Abstract
MADS-box is a key transcription factor regulating the transition to flowering and flower development. Lagerstroemia indica ‘Xiang Yun’ is a new cultivar of crape myrtle characterized by its non-fruiting nature. To study the molecular mechanism underlying the non-fruiting characteristics of ‘Xiang Yun’, 82 MADS-box genes were identified from the genome of L. indica. The physicochemical properties of these genes were examined using bioinformatics methods, and their expression as well as endogenous hormone levels at various stages of flower development were analyzed. The results showed that LiMADS genes were primarily classified into two types: type I and type II, with the majority being type II that contained an abundance of cis-acting elements in their promoters. By screening nine core proteins by predicted protein interactions and performing qRT-PCR analysis as well as in combination with transcriptome data, we found that the expression levels of most MADS genes involved in flower development were significantly lower in ‘Xiang Yun’ than in the wild type ‘Hong Ye’. Hormonal analysis indicated that ‘Xiang Yun’ had higher levels of iP, IPR, TZR, and zeatin during its early stages of flower development than ‘Hong Ye’, whereas the MeJA content was substantially lower at the late stage of flower development of ‘Hong Ye’. Finally, correlation analysis showed that JA, IAA, SA, and TZR were positively correlated with the expression levels of most type II genes. Based on these analyses, a working model for the non-fruiting ‘Xiang Yun’ was proposed. During the course of flower development, plant hormone response pathways may affect the expression of MADS genes, resulting in their low expression in flower development, which led to the abnormal development of the stamen and embryo sac and ultimately affected the fruiting process of ‘Xiang Yun’.
1. Introduction
Flowers serve as primary reproductive organs in angiosperms. A typical flower is composed of four concentric whorls of structures: the calyx, petals, stamens, and pistils. The flowering process in plants is governed by a multitude of signals and a sophisticated regulatory network involving numerous genes [1]. Currently, the prevailing molecular mechanism of plant flowering is based on the ABCDE model proposed for Arabidopsis thaliana and Antirrhinum majus [2]. These five classes of genes exhibit distinct homozygous and heterozygous functions, which are associated with various developmental processes. Specifically, genes in classes A and E operate in whorl I to establish sepals, while genes in classes A, B, and E participate in whorl II to determine petals. In whorl III, genes in classes B, C, and E contribute to the formation of the stamens, whereas genes in classes C and E are responsible for the development of the carpels in whorl IV. Lastly, genes in classes C, D, and E are involved in specifying ovules [3,4].
In the ABCDE model, all genes, excluding AP2, belong to the MADS box gene family [5]. The MADS box gene family is comprised of transcriptional regulators with vital functions and encompasses a broad group of gene families. They are extensively distributed throughout the plant kingdom and play important regulatory roles in plant growth, development, and signaling processes, such as vernalization, inflorescence meristem differentiation, embryo development, and fruit ripening [6,7]. Based on the C-terminal structure, functional role, and evolutionary rate of MADS-box proteins, the MADS-box family can be categorized into two distinct taxa: Type I and Type II [8]. Type I genes consist solely of the MADS structural domain and do not contain the K domain. These can be further divided into three subfamilies: Mα, Mβ, and Mγ. Research has demonstrated that MADS-box genes are involved in the regulation of female gametophyte and endosperm development in Arabidopsis and Gramineae [9]. Type II genes feature three more structural domains than Type I genes: a more conserved intervening structural domain (I structural domain), a moderately conserved keratin-like helical structural domain (K structural domain), and a variable C-terminal structural domain (C structural domain) [10,11]. The MADS box genes can be divided into two subfamilies, MIKC* and MIKCC. Research has demonstrated that MIKCC-type genes play a regulatory role in various aspects of sporophytic development in higher plants [12], while MIKC*-type genes are essential for male gametophyte development in the model plant A. thaliana [13,14].
Plant hormones, such as auxin, gibberellin (GA), cytokinin (CK), and abscisic acid (ABA), play crucial roles in the regulation of various cellular activities, including cell division, differentiation, reproduction, and responses to abiotic and biotic stresses. For instance, IAA as auxin controls the differentiation of the meristem into vascular tissue and promotes the development of various organs [15]. GAs, on the other hand, are mainly responsible for seed dormancy, bud elongation, seed germination, fruit, and flower maturation. For example, GA induces seed germination when exposed to cold or light [16]. ABA often interacts with other hormones, such as GA and cytokinin, to inhibit seed germination and post-germination growth, protecting plants from abiotic stresses [17]. CKs can maintain the growth potential of the stem tip meristem (pluripotency), promoting cell division and increasing cell expansion during the proliferative and amplified phases of leaf cell development [18]. Furthermore, other hormones, including jasmonic acid (JA) and salicylic acid (SA) play significant roles in plant responses to external stresses.
Lagerstroemia indica, a woody ornamental plant belonging to the family Lythraceae [19,20], is renowned for its stunning, colorful blossoms, extended flowering period, and resistance to air pollution. These features make it an ideal choice for planting in courtyards, parks, roadside areas, indoor bonsai, and cut-flower arrangements. It is a highly regarded summer ornamental tree species in China. By chance, our research team discovered a mutant of L. indica, which was named ‘Xiang Yun’ [21]. This cultivar is devoid of fruit-bearing capabilities and has a longer flowering period than the typical L. indica. Early observations of its phenotypic and physiological indicators revealed that the pollen was inactive and microspore fertility was low. The anatomic study on the process of differentiation showed that the embryo sac abortion was caused by the abnormal tapetum layer during the embryo sac development of ‘Xiang Yun’ [22]. However, the underlying mechanism by which genes are responsible for its flower organ development and interactions with plant hormones remains unclear.
In this study, we utilized bioinformatics techniques to identify the MADS gene family members in the whole genome of L. indica and analyzed their physicochemical properties, structural characteristics, and cis-acting elements. Through the comparison of differences between ‘Xiang Yun’ and wild type ‘Hong Ye’ in gene expression and endogenous hormone levels during flower organ development, a working model underlying the lack of fruit setting was proposed. The results from this study could provide a theoretical basis for understanding the developmental mechanisms of L. indica flower organs and for exploring the molecular basis of sterility observed in ‘Xiang Yun’.
2. Results
2.1. Identification of LiMADS Gene Family Members and Physicochemical Analysis
A comprehensive screening of the MADS candidate gene family in L. indica was conducted using the resources provided by NCBI and SMART. Following the elimination of inaccurate genes, 82 L. indica MADS genes were identified, and their physiochemical properties were analyzed. Each gene was subsequently named and numbered according to its respective chromosomal location and designated as LiMADS1–LiMADS82 (Table 1 and Table S1). The molecular weight and amino acid length of the MADS gene family varied. LiMADS53 is the shortest at 8.74 kDa and 75 residues, and LiMADS50 is the longest at 199.61 kDa and 1784 residues. The physicochemical properties of MADS proteins differed considerably. The isoelectric points ranged from 4.54 to 10.31, whereas the instability indices indicated that the majority of the MADS proteins were unstable, with values ranging from 34.42 to 80.52. Furthermore, the fat coefficients exhibited a broad distribution, ranging from 54.21 to 108.30. Interestingly, the hydrophilicity indices were consistent with those of the hydrophilic proteins, except for MADS22. These findings suggest that MADS proteins possess a wide range of physicochemical properties. Results from subcellular localization prediction indicated that the MADS proteins were all localized in the nucleus, which was consistent with the characteristics of transcription factors, indicating that they all functioned in the nucleus.

Table 1.
Molecular weights and physicochemical properties of 82 LiMADS proteins in L. indica.
2.2. Phylogenetic Analysis of LiMADS Gene Family Members
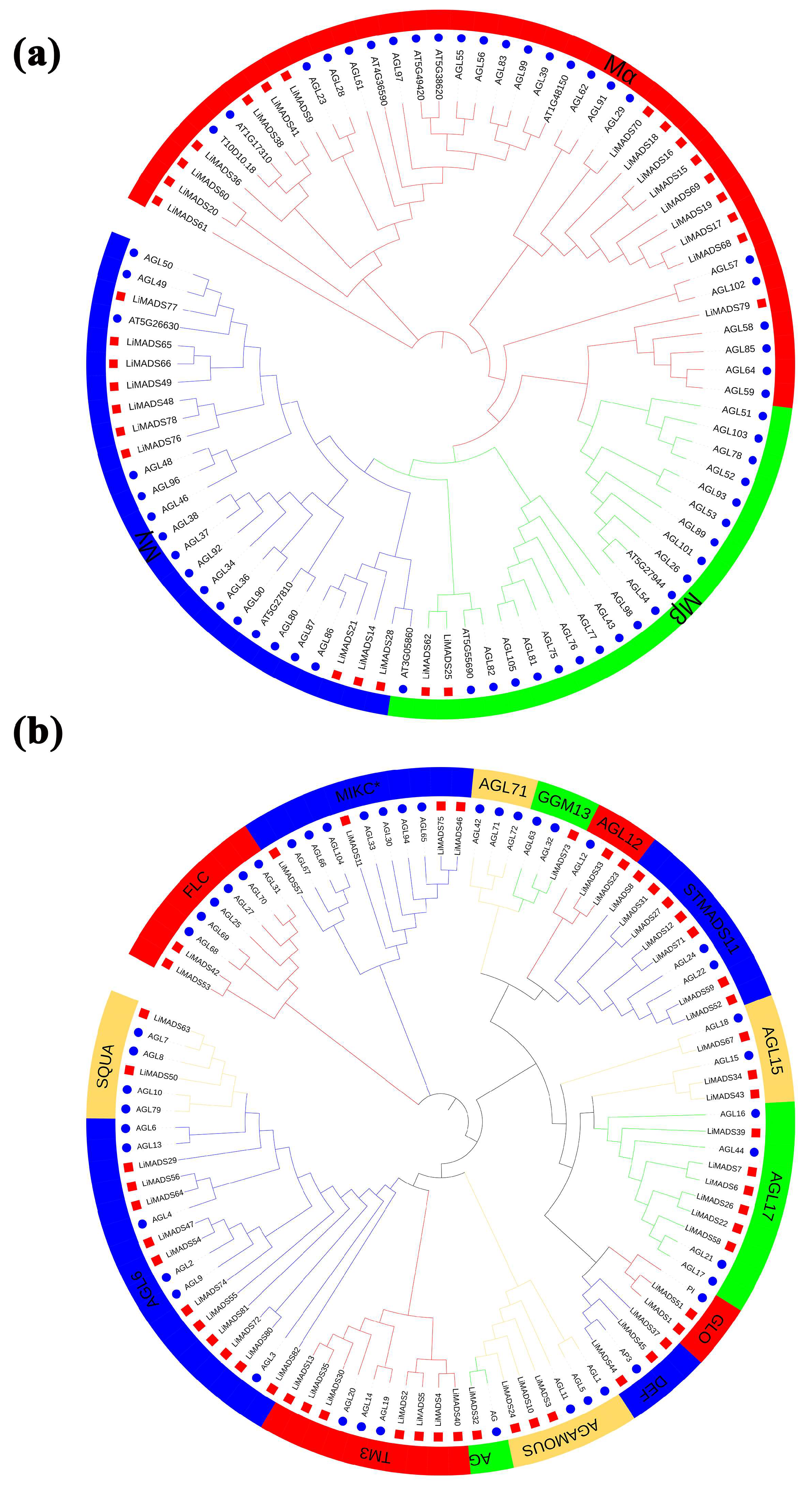
To elucidate the evolutionary relationship of LiMADS family members in L. indica, we conducted multiple sequence comparisons and clustering analyses of 82 MADS proteins isolated from L. indica with 107 MADS proteins from A. thaliana. Furthermore, their phylogenetic trees were constructed (Figure 1). Results showed that LiMADS-box members could be divided into two distinct groups, type I and type II, based on the classification of MADS-box proteins in A. thaliana. We identified three subfamilies within type I proteins, Mα, Mβ, and Mγ, based on their structural properties. In L. indica, Mα consisted of 17 out of 28 type I proteins, whereas Mβ and Mγ included 2 and 9 proteins, respectively (Figure 1a). Notably, the Mα proteins in the Mα group, which were homologous to AGL23 and AGL61 as well as to the L. indica LiMADS9 and LiMADS61, might play a role in the development of female gametophytes and embryos [23,24]. The proposed function of LiMADS25 and LiMADS62 in the Mβ subgroup was to regulate the nutrient supply to the outer testa and female gametophytes. Conversely, homologous genes of L. indica in the Mγ subgroup, such as AGL80, LiMADS14, and LiMADS28, could be responsible for promoting endosperm development with endosperm-specific functions [25,26]. The Type II proteins were characterized by the presence of MIKCC and MIKC*. Out of these Type II proteins, 49 belonged to MIKCC, while only 5 belonged to MIKC* (Figure 1b). MIKCC is a vital regulator of floral organ differentiation and was further classified into 14 subgroups based on its evolutionary history. These subgroups included FLC, AG, AGAMOUS, AGL6, GGM13, SQUA, STMADS11, DEF, and GLO, which could be important for regulating floral organ development. Additionally, subgroups, such as AGL12 [27], AGL17 [28], and TM3 [29] were involved in regulating root development, while others, such as AGL15 [30] were expressed predominantly during embryogenesis and seed development. Finally, there were specific subgroups responsible for stimulating flowering in the tips of the stems and axillary meristems. The AGL71 subpopulation [31] is crucial for the development of male gametophytes (pollen) in A. thaliana. The MIKC* complex plays a vital role in the regulation of pollen development by suppressing immature pollen genes and activating mature pollen genes. Structural domain transcription factors of the MIKC* complex are also essential [14]. Several genes expressed in the pollen of L. indica, including LiMADS11, LiMADS46, LiMADS53, LiMADS57, and LiMADS75, showed significant homology to AGL30, AGL65, AGL66, AGL67, AGL94, and AGL104.
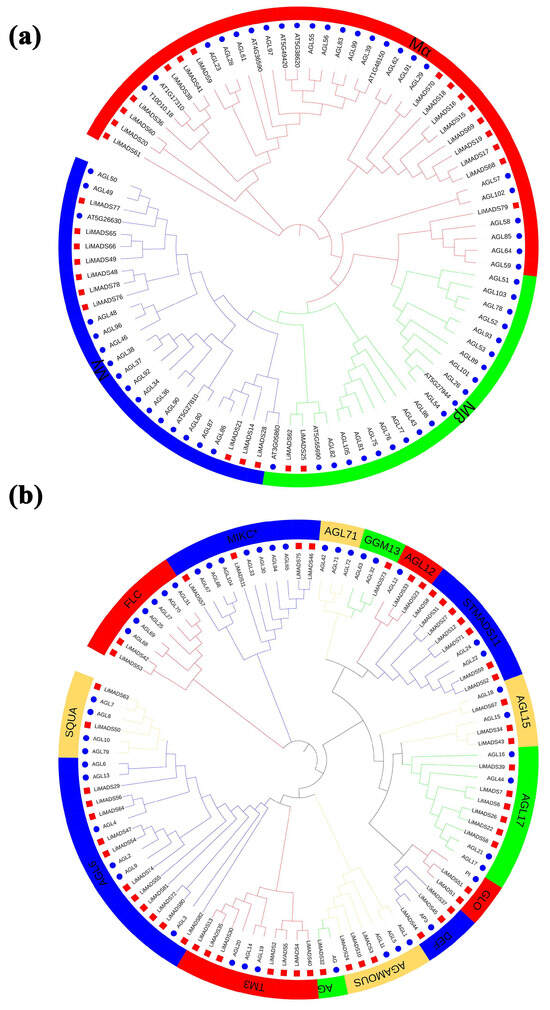
Figure 1.
