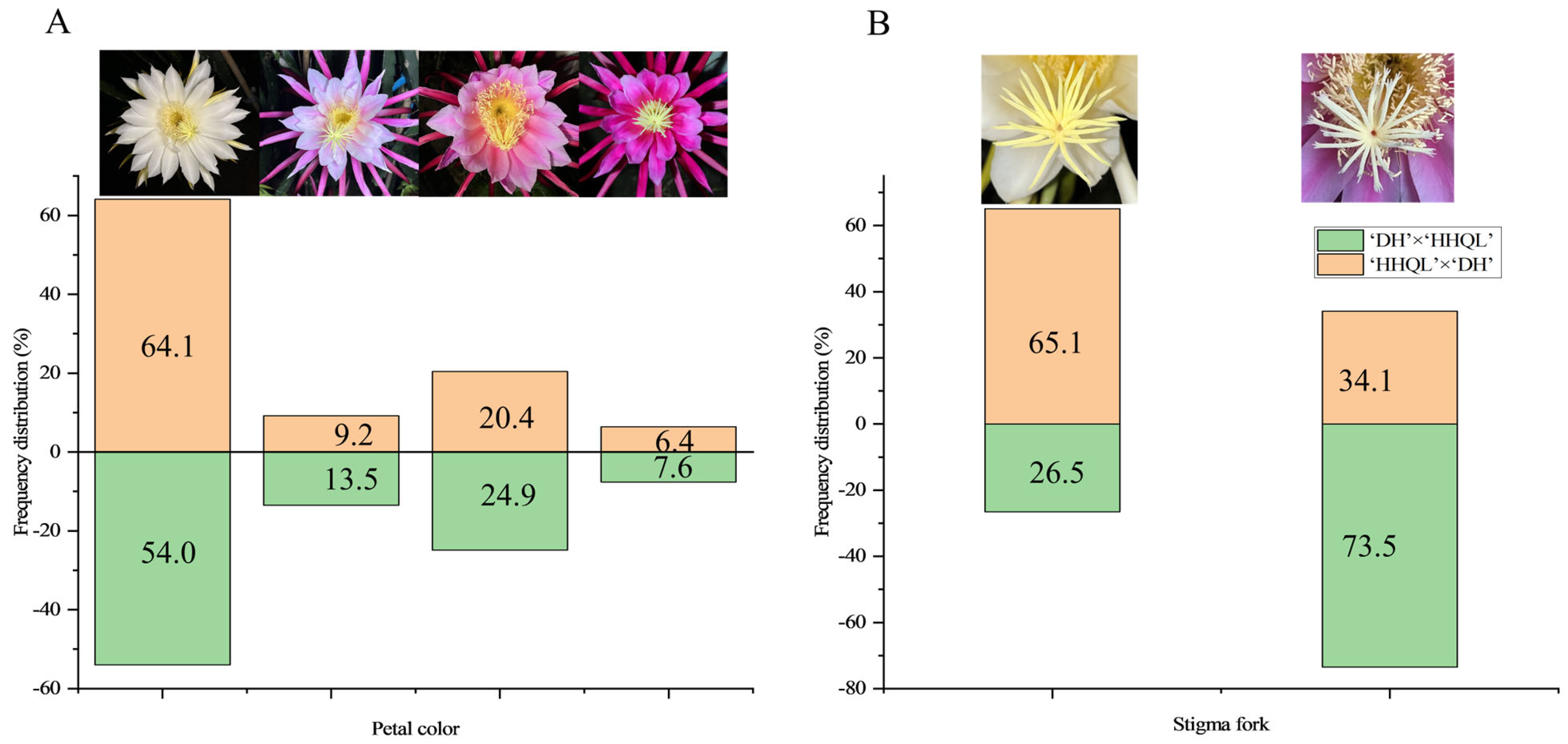
2.2. Petal Color and Stigma Fork Traits
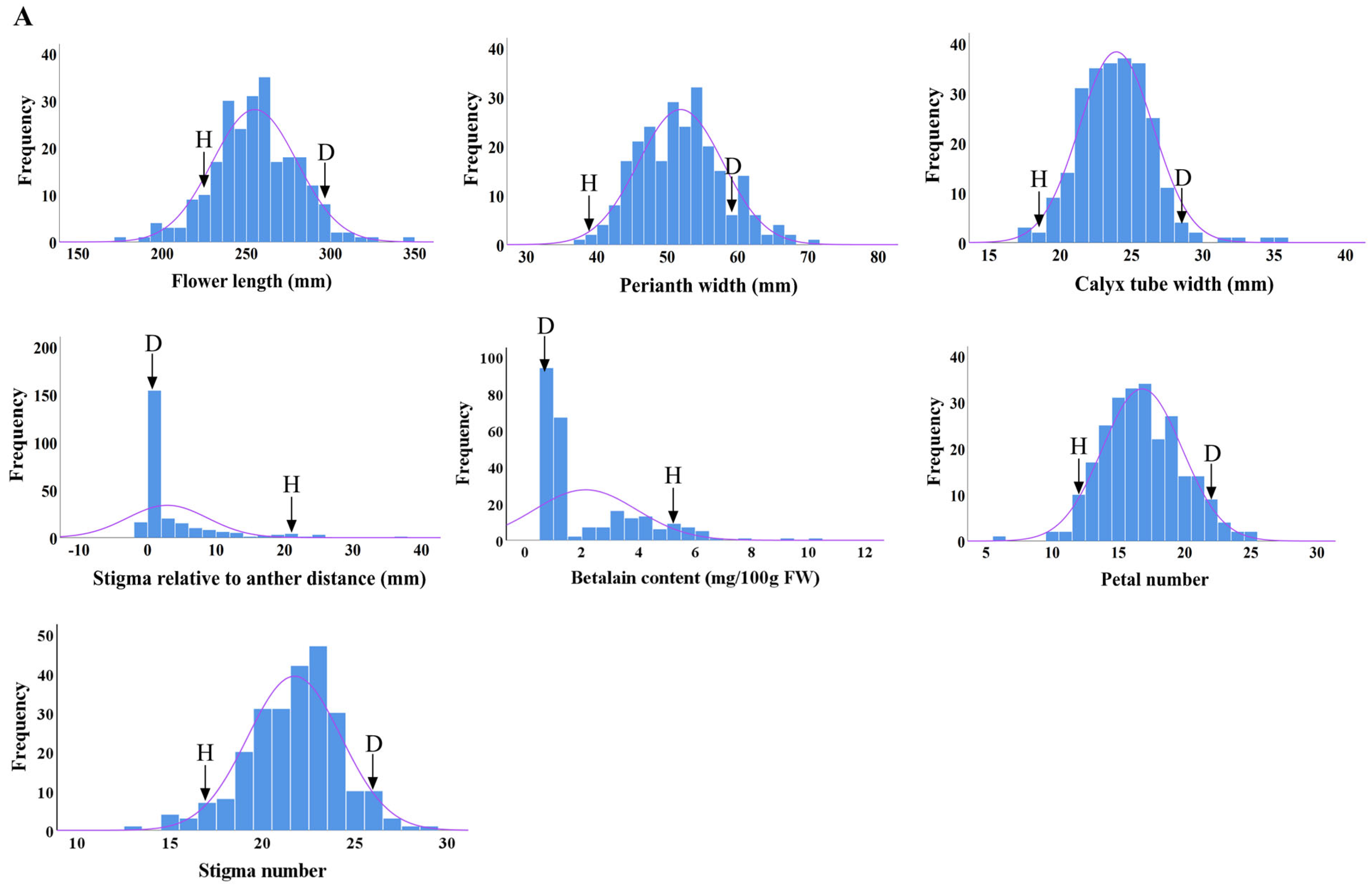
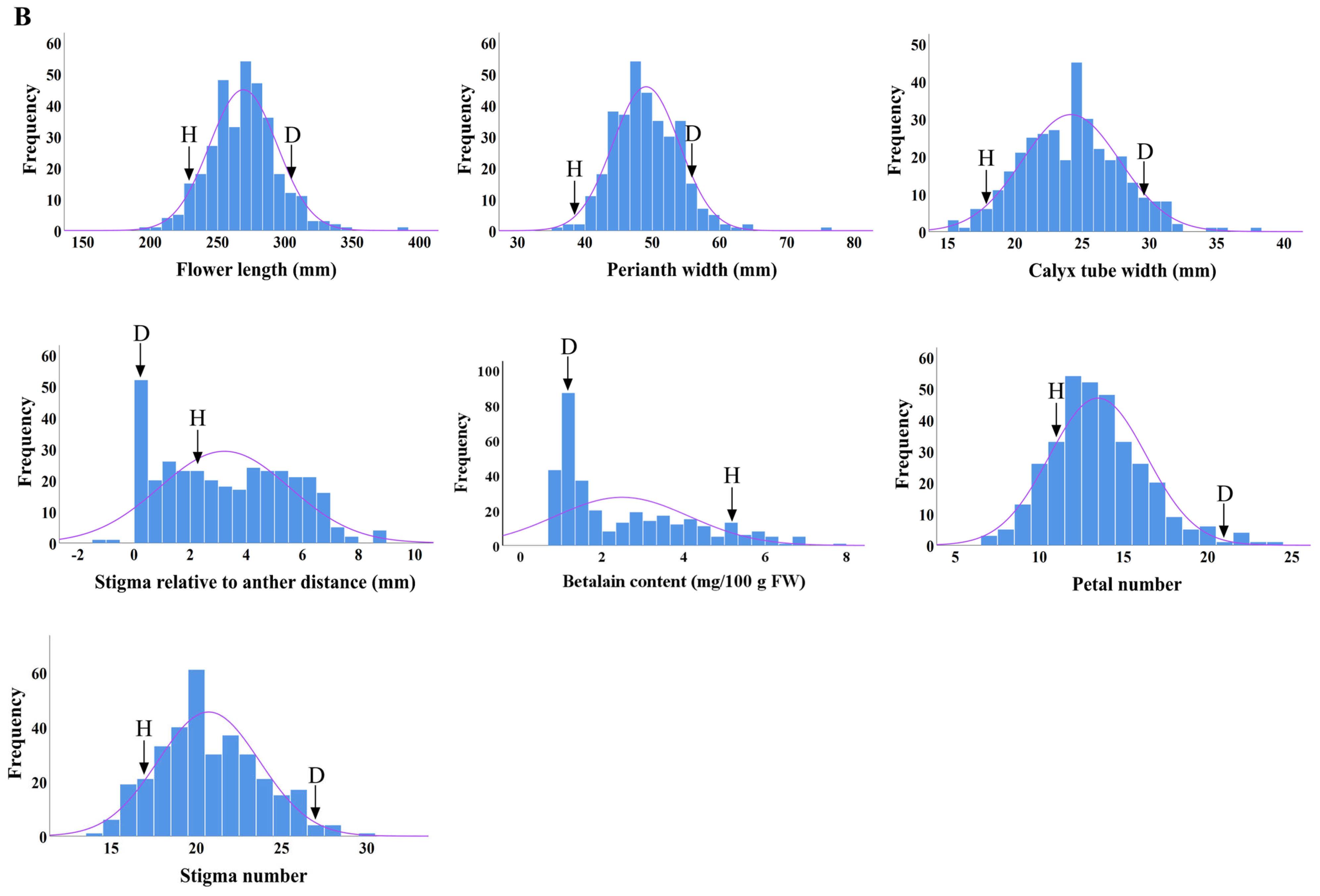
2.3. Distributions of Main Flower Traits
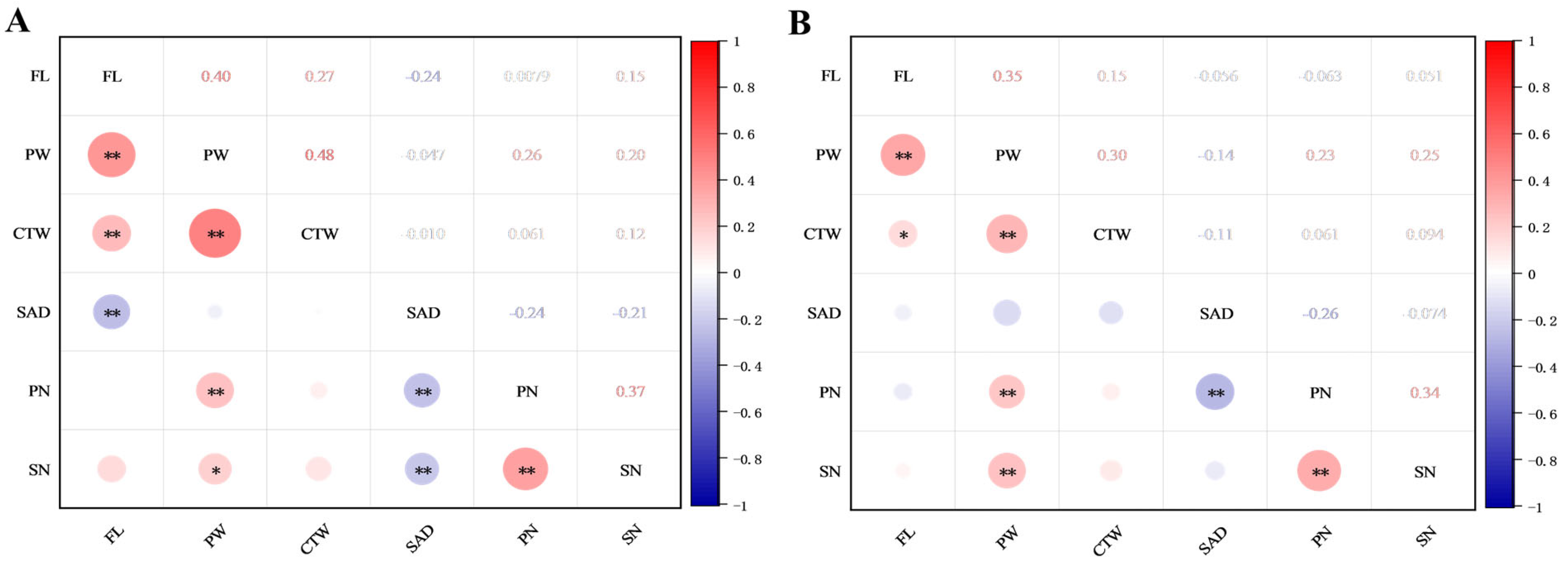
2.4. Correlation Analyses of Flower Traits
2.5. Genetic Analyses of Flower Traits
2.6. Genetic Analyses of Main Genes and Multigene of Flower Traits
2.6.1. Suitability Test of the Optimal Genetic Model for Flower-Related Traits
2.6.2. Genetic Parameter Estimation of Flower-Related Traits under the Optimal Genetic Model
3. Materials and Methods
3.1. Plant Materials
3.2. Acquirement of Hybrids
3.3. Authenticity Identification
3.4. Investigation of Flower Characteristics
3.5. Measurement of Betalains
3.6. Inheritance Analyses of Flower-Related Traits
3.7. Statistical Analysis
4. Discussion
4.1. The Genetic Effect of Flower Color
4.2. The Genetic Effect of Flower Types
4.3. The Genetic Effect of Stigmas
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ibrahim, S.R.M.; Mohamed, G.A.; Khedr, A.I.M.; Zayed, M.F.; El-Kholy, A.A.E.S. Genus Hylocereus: Beneficial phytochemicals, nutritional importance, and biological relevance-A review. Food Biochem. 2018, 42, e12491. [Google Scholar] [CrossRef]
- Trivellini, A.; Lucchesini, M.; Ferrante, A.; Massa, D.; Orlando, M.; Incrocci, L.; Mensuali-Sodi, A. Pitaya, an attractive alternative crop for Mediterranean region. Agronomy 2020, 10, 1065. [Google Scholar] [CrossRef]
- Shah, K.; Chen, J.Y.; Chen, J.X.; Qin, Y.H. Pitaya nutrition, biology, and biotechnology: A review. Int. J. Mol. Sci. 2023, 24, 13986. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.T.; Zhang, W.L.; Li, X.X.; Shu, C.; Jiang, W.B.; Cao, J.K. Nutrition, phytochemical profile, bioactivities and applications in food industry of pitaya (Hylocereus spp.) peels: A comprehensive review. Trends Food Sci. Technol. 2021, 116, 199–217. [Google Scholar] [CrossRef]
- Villalobos-Gutierrez, M.G.; Schweiggert, R.M.; Carle, R.; Esquivel, P. Chemical characterization of Central Americal pitaya (Hylocereus Sp.) seeds and seed oil. CyTA-J. Food 2012, 10, 78–83. [Google Scholar] [CrossRef]
- Adnan, L.; Osman, A.; Hamid, A.A. Antioxidant activity of different extracts of red pitaya (Hylocereus polyrhizus) seed. Food Prop. 2011, 14, 1171–1181. [Google Scholar] [CrossRef]
- Noemi, T.Z. Breeding an underutilized fruit crop: A long-term program for Hylocereus. Hortic. Res. 2022, 9, uhac078. [Google Scholar]
- Tel-Zur, N.; Mizrahi, Y.; Cisneros, A.; Mouyal, J.; Schneider, B.; Doyle, J. Phenotypic and genomic characterization of vine cactus collection (Cactaceae). Genet. Resour. Crop Evol. 2011, 58, 1075–1085. [Google Scholar] [CrossRef]
