Enhancing Salt Tolerance in Poplar Seedlings through Arbuscular Mycorrhizal Fungi Symbiosis
Abstract
1. Introduction
2. Results
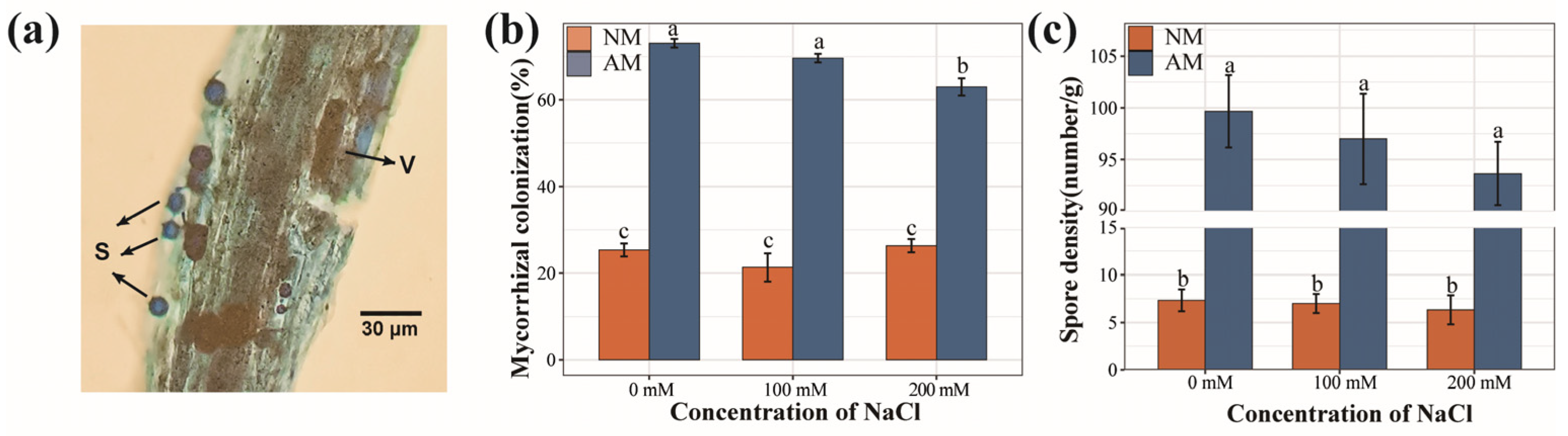
2.1. Mycorrhizal Colonization
2.2. Soil GRSP Content under Salt Stress
2.3. Plant Growth Quality
2.4. Leaf Photosynthesis and Chlorophyll Fluorescence Parameters
2.5. Antioxidant Defense of Plants
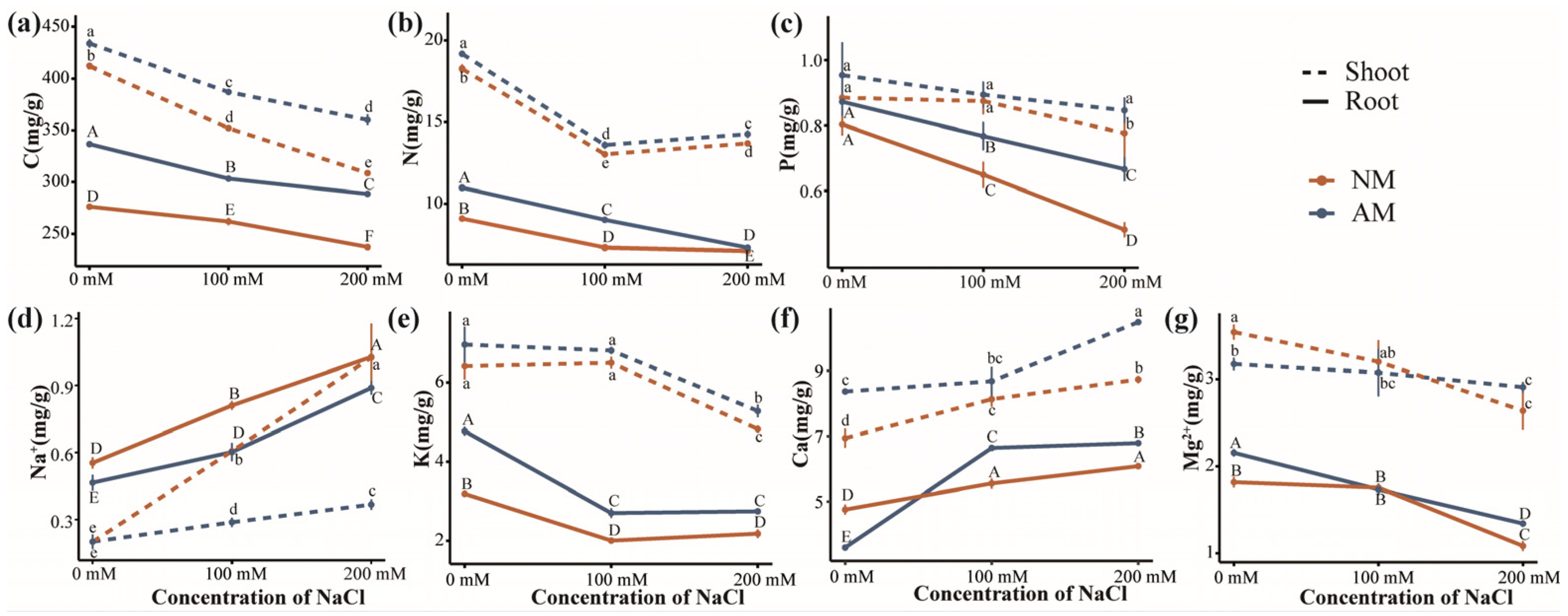
2.6. Absorption of Elements and Ions in Plants
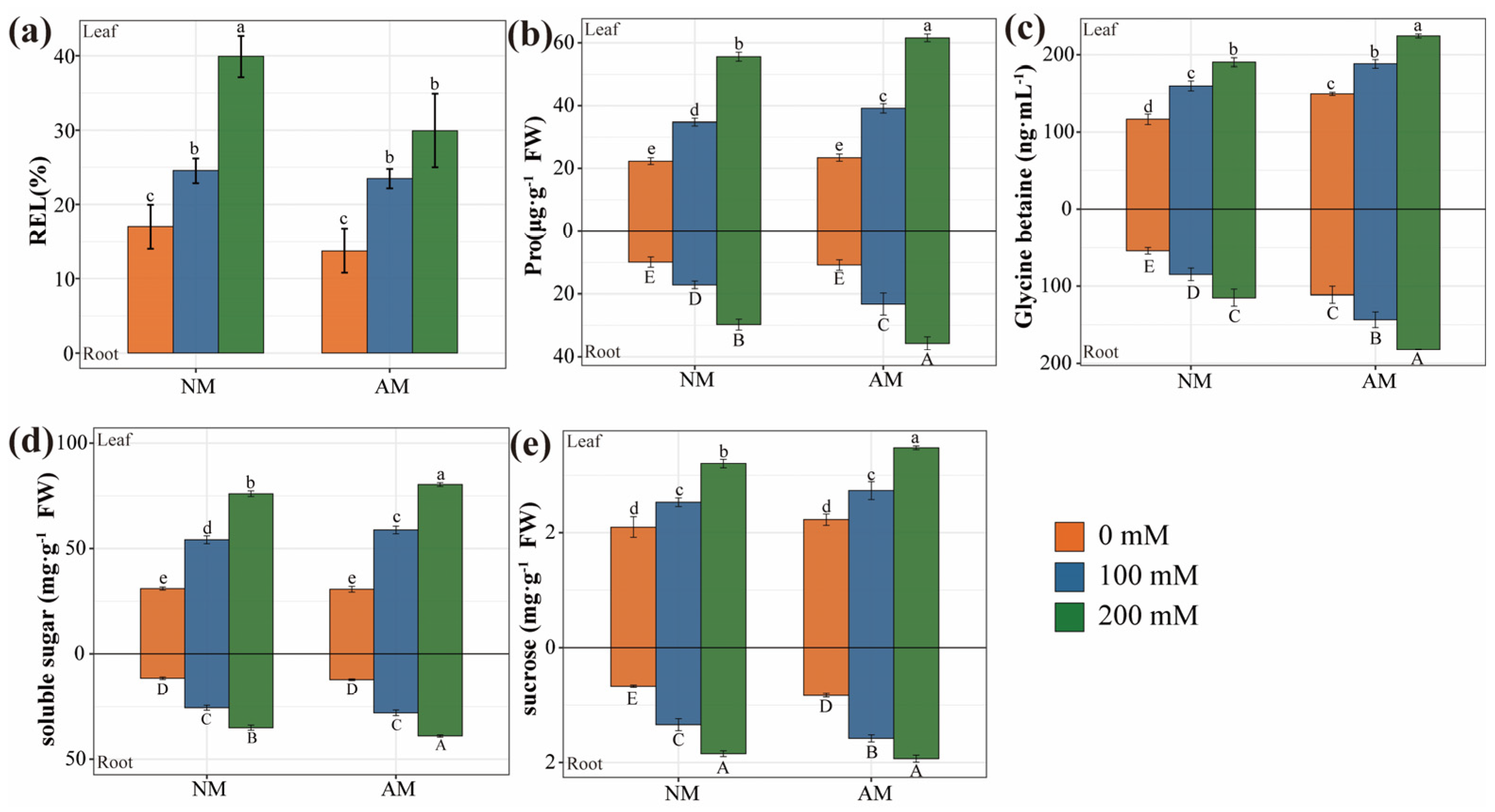
2.7. Osmotic Balance in Plants
2.8. PCA Analysis of Plants Inoculated with AMF under Salt Stress
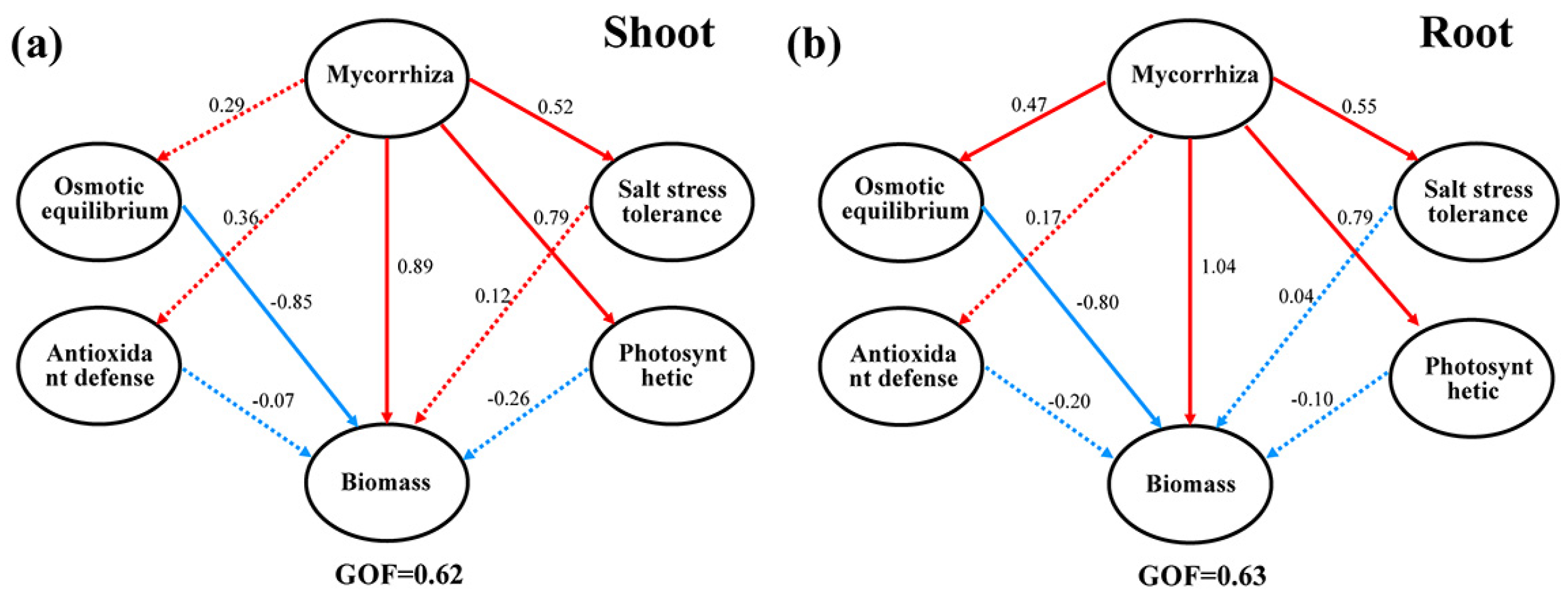
2.9. Partial Least Squares Path Modeling (PLS−PM) of AMF’s Effect on Salt Tolerance of Poplar
3. Discussion
3.1. Effects of Salt Stress and AMF Inoculation on Plant Growth and Quality
3.2. Effects of AMF Inoculation on Photosynthesis under Salt Stress
3.3. Effects of AMF Inoculation on Antioxidant Enzymes under Salt Stress
3.4. Effects of AMF Inoculation on Elemental Uptake under Salt Stress
3.5. Influence of AMF Inoculation on Osmotic Regulation under Salt Stress
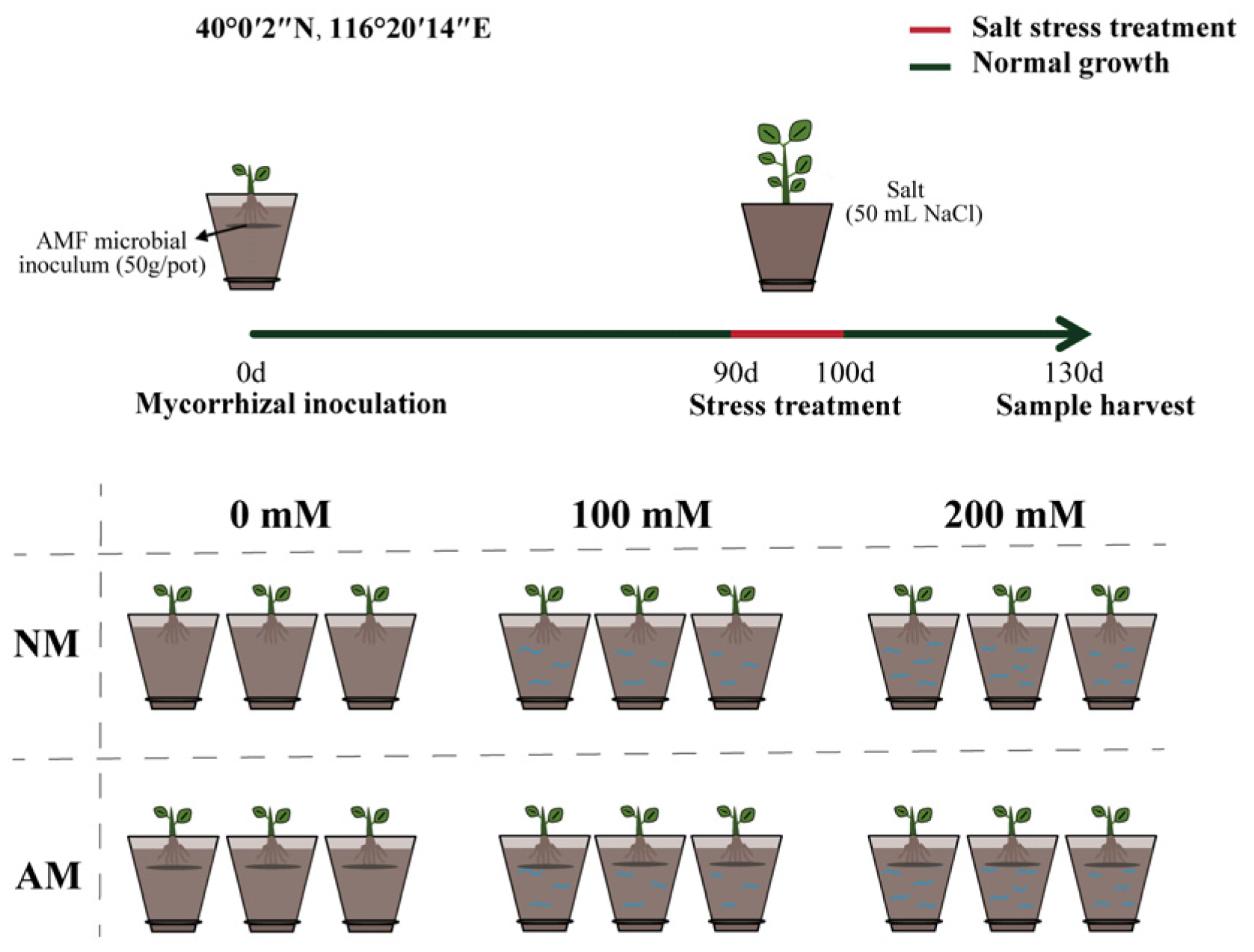
4. Materials and Methods
4.1. AMF Inoculum
4.2. Experimental Design
4.3. AMF Root Colonization and Soil Spore Density
4.4. Plant Growth Quality
4.5. Detection of Glomalin−Related Soil Protein
4.6. Photosynthetic Physiology
4.7. Antioxidant Defense
4.8. Nutrient Content
4.9. Osmotic Equilibrium
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singh, A. Soil salinization management for sustainable development: A review. J. Environ. Manag. 2021, 277, 111383. [Google Scholar] [CrossRef] [PubMed]
- Gong, Z. Plant abiotic stress: New insights into the factors that activate and modulate plant responses. J. Integr. Plant Biol. 2021, 63, 429. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Guo, Y. Unraveling salt stress signaling in plants. J. Integr. Plant Biol. 2018, 60, 796–804. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Pu, L.; Han, M.; Zhu, M.; Zhang, R.; Xiang, Y. Soil salinization research in China: Advances and prospects. J. Geogr. Sci. 2014, 24, 943–960. [Google Scholar] [CrossRef]
- Polle, A.; Janz, D.; Teichmann, T.; Lipka, V. Poplar genetic engineering: Promoting desirable wood characteristics and pest resistance. Appl. Microbiol. Biotechnol. 2013, 97, 5669–5679. [Google Scholar] [CrossRef]
- Sixto, H.; Grau, J.M.; Alba, N.; Alía, R. Response to sodium chloride in different species and clones of genus Populus L. Forestry 2005, 78, 93–104. [Google Scholar] [CrossRef]
