The Physiological and Molecular Mechanisms of Silicon Action in Salt Stress Amelioration
Abstract
1. Introduction
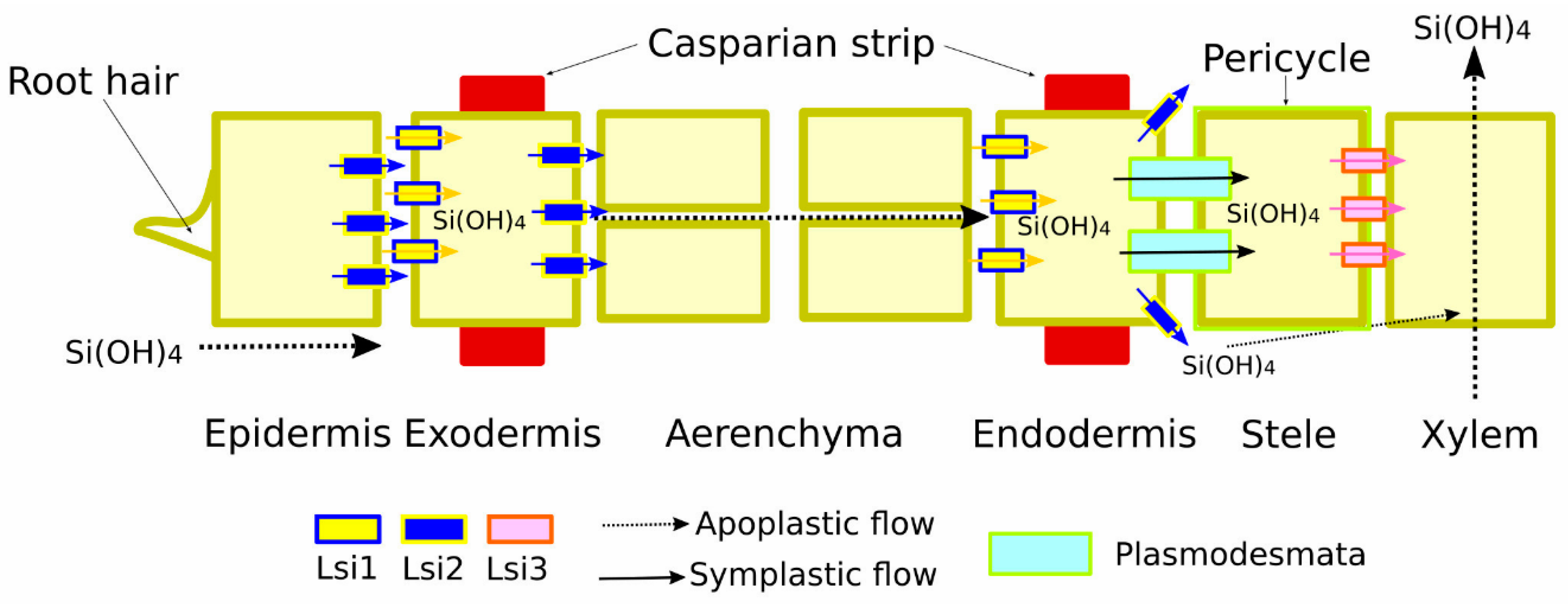
2. Mechanisms of Si Uptake, Transport, and Accumulation
2.1. Si Accumulation
- (1)
- Negative associations between Si and lignin content in several species [48];
- (2)
- Increased lignin biosynthesis in rice Si transporter mutants [49];
- (3)
- Absence of Si in hydroponic medium induced cell wall thickening in rice. The expression of lignin biosynthesis-related genes and secondary cell wall cellulose synthase genes was upregulated [50];
- (4)
- In the rice straw from many locations across South East Asia, the concentrations of Si were negatively related to the concentrations of carbon and lignin-derived phenols [51].
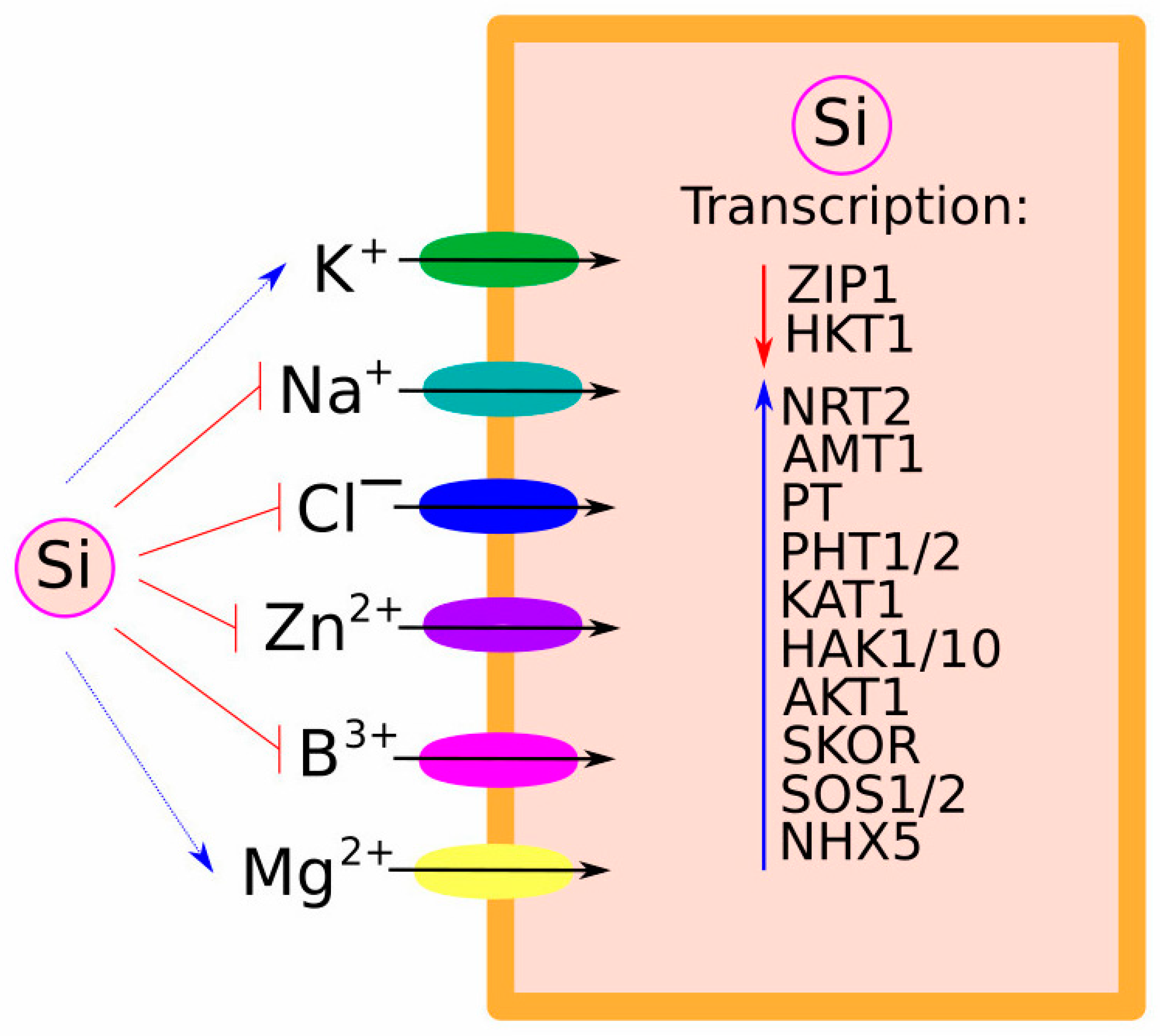
2.2. Crosstalk between Si and Other Ions
3. Effect of Si on Plant Physiology and Biochemistry
3.1. Uptake and Transport of Na+, K+, and Cl−
3.2. Protective Role of Si in Photosynthesis
- (1)
- (2)
- Si supplementation increased stomatal conductance, transpiration rate, and stomatal size and number, which resulted in efficient photosynthetic activity under salinity stress [81].