- Lichtenzveig, J.; Abbo, S.; Nerd, A.; Tel-Zur, N.; Mizrahi, Y. Cytology and mating systems in the climbing cacti Hylocereus and Selenicereus. Am. J. Bot. 2000, 87, 1058–1065. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Z.; Wang, L.; Zhao, X.Z.; Yuan, Q.F.; Xiao, T.J.; Ma, Y.H. A new pitaya cultivar ‘Qianhong’. China Fruits 2020, 2, 105–106. [Google Scholar]
- Chen, C.B.; Wu, P.Y.; Xie, F.F.; Sun, L.Y.; Xing, Y.M.; Hua, Q.Z.; Zhang, Z.K.; Chen, J.Y.; Zhao, J.T.; Hu, G.B.; et al. Breeding of ‘Hongguan No. 1’ and ‘Shuangse No. 1’ pitayas with superior quality. HortScience 2018, 53, 404–409. [Google Scholar] [CrossRef]
- Wang, Y.L.; Zheng, Y.F.; Tan, B.S.; Chen, H.P.; Sun, Q.M. A new pitaya cultivar ‘Daqiu 4’. Acta Hortic. Sin. 2022, 49 (Suppl. S1), 59–60. [Google Scholar]
- Dai, H.F.; Li, J.C.; Sun, Q.M. A new pitaya cultivar ‘Yuehong No. 5’. J. Fruit Sci. 2022, 39, 2205–2208. [Google Scholar]
- Li, J.C.; Dai, H.F.; Sun, Q.M. A new pitaya cultivar ‘Hongshuijing No. 6’. J. Fruit Sci. 2022, 39, 1973–1976. [Google Scholar]
- Tel-Zur, N. Pitahayas: Introduction, agrotechniques, and breeding. Acta Hortic. Sin. 2010, 995, 109–115. [Google Scholar] [CrossRef]
- Halder, S.; Ghosh, S.; Khan, R.; Khan, A.A.; Perween, T.; Hasan, M.A. Role of pollination in fruit crops: A review. Pharma Innov. J. 2019, 8, 695–702. [Google Scholar]
- Conner, J.K.; Sterling, A. Testing hypotheses of functional relationships: A comparative survey of correlation patterns among floral traits in five insect-pollinated plants. Am. J. Bot. 1995, 82, 1399–1406. [Google Scholar]
- Mizrahi, Y.; Mouyal, J.; Nerd, A.; Sitrit, Y. Metaxenia in the vine cacti Hylocereus polyrhizus and Selenicereus spp. Ann. Bot. 2004, 93, 469–472. [Google Scholar] [CrossRef] [PubMed]
- Weiss, J.; Nerd, A.; Mizrahi, Y. Flowering behavior and pollination requirements in climbing Cacti with fruit crop potential. HortScience 1994, 29, 1487–1492. [Google Scholar] [CrossRef]
- Pushpakumara, D.; Gunasena, H.P.M.; Karyawasam, M. Flowering and fruiting phenology, pollination vectors and breeding system of dragon fruit (Hylocereus spp.). Sri Lankan J. Agric. Sci. 2006, 42, 81–91. [Google Scholar]
- Le Bellec, F.; Vaillant, F.; Imbert, E. Pitahaya (Hylocereus spp.): A new fruit crop, a market with a future. Fruits 2006, 61, 237–250. [Google Scholar] [CrossRef]
- Choo, W.S.; Yong, W.K. Antioxidant properties of two species of Hylocereus fruits. Adv. Appl. Sci. Res. 2011, 2, 418–425. [Google Scholar]
- Woo, K.K.; Chong, Y.Y.; Li Hiong, S.K.; Tang, P. Pectin extraction and characterization from red dragon fruit (Hylocereus polyrhizus): A preliminary study. J. Biol. Sci. 2010, 10, 631–636. [Google Scholar] [CrossRef]