Phylogenetic relationships of MADS-box genes in L. indica. The MADS-box gene in Arabidopsis is indicated by a blue circle, whereas the MADS box gene in L. indica is indicated by a red square. Subfamily names are shown in different colors. (a) Type I genes are classified as Mα, Mβ, and Mγ groups; and (b) Type II genes are divided into 14 subfamilies.
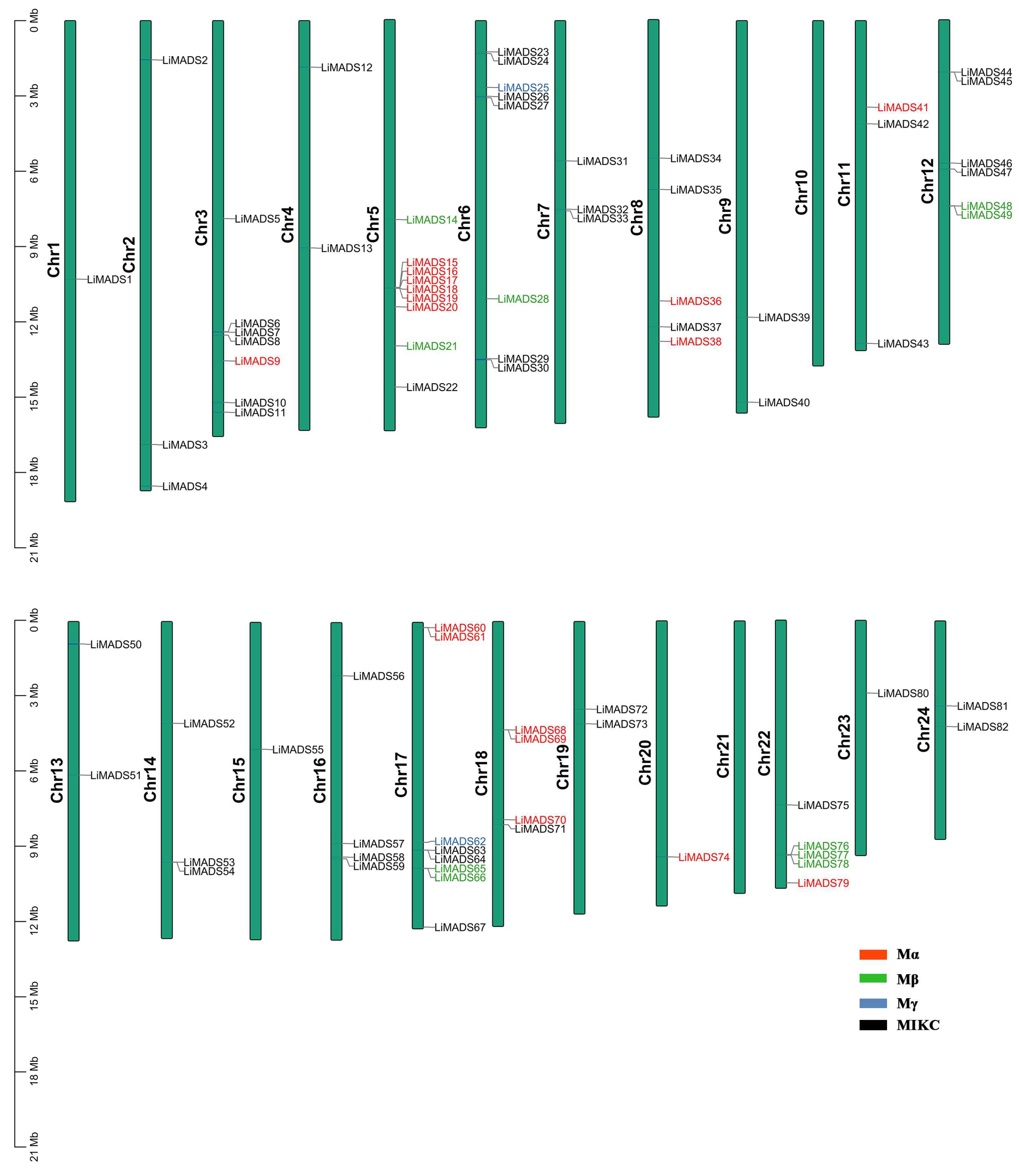
2.3. Chromosome Distribution and Gene Collinearity Analysis of LiMADS in L. indica
Employing TBtools v2.052 software, we conducted a chromosome localization analysis using the annotation data of the L. indica genome. Results showed that 82 genes were unevenly distributed across 24 chromosomes, suggesting that MADS transcription factors played multiple roles in L. indica. These genes were designated LiMADS1 to LiMADS82 according to their chromosomal locations (Figure 2). Notably, no MADS genes were present on chromosomes 10 and 21, whereas chromosome 9 harbored the highest number of MADS genes (nine genes).
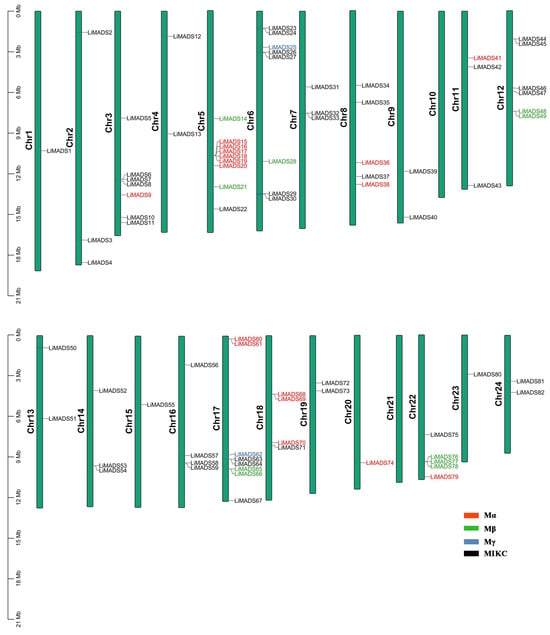
Figure 2.
LiMADS chromosome distribution in L. indica. The chromosome number is located on the left of the chromosome.
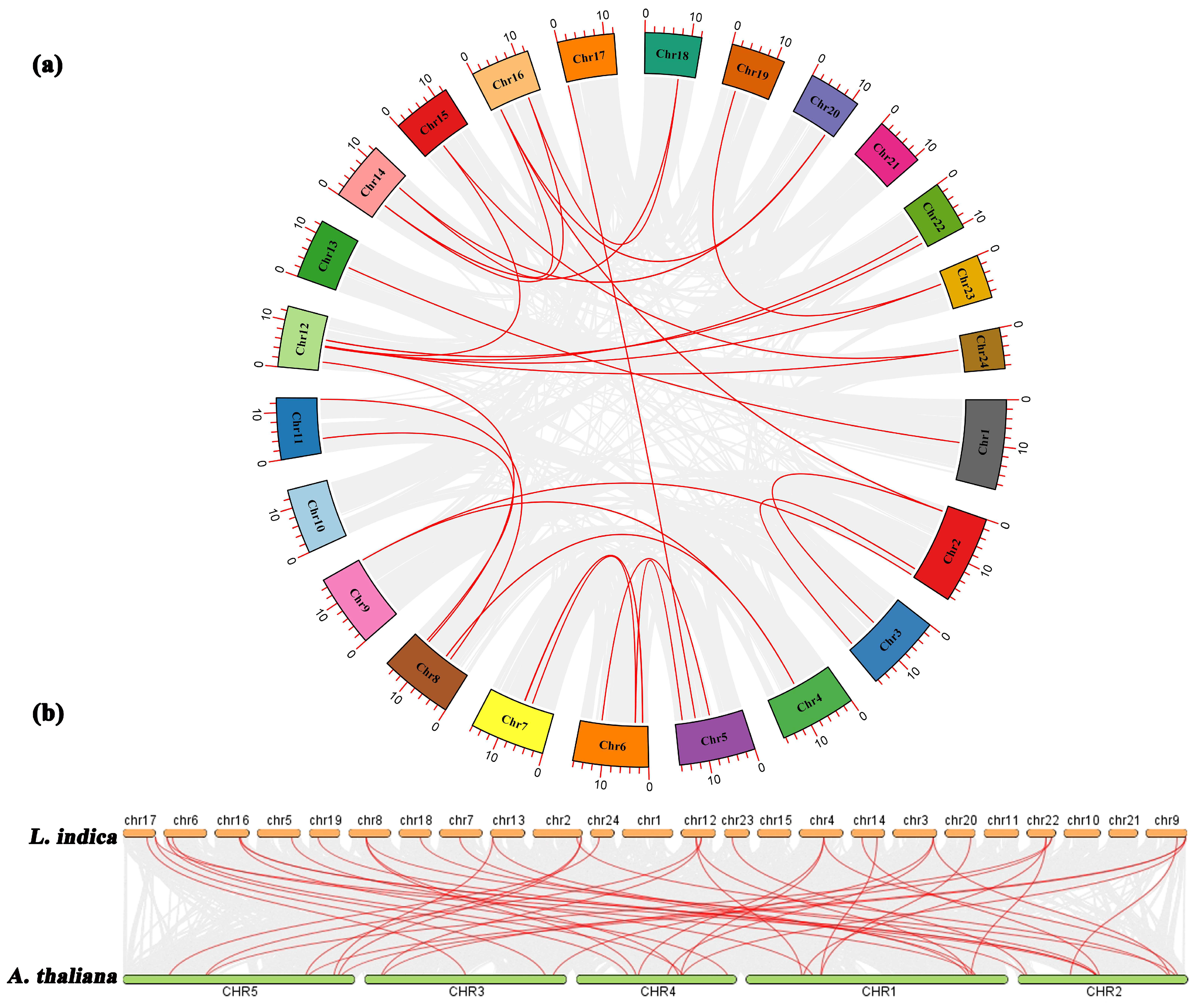
To examine the evolutionary relationships among the LiMADS genes in L. indica, we performed a BLAST comparison of the sequence similarity of all LiMADS genes (Figure 3a). The analysis revealed 29 pairs of large segment duplications and 9 pairs of closely linked genes among the 82 LiMADS genes, suggesting that tandem duplication of genes occurred. Interestingly, our analysis indicated that LiMADS6, LiMADS7, LiMADS18, LiMADS19, LiMADS44, and LiMADS45 were closely linked and could play a significant role in driving gene evolution. Moreover, covariance analysis, employed to examine the covariance between the LiMADS gene family of L. indica and the Arabidopsis genome, enabled the clarification of the origin of LiMADS genes and their evolutionary relationships (Figure 3b). In this study, we identified 45 homologous MADS genes in L. indica and A. thaliana. The MADS homologous genes were predominantly located on chromosome 22 of L. indica, whereas chromosomes 1, 10, 11, 15, and 21 did not exhibit any MADS homologues. Moreover, multiple AtMADS homologues were observed for certain LiMADS genes, and each AtMADS gene had numerous LiMADS homologues.
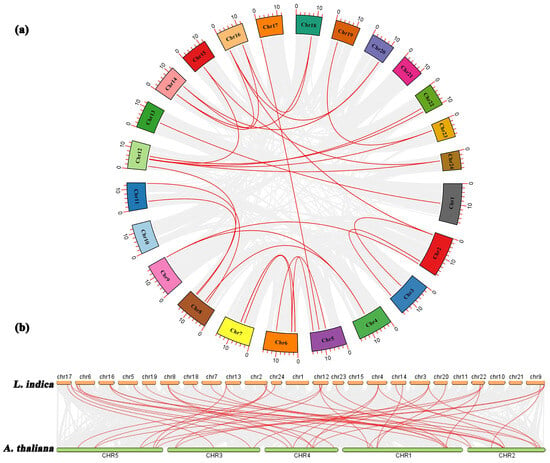
Figure 3.
Homology analysis of the MADS. (a) Collinearity analysis of the MADS gene family in L. indica, and the red line represents fragment replication within the gene family. (b) Co-linear analysis of MADS genes in L. indica and Arabidopsis. Li represents the L. indica genome. At represents the Arabidopsis genome, numbers represent chromosome numbers, gray lines represent collinear relationships between different genomes, and red lines represent collinear relationships between MADS genes.
The Ka/Ks ratio, which measures the rates of non-synonymous (Ka) and synonymous (Ks) substitutions in genetics, is a pivotal element in the evolution of nucleic acids [32]. This study demonstrated that gene pairs exhibited Ka/Ks values below 1, indicating evolution under purifying selection.