- Jansson, S.; Douglas, C.J. Populus: A model system for plant biology. Annu. Rev. Plant Biol. 2007, 58, 435–458. [Google Scholar] [CrossRef]
- Wang, R.; Chen, S.L.; Deng, L.; Fritz, E.; Hüttermann, A.; Polle, A. Leaf photosynthesis, fluorescence response to salinity and the relevance to chloroplast salt compartmentation and anti-oxidative stress in two poplars. Trees 2007, 21, 581–591. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, L.; Chen, B.; Qin, Z.; Xiao, Y.; Zhang, Y.; Yao, R.; Liu, H.; Yang, H. Progress in Understanding the Physiological and Molecular Responses of Populus to Salt Stress. Int. J. Mol. Sci. 2019, 20, 1312. [Google Scholar] [CrossRef]
- Cregger, M.A.; Carper, D.L.; Christel, S.; Doktycz, M.J.; Labbé, J.; Michener, J.K.; Dove, N.C.; Johnston, E.R.; Moore, J.A.M.; Vélez, J.M.; et al. Plant–Microbe Interactions: From Genes to Ecosystems Using Populus as a Model System. Phytobiomes J. 2021, 5, 29–38. [Google Scholar] [CrossRef]
- Chen, S.; Polle, A. Salinity tolerance of Populus. Plant Biol. 2010, 12, 317–333. [Google Scholar] [CrossRef] [PubMed]
- Evelin, H.; Kapoor, R.; Giri, B. Arbuscular mycorrhizal fungi in alleviation of salt stress: A review. Ann. Bot. 2009, 104, 1263–1280. [Google Scholar] [CrossRef] [PubMed]
- Porcel, R.; Aroca, R.; Ruiz-Lozano, J.M. Salinity stress alleviation using arbuscular mycorrhizal fungi. A review. Agron. Sustain. Dev. 2011, 32, 181–200. [Google Scholar] [CrossRef]
- Wang, J.; Yuan, J.; Ren, Q.; Zhang, B.; Zhang, J.; Huang, R.; Wang, G.G. Arbuscular mycorrhizal fungi enhanced salt tolerance of Gleditsia sinensis by modulating antioxidant activity, ion balance and P/N ratio. Plant Growth Regul. 2022, 97, 33–49. [Google Scholar] [CrossRef]
- Tekaya, M.; Dabbaghi, O.; Guesmi, A.; Attia, F.; Chehab, H.; Khezami, L.; Algathami, F.K.; Ben Hamadi, N.; Hammami, M.; Prinsen, E.; et al. Arbuscular mycorrhizas modulate carbohydrate, phenolic compounds and hormonal metabolism to enhance water deficit tolerance of olive trees (Olea europaea). Agric. Water Manag. 2022, 274, 107947. [Google Scholar] [CrossRef]
- Zhang, H.; Li, L.; Ren, W.; Zhang, W.; Tang, M.; Chen, H. Arbuscular mycorrhizal fungal colonization improves growth, photosynthesis, and ROS regulation of split-root poplar under drought stress. Acta Physiol. Plant. 2022, 44, 62. [Google Scholar] [CrossRef]
- Ma, S.; Zhu, L.; Wang, J.; Liu, X.; Jia, Z.; Li, C.; Liu, J.; Zeng, J.; Zhang, J. Arbuscular Mycorrhizal Fungi Promote Gleditsia sinensis Lam. Root Growth under Salt Stress by Regulating Nutrient Uptake and Physiology. Forests 2022, 13, 688. [Google Scholar] [CrossRef]