- Zhong, X.Y.; Zhang, S.Y.; Wang, H.; Yang, J.Y.; Li, L.; Zhu, J.; Liu, Y.J. Ultrasound-alkaline combined extraction improves the release of bound polyphenols from pitahaya (Hylocereus undatus ‘Foo-Lon’) peel: Composition, antioxidant activities and enzyme inhibitory activity. Ultrason. Sonochem. 2022, 90, 106213. [Google Scholar] [CrossRef]
- Neina, D. The role of soil pH in plant nutrition and soil remediation. Appl. Environ. Soil Sci. 2019, 2019, 5794869. [Google Scholar] [CrossRef]
- Castro, J.C.; Endo, E.H.; De Souza, M.R.; Zanqueta, E.B.; Polonio, J.C.; Pamphile, J.A.; Ueda-Nakamura, T.; Nakamura, C.V.; Dias Filho, B.P.; Abreu Filho, B.A. Bioactivity of essential oils in the control of Alternaria alternata in dragon fruit (Hylocereus undatus Haw.). Ind. Crops Prod. 2017, 97, 101–109. [Google Scholar] [CrossRef]
- Wu, J.; Zhan, R.; Liu, F.; Cang, J. First report of a stem and fruit spot of pitaya caused by Aureobasidium pullulans in China. Plant Dis. 2017, 101, 249. [Google Scholar] [CrossRef]
- Xie, F.F.; Chen, C.B.; Chen, J.Y.; Chen, J.X.; Hua, Q.Z.; Shah, K.; Zhang, Z.K.; Zhao, J.T.; Hu, G.B.; Chen, J.Y.; et al. Betalain biosynthesis in red pulp pitaya is regulated via HuMYB132: A R-R type MYB transcription factor. BMC Plant Biol. 2023, 23, 28. [Google Scholar] [CrossRef] [PubMed]
- Xiong, R.; Liu, C.; Xu, M.; Wei, S.S.; Huang, J.Q.; Tang, H. Transcriptomic analysis of flower induction for long-day pitaya by supplementary lighting in short-day winter season. BMC Genom. 2020, 21, 329. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.J.; Huang, L.F.; Huang, F.Z.; Lu, G.F.; Wei, S.T.; Liu, C.A.; Liang, G.D. Temporal transcriptome analysis provides molecular insights into flower development in red-flesh pitaya. Electron. J. Biotechnol. 2022, 58, 55–69. [Google Scholar] [CrossRef]
- Wang, Y. Identification of pitaya germplasm resources and exploitation of SCAR molecular markers for red and white pulp of pitaya. Master’s Thesis, South China Agricultural University, Guangzhou, China, 2021. [Google Scholar]
- Qin, Y.H.; Hu, G.B.; Lu, X.; Rao, D.H.; Sun, Q.M.; Wu, P.Y.; Zhang, Z.K.; Sun, L.Y.; Xie, F.F.; Wang, Y. NY/T4211-2022; Guidelines for the conduct of tests for distinctness, uniformity and stability-Hylocereus (Berger) Britt. et Rose. Ministry of Agriculture and Rural Affairs of the People’s Republic of China: Beijing, China, 2022.
- Hua, Q.Z.; Chen, C.J.; Chen, Z.; Chen, P.K.; Ma, Y.W.; Wu, J.Y.; Zheng, J.; Hu, G.B.; Zhao, J.T.; Qin, Y.H. Transcriptomic analysis reveals key genes related to betalain biosynthesis in pulp coloration of Hylocereus polyrhizus. Front. Plant Sci. 2016, 6, 1179. [Google Scholar]
- Akaike, H. On entropy maximisation principle. In Applications of Statistics; Krishnaiah, P.R., Ed.; North-Holland Publishing Company: Amsterdam, The Netherlands, 1977; pp. 27–47. [Google Scholar]
