Arthrocnemum Moq.: Unlocking Opportunities for Biosaline Agriculture and Improved Human Nutrition
Abstract
1. Introduction
2. Results
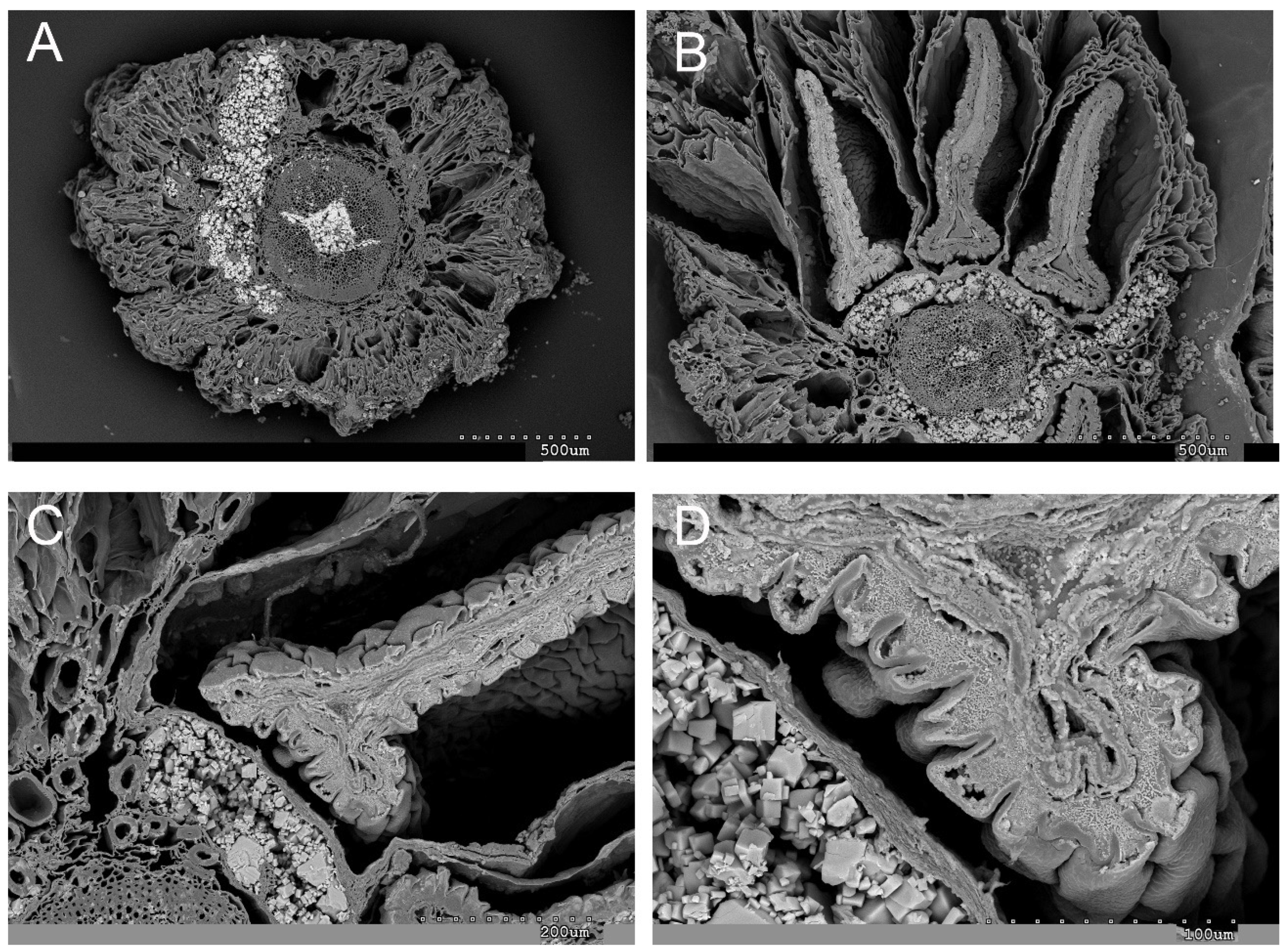
2.1. Vegetative and Floriferous Succulent Stems
2.2. Seeds
3. Discussion
3.1. Vegetative and Floriferous Succulent Stems
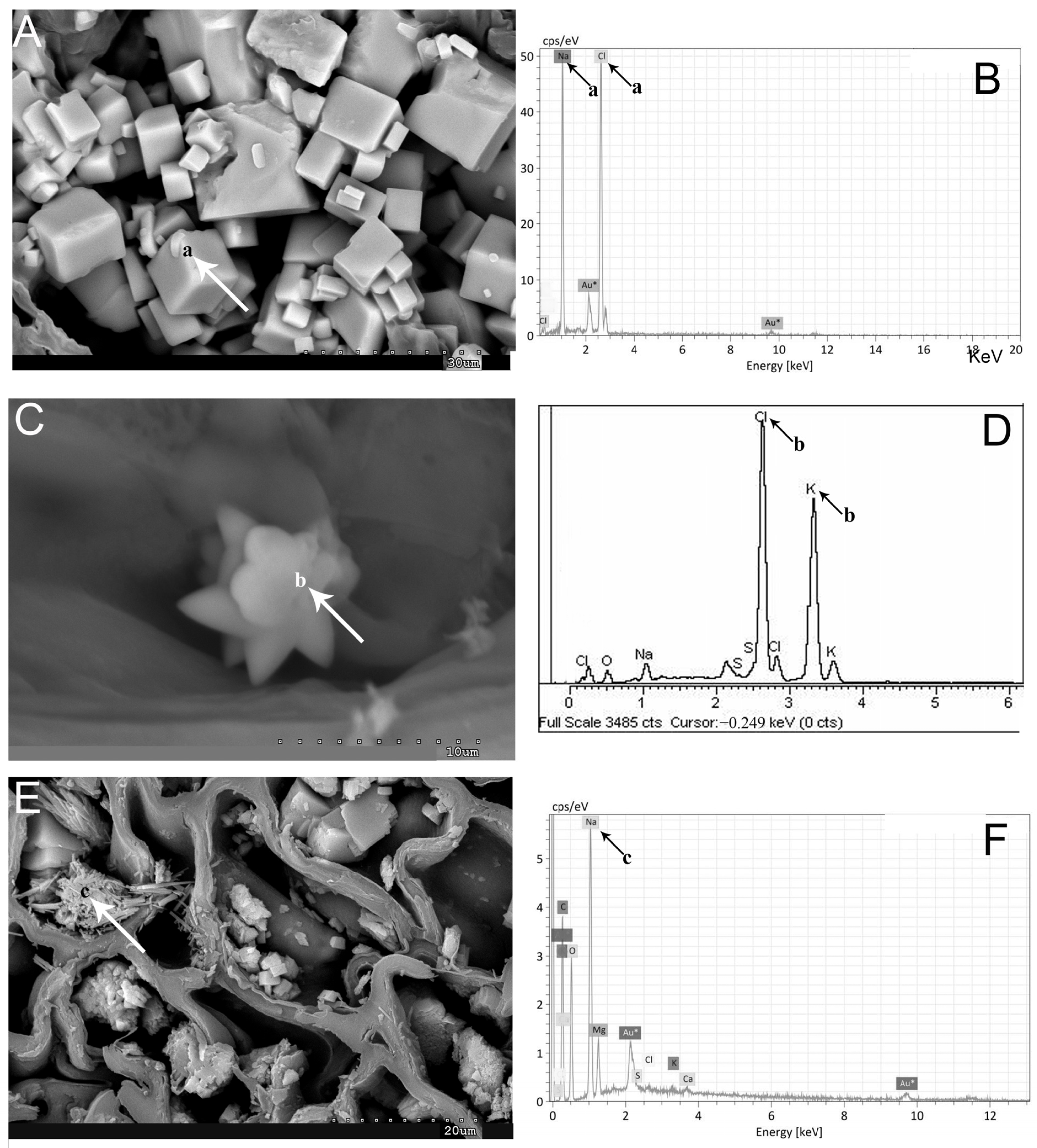
3.1.1. Salt Accumulation (Na and K) and Adaptive Mechanism
3.1.2. Influence of the Tinto River Environment
3.1.3. Exploring the Role of Other Macronutrients (Mg, Ca) and Micronutrients
3.1.4. Application in Food Products
3.2. Seeds
4. Materials and Methods
4.1. Studied Material
4.2. Inductively Coupled Plasma Mass Spectrometry (ICP-MS)
4.3. X-ray Diffraction Analysis
4.4. Scanning Electron Microscopy (SEM-EDX)
4.5. Transmission Electronic Microscopy (TEM)
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Flowers, T.J.; Colmer, T.D. Salinity tolerance in halophytes. New Phytol. 2008, 179, 945–963. [Google Scholar] [CrossRef]
- Santos, J.; Al-Azzawi, M.; Aronson, J.; Flowers, T.J. eHALOPH a Database of Salt-Tolerant Plants: Helping put Halophytes to Work. Plant Cell Physiol. 2016, 57, e10. [Google Scholar] [CrossRef]
- Al-Azzawi, M.J.; Flowers, T.J. Distribution and Potential Uses of Halophytes within the Gulf Cooperation Council States. Agronomy 2022, 12, 1030. [Google Scholar] [CrossRef]
- Yasseen, B.T.; Al-Thani, R.F. Endophytes and Halophytes to Remediate Industrial Wastewater and Saline Soils: Perspectives from Qatar. Plants 2022, 11, 1497. [Google Scholar] [CrossRef]
- Aslam, R. A critical review on halophytes: Salt tolerant plants. J. Med. Plants Res. 2011, 5, 7108–7118. [Google Scholar] [CrossRef]
- de la Fuente, V.; Rufo, L.; Sánchez-Gavilán, I.; Ramírez, E.; Rodríguez, N.; Amils, R. Plant Tissues and Embryos Biominerals in Sarcocornia pruinosa, a Halophyte from the Río Tinto Salt Marshes. Minerals 2018, 8, 505. [Google Scholar] [CrossRef]
- Flowers, T.J.; Munns, R.; Colmer, T.D. Sodium chloride toxicity and the cellular basis of salt tolerance in halophytes. Ann. Bot. 2015, 115, 419–431. [Google Scholar] [CrossRef]
- Karabourniotis, G.; Horner, H.T.; Bresta, P.; Nikolopoulos, D.; Liakopoulos, G. New insights into the functions of carbon–calcium inclusions in plants. New Phytol. 2020, 228, 845–854. [Google Scholar] [CrossRef]