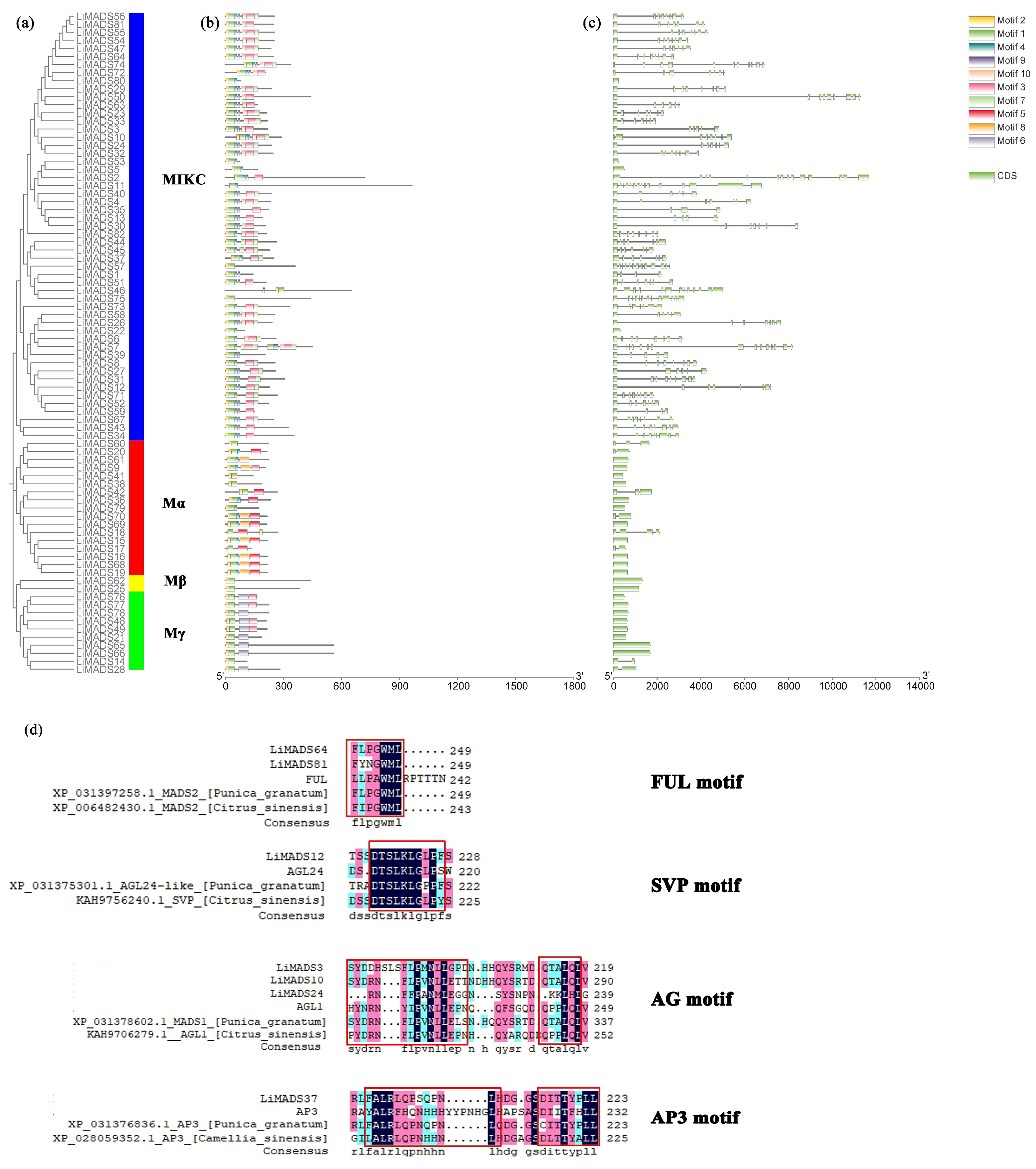
2.4. Analysis of LiMADS Gene Structure and Conserved Motifs in L. indica
Through the integration of phylogenetic trees, gene structures and motifs, we uncovered similarities and disparities in gene structures and evolutionary relationships between members of the LiMADS gene family. Our findings suggested that L. indica possesses 10 conserved motifs, with the number of conserved motifs in L. indica MADS-box proteins ranging from 2 to 12 (Figure 4b). All LiMADS proteins, comprising motif 1 and 2, comprised the typical MADS structural domains, encompassing approximately 70 amino acids. The Mα subclade of type I MADS-TF genes exhibited a predominance of motifs 5 and 8, while the Mβ subclade, comprising LiMADS25 and LiMADS62, was distinguished by the presence of only two motifs, with motifs 3 and 6 being exclusive to the Mγ subclade. The type II MADS-TF family displayed a conserved four-domain structure comprising the M, I, K, and C domains [32], with the K domain being a distinctive feature of MIKC plants and being highly conserved throughout evolution. Notably, the LiMADS11, LiMADS46, LiMADS53, LiMADS57, and LiMADS75 subfamilies within the MIKC* clade did not contain the K structural domain. The C-domain comprises various motifs that enable proteins to perform different functions (Figure 4d). On the C-terminus of the MIKC subfamily in L. indica, we identified several distinct motifs. By comparison to other plant species (Punica granatum, Citrus sinensis, and Arabidopsis), both LiMADS64 and LiMADS81 contain the FUL motif. LiMADS12 is homologous to Arabidopsis AGL24, and both contain the SVP motif in the C-terminal. LiMADS3, LiMADS10, and LiMADS24 belong to the AG subfamily and contain two AG motifs in the C-terminal. LiMADS37’s C-terminal contains a PI-derived motif with the sequence FxFRLQPSQPNLH and an euAP3 motif. The FUL, SVP, AG, and AP3 motifs are highly conserved across different species.
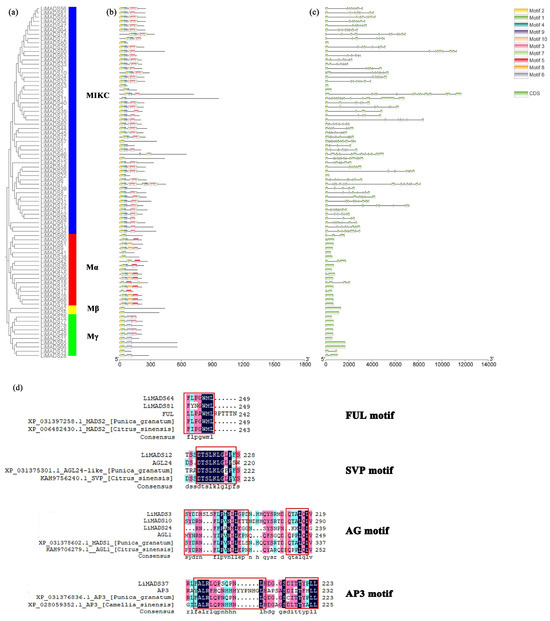
Figure 4.
Structural and conserved motif analysis of the LiMADS gene in L. indica. (a) Phylogenetic classification of LiMADS proteins; (b) Conservative motif analysis, with different patterns highlighted by colored boxes, the length of which is related to the length of the basic sequence; (c) Coding sequence of the LiMADS gene; (d) C-terminal domain differences between L. indica and different species. The red box indicates the motif’s position.
Our analysis of the gene structure suggested that most type I genes lacked introns, with only eight genes (LiMADS14, LiMADS28, LiMADS17, LiMADS18, LiMADS70, LiMADS42, LiMADS20, and LiMADS60) containing a small number of introns (Figure 4c). The results of our gene structure analysis indicated that a large proportion of type I genes lacked introns, with only eight genes (LiMADS14, LiMADS28, LiMADS17, LiMADS18, LiMADS70, LiMADS42, LiMADS20, and LiMADS60) containing a small number of them. On the other hand, all the type II genes possessed a substantial number of introns, except for four genes (LiMADS5, LiMADS22, LiMADS53, and LiMADS80) that did not have any introns. The average count of introns in the type II genes was significantly greater than that in the type I genes. LiMADS30 had the longest intron, and LiMADS2, LiMADS11, and LiMADS26 had longer introns. These genes were involved in floral transitions and had structural domains of expression typically identified in trophic tissues. Furthermore, this study revealed that intron length varied greatly among genes, indicating that type II genes have undergone increased genomic complexity during evolution.
We further cloned the MADS gene from both wild type L. indica ‘Hong Ye’ and mutant ‘Xiang Yun’ and compared their sequences (Figure S1). The results revealed that ‘Xiang Yun’ had a larger number of single nucleotide polymorphisms and base mutations compared with ‘Hong Ye’. The mutations were mainly transitions, with a few transversions, resulting in silent mutations and missense mutations in the gene sequence, which may affect the function of the protein.
2.5. Prediction of Cis-Acting Elements in the LiMADS Gene Family of L. indica
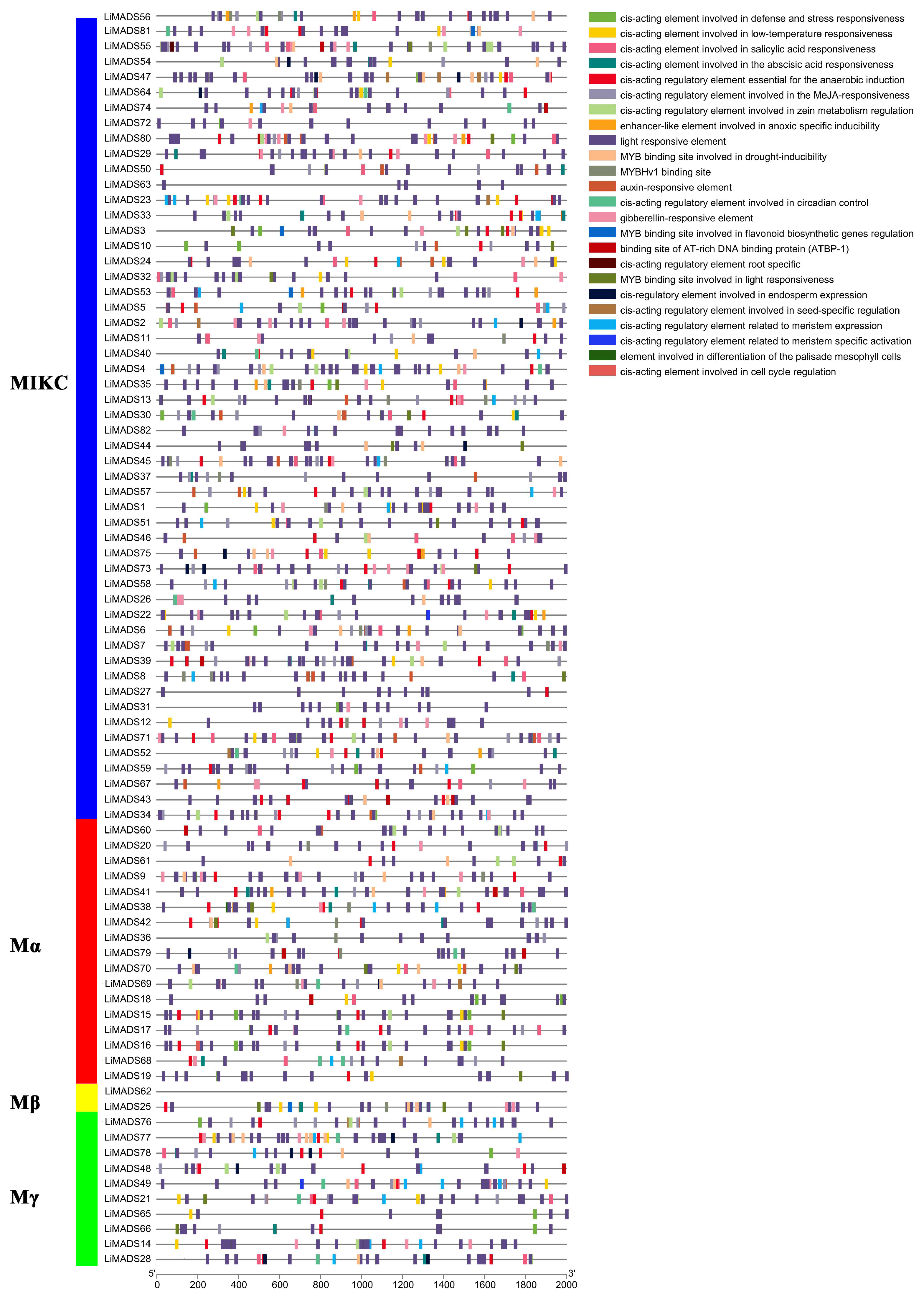
The promoter region of the LiMADS gene family of L. indica contains numerous cis-elements, which can be grouped into four categories: 25 light-responsive elements, 12 elements related to growth and development, 12 elements related to plant hormone response, and 6 elements related to biological and abiotic stresses. Among these, photoresponsive elements are the most diverse and abundant, with G-Box being present in most MADS genes (Figure 5). Light conditions may affect the expression of LiMADS, as suggested by the presence of hormone-responsive elements in LiMDAS promoters. These elements include growth factors such as AuxRE, AuxRR core, TGA elements, and TGA-box, as well as gibberellin (P-box, TATC-box, GAREmotif), ABRE, jasmonic acid (CGTCA motif, TGACG motif), and salicylic acid (SARE, TCA motif), mostly composed of abscisic acid and jasmonic acid response elements. LiMADS promoters contain stress response elements, including anaerobic induction elements (ARE, GC-motif), low temperature response elements (LTR), and drought response elements (MBS). Additionally, elements related to flower development, such as POLLEN1LAT52, GTGANTG10, and CArG, have been identified, mainly involved in pollen development (Table S2). The LiMADS gene family was anticipated to exert a substantial influence on the growth, development, and response of L. indica to external environmental factors.
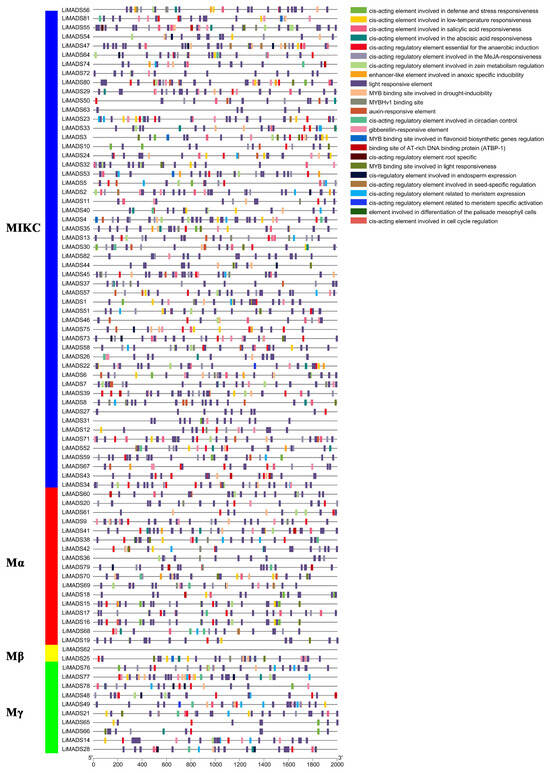
Figure 5.
Analysis of cis-acting elements in the promoter region of the LiMADS family. Different cis acting elements are displayed in different colors.
Furthermore, upon analyzing various subfamilies, it was found that type II genes had a higher number and more variety of promoters than type I genes (Table S3). Notably, the MIKCC subfamily contained a higher proportion of hormone and photoresponsive elements. Specifically, the AGL6, SQUA, AG, FLC, DEF, and GLO subfamilies exhibited a larger number of photoresponsive elements, whereas AGL15 and AGL17 had fewer. This finding may be related to their respective functions. Most of the genes are believed to be involved in hormonal pathways, as the Mα subfamily contains a smaller number of hormone elements compared to other subfamilies that have more cis-acting elements related to hormones.