- Gao, K.; Song, X.; Kong, D.; Dai, S. Genetic analysis of leaf traits in small-flower chrysanthemum (Chrysanthemum × morifolium Ramat.). Agronomy 2020, 10, 697. [Google Scholar] [CrossRef]
- Wu, C.A.; Streisfeld, M.A.; Nutter, L.I.; Cross, K.A.; Ian, D. The genetic basis of a rare flower color polymorphism in Mimulus lewisii provides insight into the repeatability of evolution. PLoS ONE 2013, 8, e81173. [Google Scholar] [CrossRef] [PubMed]
- Shen, P.; Gao, S.P.; Chen, X.; Lei, T.; Li, W.J.; Huang, Y.X.; Li, Y.R.; Jiang, M.Y.; Hu, D.; Duan, Y.F.; et al. Genetic analysis of main flower characteristics in the F1 generation derived from intraspecific hybridization between Plumbago auriculata and Plumbago auriculata f. alba. Sci. Hortic. 2020, 274, 109652. [Google Scholar] [CrossRef]
- Cai, Z.; Yue, J.Y.; Wang, Y.W.; Jing, T.H.; Lei, J.J.; Xue, L. Advances in breeding and petal coloration mechanism of red-flowered strawberry. J. Fruit Sci. 2024, 41, 155–161. [Google Scholar]
- Asen, S.; Stewart, R.N.; Norris, K.H. Anthocyanin, flavonol copigments, and pH responsible for larkspur flower color. Phytochemistry 1975, 14, 2677–2682. [Google Scholar] [CrossRef]
- Yang, Q.; Yuan, T.; Sun, X.B. Preliminary studies on the changes of flower color during the flowering period in two tree peony cultivars. Acta Hortic. Sin. 2015, 42, 930–938. [Google Scholar]
- Chen, C.; Zhou, G.; Chen, J.; Liu, X.; Lu, X.; Chen, H.; Tian, Y. Integrated metabolome and transcriptome analysis unveils novel pathway involved in the formation of yellow peel in cucumber. Int. J. Mol. Sci. 2021, 22, 1494. [Google Scholar] [CrossRef]
- Ogata, J.; Kanno, Y.; Itoh, Y.; Tsugawa, H.; Suzuki, M. Plant biochemistry: Anthocyanin biosynthesis in roses. Nature 2005, 435, 75–758. [Google Scholar] [CrossRef]
- Tanaka, Y.; Sasaki, N.; Ohmiya, A. Biosynthesis of plant pigments: Anthocyanins, betalains and carotenoids. Plant J. 2008, 54, 733–749. [Google Scholar] [CrossRef]
- Song, X.B.; Tian, Y.K.; Gao, K.; Li, J.Z.; Wang, J.Y.; Deng, C.Y.; Zhang, F.; Kong, D.Y.; Fan, G.X.; Dai, S.L. Genetic and QTL analysis of flower color and pigments in small-flowered chrysanthemum based on high-density genetic map. Ornam. Plant Res. 2023, 3, 17. [Google Scholar] [CrossRef]
- Ji, S.Q.; Wang, L.R.; Li, Y.; Zhu, G.G.; Cao, K.; Fang, W.C.; Chen, C.W.; Wang, X.W.; Zhang, Q.; Wu, J.L. Identification of peach flower genotype (Non-showy/Showy), development and utilization of trait related molecular markers. J. Fruit Sci. 2022, 11, 15. [Google Scholar]