- Rufo, L.; Iglesias-López, M.T.; de la Fuente, V. The endemic halophyte Sarcocornia carinata Fuente, Rufo & Sánchez-Mata (Chenopodiaceae) in relation to environmental variables: Elemental composition and biominerals. Plant Soil 2021, 460, 189–209. [Google Scholar] [CrossRef]
- González-Orenga, S.; Grigore, M.-N.; Boscaiu, M.; Vicente, O. Constitutive and Induced Salt Tolerance Mechanisms and Potential Uses of Limonium Mill. Species. Agronomy 2021, 11, 413. [Google Scholar] [CrossRef]
- Grigore, M.-N.; Vicente, O. Wild Halophytes: Tools for Understanding Salt Tolerance Mechanisms of Plants and for Adapting Agriculture to Climate Change. Plants 2023, 12, 221. [Google Scholar] [CrossRef]
- Accogli, R.; Tomaselli, V.; Direnzo, P.; Perrino, E.V.; Albanese, G.; Urbano, M.; Laghetti, G. Edible Halophytes and Halo-Tolerant Species in Apulia Region (Southeastern Italy): Biogeography, Traditional Food Use and Potential Sustainable Crops. Plants 2023, 12, 549. [Google Scholar] [CrossRef]
- Ahmad, F.; Hameed, M.; Ahmad, M.S.A.; Ashraf, M. Ensuring Food Security of Arid Regions through Sustainable Cultivation of Halophytes. In Handbook of Halophytes; Grigore, M.-N., Ed.; Springer International Publishing: Berlin/Heidelberg, Germany, 2021; pp. 2191–2210. [Google Scholar] [CrossRef]
- Corrêa, R.C.G.; Di Gioia, F.; Ferreira, I.C.F.R.; Petropoulos, S.A. Halophytes for Future Horticulture: The Case of Small-Scale Farming in the Mediterranean Basin. In Handbook of Halophytes; Grigore, M.-N., Ed.; Springer International Publishing: Berlin/Heidelberg, Germany, 2021; pp. 2367–2393. [Google Scholar] [CrossRef]
- de la Fuente, V.; Sánchez-Gavilán, I.; Ramírez, E.; Rufo, L.; Sánchez-Mata, D. Morphological Variability of Halophytes: Salicornioideae on Iberian Peninsula. In Handbook of Halophytes; Grigore, M.-N., Ed.; Springer International Publishing: Berlin/Heidelberg, Germany, 2020; pp. 1–36. [Google Scholar] [CrossRef]
- Piirainen, M.; Liebisch, O.; Kadereit, G. Phylogeny, biogeography, systematics and taxonomy of Salicornioideae (Amaranthaceae/Chenopodiaceae)—A cosmopolitan, highly specialized hygrohalophyte lineage dating back to the Oligocene. TAXON 2017, 66, 109–132. [Google Scholar] [CrossRef]