2.6. Analysis of Protein-Protein Interaction Network of LiMADS Gene
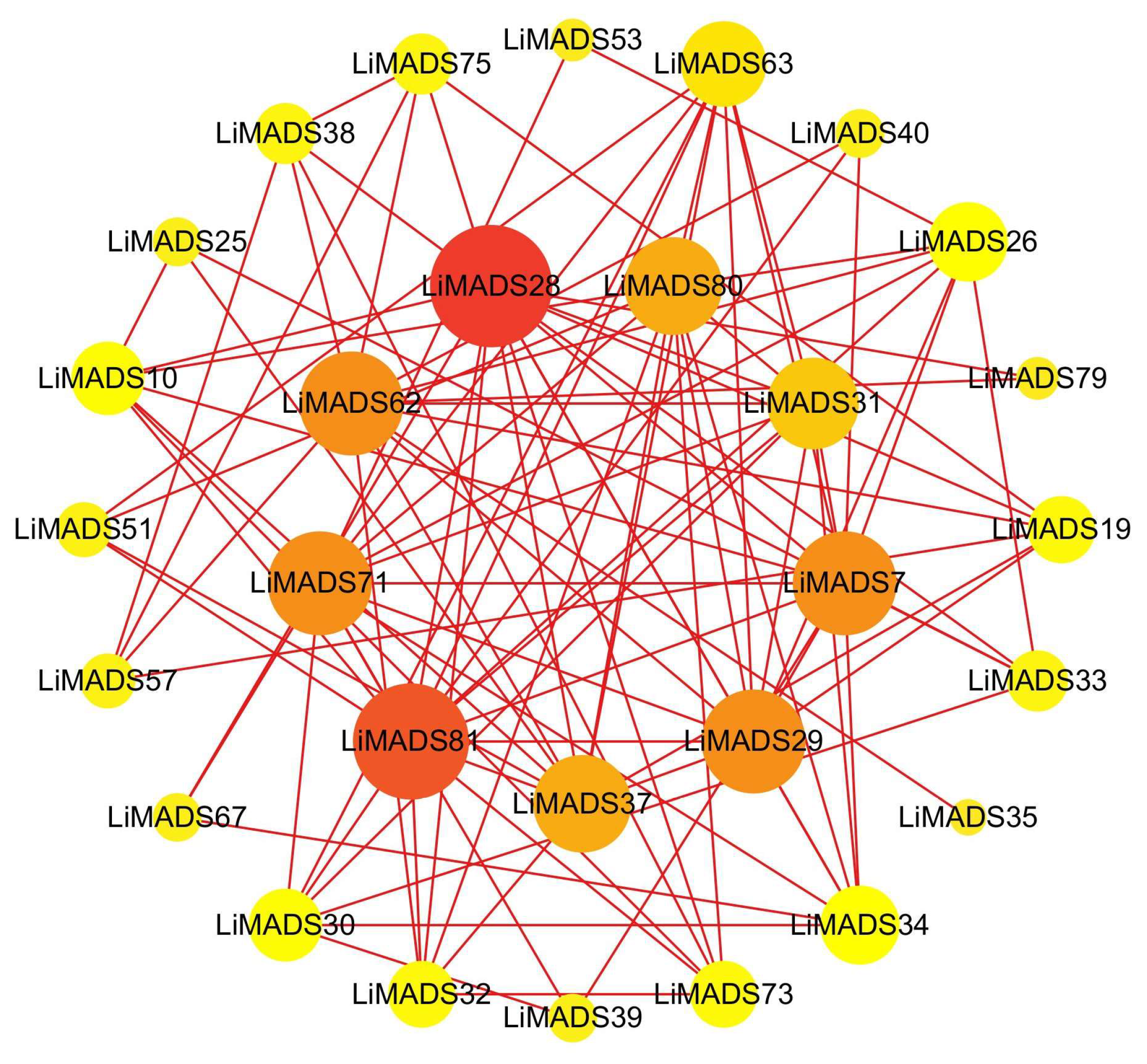
To gain information regarding the interactions among LiMADS proteins, we employed a computational approach to predict the LiMADS protein-protein interaction network using homologous MADS-box proteins from A. thaliana. This analysis was expected to enhance our understanding of LiMADS protein interactions. The study found that nine LiMADS proteins (LiMADS7, 28, 29, 31, 37, 62, 71, 80, and 81) play a central role in the protein interaction network (Figure 6). Notably, LiMADS28 and LiMADS62 are type I genes and exhibit strong interaction with LiMADS31. LiMADS37, a class B gene similar to the AP3 gene in A. thaliana, is a transcription factor essential for normal petal and stamen development. LiMADS31 forms a heterodimer with PI (LiMADS51), a gene responsible for regulating flower development. Together with LiMADS71 and LiMADS63, LiMADS31 recognizes the floral meristem and regulates the expression of class B, C, and E genes. LiMADS80 and LiMADS81 are class E genes that are active in flowers and ovules. They interact with multiple classes of genes to form complexes that ensure the normal development of petals, stamens, and carpels.
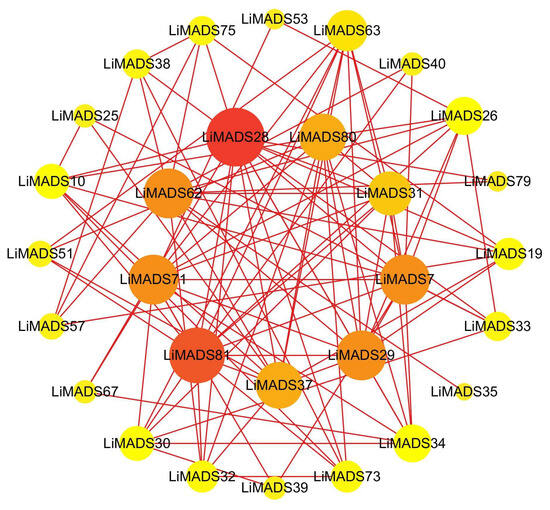
Figure 6.
Protein–protein interaction network of the MADS gene family in L. indica. The depth of the color in the figure and the size of the circle indicate the magnitude of interactions, with red indicating a large degree value and yellow indicating a small degree value.
2.7. Analysis of LiMADS Gene Expression Levels at Different Flower Development Stages
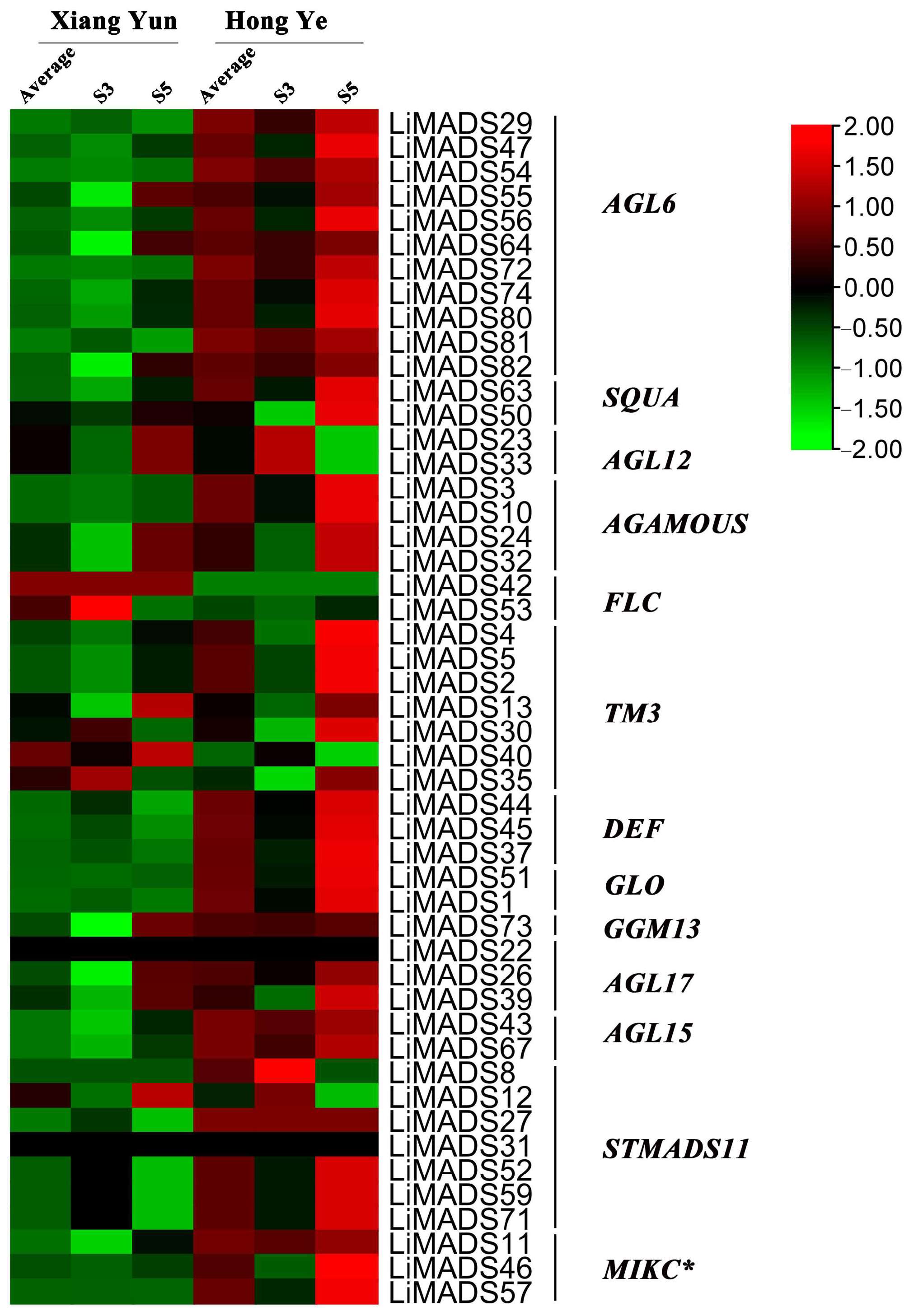
To investigate the regulation of flower organ development by the LiMADS gene family in the cultivar Xiang Yun, we conducted transcriptome sequencing on flower buds of ‘Xiang Yun’ and ‘Hong Ye’ at different stages of development (Figure 7). Our results indicated that the expression levels of AGL6, AG15, ALG15, AGL17, DEF, GLO, and STMADS11 subfamily genes, which were involved in flower development, were higher in ‘Hong Ye’ and lower in ‘Xiang Yun’ (Figure 7). Notably, AGL6, DEF, GLO, ALG15, AGL17, STMADS11, and MIKC* were related to pollen development in A. thaliana [12], and these genes were specifically highly expressed in ‘Hong Ye’. However, the expression level of ‘Xiang Yun’ was lower than the control variety ‘Hong Ye’ (Figure 7). This difference in expression was hypothesized to be associated with the development of stamens in ‘Xiang Yun’. The TM3 subfamily of genes was known to regulate the expression of egg cells and embryos during the development of flower organs [28]. The heat map results indicated that the expression of TM3 increased with the development of flowers in ‘Hong Ye’ but decreased in ‘Xiang Yun’. Therefore, it was speculated that the low expression of TM3 in ‘Xiang Yun’ was indicative of a reduction in the development of egg cells and embryos, leading to the flowering but not the fruiting phenotype observed in ‘Xiang Yun’.
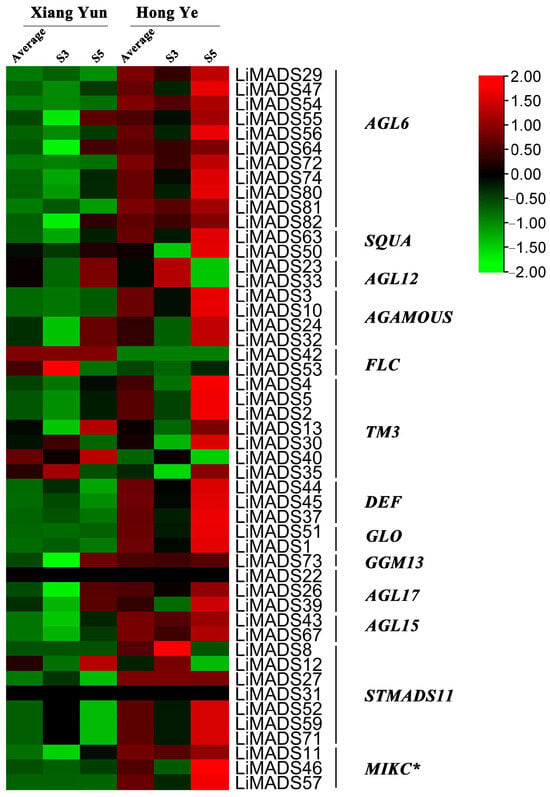
Figure 7.
Relative expression of LiMADS genes. All genes are classified into different subfamilies, and the color scale indicates relative expression levels from high (red) to low (green).
Previous research revealed that the variety ‘Xiang Yun’ possessed a more protracted flowering cycle than ‘Hong Ye’. A heat map depicting high levels of FLC gene expression in ‘Xiang Yun’ in relation to red leaves, and strong initial expression during the development of flower buds aligned with the function of FLC as a flowering inhibitor in A. thaliana (Figure 7). Whittaker [33] emphasized the importance of extended exposure to cold conditions in developing flowering ability, underscoring the crucial role of FLC as a determinant. This information supported the extended period of blooming observed in ‘Xiang Yun’.