- (3)
- Si supplementation can modulate the activities of carbohydrate metabolism enzymes, thus decreasing the content of soluble sugar and starch in leaves but increasing starch content in roots. This alleviated photosynthetic feedback repression in leaves and provided more energy for root growth [82].
3.3. Salt Stress-Mediated Surplus ROS Production and Antioxidant Response
4. Role of Osmoprotectants and Polyamines in Si-Mediated Salt Stress-Improving Effects
5. Role of Phytohormones in Si-Mediated Salt Stress-Ameliorating Effects
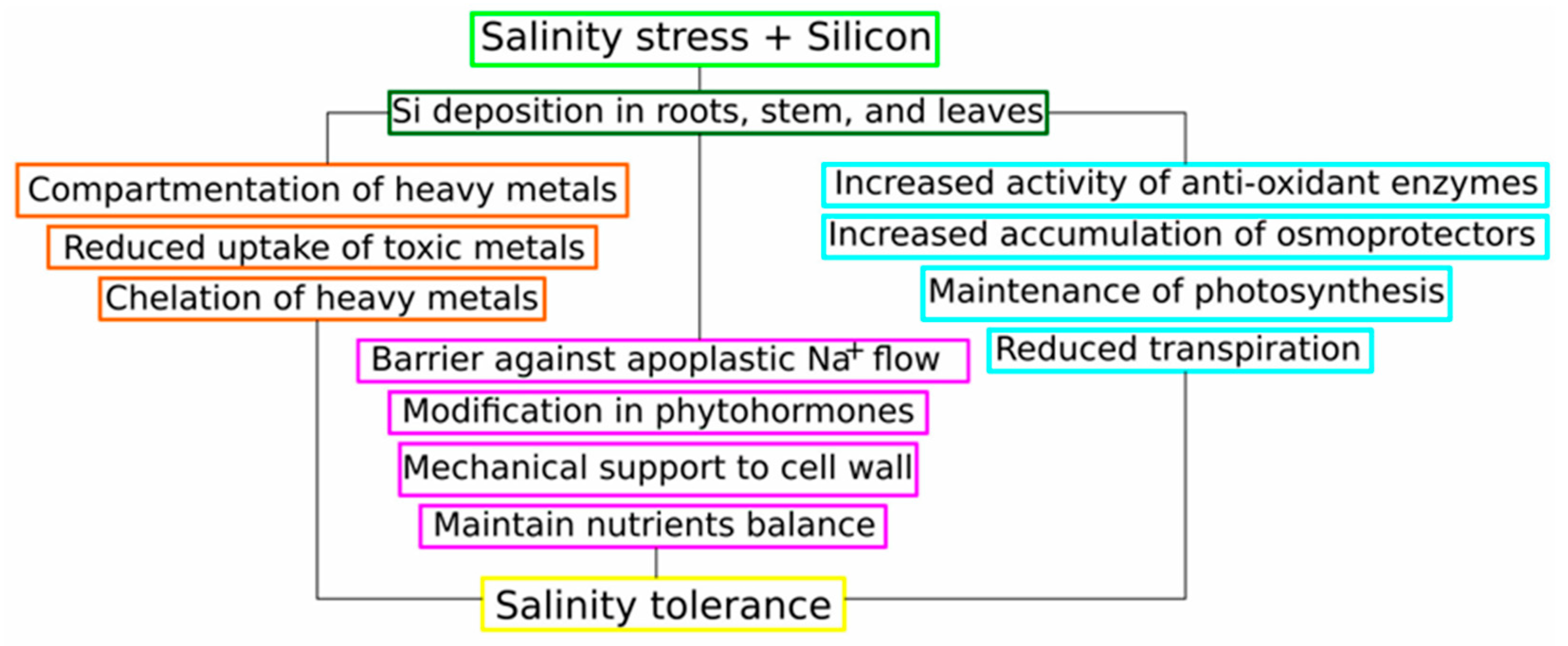
6. Conclusions and Future Prospective
- Mechanistic Understanding:
- Transcriptomics and Proteomics: Conduct more extensive and systematic transcriptomic and proteomic studies to unravel the specific genes and proteins influenced by Si supplementation under salt stress conditions. This will provide a more comprehensive understanding of the molecular mechanisms involved.
- Cellular Signalling Pathways: Investigate the intricate signalling pathways involved in Si-mediated stress tolerance. Explore how Si interacts with established stress signalling molecules, such as calcium ions (Ca2+), ROS, and nitric oxide, to modulate stress responses.
- Hormonal Crosstalk:
- Phytohormone Interactions: Elaborate on the crosstalk between Si and different phytohormones. Explore the interconnected regulatory networks, particularly focusing on how Si influences the biosynthesis, signalling, and interaction of known plant hormones.
- Temporal Dynamics: Investigate the temporal dynamics of hormonal responses to Si supplementation under salt stress. Understand how these responses evolve over time, providing insights into the short-term and long-term effects of Si on salt stress adaptation.
- Si Uptake and Transport:
- Transporter Genes: Explore the regulation of Si transporter genes (Lsi1 and Lsi2) under different stress conditions. Investigate how these transporters contribute to Si uptake and distribution within the plant and their role in salt stress tolerance.