- Yang, X.D.; Su, J.S.; Qu, Y.X.; Jiang, J.F.; Guan, Z.Y.; Fang, W.M.; Chen, F.D.; Zhang, F. Dissecting the inheritance pattern of the anemone flower type and tubular floral traits of chrysanthemum in segregating F1 populations. Euphytica 2023, 219, 16. [Google Scholar] [CrossRef]
- Meng, G.; Zhu, G.; Fang, W.; Chen, C.; Wang, X.; Wang, L.; Cao, K. Identification of loci for single/double flower trait by combining genome-wide association analysis and bulked segregant analysis in peach (Prunus persica). Plant Breed. 2019, 138, 360–367. [Google Scholar] [CrossRef]
- Yagi, M.; Yamamoto, T.; Isobe, S.; Tabata, S.; Hirakawa, H.; Yama-guchi, H.; Tanase, K.; Onozaki, T. Identification of tightly linked SSR markers for flower type in carnation (Dianthus caryophyllus L.). Euphytica 2014, 198, 175–183. [Google Scholar] [CrossRef]
- Teng, F.; Zhai, L.H.; Liu, R.X.; Bai, W.; Wang, L.Q.; Huo, D.A.; Tao, Y.S.; Zheng, Y.L.; Zhang, Z.X. ZmGA3ox2, a candidate gene fora major QTL, qPH 3.1, for plant height in maize. Plant J. 2013, 73, 405–416. [Google Scholar] [CrossRef] [PubMed]
- Tong, H.Y.; Xiao, Y.H.; Liu, D.P.; Gao, S.P.; Liu, L.C.; Yin, Y.H.; Jin, Y.; Qian, Q.; Chu, C.C. Brassinosteroid regulates cell elongation by modulating gibberellin metabolism in rice. Plant Cell 2014, 26, 4376–4393. [Google Scholar] [CrossRef] [PubMed]
- Snider, J.L.; Oosterhuis, D.M. How does timing, duration, and severity of heat stress influence pollen-pistil interactions in angiosperms? Plant Signal. Behav. 2011, 6, 930–933. [Google Scholar] [CrossRef] [PubMed]
- Vuletin Selak, G.; Perica, S.; Goreta Ban, S.; Poljak, M. The effect of temperature and genotype on pollen performance in olive (Olea europaea L.). Sci. Hortic. 2013, 156, 38–46. [Google Scholar] [CrossRef]
- Carpenedo, S.; Bassols, M.C.; Franzon, R.C.; Byrne, D.H.; Silva, J.B. Stigmatic receptivity of peach flowers submitted to heat stress. Acta Sci. Agron. 2020, 42, e42450. [Google Scholar] [CrossRef]
- Moreira, R.A.; Rodrigues, M.A.; Souza, R.C.; Silva, A.D.; Silva, F.O.; Lima, C.G.; Pasqual, M. Natural and artificial pollination of white-fleshed pitaya. An. Acad. Bras. Ciências 2022, 94, e20211200. [Google Scholar] [CrossRef] [PubMed]
- Sarah, T.K.; James, W.O. Impact of cross- and self-pollination on fruit set, fruit size, seed number, and harvest timing among 13 southern highbush blueberry cultivars. Annu. Meet. Fla. State Hortic. Soc. 2016, 26, 213–219. [Google Scholar]
- Simon-Porcar, V.I.; Pico, F.X.; Arroyo, J. Range-wide population genetics and variation in morph ratio in style-dimorphic Narcissus papyraceus. Am. J. Bot. 2015, 102, 449–456. [Google Scholar] [CrossRef]

| Flower Traits | Cross Combinations | Parents | F1 Progenies | ||||||
|---|---|---|---|---|---|---|---|---|---|
| HHQL | DH | MP | F ± S | CV (%) | Ta (%) | Hm | RHm (%) | ||