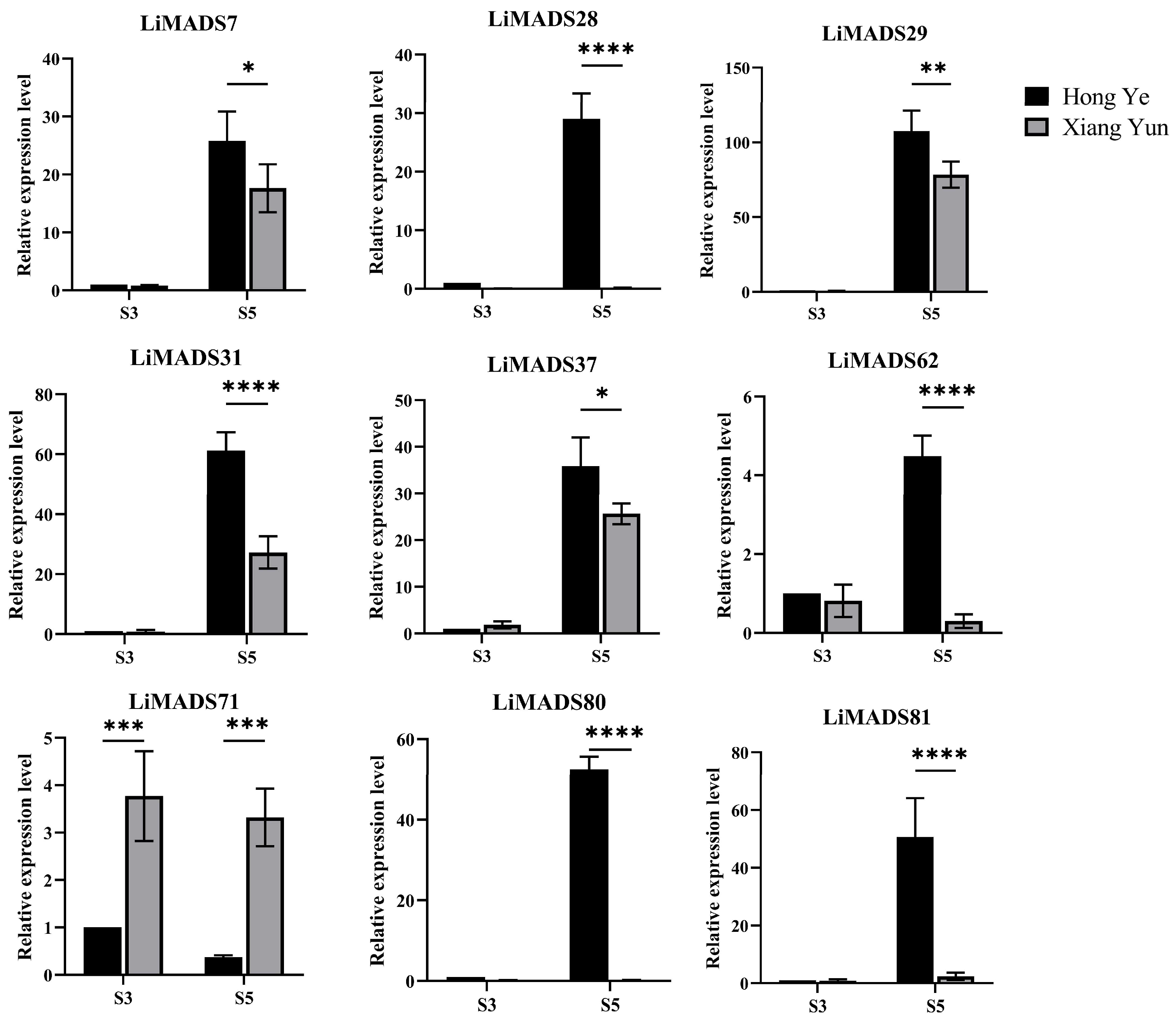
Furthermore, based on the results of protein interactions, we selected nine core MADS proteins to determine the gene expression levels of LiMADS in ‘Hong Ye’ and ‘Xiang Yun’ (Figure 8). The qRT-PCR expression pattern of most LiMADS genes was consistent with the general trend of transcriptome expression patterns. During flower development, the expression levels of LiMADS7, LiMADS29, LiMADS31, and LiMADS37 in ‘Hong Ye’ significantly increased. Additionally, there were some differences in expression levels between two cultivars during the same period. LiMADS28, LiMADS62, LiMADS80, and LiMADS81 showed low levels of expression in ‘Xiang Yun’. However, the expression levels of LiMADS71 were significantly higher in ‘Xiang Yun’ than in ‘Hong Ye’. This result differs from the transcriptome data, which may be due to differences in sequencing and qRT-PCR samples.
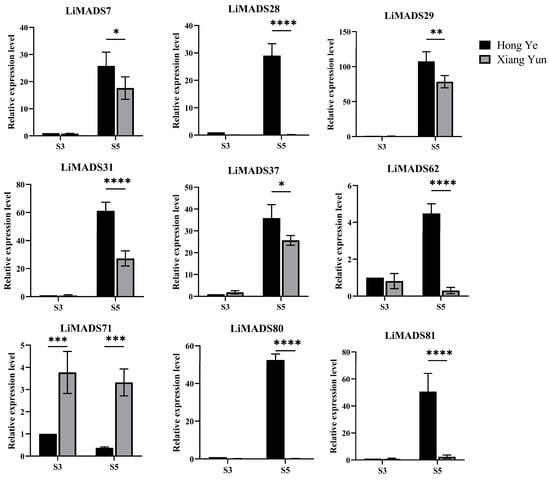
Figure 8.
Expression levels of different MADS genes in L. indica at different flower development stages. The expression analysis was normalized using Li18S gene as internal reference gene. Values are means of three independent repeats, and error bars are standard deviations, where *, **, ***, and **** represent significant differences at p < 0.05, p < 0.01, p < 0.001, and p < 0.0001 levels based on two-way ANOVA test.
2.8. Changes in Hormone Levels during Organ Development of L. indica Flowers
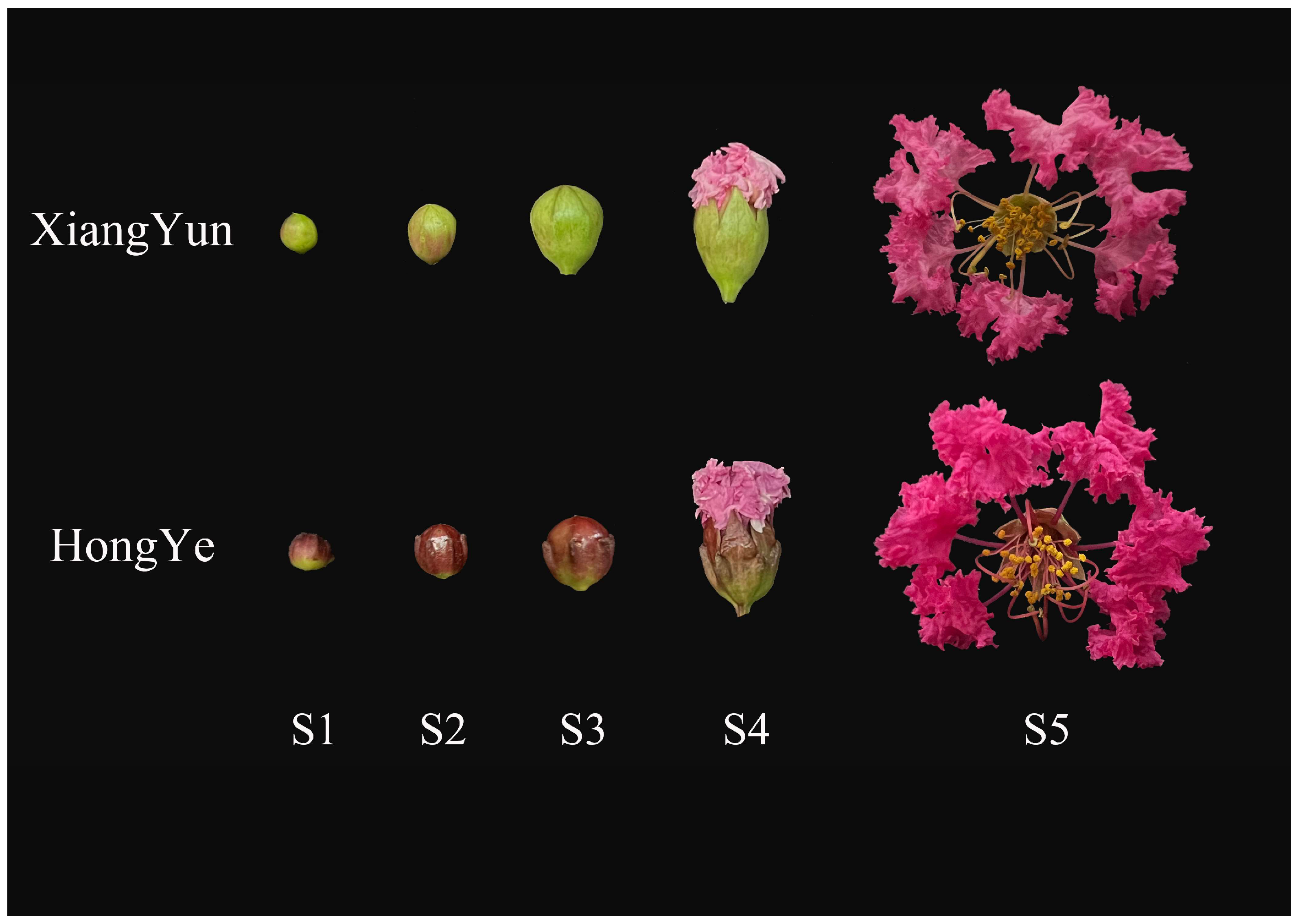
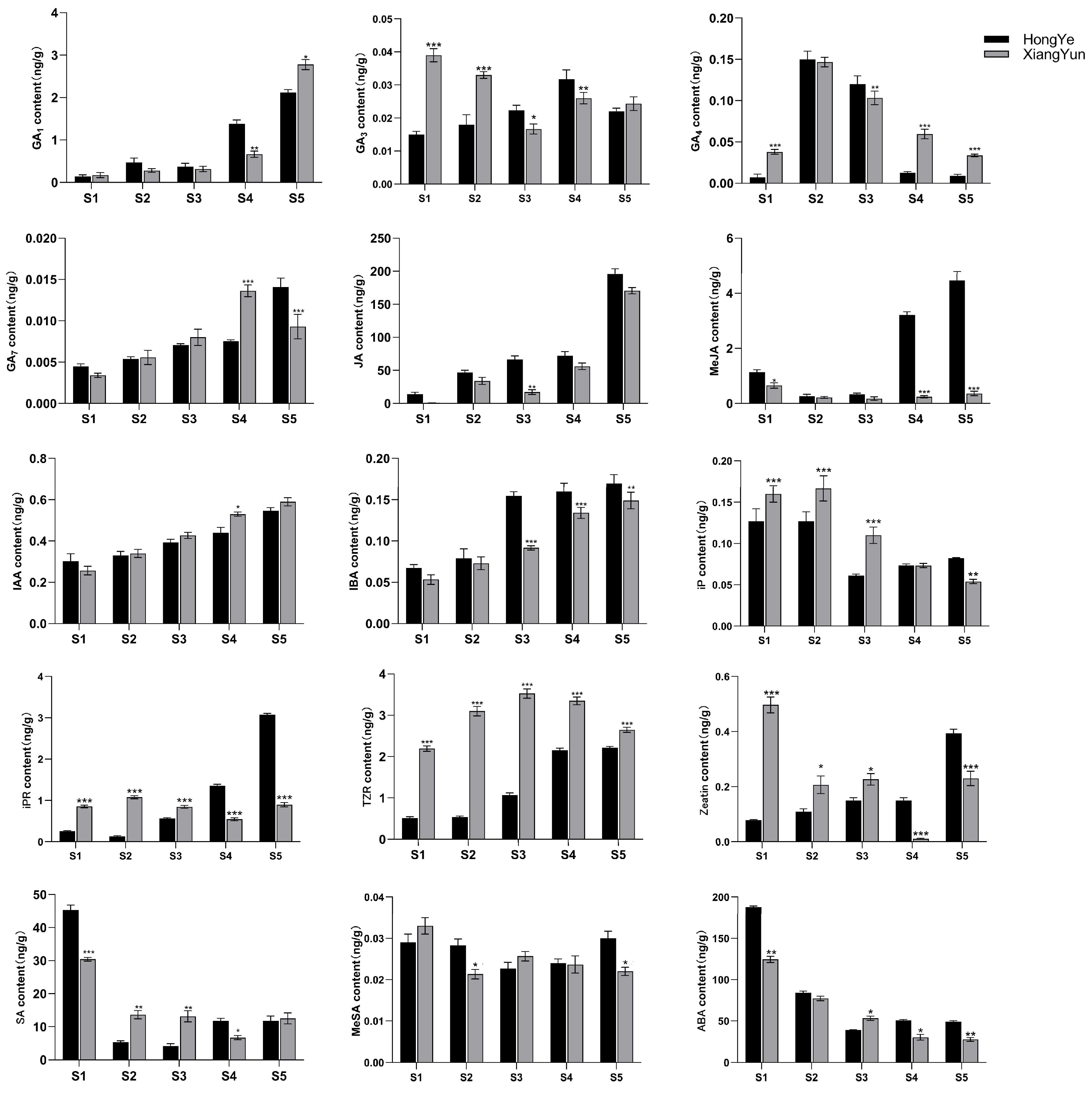
To further investigate the mechanism of sterility in ‘Xiang Yun’, we utilized ‘Hong Ye’ as a control (Figure 9) and employed HPLC to assess 15 endogenous hormones during flower development. Our findings revealed substantial disparities among hormones. The levels of IAA and IBA increase with flower development. Specifically, in the cultivar ‘Xiang Yun’, the levels of IAA and IBA increased by 1.3 and 1.75 times, respectively, during the S5 period compared with the S1 period. Similarly, the levels of IAA and IBA in red leaves increased by 0.8 and 1.54 times, respectively. In contrast, ABA and SA levels decreased with the progression of flower development. The ABA content in ‘Xiang Yun’ decreased by 77.9% and 73.8% in S5 and S1 periods, respectively, while the SA content decreased by 58.8% and 73.9% in ‘Xiang Yun’ and ‘Hong Ye’, respectively.
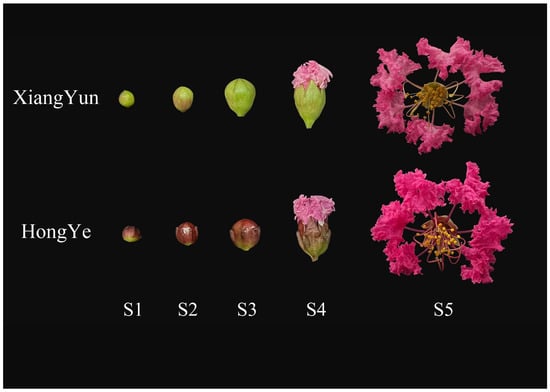
Figure 9.
Flower development of L. indica ‘Xiang Yun’ and ‘Hong Ye’ during different periods. S1–S5 is the process of flower maturation.
Biological activities of GAs are typically limited to GA1, GA3, GA4, and GA7 in higher plants [34], and they play a critical role in regulating various aspects of plant growth and development, including seed germination, seedling morphogenesis, and maturation of different organs. GA1 and GA7 contents generally increased progressively through the development of the flower organs (Figure 10). GA3 and GA4 contents fluctuated in the developmental stages. Notably, the levels of GA3 (S1 and S2 stages) and GA4 (S1, S4, and S5 stages) were significantly higher in ‘Xiang Yun’ than ‘Hong Ye’ (Figure 10). JA is a key regulatory molecule in plant growth and development as well as in response to abiotic stress. Mutants with impaired JA synthesis or signaling during flower organ development exhibit delayed stamen growth and anther unclefting [35]. In this study, we found that JA content in ‘Xiang Yun’ was significantly lower than in ‘Hong Ye’. Moreover, the content of MeJA in ‘Xiang Yun’ did not change significantly with flower development, whereas the contents of S4 and S5 in ‘Hong Ye’ were 13.4 and 12.4 times higher than in ‘Xiang Yun’, respectively (Figure 10). This suggested that JA synthesis may be affected in ‘Xiang Yun’, which could be related to its sterility. IAA and IBA contents increased with the progress of flower development. IAA levels at S4 and S5 of ‘Xiang Yun’ were significantly higher than those of ‘Hong Ye’, but IBA contents at S3–S5 of ‘Xiang Yun’ were substantially lower than those of ‘Hong Ye’. Additionally, the levels of iP, IPR, TZR, and Zeatin in ‘Xiang Yun’ were significantly greater than those in ‘Hong Ye’ from S1 to S3. Specifically, the TZR content was 4.29, 5.77, and 3.3 times higher in ‘Xiang Yun’ than in ‘Hong Ye’ at the S2, S3, and S4 stages, respectively. SA and ABA exhibited a decreased trend with the development of flowers, MeSA remained largely stable, and their levels varied in both ‘Xiang Yun’ and ‘Hong Ye’.