- Root-Soil Interactions: Study the influence of soil properties and rhizosphere interactions on Si availability and uptake. Determine the optimal conditions for Si supplementation to maximize its effectiveness in improving salt stress resistance.
- Species-Specific Responses:
- Comparative Studies: Conduct comparative studies across a diverse range of plant species to identify commonalities and variations in Si-mediated stress responses. Determine whether the effectiveness of Si supplementation is species-specific and influenced by genetic factors.
- Crop-Specific Strategies: Tailor Si supplementation strategies to different crops based on their unique physiological and genetic characteristics. Optimise Si application methods, concentrations, and timing for maximum salt stress tolerance in economically important crops.
- Interaction with Other Abiotic Stresses:
- Multiple Stressors: Investigate the combined effects of Si supplementation on plants facing multiple abiotic stresses, such as salinity, drought, and heavy metal toxicity. Understand how Si interacts with these stressors to provide holistic strategies for sustainable agriculture.
- Environmental Conditions: Explore the influence of varying environmental conditions (temperature, light intensity, humidity, etc.) on the efficacy of Si-mediated salt stress tolerance. Determine the robustness of Si-enhanced resilience under different climate scenarios.
- Field Trials and Practical Applications:
- Field-Scale Studies: Translate laboratory findings into field-scale trials to assess the practical implications of Si supplementation for stress management in real-world agricultural settings. Consider variations in soil types, climates, and cultivation practices. Application of Si depends on the plant growing stage (seed soaking, foliar, root, or soil treatment). Accordingly, working concentration would depend on the Si source (like SiO2, Si(OC2H5)4, (K2SiO3), Na2SiO3⋅5H2O, and so on) and may range from 5 mg L−1 to 400 mg L−1 [10,111].
- Economic and Environmental Impact: Evaluate the economic feasibility and environmental impact of large-scale Si supplementation. Consider factors such as cost-effectiveness, potential ecological consequences, and long-term sustainability.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhou, H.; Shi, H.; Yang, Y.; Feng, X.; Chen, X.; Xiao, F.; Lin, H.; Guo, Y. Insights into Plant Salt Stress Signaling and Tolerance. J. Genet. Genom. 2024, 51, 16–34. [Google Scholar] [CrossRef]
- Balasubramaniam, T.; Shen, G.; Esmaeili, N.; Zhang, H. Plants’ Response Mechanisms to Salinity Stress. Plants 2023, 12, 2253. [Google Scholar] [CrossRef]
- Napieraj, N.; Reda, M.; Janicka, M. The Role of NO in Plant Response to Salt Stress: Interactions with Polyamines. Funct. Plant Biol. 2020, 47, 865. [Google Scholar] [CrossRef] [PubMed]
- García-Caparrós, P.; Hasanuzzaman, M.; Lao, M.T. Oxidative Stress and Antioxidant Defense in Plants Under Salinity. In Reactive Oxygen, Nitrogen and Sulfur Species in Plants; Hasanuzzaman, M., Fotopoulos, V., Nahar, K., Fujita, M., Eds.; Wiley: Hoboken, NJ, USA, 2019; pp. 291–309. ISBN 978-1-119-46869-1. [Google Scholar]
- Shams, M.; Khadivi, A. Mechanisms of Salinity Tolerance and Their Possible Application in the Breeding of Vegetables. BMC Plant Biol. 2023, 23, 139. [Google Scholar] [CrossRef] [PubMed]
- Manivannan, A.; Soundararajan, P.; Jeong, B.R. Editorial: Silicon: A “Quasi-Essential” Element’s Role in Plant Physiology and Development. Front. Plant Sci. 2023, 14, 1157185. [Google Scholar] [CrossRef] [PubMed]
- Artyszak, A. Effect of Silicon Fertilization on Crop Yield Quantity and Quality—A Literature Review in Europe. Plants 2018, 7, 54. [Google Scholar] [CrossRef] [PubMed]
- Debona, D.; Rodrigues, F.A.; Datnoff, L.E. Silicon’s Role in Abiotic and Biotic Plant Stresses. Annu. Rev. Phytopathol. 2017, 55, 85–107. [Google Scholar] [CrossRef] [PubMed]
- Ranjan, A.; Sinha, R.; Bala, M.; Pareek, A.; Singla-Pareek, S.L.; Singh, A.K. Silicon-Mediated Abiotic and Biotic Stress Mitigation in Plants: Underlying Mechanisms and Potential for Stress Resilient Agriculture. Plant Physiol. Biochem. 2021, 163, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Dhiman, P.; Rajora, N.; Bhardwaj, S.; Sudhakaran, S.S.; Kumar, A.; Raturi, G.; Chakraborty, K.; Gupta, O.P.; Devanna, B.N.; Tripathi, D.K.; et al. Fascinating Role of Silicon to Combat Salinity Stress in Plants: An Updated Overview. Plant Physiol. Biochem. 2021, 162, 110–123. [Google Scholar] [CrossRef]
- Yassen, A.; Abdallah, E.; Gaballah, M.; Zaghloul, S. Role of Silicon Dioxide Nano Fertilizer in Mitigating Salt Stress on Growth, Yield and Chemical Composition of Cucumber (Cucumis sativus L.). Int. J. Agric. Res. 2017, 12, 130–135. [Google Scholar] [CrossRef]
- Bhardwaj, S.; Kapoor, D. Fascinating Regulatory Mechanism of Silicon for Alleviating Drought Stress in Plants. Plant Physiol. Biochem. 2021, 166, 1044–1053. [Google Scholar] [CrossRef]