| Flower length (mm) | D × H | 229.54 | 302.97 | 266.26 | 254.34 ± 26.58 | 10.05 | 95.53 | −11.29 ** | −4.47 |
| H × D | 269.09 ± 25.15 | 9.33 | 101.06 | 2.83 * | 1.06 | ||||
| Perianth width (mm) | D × H | 38.36 | 57.87 | 48.11 | 51.90 ± 6.01 | 11.57 | 107.88 | 3.79 ** | 7.88 |
| H × D | 49.07 ± 4.92 | 10.02 | 101.99 | 0.96 ** | 1.10 | ||||
| Calyx tube width (mm) | D × H | 18.72 | 29.67 | 24.19 | 24.33 ± 7.47 | 30.70 | 71.52 | −9.68 | −28.48 |
| H × D | 21.26 ± 3.19 | 15.02 | 87.89 | −9.85 ** | −12.11 | ||||
| Distance between stigma and anther (mm) | D × H | 22.21 | 0 | 11.1 | 2.88 ± 5.85 | 203.22 | 25.94 | −8.22 ** | −74.06 |
| H × D | 32.19 ± 23.16 | 71.96 | 290.00 | 21.09 ** | 190.00 | ||||
| Betalain content (mg/100 g FW) | D × H | 5.65 | 1.2 | 3.43 | 2.13 ± 1.80 | 84.60 | 62.10 | −1.30 ** | −37.90 |
| H × D | 2.49 ± 1.64 | 66.13 | 72.59 | −0.94** | −27.41 | ||||
| Petal number | D × H | 12.0 | 22.0 | 17.0 | 16.72 ± 3.00 | 17.92 | 97.86 | −0.28 | −2.15 |
| H × D | 13.47 ± 2.89 | 21.43 | 79.26 | −3.35 ** | −20.74 | ||||
| Stigma number | D × H | 17.0 | 28.0 | 23.0 | 21.64 ± 2.51 | 11.62 | 96.18 | −1.36 ** | −3.83 |
| H × D | 20.72 ± 2.97 | 14.35 | 90.08 | −2.28 ** | −9.92 | ||||
| Model | Flower Length (mm) | Perianth Width (mm) | Calyx Tube Width (mm) | Distance between Stigma and Anther (mm) | Petal Betalain Content (mg/100 g FW) | Petal Number | Stigma Number | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D × H | H × D | D × H | H × D | D × H | H × D | D × H | H × D | D × H | H × D | D × H | H × D | D × H | H × D | |
| 0MG | 2344.12 | 3160.72 | 1603.52 | 2051.85 | 1711.89 | 1844.29 | 1590.67 | 3104.81 | 1002.97 | 1306.18 | 1259.75 | 1688.98 | 1170.77 | 1709.08 |
| 1MG-AD | 2330.97 | 3155.03 | 1598.50 | 2037.49 | 1217.70 | 1844.40 | 3038.96 | 554.13 | 999.885 | 1258.30 | 1671.21 | 1166.52 | 1699.99 | |
| 1MG-A | 2329.94 | 3153.93 | 1596.84 | 2038.31 | 1223.22 | 1843.32 | 1091.66 | 3044.28 | 551.52 | 1052.40 | 1256.95 | 1669.26 | 1163.36 | 1700.90 |
| 1MG-EAD | 2331.97 | 3160.29 | 1602.79 | 2045.65 | 1439.28 | 1845.95 | 3049.49 | 551.94 | 1007.09 | 1259.58 | 1678.85 | 1167.36 | 1703.53 | |
| 1MG-NCD | 2348.12 | 3163.74 | 1603.25 | 2052.14 | 1715.49 | 1846.36 | 1439.73 | 3085.58 | 903.10 | 1205.02 | 1261.61 | 1680.62 | 1174.77 | 1701.68 |
| 2MG-ADI | 2351.10 | 3169.81 | 1610.48 | 2056.19 | 1193.38 | 1857.92 | 1343.41 | 3071.57 | 831.73 | 1104.45 | 1272.48 | 1683.09 | 1179.34 | 1713.17 |
| 2MG-AD | 2324.89 | 3145.39 | 1600.10 | 2032.45 | 1185.18 | 1843.43 | −12,002.29 | −1904.88 | −168.61 | 977.13 | 1672.80 | |||
| 2MG-A | 2328.97 | 3155.59 | 1597.05 | 2032.95 | 1188.64 | 1841.92 | −19,589.97 | 3108.65 | 651.66 | 985.18 | 1257.56 | 1670.33 | 1702.89 | |