- Cárdenas-Pérez, S.; Piernik, A.; Chanona-Pérez, J.J.; Grigore, M.N.; Perea-Flores, M.J. An overview of the emerging trends of the Salicornia L. genus as a sustainable crop. Environ. Exp. Bot. 2021, 191, 104606. [Google Scholar] [CrossRef]
- Patel, S. Salicornia: Evaluating the halophytic extremophile as a food and a pharmaceutical candidate. 3 Biotech 2016, 6, 104. [Google Scholar] [CrossRef] [PubMed]
- Lopes, M.; Silva, A.S.; Séndon, R.; Barbosa-Pereira, L.; Cavaleiro, C.; Ramos, F. Towards the Sustainable Exploitation of Salt-Tolerant Plants: Nutritional Characterisation, Phenolics Composition, and Potential Contaminants Analysis of Salicornia ramosissima and Sarcocornia perennis alpini. Molecules 2023, 28, 2726. [Google Scholar]
- Singh, D.; Buhmann, A.K.; Flowers, T.J.; Seal, C.E.; Papenbrock, J. Salicornia as a crop plant in temperate regions: Selection of genetically characterized ecotypes and optimization of their cultivation conditions. Aob Plants 2014, 6, plu071. [Google Scholar] [CrossRef]
- Urbano, M.; Tomaselli, V.; Bisignano, V.; Veronico, G.; Hammer, K.; Laghetti, G. Salicornia patula Duval-Jouve: From gathering of wild plants to some attempts of cultivation in Apulia region (southern Italy). Genet. Resour. Crop Evol. 2017, 64, 1465–1472. [Google Scholar] [CrossRef]
- Ventura, Y.; Sagi, M. Halophyte crop cultivation: The case for Salicornia and Sarcocornia. Environ. Exp. Bot. 2013, 92, 144–153. [Google Scholar] [CrossRef]
- Barcia-Piedras, J.M.; Pérez-Romero, J.A.; Mateos-Naranjo, E.; Camacho, M.; Redondo-Gómez, S. Effect of prior salt experience on desalination capacity of the halophyte Arthrocnemum macrostachyum. Desalination 2019, 463, 50–54. [Google Scholar] [CrossRef]
- Paraskevopoulou, A.T.; Ntoulas, N.; Bourtsoukli, D.; Bertsouklis, K. Effect of Seawater Irrigation on Arthrocnemum macrostachyum Growing in Extensive Green Roof Systems under Semi-Arid Mediterranean Climatic Conditions. Agronomy 2023, 13, 1198. [Google Scholar] [CrossRef]