- Hussain, M.; Khan, T.A.; Yusuf, M.; Fariduddin, Q. Silicon-Mediated Role of 24-Epibrassinolide in Wheat under High-Temperature Stress. Environ. Sci. Pollut. Res. 2019, 26, 17163–17172. [Google Scholar] [CrossRef] [PubMed]
- Soundararajan, P.; Manivannan, A.; Cho, Y.S.; Jeong, B.R. Exogenous Supplementation of Silicon Improved the Recovery of Hyperhydric Shoots in Dianthus caryophyllus L. by Stabilizing the Physiology and Protein Expression. Front. Plant Sci. 2017, 8, 738. [Google Scholar] [CrossRef] [PubMed]
- Islam, W.; Tayyab, M.; Khalil, F.; Hua, Z.; Huang, Z.; Chen, H.Y.H. Silicon-Mediated Plant Defense against Pathogens and Insect Pests. Pestic. Biochem. Physiol. 2020, 168, 104641. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.-X.; Gong, H.-J.; Yin, J.-L. Role of Silicon in Mediating Salt Tolerance in Plants: A Review. Plants 2019, 8, 147. [Google Scholar] [CrossRef] [PubMed]
- Zargar, S.M.; Mahajan, R.; Bhat, J.A.; Nazir, M.; Deshmukh, R. Role of Silicon in Plant Stress Tolerance: Opportunities to Achieve a Sustainable Cropping System. 3 Biotech 2019, 9, 73. [Google Scholar] [CrossRef]
- Song, A.; Ning, D.; Fan, F.; Li, Z.; Provance-Bowley, M.; Liang, Y. The Potential for Carbon Bio-Sequestration in China’s Paddy Rice (Oryza sativa L.) as Impacted by Slag-Based Silicate Fertilizer. Sci. Rep. 2015, 5, 17354. [Google Scholar] [CrossRef]
- Kaur, H.; Greger, M. A Review on Si Uptake and Transport System. Plants 2019, 8, 81. [Google Scholar] [CrossRef]
- Gaur, S.; Kumar, J.; Kumar, D.; Chauhan, D.K.; Prasad, S.M.; Srivastava, P.K. Fascinating Impact of Silicon and Silicon Transporters in Plants: A Review. Ecotoxicol. Environ. Saf. 2020, 202, 110885. [Google Scholar] [CrossRef]
- Ma, J.F.; Tamai, K.; Yamaji, N.; Mitani, N.; Konishi, S.; Katsuhara, M.; Ishiguro, M.; Murata, Y.; Yano, M. A Silicon Transporter in Rice. Nature 2006, 440, 688–691. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, Z.; Liu, F.; Sun, L.; Hao, F. Versatile Roles of Aquaporins in Plant Growth and Development. Int. J. Mol. Sci. 2020, 21, 9485. [Google Scholar] [CrossRef]
- Ma, J.F.; Yamaji, N.; Mitani, N.; Tamai, K.; Konishi, S.; Fujiwara, T.; Katsuhara, M.; Yano, M. An Efflux Transporter of Silicon in Rice. Nature 2007, 448, 209–212. [Google Scholar] [CrossRef]
- Mitani, N.; Chiba, Y.; Yamaji, N.; Ma, J.F. Identification and Characterization of Maize and Barley Lsi2-Like Silicon Efflux Transporters Reveals a Distinct Silicon Uptake System from That in Rice. Plant Cell 2009, 21, 2133–2142. [Google Scholar] [CrossRef] [PubMed]
- Montpetit, J.; Vivancos, J.; Mitani-Ueno, N.; Yamaji, N.; Rémus-Borel, W.; Belzile, F.; Ma, J.F.; Bélanger, R.R. Cloning, Functional Characterization and Heterologous Expression of TaLsi1, a Wheat Silicon Transporter Gene. Plant Mol. Biol. 2012, 79, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Mitani, N.; Yamaji, N.; Ago, Y.; Iwasaki, K.; Ma, J.F. Isolation and Functional Characterization of an Influx Silicon Transporter in Two Pumpkin Cultivars Contrasting in Silicon Accumulation. Plant J. 2011, 66, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Noronha, H.; Silva, A.; Mitani-Ueno, N.; Conde, C.; Sabir, F.; Prista, C.; Soveral, G.; Isenring, P.; Ma, J.F.; Bélanger, R.R.; et al. The Grapevine NIP2;1 Aquaporin Is a Silicon Channel. J. Exp. Bot. 2020, 71, 6789–6798. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Guo, J.; Duan, Y.; Zhang, T.; Huo, H.; Gong, H. Isolation and Functional Characterization of CsLsi1, a Silicon Transporter Gene in Cucumis sativus. Physiol. Plant 2017, 159, 201–214. [Google Scholar] [CrossRef]
- Sun, H.; Duan, Y.; Qi, X.; Zhang, L.; Huo, H.; Gong, H. Isolation and Functional Characterization of CsLsi2, a Cucumber Silicon Efflux Transporter Gene. Ann. Bot. 2018, 122, 641–648. [Google Scholar] [CrossRef]
- Sun, H.; Duan, Y.; Mitani-Ueno, N.; Che, J.; Jia, J.; Liu, J.; Guo, J.; Ma, J.F.; Gong, H. Tomato Roots Have a Functional Silicon Influx Transporter but Not a Functional Silicon Efflux Transporter. Plant Cell Environ. 2020, 43, 732–744. [Google Scholar] [CrossRef] [PubMed]
- Mandlik, R.; Thakral, V.; Raturi, G.; Shinde, S.; Nikolić, M.; Tripathi, D.K.; Sonah, H.; Deshmukh, R. Significance of Silicon Uptake, Transport, and Deposition in Plants. J. Exp. Bot. 2020, 71, 6703–6718. [Google Scholar] [CrossRef]
- Saitoh, Y.; Mitani-Ueno, N.; Saito, K.; Matsuki, K.; Huang, S.; Yang, L.; Yamaji, N.; Ishikita, H.; Shen, J.-R.; Ma, J.F.; et al. Structural Basis for High Selectivity of a Rice Silicon Channel Lsi1. Nat. Commun. 2021, 12, 6236. [Google Scholar] [CrossRef]
- Van Den Berg, B.; Pedebos, C.; Bolla, J.R.; Robinson, C.V.; Baslé, A.; Khalid, S. Structural Basis for Silicic Acid Uptake by Higher Plants. J. Mol. Biol. 2021, 433, 167226. [Google Scholar] [CrossRef]
- Rougé, P.; Barre, A. A Molecular Modeling Approach Defines a New Group of Nodulin 26-like Aquaporins in Plants. Biochem. Biophys. Res. Commun. 2008, 367, 60–66. [Google Scholar] [CrossRef]
- Vatansever, R.; Ozyigit, I.I.; Filiz, E.; Gozukara, N. Genome-Wide Exploration of Silicon (Si) Transporter Genes, Lsi1 and Lsi2 in Plants; Insights into Si-Accumulation Status/Capacity of Plants. Biometals 2017, 30, 185–200. [Google Scholar] [CrossRef] [PubMed]