| 2MG-EA | 2327.04 | 3151.64 | 1597.27 | 2030.63 | 1186.92 | 1841.71 | −14,152.0 | −1398.03 | 648.13 | 1096.52 | 1256.36 | 1669.47 | −1706.02 | 1694.80 |
| 2MG-CD | 2347.41 | 3164.72 | 1607.53 | 2055.85 | 1715.88 | 1848.30 | 1594.67 | 3108.82 | 1006.97 | 1310.18 | 1263.76 | 1692.98 | 1170.81 | 1713.08 |
| 2MG-EAD | 2345.41 | 3162.72 | 1605.53 | 2053.85 | 1713.88 | 1846.30 | 1592.67 | 3106.82 | 1004.97 | 1308.18 | 1261.76 | 1690.98 | 1169.07 | 1711.08 |
| Characteristics | Cross Combinations | Model | U12 | U22 | U32 | nW2 | Dn |
|---|---|---|---|---|---|---|---|
| Flower length (mm) | D × H | 2MG-AD | 0.0009 (0.9761) | 0.0017 (0.9676) | 0.0022 (0.9629) | 0.0081 (1.0005) | 0.0205 (>0.05) |
| H × D | 2MG-AD | 0.0003 (0.9867) | 0.0003 (0.9871) | 0.0000 (0.9999) | 0.008 (1.0006) | 0.0144 (>0.05) | |
| Perianth width (mm) | D × H | 1MG-A | 0.006 (0.9383) | 0.0052 (0.9427) | 0.0002 (0.9901) | 0.025 (0.9896) | 0.0289 (>0.05) |
| H × D | 2MG-EA | 0.0013 (0.9708) | 0.0006 (0.9799) | 0.0017 (0.9672) | 0.0268 (09855) | 0.0225 (>0.05) | |
| Calyx tube width (mm) | D × H | 2MG-AD | 0.0033 (0.9544) | 0.0005 (0.9815) | 0.0165 (0.8978) | 0.0147 (0.9997) | 0.0207 (>0.05) |
| H × D | 2MG-EA | 0.0000 (0.9997) | 0.0000 (0.9996) | 0.0000 (0.9973) | 0.0325 (0.9672) | 0.0373 (>0.05) | |
| Stigma relative to anther distance (mm) | D × H | 2MG-A | 72.5467 (0.0000) | 54.2533 (0.0000) | 12.4266 (0.0000) | 12.7164 (0.0000) | 0.5325 (<0.05) |
| H × D | 2MG-AD | 0.1137 (0.7359) | 0.0028 (0.9581) | 2.2991 (0.1295) | 0.1861 (0.2973) | 0.112 (<0.05) | |
| Betalain content (mg/100 g FW) | D × H | 2MG-AD | 0.6366 (0.4249) | 0.3363 (0.5620) | 0.5936 (0.4410) | 0.1015 (0.5880) | 0.0463 (<0.05) |
| H × D | 2MG-AD | 0.0049 (0.9444) | 0.0017 (0.9669) | 0.1904 (0.6626) | 0.0736 (0.738) | 0.0418 (>0.05) | |
| Petal number | D × H | 2MG-EA | 0.0014 (0.9699) | 0.0001 (0.993) | 0.0124 (0.9113) | 0.2215 (0.2328) | 0.0716 (>0.05) |
| H × D | 2MG-EA | 0.0006 (0.9803) | 0.0029 (0.9568) | 0.0146 (0.9038) | 0.3832 (0.0836) | 0.0810 (<0.05) | |
| Stigma number | D × H | 2MG-EA | 0.3124 (0.5762) | 0.7375 (0.3905) | 1.6138 (0.204) | 0.6253 (0.0195) | 0.1565 (<0.05) |
| H × D | 2MG-EA | 0.0000 (0.9958) | 0.0019 (0.9654) | 0.0376 (0.8463) | 0.3495 (0.1037) | 0.0978 (<0.05) |
| Genetic Parameters | Flower Length (mm) | Perianth Width (mm) | Calyx Tube Width (mm) | Distance between Stigma and Anther (mm) | Betalain Content (mg/100 g FW) | Petal Number | Stigma Number | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D × H | H × D | D × H | H × D | D × H | H × D | D × H | H × D | D × H | H × D | D × H | H × D | D × H | H × D | |
| M | 261.207 | 271.055 | 51.942 | 49.116 | 25.310 | 24.170 | 3.176 | 28.483 | 2.627 | 3.042 | 16.79 | 13.48 | 21.51 | 20.72 |