- Ramírez, E.; Sánchez-Gavilán, I.; Rufo, L.; Sánchez-Mata, D.; de la Fuente, V. Morphology, anatomy and phylogeny of the two sister halophytic genera Microcnemum and Arthrocnemum (Salicornioideae/Amaranthaceae). Plant Biosyst. Int. J. Deal. Asp. Plant Biol. 2022, 156, 1422–1437. [Google Scholar] [CrossRef]
- Barreira, L.; Resek, E.; Rodrigues, M.J.; Rocha, M.I.; Pereira, H.; Bandarra, N.; da Silva, M.M.; Varela, J.; Custódio, L. Halophytes: Gourmet food with nutritional health benefits? J. Food Compos. Anal. 2017, 59, 35–42. [Google Scholar] [CrossRef]
- Chekroun-Bechlaghem, N.; Belyagoubi-Benhammou, N.; Belyagoubi, L.; Gismondi, A.; Nanni, V.; Di Marco, G.; Canuti, L.; Canini, A.; El Haci, I.A.; Atik Bekkara, F. Phytochemical analysis and antioxidant activity of Tamarix africana, Arthrocnemum macrostachyum and Suaeda fruticosa, three halophyte species from Algeria. Plant Biosyst. Int. J. Deal. Asp. Plant Biol. 2019, 153, 843–852. [Google Scholar] [CrossRef]
- El Naker, N.A.; Yousef, A.F.; Yousef, L.F. A review of Arthrocnemum (Arthrocaulon) macrostachyum chemical content and bioactivity. Phytochem. Rev. 2020, 19, 1427–1448. [Google Scholar] [CrossRef]
- Jitan, S.A.; AlKhoori, S.; Ochsenkühn, M.; Amin, S.A.; Yousef, L.F. Ethanol/water extracts from halophyte species Arthrocnemum macrostachyum and Tetraena qatarensis. Cogent Chem. 2018, 4, 1536311. [Google Scholar] [CrossRef]
- Ramírez, E.; Chaâbene, Z.; Hernández-Apaolaza, L.; Rekik, M.; Elleuch, A.; de la Fuente, V. Seed priming to optimize germination in Arthrocnemum Moq. BMC Plant Biol. 2022, 22, 527. [Google Scholar] [CrossRef]
- Khan, M.; Gul, B. Arthrocnemum macrostachyum: A potential case for agriculture using above seawater salinity. In Prospects for Saline Agriculture; Ahmad, R., Malik, K.A., Eds.; Springer: Dordrecht, The Netherlands, 2002; Volume 37, pp. 353–364. [Google Scholar]
- Khan, M.; Gul, B. Halophyte Seed Germination. In Ecophysiology of High Salinity Tolerant Plants; Khan, M., Weber, D.J., Eds.; Springer: Dordrecht, The Netherlands, 2006; Volume 40, pp. 11–30. [Google Scholar] [CrossRef]
- Loconsole, D.; Cristiano, G.; De Lucia, B. Glassworts: From Wild Salt Marsh Species to Sustainable Edible Crops. Agriculture 2019, 9, 14. [Google Scholar] [CrossRef]