- Mitani, N.; Yamaji, N.; Ma, J.F. Characterization of Substrate Specificity of a Rice Silicon Transporter, Lsi1. Pflug. Arch. Eur. J. Physiol. 2008, 456, 679–686. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, R.K.; Vivancos, J.; Ramakrishnan, G.; Guérin, V.; Carpentier, G.; Sonah, H.; Labbé, C.; Isenring, P.; Belzile, F.J.; Bélanger, R.R. A Precise Spacing between the NPA Domains of Aquaporins Is Essential for Silicon Permeability in Plants. Plant J. 2015, 83, 489–500. [Google Scholar] [CrossRef]
- Coskun, D.; Deshmukh, R.; Sonah, H.; Shivaraj, S.M.; Frenette-Cotton, R.; Tremblay, L.; Isenring, P.; Bélanger, R.R. Si Permeability of a Deficient Lsi1 Aquaporin in Tobacco Can Be Enhanced through a Conserved Residue Substitution. Plant Direct 2019, 3, e00163. [Google Scholar] [CrossRef] [PubMed]
- Konishi, N.; Mitani-Ueno, N.; Yamaji, N.; Ma, J.F. Polar Localization of a Rice Silicon Transporter Requires Isoleucine at both C- and N-Termini as well as Positively Charged Residues. Plant Cell 2023, 35, 2232–2250. [Google Scholar] [CrossRef]
- Huang, S.; Yamaji, N.; Sakurai, G.; Mitani-Ueno, N.; Konishi, N.; Ma, J.F. A Pericycle-localized Silicon Transporter for Efficient Xylem Loading in Rice. New Phytol. 2022, 234, 197–208. [Google Scholar] [CrossRef]
- Vivancos, J.; Deshmukh, R.; Grégoire, C.; Rémus-Borel, W.; Belzile, F.; Bélanger, R.R. Identification and Characterization of Silicon Efflux Transporters in Horsetail (Equisetum arvense). J. Plant Physiol. 2016, 200, 82–89. [Google Scholar] [CrossRef]
- Guerriero, G.; Deshmukh, R.; Sonah, H.; Sergeant, K.; Hausman, J.-F.; Lentzen, E.; Valle, N.; Siddiqui, K.S.; Exley, C. Identification of the Aquaporin Gene Family in Cannabis sativa and Evidence for the Accumulation of Silicon in Its Tissues. Plant Sci. 2019, 287, 110167. [Google Scholar] [CrossRef] [PubMed]
- Abe, J. Silicon Deposition in Leaf Trichomes of Cucurbitaceae Horticultural Plants: A Short Report. Am. J. Plant Sci. 2019, 10, 486–490. [Google Scholar] [CrossRef]
- Kumar, S.; Milstein, Y.; Brami, Y.; Elbaum, M.; Elbaum, R. Mechanism of Silica Deposition in Sorghum Silica Cells. New Phytol. 2017, 213, 791–798. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Adiram-Filiba, N.; Blum, S.; Sanchez-Lopez, J.A.; Tzfadia, O.; Omid, A.; Volpin, H.; Heifetz, Y.; Goobes, G.; Elbaum, R. Siliplant1 Protein Precipitates Silica in Sorghum Silica Cells. J. Exp. Bot. 2020, 71, 6830–6843. [Google Scholar] [CrossRef] [PubMed]
- Bokor, B.; Soukup, M.; Vaculík, M.; Vd’ačný, P.; Weidinger, M.; Lichtscheidl, I.; Vávrová, S.; Šoltys, K.; Sonah, H.; Deshmukh, R.; et al. Silicon Uptake and Localisation in Date Palm (Phoenix Dactylifera)—A Unique Association with Sclerenchyma. Front. Plant Sci. 2019, 10, 988. [Google Scholar] [CrossRef]
- Bauer, P.; Elbaum, R.; Weiss, I.M. Calcium and Silicon Mineralization in Land Plants: Transport, Structure and Function. Plant Sci. 2011, 180, 746–756. [Google Scholar] [CrossRef]
- Schoelynck, J.; Bal, K.; Backx, H.; Okruszko, T.; Meire, P.; Struyf, E. Silica Uptake in Aquatic and Wetland Macrophytes: A Strategic Choice between Silica, Lignin and Cellulose? New Phytol. 2010, 186, 385–391. [Google Scholar] [CrossRef]
- Suzuki, S.; Ma, J.F.; Yamamoto, N.; Hattori, T.; Sakamoto, M.; Umezawa, T. Silicon Deficiency Promotes Lignin Accumulation in Rice. Plant Biotechnol. 2012, 29, 391–394. [Google Scholar] [CrossRef]
- Yamamoto, T.; Nakamura, A.; Iwai, H.; Ishii, T.; Ma, J.F.; Yokoyama, R.; Nishitani, K.; Satoh, S.; Furukawa, J. Effect of Silicon Deficiency on Secondary Cell Wall Synthesis in Rice Leaf. J. Plant Res. 2012, 125, 771–779. [Google Scholar] [CrossRef]
- Klotzbücher, T.; Klotzbücher, A.; Kaiser, K.; Vetterlein, D.; Jahn, R.; Mikutta, R. Variable Silicon Accumulation in Plants Affects Terrestrial Carbon Cycling by Controlling Lignin Synthesis. Glob. Change Biol. 2018, 24, e183–e189. [Google Scholar] [CrossRef]
- Rivai, R.R.; Miyamoto, T.; Awano, T.; Yoshinaga, A.; Chen, S.; Sugiyama, J.; Tobimatsu, Y.; Umezawa, T.; Kobayashi, M. Limiting Silicon Supply Alters Lignin Content and Structures of Sorghum Seedling Cell Walls. Plant Sci. 2022, 321, 111325. [Google Scholar] [CrossRef]
- Soukup, M.; Martinka, M.; Bosnić, D.; Čaplovičová, M.; Elbaum, R.; Lux, A. Formation of Silica Aggregates in Sorghum Root Endodermis Is Predetermined by Cell Wall Architecture and Development. Ann. Bot. 2017, 120, 739–753. [Google Scholar] [CrossRef]
- Zexer, N.; Elbaum, R. Unique Lignin Modifications Pattern the Nucleation of Silica in Sorghum Endodermis. J. Exp. Bot. 2020, 71, 6818–6829. [Google Scholar] [CrossRef]
- Soukup, M.; Rodriguez Zancajo, V.M.; Kneipp, J.; Elbaum, R. Formation of Root Silica Aggregates in Sorghum Is an Active Process of the Endodermis. J. Exp. Bot. 2020, 71, 6807–6817. [Google Scholar] [CrossRef]
- Zexer, N.; Elbaum, R. Hydrogen Peroxide Modulates Silica Deposits in Sorghum Roots. J. Exp. Bot. 2022, 73, 1450–1463. [Google Scholar] [CrossRef]