- Wang, J.; Fu, Z.; Ren, Q.; Zhu, L.; Lin, J.; Zhang, J.; Cheng, X.; Ma, J.; Yue, J. Effects of Arbuscular Mycorrhizal Fungi on Growth, Photosynthesis, and Nutrient Uptake of Zelkova serrata (Thunb.) Makino Seedlings under Salt Stress. Forests 2019, 10, 186. [Google Scholar] [CrossRef]
- Kulczyk-Skrzeszewska, M.; Kieliszewska-Rokicka, B. Influence of drought and salt stress on the growth of young Populus nigra ‘Italica’ plants and associated mycorrhizal fungi and non-mycorrhizal fungal endophytes. New For. 2021, 53, 679–694. [Google Scholar] [CrossRef]
- Quoreshi, A.M.; Khasa, D.P. Effectiveness of mycorrhizal inoculation in the nursery on root colonization, growth, and nutrient uptake of aspen and balsam poplar. Biomass Bioenergy 2008, 32, 381–391. [Google Scholar] [CrossRef]
- Deyou, Q.; Shenglong, B.; Jianchao, M.; Lisha, Z.; Fenjuan, S.; Kaikai, Z.; Yanfang, Y.; Ting, S.; Jinling, H.; Yun, Z. The genome of Populus alba x Populus tremula var. glandulosa clone 84K. DNA Res. 2019, 5, 423–431. [Google Scholar] [CrossRef]
- Tikhomirova, T.S.; Krutovsky, K.V.; Shestibratov, K.A. Molecular Traits for Adaptation to Drought and Salt Stress in Birch, Oak and Poplar Species. Forests 2022, 14, 7. [Google Scholar] [CrossRef]
- Cicatelli, A.; Torrigiani, P.; Todeschini, V.; Biondi, S.; Castiglione, S.; Lingua, G. Arbuscular mycorrhizal fungi as a tool to ameliorate the phytoremediation potential of poplar: Biochemical and molecular aspects. Ifor. Biogeosci. For. 2014, 7, 333. [Google Scholar] [CrossRef]
- Klinsukon, C.; Lumyong, S.; Kuyper, T.W.; Boonlue, S. Colonization by arbuscular mycorrhizal fungi improves salinity tolerance of eucalyptus (Eucalyptus camaldulensis) seedlings. Sci. Rep. 2021, 11, 4362. [Google Scholar] [CrossRef] [PubMed]
- Garg, N.; Chandel, S. The Effects of Salinity on Nitrogen Fixation and Trehalose Metabolism in Mycorrhizal Cajanus cajan (L.) Millsp. Plants. J. Plant Growth Regul. 2011, 30, 490–503. [Google Scholar] [CrossRef]
- Wang, Q.; Lu, H.; Chen, J.; Jiang, Y.; Williams, M.A.; Wu, S.; Li, J.; Liu, J.; Yang, G.; Yan, C. Interactions of soil metals with glomalin-related soil protein as soil pollution bioindicators in mangrove wetland ecosystems. Sci. Total Environ. 2020, 709, 136051. [Google Scholar] [CrossRef] [PubMed]
- Ben-Laouane, R.; Baslam, M.; Ait-El-Mokhtar, M.; Anli, M.; Boutasknit, A.; Rahou, Y.A.; Toubali, S.; Mitsui, T.; Oufdou, K.; Wahbi, S.; et al. Potential of Native Arbuscular Mycorrhizal Fungi, Rhizobia, and/or Green Compost as Alfalfa (Medicago sativa) Enhancers under Salinity. Microorganisms 2020, 8, 1695. [Google Scholar] [CrossRef] [PubMed]