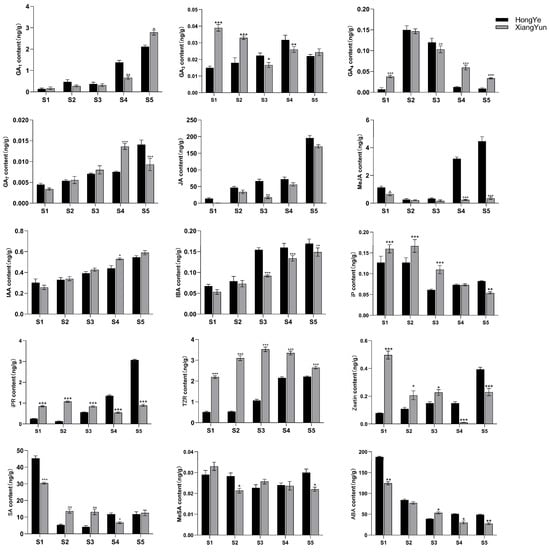
Figure 10.
Levels of 15 hormones at different flower development stages of L. indica. All data were derived from at least three biological replicates and are expressed as the mean ± standard deviation. (*: p < 0.05, **: p < 0.01, ***: p < 0.001, two-way ANOVA test).
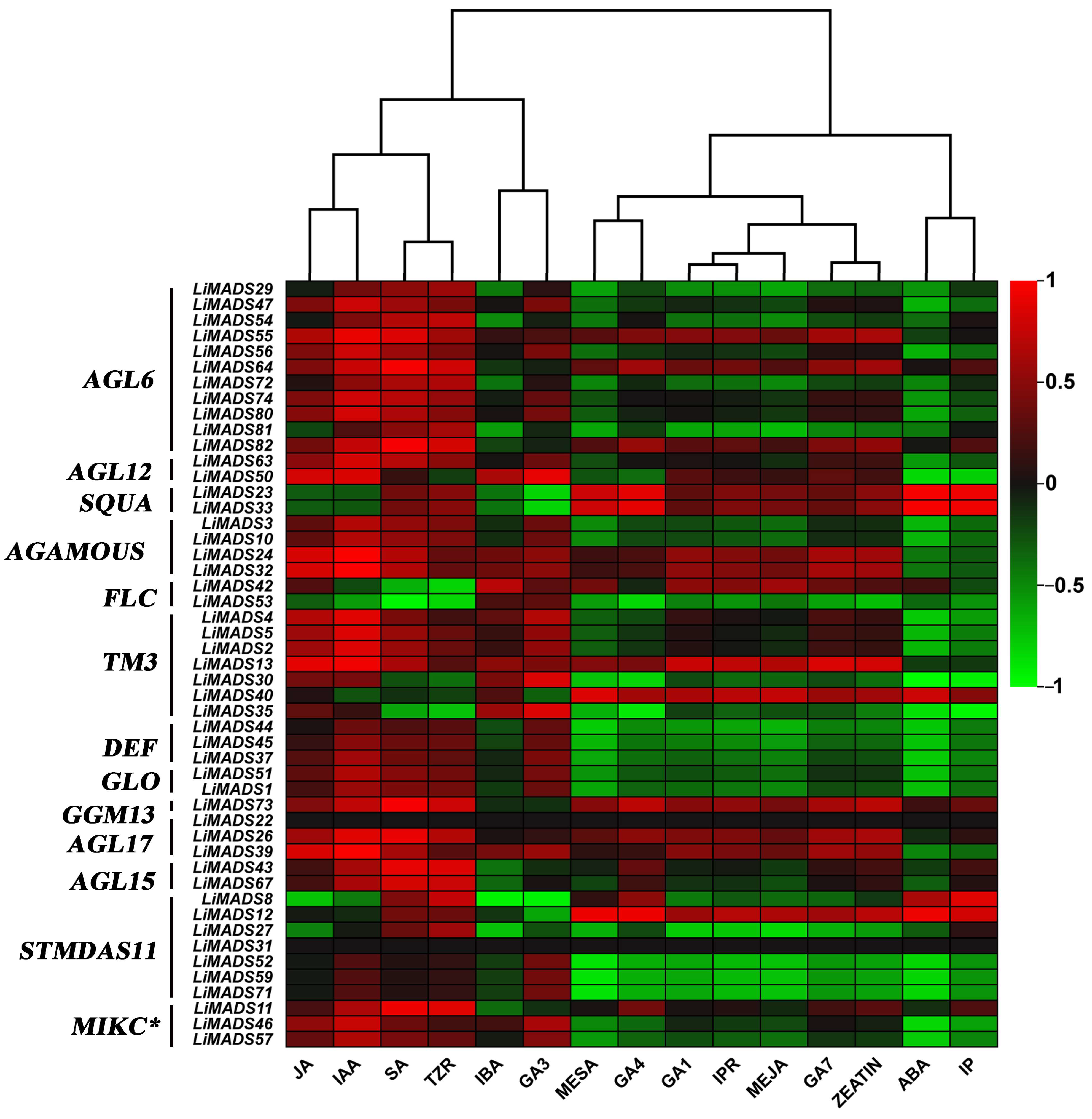
Finally, we analyzed the correlation between hormone concentrations and gene expression levels in the transcriptome (Figure 11). The results showed that JA, IAA, SA, and TZR were positively correlated with the expression levels of most type II genes. Additionally, genes in AGL6, AG, and TM3 were positively correlated with GA3, GA4, and GA7. On the other hand, ABA was negatively correlated with most genes.
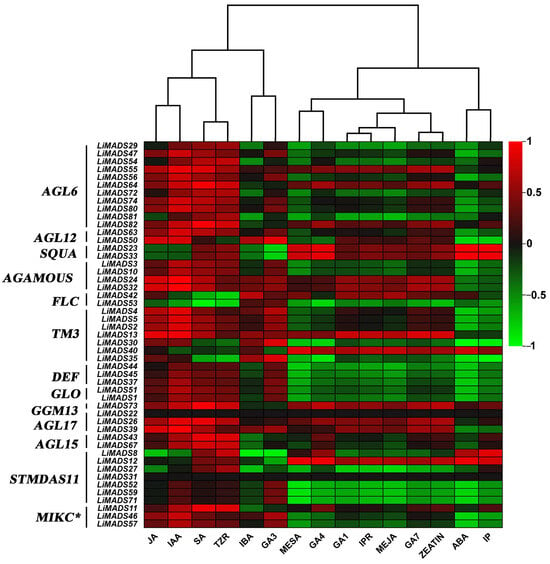
Figure 11.
The correlation analysis between different hormones and MADS type II gene. All genes were grouped into different subfamilies, with color scales indicating correlation coefficients from high (red) to low (green).
3. Discussion
MADS-box genes are of paramount importance in various plant activities, including the formation of flowers, regulation of flowering time, and flower development [36]. As a result of the completion of genome sequencing in multiple species, there has been an increased understanding of the role of MADS-box genes in plant development. However, research on the function of the MADS gene family in L. indica is limited. In this study, we analyzed the MADS gene family in L. indica and identified 82 LiMADS genes, the number is similar to that reported by Zhao et al. [37] in pomegranate. A comparative phylogenetic analysis of L. indica and Arabidopsis resulted in the classification of L. indica into five subfamilies, with the MIKCC subfamily comprising 60% of the genes and exhibiting the most homologous genes compared to Arabidopsis. This finding suggested that the MIKCC subfamily was conserved across species. The MIKCC gene class is crucial for regulating floral organ characteristics and plays a vital role in the evolution of diploid sporophytic reproductive structures in seed plants [11]. The data indicated that only two Mβ genes were present in L. indica, which were significantly fewer in number than in Arabidopsis. This suggested that the Mβ gene in L. indica could be lost during evolution. Similar observations were reported in Camellia sinensis [38]. Consequently, the MIKCC gene underwent greater proliferation and diversification in L. indica than other LiMADS genes, potentially enabling it to perform multiple functions.
The function of a gene is dictated by its structure within the organism. The greater the number and length of introns in a gene sequence, the more diverse are the methods by which genes can be spliced, which subsequently affects gene expression and protein activity [39]. The intron average of type II genes in the MADS gene structure was significantly higher than that of the type I genes. This is similar to the MADS gene family in orchids [40], grapes [41], walnuts [42], and other plants, leading us to hypothesize that type II genes could play a critical role in the development of L. indica flower organs. Examination of cis-acting components within the L. indica MADS gene family revealed the presence of cis-acting elements associated with hormonal responses and abiotic stresses, specifically the methyl jasmonate response element, gibberellin response elements, light response elements, and flower development-related elements such as CArG. Comparable cis-acting elements have been identified in maize and lettuce [43,44], suggesting that the regulatory promoter elements of MADS-box genes are conserved across species. Consequently, the findings indicate that the MADS-box can respond to hormonal regulation. The hormones in question are of paramount importance for plant growth and development as well as in the response of plants to stress. Furthermore, the MADS-box family protein network was analyzed, and it was found that most MADS-box members interact with each other. LiMADS28 (AGL80) plays a crucial role in the protein interaction network and exhibits strong interaction with LiMADS19 (AGL61). In Arabidopsis, AGL80 and AGL61 are expressed in central cells through the formation of heterogenic complexes [24]. The Class B transcription factor LiMADS37 (AP3) consistently forms heterodimers with LiMADS51 (PI). The AP3/PI complex can also form ternary complexes with the class E transcription factor LiMADS80 (SEP3) to contribute to the development of petals and stamens [45]. Additionally, gene duplication is a critical mechanism in the development and evolution of gene families in plants [46]. Duplicated genes can evolve novel functions and enhance the capacity of plants to adapt to their environments [47]. In L. indica, tandem duplications were identified in nine gene pairs. The calculation of Ka and Ks values indicated that LiMADS genes were subjected to purifying selection, suggesting that their structure has contributed to the expansion of the L. indica MADS gene family, which plays a crucial role in increasing gene function diversity.
The primary causes of plant failure to reproduce have been ascribed to male or female sterility, and extensive research has been conducted on fertility genes and their associated mechanisms in model plants. To investigate the sterility mechanism of ‘Xiang Yun’, we analyzed gene expression levels using transcriptome data and qPCR. The results indicate that LiMADS71 gene expression is high in ‘Xiang Yun’. We also found that LiMADS71 may be homologous to SVP in A. thaliana, which can inhibit class B and class C flower homologous genes [48]. Further analysis revealed that genes responsible for flower meristem development (LiMADS29, and LiMADS31), stamen development (LiMADS37, LiMADS62, LiMADS80, and LiMADS81), and pistil development (LiMADS28) were expressed at lower levels in ‘Xiang Yun’. This suggests that the sterility of ‘Xiang Yun’ may be linked to the underdevelopment of its reproductive organs. Male sterility, which encompasses anther morphological defects, microsporogenesis issues, impaired pollen development, and disrupted pollen function, may also be a contributing factor [49]. Similarly, female sterility can arise from defects in the ovaries and ovules [50,51]. A previous study exploring the morphology of L. indica identified anther dehiscence and abnormal embryonic sac development as the primary causes of L. indica sterility. Furthermore, it is often observed that senescence-triggering hormones originate from the maturation of plants, and a deficiency in these hormones can extend their lifespan [52]. FLC, a suppressor of flowering, is significantly expressed during the early stages of flower bud development in ‘Xiang Yun’. This finding lends credence to the idea that ‘Xiang Yun’ has a longer flowering period compared to its ‘Hong Ye’ counterpart.
During flower development in angiosperms, sepal opening, petal growth, stamen development, style elongation, anther dehiscence (which releases pollen), and stigma maturation (which facilitates pollen germination and pollen tube growth) are all regulated by endogenous hormones [53]. MeJA levels were significantly higher in ‘Hong Ye’ than ‘Xiang Yun’. Furthermore, low levels of MeJA were detected in male sterile cotton [54], rice [55], and tomato [56], where the application of exogenous MeJA to early buds reversed anther sterility. Moreover, our correlation analysis results showed that JA was positively correlated with MADS gene expression. Therefore, we hypothesized that the synthesis of jasmonic acid in ‘Xiang Yun’ was associated with the development of stamens, which ultimately resulted in flowering failure. JA is known to co-regulate various developmental processes in plants with other plant hormones, such as cytokinin [57]. Our findings revealed that TZR, zeatin, and iP, were lower in ‘Hong Ye’ than in ‘Xiang Yun’. This may be attributed to the antagonistic relationship between JA and TZR, Zeatin, and iP during the development of flower organs, where high levels of JA inhibit TZR, Zeatin, and iP production.