- Berni, R.; Mandlik, R.; Hausman, J.; Guerriero, G. Silicon-induced Mitigatory Effects in Salt-stressed Hemp Leaves. Physiol. Plant. 2021, 171, 476–482. [Google Scholar] [CrossRef]
- Chaiwong, N.; Bouain, N.; Prom-u-thai, C.; Rouached, H. Interplay Between Silicon and Iron Signaling Pathways to Regulate Silicon Transporter Lsi1 Expression in Rice. Front. Plant Sci. 2020, 11, 1065. [Google Scholar] [CrossRef]
- Huang, S.; Ma, J.F. Silicon Suppresses Zinc Uptake through Down-regulating Zinc Transporter Gene in Rice. Physiol. Plant. 2020, 170, 580–591. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Cai, X.; Jeong, B.R. Silicon Affects Root Development, Tissue Mineral Content, and Expression of Silicon Transporter Genes in Poinsettia (Euphorbia pulcherrima Willd.) Cultivars. Plants 2019, 8, 180. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Li, Y.; Jeong, B.R. Putative Silicon Transporters and Effect of Temperature Stresses and Silicon Supplementation on Their Expressions and Tissue Silicon Content in Poinsettia. Plants 2020, 9, 569. [Google Scholar] [CrossRef] [PubMed]
- Khan, E.; Gupta, M. Arsenic–Silicon Priming of Rice (Oryza sativa L.) Seeds Influence Mineral Nutrient Uptake and Biochemical Responses through Modulation of Lsi-1, Lsi-2, Lsi-6 and Nutrient Transporter Genes. Sci. Rep. 2018, 8, 10301. [Google Scholar] [CrossRef]
- Zhu, Y.; Yin, J.; Liang, Y.; Liu, J.; Jia, J.; Huo, H.; Wu, Z.; Yang, R.; Gong, H. Transcriptomic Dynamics Provide an Insight into the Mechanism for Silicon-Mediated Alleviation of Salt Stress in Cucumber Plants. Ecotoxicol. Environ. Saf. 2019, 174, 245–254. [Google Scholar] [CrossRef]
- Soundararajan, P.; Manivannan, A.; Ko, C.H.; Muneer, S.; Jeong, B.R. Leaf Physiological and Proteomic Analysis to Elucidate Silicon Induced Adaptive Response under Salt Stress in Rosa Hybrida ‘Rock Fire’. Int. J. Mol. Sci. 2017, 18, 1768. [Google Scholar] [CrossRef]
- Jana, G.A.; Al Kharusi, L.; Sunkar, R.; Al-Yahyai, R.; Yaish, M.W. Metabolomic Analysis of Date Palm Seedlings Exposed to Salinity and Silicon Treatments. Plant Signal. Behav. 2019, 14, 1663112. [Google Scholar] [CrossRef] [PubMed]
- Chele, K.H.; Steenkamp, P.; Piater, L.A.; Dubery, I.A.; Huyser, J.; Tugizimana, F. A Global Metabolic Map Defines the Effects of a Si-Based Biostimulant on Tomato Plants under Normal and Saline Conditions. Metabolites 2021, 11, 820. [Google Scholar] [CrossRef]
- Yin, L.; Wang, S.; Li, J.; Tanaka, K.; Oka, M. Application of Silicon Improves Salt Tolerance through Ameliorating Osmotic and Ionic Stresses in the Seedling of Sorghum Bicolor. Acta Physiol. Plant 2013, 35, 3099–3107. [Google Scholar] [CrossRef]
- Hurtado, A.C.; Chiconato, D.A.; De Mello Prado, R.; Da Silveira Sousa, G., Jr.; Viciedo, D.O.; Díaz, Y.P.; Calzada, K.P.; Gratão, P.L. Silicon Alleviates Sodium Toxicity in Sorghum and Sunflower Plants by Enhancing Ionic Homeostasis in Roots and Shoots and Increasing Dry Matter Accumulation. Silicon 2021, 13, 475–486. [Google Scholar] [CrossRef]
- Calero Hurtado, A.; Aparecida Chiconato, D.; De Mello Prado, R.; Da Silveira Sousa, G., Jr.; Felisberto, G. Silicon Attenuates Sodium Toxicity by Improving Nutritional Efficiency in Sorghum and Sunflower Plants. Plant Physiol. Biochem. 2019, 142, 224–233. [Google Scholar] [CrossRef]
- Javaid, T.; Farooq, M.A.; Akhtar, J.; Saqib, Z.A.; Anwar-ul-Haq, M. Silicon Nutrition Improves Growth of Salt-Stressed Wheat by Modulating Flows and Partitioning of Na+, Cl− and Mineral Ions. Plant Physiol. Biochem. 2019, 141, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Faiyue, B.; Al-Azzawi, M.J.; Flowers, T.J. A New Screening Technique for Salinity Resistance in Rice (Oryza sativa L.) Seedlings Using Bypass Flow. Plant Cell Environ. 2012, 35, 1099–1108. [Google Scholar] [CrossRef] [PubMed]
- Yan, G.; Fan, X.; Tan, L.; Yin, C.; Li, T.; Liang, Y. Root Silicon Deposition and Its Resultant Reduction of Sodium Bypass Flow Is Modulated by OsLsi1 and OsLsi2 in Rice. Plant Physiol. Biochem. 2021, 158, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Flam-Shepherd, R.; Huynh, W.Q.; Coskun, D.; Hamam, A.M.; Britto, D.T.; Kronzucker, H.J. Membrane Fluxes, Bypass Flows, and Sodium Stress in Rice: The Influence of Silicon. J. Exp. Bot. 2018, 69, 1679–1692. [Google Scholar] [CrossRef]
- Yan, G.; Fan, X.; Zheng, W.; Gao, Z.; Yin, C.; Li, T.; Liang, Y. Silicon Alleviates Salt Stress-Induced Potassium Deficiency by Promoting Potassium Uptake and Translocation in Rice (Oryza sativa L.). J. Plant Physiol. 2021, 258–259, 153379. [Google Scholar] [CrossRef] [PubMed]
- Gupta, B.K.; Sahoo, K.K.; Anwar, K.; Nongpiur, R.C.; Deshmukh, R.; Pareek, A.; Singla-Pareek, S.L. Silicon Nutrition Stimulates Salt-Overly Sensitive (SOS) Pathway to Enhance Salinity Stress Tolerance and Yield in Rice. Plant Physiol. Biochem. 2021, 166, 593–604. [Google Scholar] [CrossRef] [PubMed]
- Bosnic, P.; Bosnic, D.; Jasnic, J.; Nikolic, M. Silicon Mediates Sodium Transport and Partitioning in Maize under Moderate Salt Stress. Environ. Exp. Bot. 2018, 155, 681–687. [Google Scholar] [CrossRef]