- Chandra, P.; Singh, A.; Prajapat, K.; Rai, A.K.; Yadav, R.K. Native arbuscular mycorrhizal fungi improve growth, biomass yield, and phosphorus nutrition of sorghum in saline and sodic soils of the semi–arid region. Environ. Exp. Bot. 2022, 201, 104982. [Google Scholar] [CrossRef]
- Garcia, C.L.; Dattamudi, S.; Chanda, S.; Jayachandran, K. Effect of salinity stress and microbial inoculations on glomalin production and plant growth parameters of snap bean (Phaseolus vulgaris). Agronomy 2019, 9, 545. [Google Scholar] [CrossRef]
- Abdal, M.; Etemadi, N.; Nikbakht, A.; Amirikhah, R. The arbuscular mycorrhizal symbiosis alleviating long-term salt stress through the modulation of nutrient elements, osmolytes, and antioxidant capacity in rosemary. Biologia 2022, 78, 993–1010. [Google Scholar] [CrossRef]
- Hashem, A.; Abd Allah, E.F.; Alqarawi, A.A.; Wirth, S.; Egamberdieva, D. Comparing symbiotic performance and physiological responses of two soybean cultivars to arbuscular mycorrhizal fungi under salt stress. Saudi J. Biol. Sci. 2019, 26, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.; Li, Z.; Liu, H.; Tang, M. Influence of arbuscular mycorrhiza on photosynthesis and water status of Populus cathayana Rehder males and females under salt stress. Acta Physiol. Plant. 2015, 37, 287–296. [Google Scholar] [CrossRef]
- Bárzana, G.; Aroca, R.; Paz, J.A.; Chaumont, F.; Martinez-Ballesta, M.C.; Carvajal, M.; Ruiz-Lozano, J.M. Arbuscular mycorrhizal symbiosis increases relative apoplastic water flow in roots of the host plant under both well-watered and drought stress conditions. Ann. Bot. 2012, 109, 1009–1017. [Google Scholar] [CrossRef]
- Hajiboland, R.; Sadeghzadeh, N.; Moradtalab, N.; Aliasgharzad, N.; Schweikert, K.; Poschenrieder, C. The arbuscular mycorrhizal mycelium from barley differentially influences various defense parameters in the non-host sugar beet under co-cultivation. Mycorrhiza 2020, 30, 647–661. [Google Scholar] [CrossRef]
- Wang, Y.; Dong, F.; Tang, M. Transcriptome Analysis of Arbuscular Mycorrhizal Casuarina glauca in Damage Mitigation of Roots on NaCl Stress. Microorganisms 2021, 10, 15. [Google Scholar] [CrossRef]
- Chen, X.W.; Kang, Y.; So, P.S.; Ng, C.W.W.; Wong, M.H. Arbuscular mycorrhizal fungi increase the proportion of cellulose and hemicellulose in the root stele of vetiver grass. Plant Soil 2018, 425, 309–319. [Google Scholar] [CrossRef]