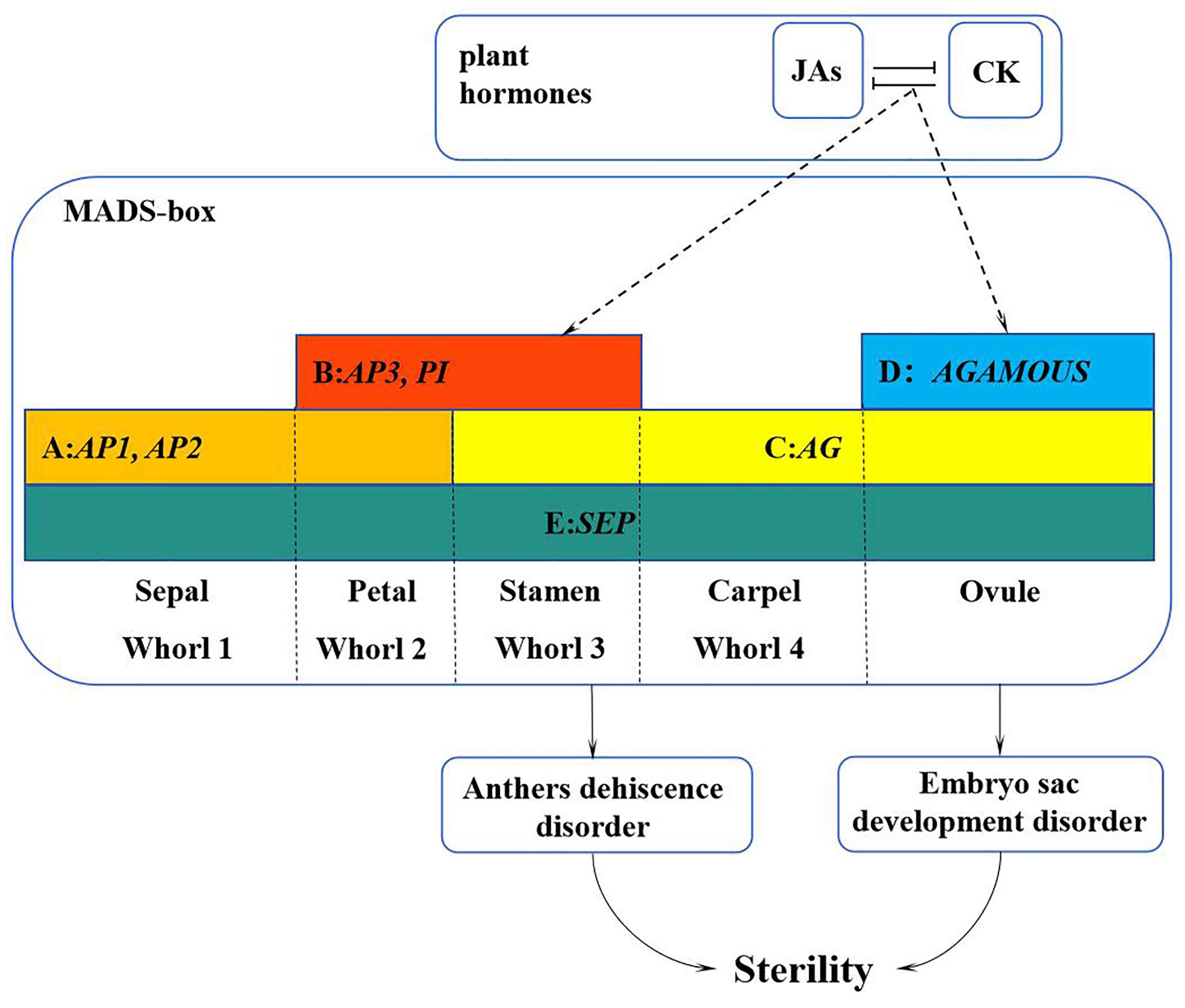
In summary, we proposed a working model to illustrate how the MADS-box family genes interact with hormones to regulate the development of flower organs in L. indica ‘Xiang Yun’ (Figure 12). Specifically, plant hormone response pathways affect the expression of MADS genes, resulting in their low expression during the course of flower development. The reduced expression may lead to the abnormal development of stamen and embryo sac and ultimately hamper the fruiting process of ‘Xiangyun’. Obviously, the regulation mechanism underlying the sterility of ‘Xiang Yun’ is complex. This study only discusses genes in the MADS-box family and their interactions with hormones in flower development. It is known that flower development also depends on the regulation of proteins, such as LFY and UFO, and their involvement in the regulation of ‘Xiang Yun’ has not been studied. Thus, further research is warranted to verify the function of the MADS gene in L. indica flowering and specific mechanism underlying the sterility of ‘Xiang Yun’.
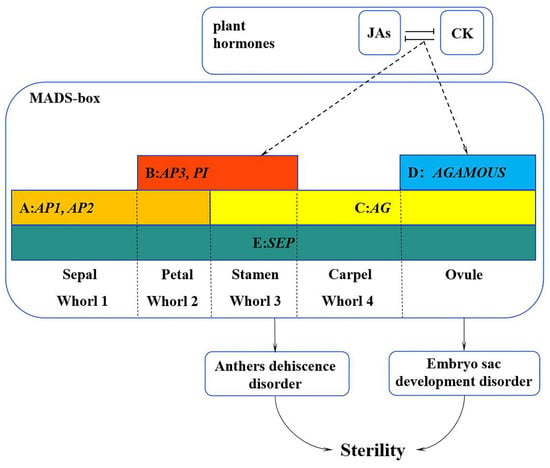
Figure 12.
A working model of the MADS-box family genes in regulation of flower development of L. indica ‘Xiang Yun’. The sterility of ‘Xiang Yun’ was characterized by pollen inactivity, another dehiscent disorder, and an abnormal tapetum layer leading to embryo sac abortion. The dashed line represents the procedure proposed based on this study.
4. Materials and Methods
4.1. Identification of MADS Gene in the Genome of L. indica
To identify members of the MADS family within the unpublished genome of L. indica, we obtained a conserved domain Hidden Markov Model for MADS (PF00319) from the Pfam protein database. Using Hmmer software (version 3.0) with default parameters, L. indica protein sequences were analyzed. Furthermore, we verified the structural domains of the MADS family using the online portals SMART (http://smart.embl-heidelberg.de/, accessed on 22 October 2023) and NCBI CDsearch (https://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi, accessed on 22 October 2023). Genes with incomplete predictions were excluded from the analysis. The distribution of LiMADS genes across chromosomes was illustrated using the “Gene Location” feature in TBtools. The key properties of the LiMADS, including the theoretical isoelectric point (pI), molecular weight (MW), instability index, and hydrophobicity index, were estimated using Expasy (https://web.expasy.org/protparam/, accessed on 22 October 2023).
4.2. Phylogenetic Analysis of LiMADS
The protein sequences of Arabidopsis MADS were obtained from the Plant Transcription Factor Database (http://planttfdb.gao-lab.org/index.php/, accessed on 22 October 2023). Subsequently, L. indica and Arabidopsis MADS protein sequences were analyzed using MEGA v11 software and the NJ clustering method with default parameters. Finally, the resulting phylogenetic tree was visualized using iTOL v6 (https://itol.embl.de/, accessed on 25 January 2024).
4.3. Chromosome Location and Synteny Analysis of the MADS-Box Gene Family
The MADS-box gene family was located on the chromosome using Gene Location Visualize in TBtools [58], and the genes were named according to their respective positions on the chromosome.
The analysis of homology between MADS L. indica and Arabidopsis was conducted using the “MCScanX” tool from TBtools. Subsequently, homologous L. indica genes were analyzed using the “Advanced Circos” tool. The duplicated gene pairs were identified, and the identity substitution rate (Ks) and non-identity substitution rate (Ka) of these pairs were calculated using the “Simple Ka/Ks calculator” tool. The time of differentiation for the duplicate gene pairs was calculated using the Arabidopsis molecular clock (λ) at a rate of 1.5 × 10−8 substitutions per site per year, as T = Ks/2λ.
4.4. LiMADS Gene Structure Analysis and Cloning
The coding sequence and genome annotation information for the LiMADS gene family were derived from the L. indica genome database. The MEME v5.5.2 (https://meme-suite.org/meme/tools/meme, accessed on 5 November 2023) online tool was employed to predict the conserved motifs of LiMADS, with the motif count set to 10 and all other parameters set to their default settings. The motif data obtained from MEME, along with the genomic GFF data from L. indica, were visualized using the “Gene Structure View” feature in TBTools.
The method of gene cloning was to design primers on the CDS sequence of L. indica (Table S4), purify the product after PCR amplification, and determine the sequence size by agarose gel electrophoresis. It was then linked to the pTOPO-Blunt cloning vector (Aidlab Biotechnologies Co., Ltd. Beijing, China), transformed into DH5α, and finally sent to the BGI Genomics company for sequencing. We then uploaded the correct gene sequences to NCBI and obtained the following accession numbers: LiMADS81-Hong Ye (PP388995), LiMADS81-Xiang Yun (PP388996), LiMADS37-Hong Ye (PP388997), and LiMADS37-Xiang Yun (PP388998).
4.5. Prediction of Cis-Regulatory Elements in the Promoter Region
The prediction of cis-acting elements for a 2000 bp sequence located upstream of the LiMADS start site was performed using the PlantCARE (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/, accessed on 23 October 2023) and New PLACE (https://www.dna.affrc.go.jp/PLACE/, accessed on 25 January 2024) online software. The resulting data was subsequently visualized through the TBtools v2.052 “Basic Biosequence View” visualization tool.
4.6. Analysis of LiMADS Protein-Protein Interaction Network
To investigate protein interactions, the protein sequences derived from the L. indica genome were submitted to the STRING database v12.0 (https://string-db.org/, accessed on 3 November 2023), which led to the analysis of the relationships between LiMADS proteins using Arabidopsis as a reference, and the results were visualized via the Cytoscape v3.10.1 (https://cytoscape.org/, accessed on 3 November 2023).
4.7. Expression Analysis of MADS Gene at Different Stages of L. indica Flower Development
To investigate the expression level of the MADS-box gene during flower development of L. indica, we extracted total RNA from ‘Xiang Yun’ and ‘Hong Ye’ flowers at two stages: the bud stage (S3) and the mature flowering stage (S5). Each sample was replicated three times. Novogene Co., Ltd. (Beijing, China) performed transcriptome sequencing. We uploaded the transcriptome data to the NCBI and obtained a login number, PRJNA1071351. Fragments per kilobase per million from mapped readings (FPKM) were used to compare the expression levels of LiMADS during two stages of flower development. A cluster heatmap was created using the cluster heatmap tool of TBtools to visually analyze gene expression levels.
We further determined the expression of ‘Xiang Yun’ and ‘Hong Ye’ flower genes at S3 and S5 stages using fluorescence quantitative PCR (Applied Biosystems QuantStudio 6 Flex). ‘Hong Ye’ was used as a control, and the 18S gene was used as a reference gene. Each sample was repeated three times. The reaction system and amplification procedures were set up according to the indicated protocols using standard procedures, and the data were analyzed using the 2−ΔΔCt method. Primers used for vector construction are listed in Table S4.
4.8. Analysis of Hormone Levels at Different Stages of L. indica Flower Development
Flower buds or flowers were collected from ‘Xiang Yun’ and ‘Hong Ye’ grown on the Experimental Forest Farm of the Hunan Academy of Forestry. They were picked at 72 h (S1), 48 h (S2), 24 h (S3), 1 h (S4), and 0 h (S5) before flowers opened. Sam The samples were cleaned with deionized water, frozen in liquid nitrogen, and stored at −80 °C. Fifteen plant hormones (ABA, MeSA, MeJA, GA3, GA4, JA, IAA, IBA, c-Zeatin, TZR, iP, and iPR, sourced from Sigma, Shanghai, China; SA, GA1, and GA7, purchased from TRC, Toronto, Canada) were subjected to high-performance liquid chromatography (HPLC). Freeze-dried flower bud samples (0.5 g) were mixed with an acetonitrile volume of 10 times and left to extract overnight at 4 °C. The mixture was then centrifuged, and acetonitrile was added to the supernatant. Centrifugation was performed at 10,000× g for 5 min after adding 40 mg of C18 filler to the supernatant, followed by drying with nitrogen and redissolving in methanol. Subsequently, an organic phase filter membrane with a pore size of 0.22 μm and filter size of 200 μ was employed, and the resulting mixture was stored at −20 °C. The SCIEX-Qtrap6500 instrument (AB SCIEX, Redwood City, CA, USA)was utilized for the analysis of phytohormones, with each analyte identified and quantified using the multiple reaction monitoring (MRM) mode. Three independent replicates were used for each phytohormone extraction.
4.9. Data Processing
All experimental data were repeatedly measured three times, subsequently averaged, and presented as mean ± standard deviation (SD). To perform a two-way ANOVA multi-factor analysis of variance and generate bar charts, the software program GraphPad Prism 10 was utilized.
5. Conclusions
This study identified a total of 82 LiMADS genes in L. indica, which were classified into five subfamilies. Among them, the MIKCC subfamily comprises 60% of the genes. The MIKCC genes are crucial for regulating floral organ development. Analyzing upstream sequences of LiMADS indicated cis-acting elements associated with hormonal responses, specifically the methyl JA response elements and gibberellin response elements, occurred in the promoter regions. Endogenous hormones, particularly JAs, IPR, TZR, Zeatin, and Gas, showed dynamic changes during the course of flower development. Correlation analysis showed that JA, IAA, and GAs were positively correlated with most MIKCC genes. A working model for the development of L. indica ‘Xiang Yun’ was proposed. The hormone response pathway may affect the MADS gene family related to flower development and cause its low expression, resulting in abnormal development of stamen and embryo sac, and ultimately affect the outcome process of ‘Xiangyun’. It is anticipated that the findings from this study could serve as a valuable reference for investigating the function of the MADS-box gene family in L. indica and mechanisms underlying the sterility of ‘Xiang Yun’.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/plants13050709/s1, Figure S1: Sequence differences between Xiang Yun and Hong Ye genes; Table S1: Molecular weights and physicochemical properties of 82 LiMADS proteins in L. indica; Table S2: Prediction of cis-acting elements of L. indica; Table S3: Visualization of cis-acting elements in the LiMADS family promoter region; Table S4: Primers were used in this research.
Author Contributions
Conceptualization, F.D.; methodology, H.Z.; software, Y.L. and L.L. (Lu Li); validation, X.L. and L.L. (Lu Li); formal analysis, X.L.; investigation, L.L. (Liushu Lu); resources, J.C.; data curation, Y.L.; writing—original draft preparation, F.D.; writing—review and editing, Z.Q., F.D. and J.C.; visualization, J.C.; supervision, J.C. and Y.C.; project administration, Z.Q. and Y.C.; funding acquisition, Z.Q. All authors have read and agreed to the published version of the manuscript.
Funding
This research is jointly funded by the Hunan Provincial Natural Science Foundation of China (grant no. 2023JJ30338), the Science and Technology Innovation Program of Hunan Province (grant no. 2022RC1012), and the Training Program for Excellent Young Innovators of Changsha (grant no. kq2106090).
Data Availability Statement
The data presented in this study are available in Supplementary Materials.