| da | 15.130 | 19.552 | 6.674 | 2.884 | 1.033 | 2.220 | 2.625 | 19.629 | 1.704 | 1.910 | 1.49 | 1.65 | 0.74 | 2.33 |
| db | 8.918 | −3.737 | 1.798 | 2.890 | 8.855 | 0.154 | 0.216 | |||||||
| ha | −10.876 | 4.488 | −1.309 | 10.335 | −1.537 | −0.635 | ||||||||
| hb | −3.179 | −8.449 | −1.071 | 3.038 | −0.032 | −0.023 | ||||||||
| I | ||||||||||||||
| jab | ||||||||||||||
| jba | ||||||||||||||
| L | ||||||||||||||
| σ2mg | 646.179 | 0.000 | 13.072 | 0.000 | 0.000 | 0.000 | 0.000 | 463.822 | 0.000 | 1.255 | 2.53 | 0.00 | 6.37 | 7.49 |
| h2mg (%) | 91.108 | 0.000 | 36.080 | 0.000 | 0.000 | 0.000 | 0.000 | 86.452 | 0.000 | 46.404 | 27.77 | 0.00 | 100.00 | 84.67 |
| Cultivars Traits | Petal Color | Stigma Color | Stigma Number | Stigma Split | Stigma–Anther Relative Position | Petal Number | Compatible/ Incompatible |
|---|---|---|---|---|---|---|---|
| DH | white | Yellow–green | 28.0 | absent | Equal | 22.0 | compatible |
| HHQL | red | faint yellow | 17.0 | present | Higher | 12.0 | incompatible |
| Primer Names | Sequences (5′-3′) | Primer Names | Sequences (5′-3′) |
|---|---|---|---|
| SCoT-12 | ACGACATGGCGACCAACG | SCoT-56 | ACAATGGCTACCACTAGC |
| SCoT-13 | ACGACATGGCGACCATCG | SCoT-58 | ACAATGGCTACCACTAGG |
| SCoT-19 | ACCATGGCTACCACCGGC | SCoT-61 | CAACAATGGCTACCACCG |
| SCoT-21 | ACGACATGGCGACCCACA | SCoT-62 | ACCATGGCTACCACGGAG |
| SCoT-36 | GCAACAATGGCTACCACC | SCoT-63 | ACCATGGCTACCACGGGC |
| SCoT-42 | ACCATGGCTACCACCGAT | SCoT-64 | ACCATGGCTACCACGGTC |
| SCoT-47 | ACAATGGCTACCACTGCC | SCoT-67 | ACCATGGCTACCAGCGGC |
| SCoT-49 | ACAATGGCTACCACTGCG | SCoT-73 | CCATGGCTACCACCGGCT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, T.; Xing, K.; Chen, J.; Sabir, I.A.; Shah, K.; Chen, J.; Zhang, Z.; Zhao, J.; Hu, G.; Qin, Y. Genetic Analyses of Flower Main Traits from Two Pitayas and Their Progenies: A Cactus Plant. Plants 2024, 13, 699. https://doi.org/10.3390/plants13050699
Zhang T, Xing K, Chen J, Sabir IA, Shah K, Chen J, Zhang Z, Zhao J, Hu G, Qin Y. Genetic Analyses of Flower Main Traits from Two Pitayas and Their Progenies: A Cactus Plant. Plants. 2024; 13(5):699. https://doi.org/10.3390/plants13050699
Chicago/Turabian StyleZhang, Tiantian, Kangmin Xing, Jiayi Chen, Irfan Ali Sabir, Kamran Shah, Jiaxuan Chen, Zhike Zhang, Jietang Zhao, Guibing Hu, and Yonghua Qin. 2024. "Genetic Analyses of Flower Main Traits from Two Pitayas and Their Progenies: A Cactus Plant" Plants 13, no. 5: 699. https://doi.org/10.3390/plants13050699
APA StyleZhang, T., Xing, K., Chen, J., Sabir, I. A., Shah, K., Chen, J., Zhang, Z., Zhao, J., Hu, G., & Qin, Y. (2024). Genetic Analyses of Flower Main Traits from Two Pitayas and Their Progenies: A Cactus Plant. Plants, 13(5), 699. https://doi.org/10.3390/plants13050699