- Cheeseman, J.M. The evolution of halophytes, glycophytes and crops, and its implications for food security under síaline conditions. New Phytol. 2015, 206, 557–570. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Gavilán, I.; Ramírez, E.; de la Fuente, V. Bioactive Compounds in Sarcocornia and Arthrocnemum, Two Wild Halophilic Genera from the Iberian Peninsula. Plants 2021, 10, 2218. [Google Scholar] [CrossRef] [PubMed]
- Abideen, Z.; Qasim, M.; Rizvi, R.F.; Gul, B.; Ansari, R.; Khan, M.A. Oilseed halophytes: A potential source of biodiesel using saline degraded lands. Biofuels 2015, 6, 241–248. [Google Scholar] [CrossRef]
- de la Fuente, V.; Rufo, L.; Rodríguez, N.; Amils, R.; Zuluaga, J. Metal Accumulation Screening of the Río Tinto Flora (Huelva, Spain). Biol. Trace Elem. Res. 2010, 134, 318–341. [Google Scholar] [CrossRef]
- Lowenstam, H.A. Minerals Formed by Organisms. Science 1981, 211, 1126–1131. [Google Scholar] [CrossRef] [PubMed]
- Monje, P.V.; Baran, E.J. Evidence of formation of glushinskite as a biomineral in a Cactaceae species. Phytochemistry 2005, 66, 611–614. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, N.; Menéndez, N.; Tornero, J.; Amils, R.; De La Fuente, V. Internal iron biomineralization in Imperata cylindrica, a perennial grass: Chemical composition, speciation and plant localization. New Phytol. 2005, 165, 781–789. [Google Scholar] [CrossRef] [PubMed]
- Lagasca, M. Memoria Sobre las Plantas Barrilleras de España; Imprenta Real: Madrid, Spain, 1817. [Google Scholar]
- Grigore, M.; Toma, C. Anatomical Adaptations of Halophytes; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Maatallah Zaier, M.; Ciudad-Mulero, M.; Cámara, M.; Pereira, C.; Ferreira, I.C.; Achour, L.; Kacem, A.; Morales, P. Revalorization of Tunisian wild Amaranthaceae halophytes: Nutritional composition variation at two different phenotypes stages. J. Food Compos. Anal. 2020, 89, 103463. [Google Scholar] [CrossRef]
- Sánchez-Gavilán, I.; Rufo, L.; Rodríguez, N.; de La Fuente, V. On the elemental composition of the Mediterranean euhalophyte Salicornia patula Duval-Jouve (Chenopodiaceae) from saline habitats in Spain (Huelva, Toledo and Zamora). Environ. Sci. Pollut. Res. 2021, 28, 2719–2727. [Google Scholar] [CrossRef] [PubMed]