- Chen, W.; Zou, D.; Guo, W.; Xu, H.; Shi, D.; Yang, C. Effects of salt stress on growth, photosynthesis and solute accumulation in three poplar cultivars. Photosynthetica 2009, 47, 415–421. [Google Scholar] [CrossRef]
- Zhang, T.; Hu, Y.; Zhang, K.; Tian, C.; Guo, J. Arbuscular mycorrhizal fungi improve plant growth of Ricinus communis by altering photosynthetic properties and increasing pigments under drought and salt stress. Ind. Crops Prod. 2018, 117, 13–19. [Google Scholar] [CrossRef]
- Pintó-Marijuan, M.; Munné-Bosch, S. Photo-oxidative stress markers as a measure of abiotic stress-induced leaf senescence: Advantages and limitations. J. Exp. Bot. 2014, 65, 3845–3857. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Song, F.; Xu, H. Arbuscular mycorrhizae improves low temperature stress in maize via alterations in host water status and photosynthesis. Plant Soil 2009, 331, 129–137. [Google Scholar] [CrossRef]
- Sharma, D.K.; Andersen, S.B.; Ottosen, C.O.; Rosenqvist, E. Wheat cultivars selected for high Fv/Fm under heat stress maintain high photosynthesis, total chlorophyll, stomatal conductance, transpiration and dry matter. Physiol. Plant. 2015, 153, 284–298. [Google Scholar] [CrossRef] [PubMed]
- Mo, Y.; Wang, Y.; Yang, R.; Zheng, J.; Liu, C.; Li, H.; Ma, J.; Zhang, Y.; Wei, C.; Zhang, X. Regulation of Plant Growth, Photosynthesis, Antioxidation and Osmosis by an Arbuscular Mycorrhizal Fungus in Watermelon Seedlings under Well-Watered and Drought Conditions. Front. Plant Sci. 2016, 7, 644. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Mu, X.; Shao, H.; Wang, H.; Brestic, M. Global plant-responding mechanisms to salt stress: Physiological and molecular levels and implications in biotechnology. Crit. Rev. Biotechnol. 2015, 35, 425–437. [Google Scholar] [CrossRef]
- Yan, K.; Shao, H.; Shao, C.; Chen, P.; Zhao, S.; Brestic, M.; Chen, X. Physiological adaptive mechanisms of plants grown in saline soil and implications for sustainable saline agriculture in coastal zone. Acta Physiol. Plant. 2013, 35, 2867–2878. [Google Scholar] [CrossRef]
- El-Amery, E.M.; Kasem, A.M.M.A.; El-Khatib, A.A. Allelopathic potential of Egyptian halophytes Arthrocnemum macrostachyum and Halocnemum strobilaceum from two coastal areas. Allelopath. J. 2020, 50, 225–242. [Google Scholar] [CrossRef]
- Ghanem, A.-M.F.M.; Mohamed, E.; Kasem, A.M.M.A.; El-Ghamery, A.A. Differential Salt Tolerance Strategies in Three Halophytes from the Same Ecological Habitat: Augmentation of Antioxidant Enzymes and Compounds. Plants 2021, 10, 1100. [Google Scholar] [CrossRef]