- Muhammad, I.; Shalmani, A.; Ali, M.; Yang, Q.-H.; Ahmad, H.; Li, F.B. Mechanisms Regulating the Dynamics of Photosynthesis under Abiotic Stresses. Front. Plant Sci. 2021, 11, 615942. [Google Scholar] [CrossRef]
- Hameed, A.; Ahmed, M.Z.; Hussain, T.; Aziz, I.; Ahmad, N.; Gul, B.; Nielsen, B.L. Effects of Salinity Stress on Chloroplast Structure and Function. Cells 2021, 10, 2023. [Google Scholar] [CrossRef]
- Cao, B.; Ma, Q.; Zhao, Q.; Wang, L.; Xu, K. Effects of Silicon on Absorbed Light Allocation, Antioxidant Enzymes and Ultrastructure of Chloroplasts in Tomato Leaves under Simulated Drought Stress. Sci. Hortic. 2015, 194, 53–62. [Google Scholar] [CrossRef]
- Rastogi, A.; Yadav, S.; Hussain, S.; Kataria, S.; Hajihashemi, S.; Kumari, P.; Yang, X.; Brestic, M. Does Silicon Really Matter for the Photosynthetic Machinery in Plants…? Plant Physiol. Biochem. 2021, 169, 40–48. [Google Scholar] [CrossRef]
- Abbas, T.; Balal, R.M.; Shahid, M.A.; Pervez, M.A.; Ayyub, C.M.; Aqueel, M.A.; Javaid, M.M. Silicon-Induced Alleviation of NaCl Toxicity in Okra (Abelmoschus esculentus) Is Associated with Enhanced Photosynthesis, Osmoprotectants and Antioxidant Metabolism. Acta Physiol. Plant 2015, 37, 6. [Google Scholar] [CrossRef]
- Zhu, Y.; Guo, J.; Feng, R.; Jia, J.; Han, W.; Gong, H. The Regulatory Role of Silicon on Carbohydrate Metabolism in Cucumis sativus L. under Salt Stress. Plant Soil. 2016, 406, 231–249. [Google Scholar] [CrossRef]
- Silveira, J.A.G.; Carvalho, F.E.L. Proteomics, Photosynthesis and Salt Resistance in Crops: An Integrative View. J. Proteom. 2016, 143, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.-X.; Xu, X.-B.; Hu, Y.-H.; Han, W.-H.; Yin, J.-L.; Li, H.-L.; Gong, H.-J. Silicon Improves Salt Tolerance by Increasing Root Water Uptake in Cucumis sativus L. Plant Cell Rep. 2015, 34, 1629–1646. [Google Scholar] [CrossRef] [PubMed]
- Khan, W.; Aziz, T.; Hussain, I.; Ramzani, P.M.A.; Reichenauer, T.G. Silicon: A Beneficial Nutrient for Maize Crop to Enhance Photochemical Efficiency of Photosystem II under Salt Stress. Arch. Agron. Soil. Sci. 2017, 63, 599–611. [Google Scholar] [CrossRef]
- Alam, P.; Balawi, T.A.; Qadir, S.U.; Ahmad, P. Gibberellic Acid and Silicon Ameliorate NaCl Toxicity in Brassica Juncea: Possible Involvement of Antioxidant System and Ascorbate-Glutathione Cycle. Plants 2023, 12, 1210. [Google Scholar] [CrossRef] [PubMed]
- Kesawat, M.S.; Satheesh, N.; Kherawat, B.S.; Kumar, A.; Kim, H.-U.; Chung, S.-M.; Kumar, M. Regulation of Reactive Oxygen Species during Salt Stress in Plants and Their Crosstalk with Other Signaling Molecules—Current Perspectives and Future Directions. Plants 2023, 12, 864. [Google Scholar] [CrossRef]
- Liu, P.; Yin, L.; Wang, S.; Zhang, M.; Deng, X.; Zhang, S.; Tanaka, K. Enhanced Root Hydraulic Conductance by Aquaporin Regulation Accounts for Silicon Alleviated Salt-Induced Osmotic Stress in Sorghum bicolor L. Environ. Exp. Bot. 2015, 111, 42–51. [Google Scholar] [CrossRef]
- Das, P.; Manna, I.; Biswas, A.K.; Bandyopadhyay, M. Exogenous Silicon Alters Ascorbate-Glutathione Cycle in Two Salt-Stressed Indica Rice Cultivars (MTU 1010 and Nonabokra). Environ. Sci. Pollut. Res. 2018, 25, 26625–26642. [Google Scholar] [CrossRef]
- Li, Y.-T.; Zhang, W.-J.; Cui, J.-J.; Lang, D.-Y.; Li, M.; Zhao, Q.-P.; Zhang, X.-H. Silicon Nutrition Alleviates the Lipid Peroxidation and Ion Imbalance of Glycyrrhiza Uralensis Seedlings under Salt Stress. Acta Physiol. Plant 2016, 38, 96. [Google Scholar] [CrossRef]
- Zhang, W.; Xie, Z.; Wang, L.; Li, M.; Lang, D.; Zhang, X. Silicon Alleviates Salt and Drought Stress of Glycyrrhiza Uralensis Seedling by Altering Antioxidant Metabolism and Osmotic Adjustment. J. Plant Res. 2017, 130, 611–624. [Google Scholar] [CrossRef]
- Akhter, M.; Noreen, S.; Ummara, U.; Aqeel, M.; Saleem, N.; Ahmed, M.; Mahmood, S.; Athar, H.-R.; Alyemeni, M.; Kaushik, P.; et al. Silicon-Induced Mitigation of NaCl Stress in Barley (Hordeum vulgare L.), Associated with Enhanced Enzymatic and Non-Enzymatic Antioxidant Activities. Plants 2022, 11, 2379. [Google Scholar] [CrossRef]
- Meng, Y.; Yin, Q.; Yan, Z.; Wang, Y.; Niu, J.; Zhang, J.; Fan, K. Exogenous Silicon Enhanced Salt Resistance by Maintaining K+/Na+ Homeostasis and Antioxidant Performance in Alfalfa Leaves. Front. Plant Sci. 2020, 11, 1183. [Google Scholar] [CrossRef]
- El-Serafy, R.S.; El-Sheshtawy, A.-N.A.; Atteya, A.K.G.; Al-Hashimi, A.; Abbasi, A.M.; Al-Ashkar, I. Seed Priming with Silicon as a Potential to Increase Salt Stress Tolerance in Lathyrus odoratus. Plants 2021, 10, 2140. [Google Scholar] [CrossRef]