- Abramov, S.M.; Tejada, J.; Grimm, L.; Schädler, F.; Bulaev, A.; Tomaszewski, E.J.; Byrne, J.M.; Straub, D.; Thorwarth, H.; Kappler, A.; et al. Role of biogenic Fe(III) minerals as a sink and carrier of heavy metals in the Rio Tinto, Spain. Sci. Total Environ. 2020, 718, 137294. [Google Scholar] [CrossRef] [PubMed]
- Amils, R. Lessons learned from thirty years of geomicrobiological studies of Río Tinto. Res. Microbiol. 2016, 167, 539–545. [Google Scholar] [CrossRef] [PubMed]
- Amils, R.; González-Toril, E.; Fernández-Remolar, D.; Gómez, F.; Aguilera, Á.; Rodríguez, N.; Malki, M.; Garcia-Moyano, A.; Fairen, A.G.; Sanz, J.L.; et al. Extreme environments as Mars terrestrial analogs: The Rio Tinto case. Planet. Space Sci. 2007, 55, 370–381. [Google Scholar] [CrossRef]
- Davis, R.A., Jr.; Welty, A.T.; Borrego, J.; Morales, J.A.; Pendon, J.G.; Ryan, J.G. Rio Tinto estuary (Spain): 5000 years of pollution. Environ. Geol. 2000, 39, 1107–1116. [Google Scholar] [CrossRef]
- Rufo, L.; Rodríguez, N.; Amils, R.; de la Fuente, V.; Jiménez-Ballesta, R. Surface geochemistry of soils associated to the Tinto River (Huelva, Spain). Sci. Total Environ. 2007, 378, 223–227. [Google Scholar] [CrossRef]
- Rufo, L.; Rodríguez, N.; de la Fuente, V. Chemical and mineralogical characterization of the soils of the main plant communities of the Río Tinto basin. Schironia 2010, 9, 5–12. [Google Scholar]
- Sanjosé, I.; Navarro Roldán, F.J.; Montero, Y.; Ramírez Acosta, S.; Jiménez Nieva, F.J.; Infante Izquierdo, M.D.; Polo-Ávila, A.; Muñoz Rodríguez, A.F. The Bioconcentration and the Translocation of Heavy Metals in Recently Consumed Salicornia ramosissima J. Woods in Highly Contaminated Estuary Marshes and Its Food Risk. Diversity 2022, 14, 452. [Google Scholar] [CrossRef]
- Redondo-Gómez, S.; Mateos-Naranjo, E.; Andrades-Moreno, L. Accumulation and tolerance characteristics of cadmium in a halophytic Cd-hyperaccumulator, Arthrocnemum macrostachyum. J. Hazard. Mater. 2010, 184, 299–307. [Google Scholar] [CrossRef]
- He, H.; Veneklaas, E.J.; Kuo, J.; Lambers, H. Physiological and ecological significance of biomineralization in plants. Trends Plant Sci. 2014, 19, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Monje, P.V.; Baran, E.J. Characterization of Calcium Oxalates Generated as Biominerals in Cacti. Plant Physiol. 2002, 128, 707–713. [Google Scholar] [CrossRef] [PubMed]