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Wils, C.R.; Kaufmann, K. Gene-regulatory networks controlling inflorescence and flower development in Arabidopsis thaliana. Biochim. Biophys. Acta. Gene Regul. Mech. 2017, 1860, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Coen, E.S.; Meyerowitz, E.M. The war of the whorls: Genetic interactions controlling flower development. Nature 1991, 353, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Liu, Z.; Lan, S. Genome sequencing reveals the role of mads-box gene families in the floral morphology evolution of orchids. Hortic. Plant J. 2019, 5, 8. [Google Scholar] [CrossRef]
- Kieffer, M.; Davies, B. Developmental programmes in floral organ formation. Semin. Cell Dev. Biol. 2001, 12, 373–380. [Google Scholar] [CrossRef]
- Chandler, J.W. Class VIIIb APETALA2 ethylene response factors in plant development. Trends Plant Sci. 2018, 23, 151–162. [Google Scholar] [CrossRef]
- Theissen, G.; Kim, J.T.; Saedler, H. Classification and phylogeny of the MADS-box multigene family suggest defined roles of MADS-box gene subfamilies in the morphological evolution of eukaryotes. J. Mol. Evol. 1996, 43, 484–516. [Google Scholar] [CrossRef]
- Liljegren, S.J.; Ditta, G.S.; Eshed, Y.; Savidge, B.; Bowman, J.L.; Yanofsky, M.F. SHATTERPROOF MADS-box genes control seed dispersal in Arabidopsis. Nature 2000, 404, 766–770. [Google Scholar] [CrossRef]
- Nam, J.; dePamphilis, C.W.; Ma, H.; Nei, M. Antiquity and evolution of the MADS-box gene family controlling flower development in plants. Mol. Biol. Evol. 2003, 20, 1435–1447. [Google Scholar] [CrossRef]
- Qiu, Y.C.; Köhler, C. Endosperm evolution by duplicated and neofunctionalized type I MADS-Box transcription factors. Mol. Biol. Evol. 2022, 39, msab355. [Google Scholar] [CrossRef]
- Lai, X.L.; Daher, H.; Galien, A.; Hugouvieux, V.; Zubieta, C. Structural basis for plant MADS transcription factor oligomerization. Comput. Struct. Biotechnol. J. 2019, 17, 946–953. [Google Scholar] [CrossRef]
- Gramzow, L.; Theissen, G. A hitchhiker’s guide to the MADS world of plants. Genome Biol. 2010, 11, 214. [Google Scholar] [CrossRef]
- Becker, A.; Theissen, G. The major clades of MADS-box genes and their role in the development and evolution of flowering plants. Mol. Phylogenet. Evol. 2003, 29, 464–489. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Cui, S.J.; Wu, F.; Yan, S.; Lin, X.L.; Du, X.Q.; Chong, K.; Schilling, S.; Theißen, G.; Meng, Z. Functional conservation of MIKC*-Type MADS box genes in Arabidopsis and rice pollen maturation. Plant Cell 2013, 25, 1288–1303. [Google Scholar] [CrossRef]
- Kwantes, M.; Liebsch, D.; Verelst, W. How MIKC* MADS-box genes originated and evidence for their conserved function throughout the evolution of vascular plant gametophytes. Mol. Biol. Evol. 2012, 29, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, N.; Huang, J.B.; Xu, Y.F.; Tanoi, K.; Ito, T. Fine-tuning of auxin homeostasis governs the transition from floral stem cell maintenance to gynoecium formation. Nat. Commun. 2017, 8, 1125. [Google Scholar] [CrossRef]
- Lymperopoulos, P.; Msanne, J.; Rabara, R. Phytochrome and Phytohormones: Working in tandem for plant growth and development. Front. Plant Sci. 2018, 9, 1037. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.J.; Ren, Z.Y.; Cheng, C.H.; Wang, T.; Ji, H.T.; Zhao, Y. Counteraction of ABA-mediated inhibition of seed germination and seedling establishment by ABA signaling terminator in Arabidopsis. Mol. Plant 2020, 13, 1284–1297. [Google Scholar] [CrossRef]
- Wu, W.Q.; Du, K.; Kang, X.Y.; Wei, H.R. The diverse roles of cytokinins in regulating leaf development. Hortic. Res. 2021, 8, 118. [Google Scholar] [CrossRef]
- Qiao, Z.Q.; Liu, S.S.; Zeng, H.J.; Li, Y.X.; Wang, X.M.; Chen, Y.; Wang, X.; Cai, N. Exploring the molecular mechanism underlying the stable purple-red leaf phenotype in Lagerstroemia indica cv. Ebony Embers. Int. J. Mol. Sci. 2019, 20, 5636. [Google Scholar] [CrossRef]
- Hu, L.; Zheng, T.; Cai, M.; Pan, H.; Zhang, Q. Transcriptome analysis during floral organ development provides insights into stamen petaloidy in Lagerstroemia speciosa. Plant Physiol. Biochem. 2019, 142, 510–518. [Google Scholar] [CrossRef]
- Wang, X.; Chen, J.; Zeng, H.J.; Qiao, Z.Q.; Li, Y.; Cai, N.; Wang, X. Lagerstroemia indica ‘Xiangyun’, a seedless crape myrtle. HortScience 2014, 49, 1590–1592. [Google Scholar] [CrossRef]
- Zhou, W.; Chen, J.H.; Wang, X.M.; Zeng, H.J.; Qiao, Z.Q. Germination characteristics of fruiting and nonfruiting Lagerstroemia indica pollen. J. Cent. South Univ. For. Technol. 2023, 43, 74–81. (In Chinese) [Google Scholar]
- Colombo, M.; Masiero, S.; Vanzulli, S.; Lardelli, P.; Kater, M.M.; Colombo, L. AGL23, a type I MADS-box gene that controls female gametophyte and embryo development in Arabidopsis. Plant J. 2008, 54, 1037–1048. [Google Scholar] [CrossRef] [PubMed]
- Steffen, J.G.; Kang, I.; Portereiko, M.F.; Lloyd, A.; Drews, G.N. AGL61 interacts with AGL80 and is required for central cell development in Arabidopsis. Plant Physiol. 2008, 148, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Portereiko, M.F.; Lloyd, A.; Steffen, J.G.; Punwani, J.A.; Otsuga, D.; Drews, G.N. AGL80 is required for central cell and endosperm development in Arabidopsis. Plant Cell 2006, 18, 1862–1872. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.S.; Wang, D.F.; Zhang, H.J.; Skaggs, M.I.; Lloyd, A.; Ran, D.; An, L.; Schumaker, K.S.; Drews, G.N.; Yadegari, R. FERTILIZATION-INDEPENDENT SEED-polycomb repressive complex 2 plays a dual role in regulating type I MADS-box genes in early endosperm development. Plant Physiol. 2018, 177, 285–299. [Google Scholar] [CrossRef] [PubMed]
- Tapia-López, R.; García-Ponce, B.; Dubrovsky, J.G.; Garay-Arroyo, A.; Pérez-Ruíz, R.V.; Kim, S.H.; Acevedo, F.; Pelaz, S.; Alvarez-Buylla, E.R. An AGAMOUS-related MADS-box gene, XAL1 (AGL12), regulates root meristem cell proliferation and flowering transition in Arabidopsis. Plant Physiol. 2008, 146, 1182–1192. [Google Scholar] [CrossRef]
- Han, P.; García-Ponce, B.; Fonseca-Salazar, G.; Álvarez-Buylla, E.; Yu, H. Agamous-like 17, a novel flowering promoter, acts in a FT-independent photoperiod pathway. Plant J. 2008, 55, 253–265. [Google Scholar] [CrossRef]
- Rounsley, S.D.; Ditta, G.S.; Yanofsky, M.F. Diverse roles for MADS box genes in Arabidopsis development. Plant Cell 1995, 7, 1259–1269. [Google Scholar] [CrossRef]
- Joshi, S.; Paul, P.; Hartman, J.M.; Perry, S.E. AGL15 Promotion of somatic embryogenesis: Role and molecular mechanism. Front. Plant Sci. 2022, 13, 861556. [Google Scholar] [CrossRef]
- Dorca, F.C.; Gregis, V.; Grandi, V.; Coupland, G.; Colombo, L.; Kater, M.M. The Arabidopsis SOC1-like genes AGL42, AGL71 and AGL72 promote flowering in the shoot apical and axillary meristems. Plant J. 2011, 67, 1006–1017. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, J.; Zhao, X.Q.; Wang, J.; Wong, G.K.; Yu, J. KaKs_Calculator: Calculating Ka and Ks through model selection and model averaging. Genom. Proteom. Bioinform. 2006, 4, 259–263. [Google Scholar] [CrossRef] [PubMed]
- Whittaker, C.; Dean, C. The FLC Locus: A Platform for Discoveries in Epigenetics and Adaptation. Annu. Rev. Cell Dev. Biol. 2017, 33, 555–575. [Google Scholar] [CrossRef] [PubMed]
- Hedden, P.; Phillips, A.L. Gibberellin metabolism: New insights revealed by the genes. Trends Plant Sci. 2000, 5, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Stintzi, A.; Browse, J. The Arabidopsis male-sterile mutant, opr3, lacks the 12-oxophytodienoic acid reductase required for jasmonate synthesis. Proc. Natl. Acad. Sci. USA 2000, 97, 10625–10630. [Google Scholar] [CrossRef] [PubMed]
- Ng, M.; Yanofsky, M.F. Function and evolution of the plant MADS-box gene family. Nat. Rev. Genet. 2001, 2, 186–195. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, H.; Wang, Y.; Zhang, X.; Zhao, X.; Yuan, Z. Genome-wide identification and expression analysis of MIKC-type MADS-Box gene family in Punica granatum L. Agronomy 2020, 10, 1197. [Google Scholar] [CrossRef]
- Zhou, P.Y.; Qu, Y.S.; Wang, Z.W.; Huang, B.; Wen, Q.; Xin, Y.; Xu, L. Gene structural specificity and expression of gene family in Camellia chekiangoleosa. Int. J. Mol. Sci. 2023, 24, 3434. [Google Scholar] [CrossRef]
- Hong, X.; Scofield, D.G.; Lynch, M. Intron size, abundance, and distribution within untranslated regions of genes. Mol. Biol. Evol. 2006, 23, 2392–2404. [Google Scholar] [CrossRef]
- Lin, C.S.; Hsu, C.T.; Liao, D.C.; Chang, W.J.; Chou, M.L.; Huang, Y.T.; Chen, J.J.W.; Ko, S.; Chan, M.; Shih, M. Transcriptome-wide analysis of the MADS-box gene family in the orchid Erycina pusilla. Plant Biotechnol. J. 2016, 14, 284–298. [Google Scholar] [CrossRef]
- José, D.; Diego, L.; José, M.M.; José, C.M. Genome-wide analysis of MIKCc-type MADS box genes in grapevine. Plant Physiol. 2009, 149, 354–369. [Google Scholar] [CrossRef]
- Li, H.X.; Li, Y.X.; Zhang, X.X.; Cai, K.W.; Li, Y.; Wang, Q.C.; Qu, G.; Han, R.; Zhao, X. Genome-wide identification and expression analysis of the MADS-box gene family during female and male flower development in Juglans mandshurica. Front. Plant Sci. 2022, 13, 1020706. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Chen, Z.; Xu, L.; Zhang, L.; Zou, Q. Genome-wide analysis of the MADS-Box gene family in maize: Gene structure, evolution, and relationships. Genes 2021, 12, 1956. [Google Scholar] [CrossRef]
- Ning, K.; Han, Y.Y.; Chen, Z.J.; Luo, C.; Wang, S.L.; Zhang, W.J.; Li, L.; Zhang, X.; Fan, S.; Wang, Q. Genome-wide analysis of MADS-box family genes during flower development in lettuce. Plant Cell Environ. 2019, 42, 1868–1881. [Google Scholar] [CrossRef] [PubMed]
- Wuest, S.E.; O’Maoileidigh, D.S.; Rae, L.; Kwasniewska, K.; Raganelli, A.; Hanczaryk, K.; Lohan, A.J.; Loftus, B.; Graciet, E.; Wellmer, F. Molecular basis for the specification of floral organs by APETALA3 and PISTILLATA. Proc. Natl. Acad. Sci. USA 2012, 109, 13452–13457. [Google Scholar] [CrossRef]
- Kong, H.Z.; Landherr, L.L.; Frohlich, M.W.; Leebens-Mack, J.; Ma, H.; dePamphilis, C.W. Patterns of gene duplication in the plant SKP1 gene family in angiosperms: Evidence for multiple mechanisms of rapid gene birth. Plant J. 2007, 50, 873–885. [Google Scholar] [CrossRef] [PubMed]
- Airoldi, C.A.; Davies, B. Gene duplication and the evolution of plant MADS-box transcription factors. J. Genet. Genom. 2012, 39, 157–165. [Google Scholar] [CrossRef]
- Gregis, V.; Sessa, A.; Dorca-Fornell, C.; Kater, M.M. The Arabidopsis floral meristem identity genes AP1, AGL24 and SVP directly repress class B and C floral homeotic genes. Plant J. 2009, 60, 626–637. [Google Scholar] [CrossRef]
- Brownfield, L. Plant breeding: Revealing the secrets of cytoplasmic male sterility in wheat. Curr. Biol. 2021, 31, R724–R726. [Google Scholar] [CrossRef]
- Cheng, Z.H.; Song, W.Y.; Zhang, X.L. Genic male and female sterility in vegetable crops. Hortic. Res. 2023, 10, uhac232. [Google Scholar] [CrossRef]
- Farinati, S.; Draga, S.; Betto, A.; Palumbo, F.; Vannozzi, A.; Lucchin, M.; Barcaccia, G. Current insights and advances into plant male sterility: New precision breeding technology based on genome editing applications. Front. Plant Sci. 2023, 14, 1223861. [Google Scholar] [CrossRef] [PubMed]
- García-Sogo, B.; Pineda, B.; Castelblanque, L.; Antón, T.; Medina, M.; Roque, E.; Torresi, C.; Beltrán, J.P.; Moreno, V.; Cañas, L.A. Efficient transformation of Kalanchoe blossfeldiana and production of male-sterile plants by engineered anther ablation. Plant Cell Rep. 2010, 29, 61–77. [Google Scholar] [CrossRef]
- Reeves, P.H.; Ellis, C.M.; Ploense, S.E.; Wu, M.F.; Yadav, V.; Tholl, D.; Chételat, A.; Haupt, I.; Kennerley, B.J.; Hodgens, C.; et al. A regulatory network for coordinated flower maturation. PLoS Genet. 2012, 8, e1002506. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.H.; Ma, Y.; Wu, Y.; Akbar, A.; Shaban, M.; Ullah, A.; Deng, J.; Khan, A.S.; Chi, H.; Zhu, L.; et al. High-temperature stress suppresses allene oxide cyclase 2 and causes male sterility in cotton by disrupting jasmonic acid signaling. Crop J. 2023, 11, 33–45. [Google Scholar] [CrossRef]
- He, Y.; Liu, C.; Zhu, L.; Fu, M.; Sun, Y.; Zeng, H. Jasmonic acid plays a pivotal role in pollen development and fertility regulation in different types of P(T)GMS rice lines. Int. J. Mol. Sci. 2021, 22, 7926. [Google Scholar] [CrossRef] [PubMed]
- Schubert, R.; Dobritzsch, S.; Gruber, C.; Hause, G.; Athmer, B.; Schreiber, T.; Marillonnet, S.; Okabe, Y.; Ezura, H.; Acosta, I.F.; et al. Tomato MYB21 Acts in Ovules to Mediate Jasmonate-Regulated Fertility. Plant Cell 2019, 31, 1043–1062. [Google Scholar] [CrossRef]
- Jang, G.; Yoon, Y.; Choi, Y.D. Crosstalk with jasmonic acid integrates multiple responses in plant development. Int. J. Mol. Sci. 2020, 21, 305. [Google Scholar] [CrossRef]
- Chen, C.J.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.H.; Xia, R. TBtools: An integrative toolkit developed for interactive analyses of big biological data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).