- Yin, J.; Jia, J.; Lian, Z.; Hu, Y.; Guo, J.; Huo, H.; Zhu, Y.; Gong, H. Silicon Enhances the Salt Tolerance of Cucumber through Increasing Polyamine Accumulation and Decreasing Oxidative Damage. Ecotoxicol. Environ. Saf. 2019, 169, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Wang, S.; Tanaka, K.; Fujihara, S.; Itai, A.; Den, X.; Zhang, S. Silicon-mediated Changes in Polyamines Participate in Silicon-induced Salt Tolerance in S Orghum Bicolor L. Plant Cell Environ. 2016, 39, 245–258. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Liu, P.; Chen, D.; Yin, L.; Li, H.; Deng, X. Silicon Enhanced Salt Tolerance by Improving the Root Water Uptake and Decreasing the Ion Toxicity in Cucumber. Front. Plant Sci. 2015, 6, 759. [Google Scholar] [CrossRef] [PubMed]
- Das, P.; Manna, I.; Sil, P.; Bandyopadhyay, M.; Biswas, A.K. Silicon Augments Salt Tolerance through Modulation of Polyamine and GABA Metabolism in Two Indica Rice (Oryza sativa L.) Cultivars. Plant Physiol. Biochem. 2021, 166, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Zhang, E.; Zhang, X.; Wang, Q. Silicon Alleviates Salinity Stress in Licorice (Glycyrrhiza uralensis) by Regulating Carbon and Nitrogen Metabolism. Sci. Rep. 2021, 11, 1115. [Google Scholar] [CrossRef] [PubMed]
- Guerriero, G.; Sutera, F.M.; Torabi-Pour, N.; Renaut, J.; Hausman, J.-F.; Berni, R.; Pennington, H.C.; Welsh, M.; Dehsorkhi, A.; Zancan, L.R.; et al. Phyto-Courier, a Silicon Particle-Based Nano-Biostimulant: Evidence from Cannabis sativa Exposed to Salinity. ACS Nano 2021, 15, 3061–3069. [Google Scholar] [CrossRef] [PubMed]
- Farouk, S.; Elhindi, K.M.; Alotaibi, M.A. Silicon Supplementation Mitigates Salinity Stress on Ocimum basilicum L. via Improving Water Balance, Ion Homeostasis, and Antioxidant Defense System. Ecotoxicol. Environ. Saf. 2020, 206, 111396. [Google Scholar] [CrossRef]
- Alam, P.; Arshad, M.; Al-Kheraif, A.A.; Azzam, M.A.; Al Balawi, T. Silicon Nanoparticle-Induced Regulation of Carbohydrate Metabolism, Photosynthesis, and ROS Homeostasis in Solanum lycopersicum Subjected to Salinity Stress. ACS Omega 2022, 7, 31834–31844. [Google Scholar] [CrossRef]
- Yan, G.; Fan, X.; Peng, M.; Yin, C.; Xiao, Z.; Liang, Y. Silicon Improves Rice Salinity Resistance by Alleviating Ionic Toxicity and Osmotic Constraint in an Organ-Specific Pattern. Front. Plant Sci. 2020, 11, 260. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Wu, Z.; Yin, L.; Chen, S.; Cai, Z.; Geng, X.; Wang, D. Physiological Basis and Differentially Expressed Genes in the Salt Tolerance Mechanism of Thalassia hemprichii. Front. Plant Sci. 2022, 13, 975251. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhu, L.; Zhao, F.; Li, J.; Zhang, X.; Kong, X.; Wu, H.; Zhang, Z. Plant Salinity Stress Response and Nano-Enabled Plant Salt Tolerance. Front. Plant Sci. 2022, 13, 843994. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Jiang, X.; Zhang, J.; He, Y.; Zhu, X.; Zhou, X.; Gong, H.; Yin, J.; Liu, Y. Silicon Confers Cucumber Resistance to Salinity Stress through Regulation of Proline and Cytokinins. Plant Physiol. Biochem. 2020, 156, 209–220. [Google Scholar] [CrossRef] [PubMed]
- Markovich, O.; Steiner, E.; Kouřil, Š.; Tarkowski, P.; Aharoni, A.; Elbaum, R. Silicon Promotes Cytokinin Biosynthesis and Delays Senescence in Arabidopsis and Sorghum. Plant Cell Environ. 2017, 40, 1189–1196. [Google Scholar] [CrossRef] [PubMed]
- Lang, D.Y.; Fei, P.X.; Cao, G.Y.; Jia, X.X.; Li, Y.T.; Zhang, X.H. Silicon Promotes Seedling Growth and Alters Endogenous IAA, GA3 and ABA Concentrations in Glycyrrhiza uralensis under 100 mM NaCl Stress. J. Hortic. Sci. Biotechnol. 2019, 94, 87–93. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, W.; Lang, D.; Cui, J.; Li, Y. Silicon Improves Salt Tolerance of Glycyrrhiza Uralensis Fisch. by Ameliorating Osmotic and Oxidative Stresses and Improving Phytohormonal Balance. Environ. Sci. Pollut. Res. 2018, 25, 25916–25932. [Google Scholar] [CrossRef]
- Khan, A.; Bilal, S.; Khan, A.L.; Imran, M.; Al-Harrasi, A.; Al-Rawahi, A.; Lee, I.-J. Silicon-Mediated Alleviation of Combined Salinity and Cadmium Stress in Date Palm (Phoenix dactylifera L.) by Regulating Physio-Hormonal Alteration. Ecotoxicol. Environ. Saf. 2020, 188, 109885. [Google Scholar] [CrossRef]
- Mukarram, M.; Petrik, P.; Mushtaq, Z.; Khan, M.M.A.; Gulfishan, M.; Lux, A. Silicon Nanoparticles in Higher Plants: Uptake, Action, Stress Tolerance, and Crosstalk with Phytohormones, Antioxidants, and Other Signalling Molecules. Environ. Pollut. 2022, 310, 119855. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dabravolski, S.A.; Isayenkov, S.V. The Physiological and Molecular Mechanisms of Silicon Action in Salt Stress Amelioration. Plants 2024, 13, 525. https://doi.org/10.3390/plants13040525
Dabravolski SA, Isayenkov SV. The Physiological and Molecular Mechanisms of Silicon Action in Salt Stress Amelioration. Plants. 2024; 13(4):525. https://doi.org/10.3390/plants13040525
Chicago/Turabian StyleDabravolski, Siarhei A., and Stanislav V. Isayenkov. 2024. "The Physiological and Molecular Mechanisms of Silicon Action in Salt Stress Amelioration" Plants 13, no. 4: 525. https://doi.org/10.3390/plants13040525
APA StyleDabravolski, S. A., & Isayenkov, S. V. (2024). The Physiological and Molecular Mechanisms of Silicon Action in Salt Stress Amelioration. Plants, 13(4), 525. https://doi.org/10.3390/plants13040525