- Amils, R.; de la Fuente, V.; Rodríguez, N.; Zuluaga, J.; Menéndez, N.; Tornero, J. Composition, speciation and distribution of iron minerals in Imperata cylindrica. Plant Physiol. Biochem. 2007, 45, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Ducic, T.; Polle, A. Transport and detoxification of manganese and copper in plants. Braz. J. Plant Physiol. 2005, 17, 103–112. [Google Scholar] [CrossRef]
- Cambrollé, J.; Mancilla-Leytón, J.M.; Muñoz-Vallés, S.; Luque, T.; Figueroa, M.E. Zinc tolerance and accumulation in the salt-marsh shrub Halimione portulacoides. Chemosphere 2012, 86, 867–874. [Google Scholar] [CrossRef]
- Duarte, B.; Caetano, M.; Almeida, P.R.; Vale, C.; Caçador, I. Accumulation and biological cycling of heavy metal in four salt marsh species, from Tagus estuary (Portugal). Environ. Pollut. 2010, 158, 1661–1668. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Chen, G.; Shi, C.; Chai, M.; Liu, J.; Cheng, S.; Shi, F. Effects of Zn Stress on Growth, Zn Accumulation, Translocation, and Subcellular Distribution of Spartina alterniflora Loisel: General. Clean. Soil Air Water 2016, 44, 579–585. [Google Scholar] [CrossRef]
- Institute of Medicine (US) Panel on Micronutrients. DRI: Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc: A Report of the Panel on Micronutrients… and the Standing Committee on the Scientific Evaluation of Dietary Reference Intakes, Food and Nutrition Board, Institute of Medicine; National Academy Press: Cambridge, MA, USA, 2001.
- Burger, A.; Lichtscheidl, I. Strontium in the environment: Review about reactions of plants towards stable and radioactive strontium isotopes. Sci. Total Environ. 2019, 653, 1458–1512. [Google Scholar] [CrossRef] [PubMed]
- Barroca, M.J.; Flores, C.; Ressurreição, S.; Guiné, R.; Osório, N.; Moreira da Silva, A. Re-Thinking Table Salt Reduction in Bread with Halophyte Plant Solutions. Appl. Sci. 2023, 13, 5342. [Google Scholar] [CrossRef]
- Clavel-Coibrié, E.; Sales, J.R.; da Silva, A.M.; Barroca, M.J.; Sousa, I.; Raymundo, A. Sarcocornia perennis: A Salt Substitute in Savory Snacks. Foods 2021, 10, 3110. [Google Scholar] [CrossRef] [PubMed]