- Ahanger, M.A.; Agarwal, R.M.; Tomar, N.S.; Shrivastava, M. Potassium induces positive changes in nitrogen metabolism and antioxidant system of oat (Avena sativa L cultivar Kent). J. Plant Interact. 2015, 10, 211–223. [Google Scholar] [CrossRef]
- Yilmaz, A.; Yildirim, E.; Yilmaz, H.; Soydemir, H.E.; Güler, E.; Ciftci, V.; Yaman, M. Use of Arbuscular Mycorrhizal Fungi for Boosting Antioxidant Enzyme Metabolism and Mitigating Saline Stress in Sweet Basil (Ocimum basilicum L.). Sustainability 2023, 15, 5982. [Google Scholar] [CrossRef]
- He, J.D.; Zou, Y.N.; Wu, Q.S.; Kuča, K. Mycorrhizas enhance drought tolerance of trifoliate orange by enhancing activities and gene expression of antioxidant enzymes. Sci. Hortic. 2020, 262, 108745. [Google Scholar] [CrossRef]
- Spohn, M.; Novák, T.J.; Incze, J.; Giani, L. Dynamics of soil carbon, nitrogen, and phosphorus in calcareous soils after land-use abandonment—A chronosequence study. Plant Soil 2015, 401, 185–196. [Google Scholar] [CrossRef]
- Frosi, G.; Barros, V.A.; Oliveira, M.T.; Santos, M.; Ramos, D.G.; Maia, L.C.; Santos, M.G. Arbuscular mycorrhizal fungi and foliar phosphorus inorganic supply alleviate salt stress effects in physiological attributes, but only arbuscular mycorrhizal fungi increase biomass in woody species of a semiarid environment. Tree Physiol. 2018, 38, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Diao, F.; Jia, B.; Wang, X.; Luo, J.; Hou, Y.; Li, F.Y.; Guo, W. Proteomic analysis revealed modulations of carbon and nitrogen by arbuscular mycorrhizal fungi associated with the halophyte Suaeda salsa in a moderately saline environment. Land Degrad. Dev. 2022, 33, 1933–1943. [Google Scholar] [CrossRef]
- Dodd, J. Arbuscular mycorrhizas: Physiology and function. Geofis. Int. 2010, 104, 57–71. [Google Scholar] [CrossRef]
- Greco, M.; Chiappetta, A.; Bruno, L.; Bitonti, M.B. In Posidonia oceanica cadmium induces changes in DNA methylation and chromatin patterning. J. Exp. Bot. 2012, 63, 695–709. [Google Scholar] [CrossRef] [PubMed]
- Almeida, D.M.; Oliveira, M.M.; Saibo, N.J. Regulation of Na+ and K+ homeostasis in plants: Towards improved salt stress tolerance in crop plants. Genet. Mol. Biol. 2017, 40, 326–345. [Google Scholar] [CrossRef] [PubMed]
- Romero-Munar, A.; Baraza, E.; Gulías, J.; Cabot, C. Arbuscular Mycorrhizal Fungi Confer Salt Tolerance in Giant Reed (Arundo donax L.) Plants Grown Under Low Phosphorus by Reducing Leaf Na+ Concentration and Improving Phosphorus Use Efficiency. Front. Plant Sci. 2019, 10, 843. [Google Scholar] [CrossRef] [PubMed]
- Khalediyan, N.; Weisany, W.; Schenk, P.M. Arbuscular mycorrhizae and rhizobacteria improve growth, nutritional status and essential oil production in Ocimum basilicum and Satureja hortensis. Ind. Crops Prod. 2021, 160, 113163. [Google Scholar] [CrossRef]
- Ismail, A.M.; Horie, T. Genomics, Physiology, and Molecular Breeding Approaches for Improving Salt Tolerance. Annu. Rev. Plant Biol. 2017, 68, 405–434. [Google Scholar] [CrossRef]
- Hashem, A.; Alqarawi, A.A.; Radhakrishnan, R.; Al-Arjani, A.-B.F.; Aldehaish, H.A.; Egamberdieva, D.; Abd Allah, E.F. Arbuscular mycorrhizal fungi regulate the oxidative system, hormones and ionic equilibrium to trigger salt stress tolerance in Cucumis sativus L. Saudi J. Biol. Sci. 2018, 25, 1102–1114. [Google Scholar] [CrossRef] [PubMed]
- Yousuf, P.Y.; Ahmad, A.; Kardam, H.; Ganie, A.H.; Aref, I.; Iqbal, M. Potassium and calcium application ameliorates growth and oxidative homeostasis in salt-stressed Indian mustard (Brassica juncea) plants. Pak. J. Bot. 2015, 47, 1629–1639. [Google Scholar]
- Cuin, T.A.; Zhou, M.; Parsons, D.; Shabala, S. Genetic behaviour of physiological traits conferring cytosolic K+/Na+ homeostasis in wheat. Plant Biol. 2012, 14, 438–446. [Google Scholar] [CrossRef] [PubMed]
- Zakery-Asl, M.A.; Bolandnazar, S.; Oustan, S. Effect of salinity and nitrogen on growth, sodium, potassium accumulation, and osmotic adjustment of halophyte Suaeda aegyptiaca (Hasselq.) Zoh. Arch. Agron. Soil Sci. 2014, 60, 785–792. [Google Scholar] [CrossRef]
- Teh, C.Y.; Shaharuddin, N.A.; Ho, C.L.; Mahmood, M. Exogenous proline significantly affects the plant growth and nitrogen assimilation enzymes activities in rice (Oryza sativa) under salt stress. Acta Physiol. Plant. 2016, 38, 151. [Google Scholar] [CrossRef]
- Zhang, T.; Yang, X. Exogenous Glycinebetaine-Mediated Modulation of Abiotic Stress Tolerance in Plants: Possible Mechanisms. In Osmoprotectant-Mediated Abiotic Stress Tolerance in Plants; Springer: Berlin/Heidelberg, Germany, 2019; pp. 141–152. [Google Scholar] [CrossRef]
- Abdel Latef, A.A.H.; Chaoxing, H. Does Inoculation with Glomus mosseae Improve Salt Tolerance in Pepper Plants? J. Plant Growth Regul. 2014, 33, 644–653. [Google Scholar] [CrossRef]
- Wright, D.P.; Read, D.J.; Scholes, J.D. Mycorrhizal sink strength influences whole-plant carbon balance of Trifolium repens L. Plant Cell Environ. 2002, 21, 881–891. [Google Scholar] [CrossRef]
- Zhu, X.C.; Song, F.B.; Liu, F.L.; Liu, S.Q.; Tian, C.J. Carbon and nitrogen metabolism in arbuscular mycorrhizal maize plants under low-temperature stress. Crop Pasture Sci. 2015, 66, 62–70. [Google Scholar] [CrossRef]
- Masson, P.; Dalix, T.; Bussière, S. Determination of Major and Trace Elements in Plant Samples by Inductively Coupled Plasma–Mass Spectrometry. Commun. Soil Sci. Plant Anal. 2010, 41, 231–243. [Google Scholar] [CrossRef]
- McGonigle, T.P.; Miller, M.H.; Evans, D.; Fairchild, G.; Swan, J.A. A new method which gives an objective measure of colonization of roots by vesicular–Arbuscular mycorrhizal fungi. New Phytol. 1990, 115, 495–501. [Google Scholar] [CrossRef]
- Liu, R.; Chen, Y. Mycorrhizology; Science Press: Beijing, China, 2007; p. 388. [Google Scholar]
- Kapat, A.; Dey, S. An alternative approach to the detection of lignin: A note on the application of ELISA using polyclonal antibodies. Bioprocess Eng. 2000, 22, 75–77. [Google Scholar] [CrossRef]
- Wright, S.F.; Upadhyaya, A. A survey of soils for aggregate stability and glomalin, a glycoprotein produced by hyphae of arbuscular mycorrhizal fungi. Plant Soil 1998, 198, 97–107. [Google Scholar] [CrossRef]
- Rillig, M.C. Arbuscular mycorrhizae, glomalin, and soil aggregation. Can. J. Soil Sci. 2004, 84, 355–363. [Google Scholar] [CrossRef]
- He, H.; Yang, R.; Jia, B.; Chen, L.; Fan, H.; Cui, J.; Yang, D.; Li, M.; Ma, F.-Y. Rice Photosynthetic Productivity and PSII Photochemistry under Nonflooded Irrigation. Sci. World J. 2014, 2014, 839658. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.-M.; Zhang, J.-H. Heat-induced Multiple Effects on PSII in Wheat Plants. J. Plant Physiol. 2000, 156, 259–265. [Google Scholar] [CrossRef]
- Kirk, P.L. Kjeldahl method for total nitrogen. Anal. Chem. 1950, 22, 354–358. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, S.; Cheng, Y.; Wu, G.; He, X.; Zhao, G. Enhancing Salt Tolerance in Poplar Seedlings through Arbuscular Mycorrhizal Fungi Symbiosis. Plants 2024, 13, 233. https://doi.org/10.3390/plants13020233
Han S, Cheng Y, Wu G, He X, Zhao G. Enhancing Salt Tolerance in Poplar Seedlings through Arbuscular Mycorrhizal Fungi Symbiosis. Plants. 2024; 13(2):233. https://doi.org/10.3390/plants13020233
Chicago/Turabian StyleHan, Shuo, Yao Cheng, Guanqi Wu, Xiangwei He, and Guozhu Zhao. 2024. "Enhancing Salt Tolerance in Poplar Seedlings through Arbuscular Mycorrhizal Fungi Symbiosis" Plants 13, no. 2: 233. https://doi.org/10.3390/plants13020233
APA StyleHan, S., Cheng, Y., Wu, G., He, X., & Zhao, G. (2024). Enhancing Salt Tolerance in Poplar Seedlings through Arbuscular Mycorrhizal Fungi Symbiosis. Plants, 13(2), 233. https://doi.org/10.3390/plants13020233