- Pires-Cabral, P.; Pires-Cabral, P.; Quintas, C. Salicornia ramosissima as a salt substitute in the fermentation of white cabbage. J. Food Sci. Technol. 2022, 59, 597–605. [Google Scholar] [CrossRef] [PubMed]
- Savage, G.; Klunklin, W. Oxalates are Found in Many Different European and Asian Foods—Effects of Cooking and Processing. JFR 2018, 7, 76. [Google Scholar] [CrossRef]
- Grauso, L.; De Falco, B.; Motti, R.; Lanzotti, V. Corn poppy, Papaver rhoeas L.: A critical review of its botany, phytochemistry and pharmacology. Phytochem. Rev. 2021, 20, 227–248. [Google Scholar] [CrossRef]
- Pathan, S.; Siddiqui, R.A. Nutritional Composition and Bioactive Components in Quinoa (Chenopodium quinoa Willd.) Greens: A Review. Nutrients 2022, 14, 558. [Google Scholar] [CrossRef]
- Reguera, M.; Conesa, C.M.; Gil-Gómez, A.; Haros, C.M.; Pérez-Casas, M.Á.; Briones-Labarca, V.; Bolanos, L.; Bonilla, I.; Alvarez, R.; Bascuñán-Godoy, L.; et al. The impact of different agroecological conditions on the nutritional composition of quinoa seeds. PeerJ 2018, 6, e4442. [Google Scholar] [CrossRef]
- Rodríguez, M.; Menéndez, N.; Tornero, J.; Amils, R.; De La Fuente, V. Unique nutritional features that distinguish Amaranthus cruentus L. and Chenopodium quinoa Willd seeds. Food Res. Int. 2023, 164, 112160. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, K.A.; Macfarlane, T.D.; Colmer, T.D. Morphology, Anatomy and Histochemistry of Salicornioideae (Chenopodiaceae) Fruits and Seeds. Ann. Bot. 2005, 95, 917–933. [Google Scholar] [CrossRef]
- Montserrat-Marti, J. Morfología de las semillas de Moehringia intricata (Caryophyllaceae). Lagascalia 1988, 15, 195–203. [Google Scholar]
- Zuluaga, J.; Rodríguez, N.; Rivas-Ramirez, I.; de la Fuente, V.; Rufo, L.; Amils, R. An Improved Semiquantitative Method for Elemental Analysis of Plants Using Inductive Coupled Plasma Mass Spectrometry. Biol. Trace Elem. Res. 2011, 144, 1302–1317. [Google Scholar] [CrossRef] [PubMed]
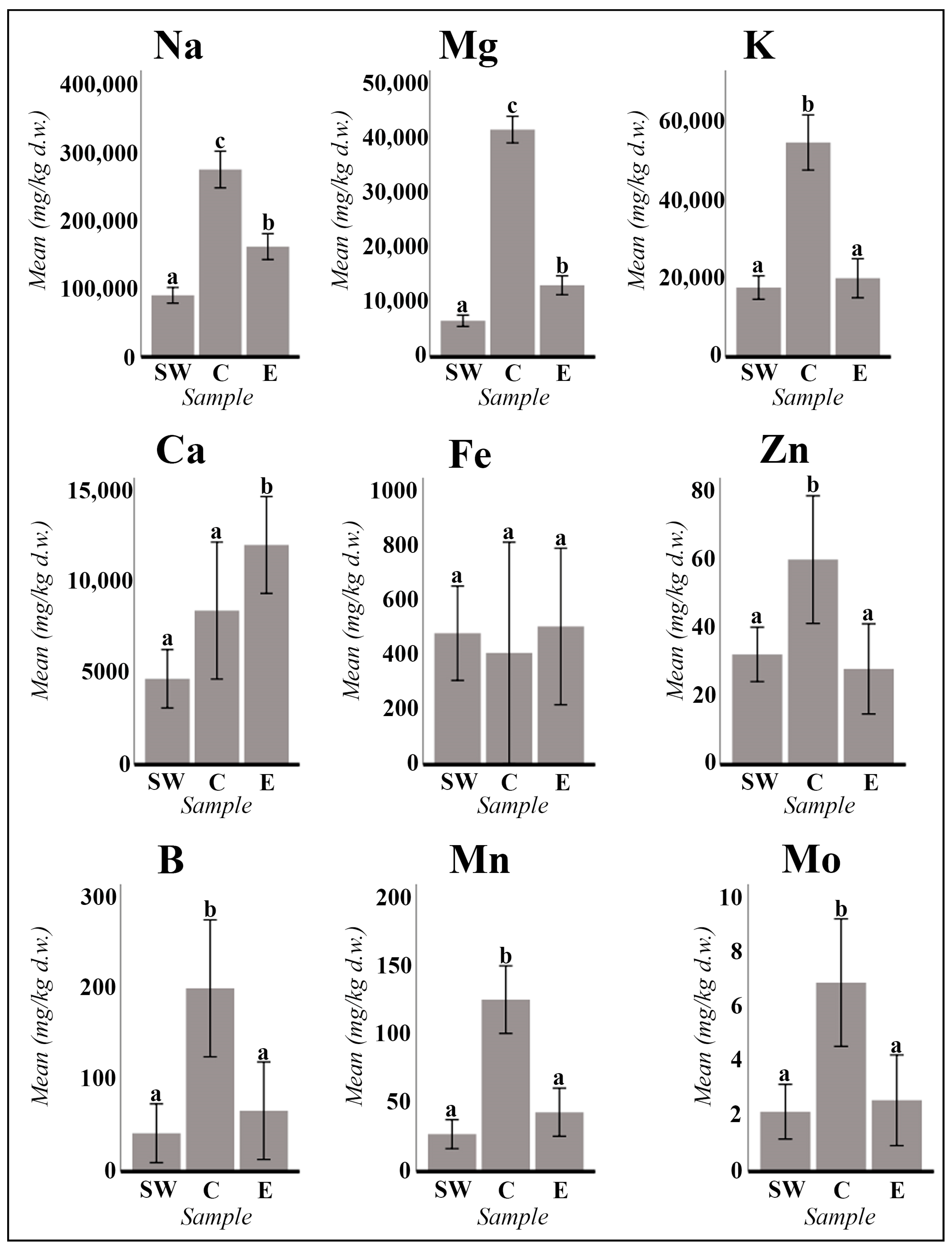
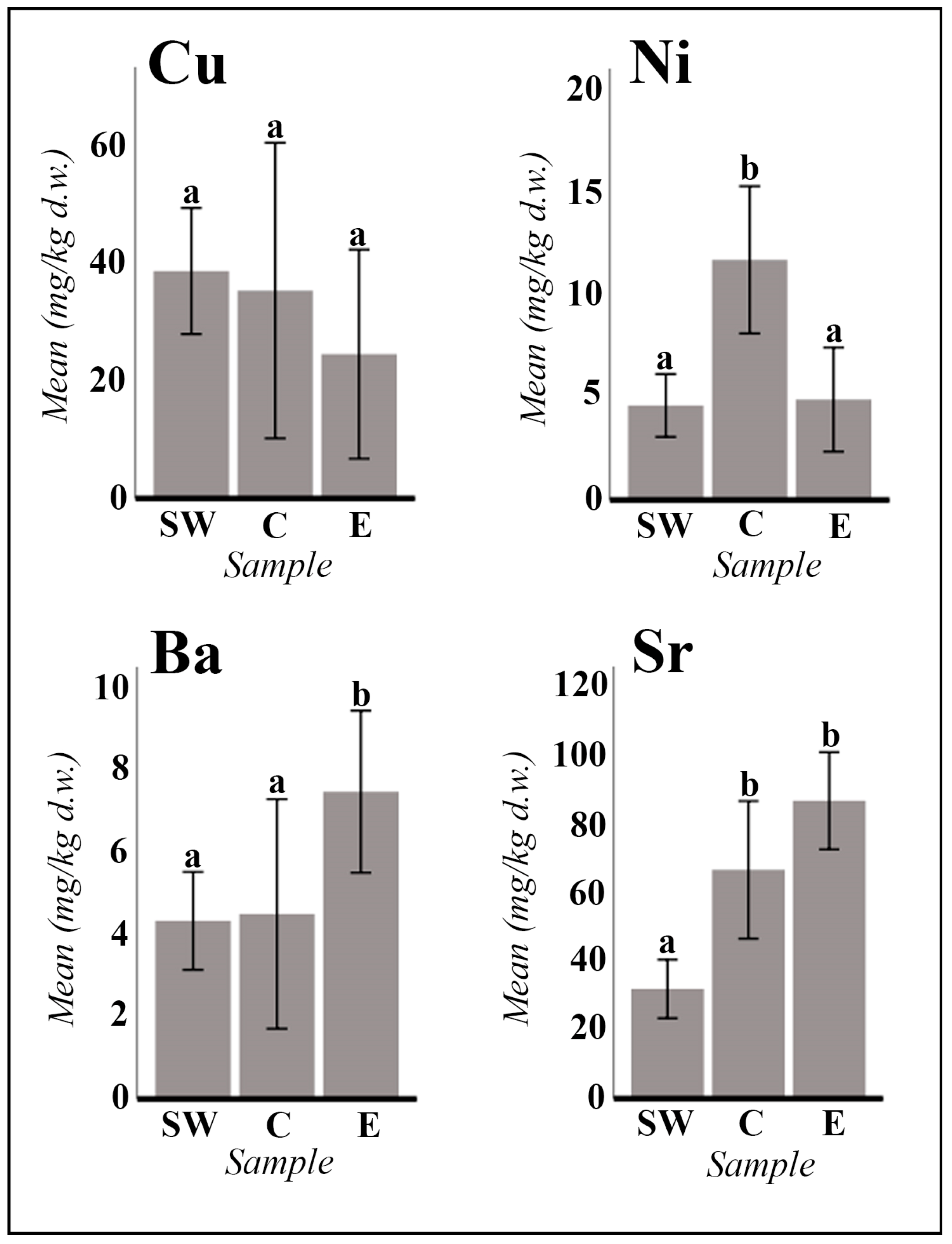

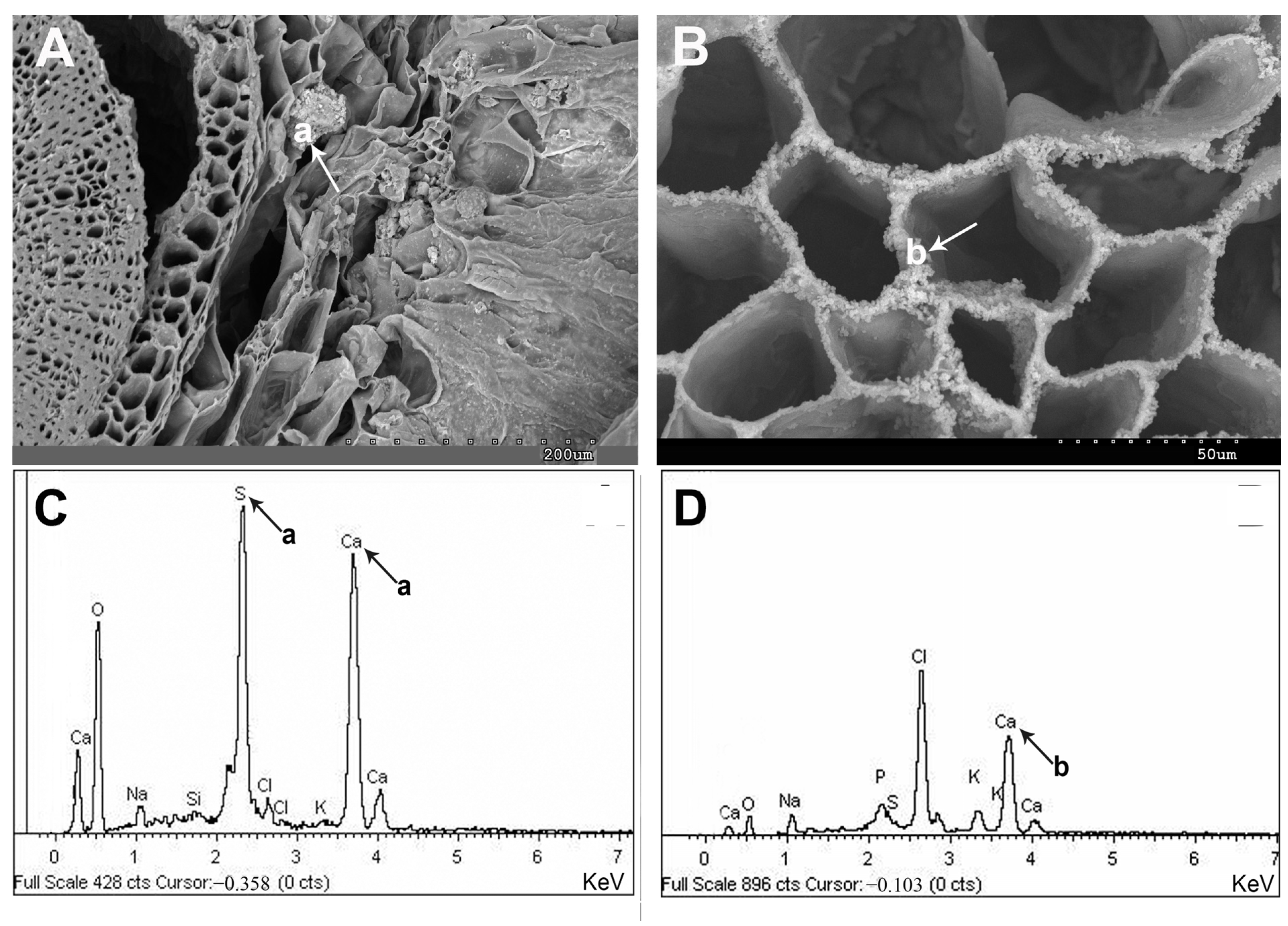
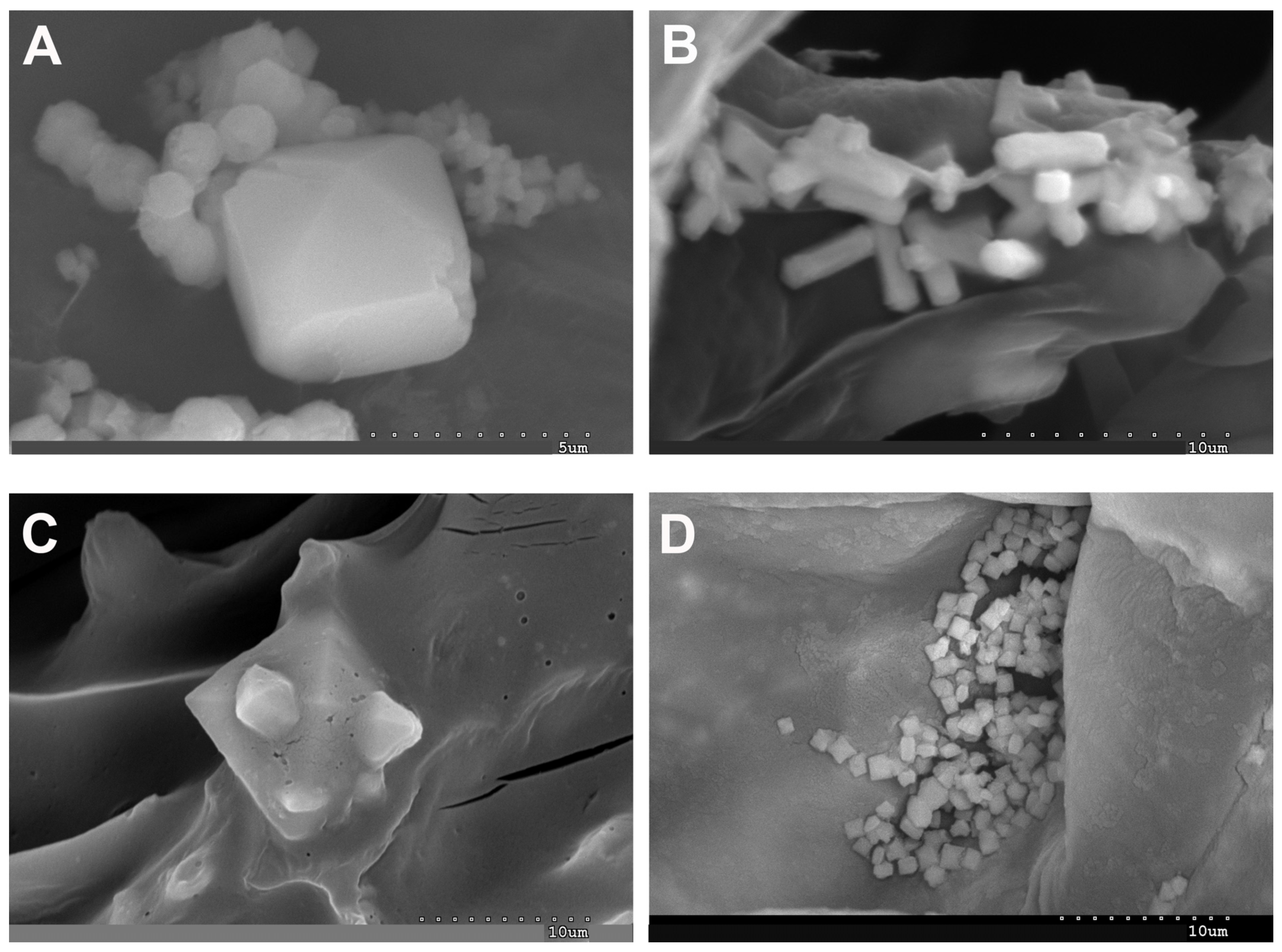
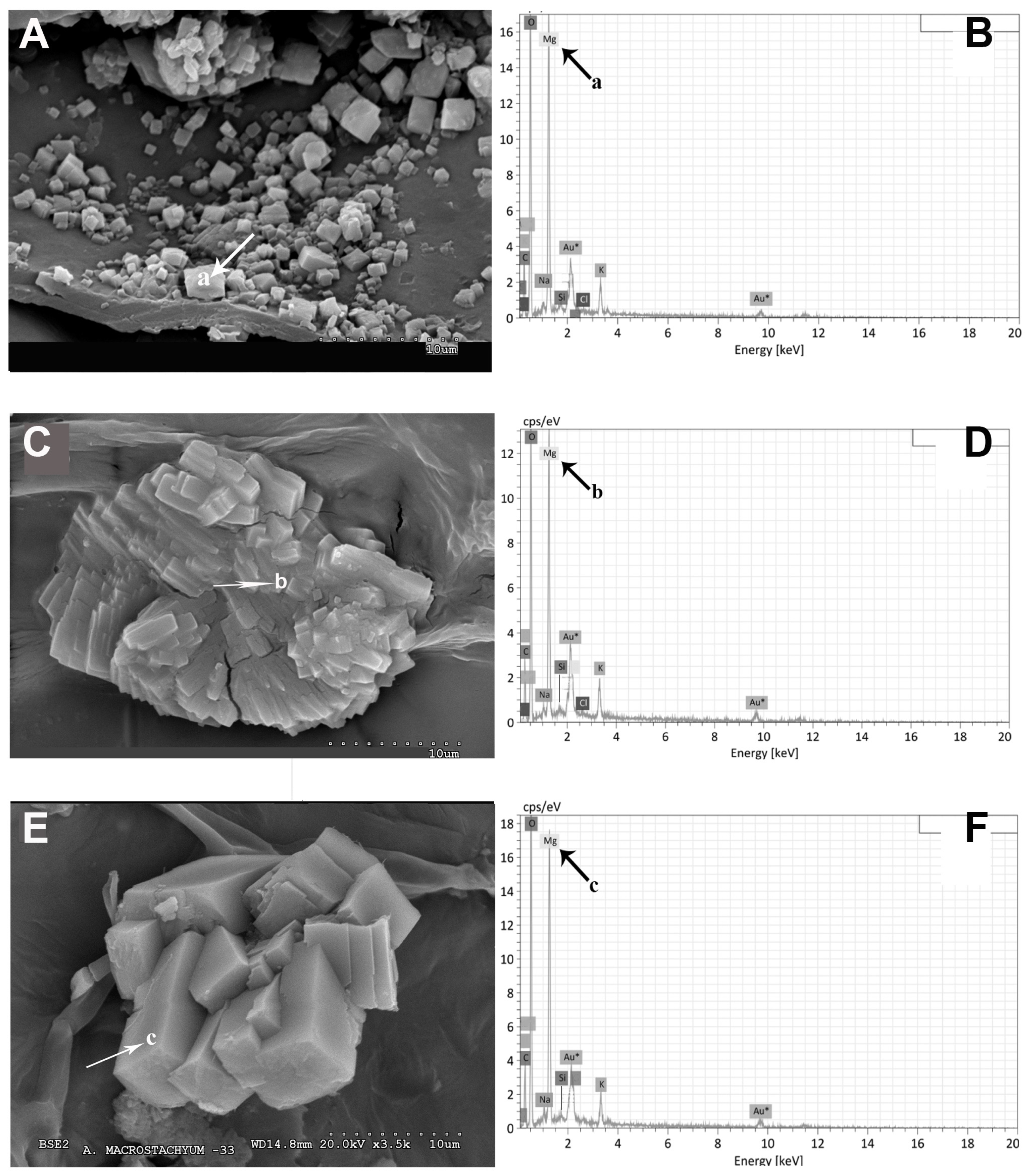
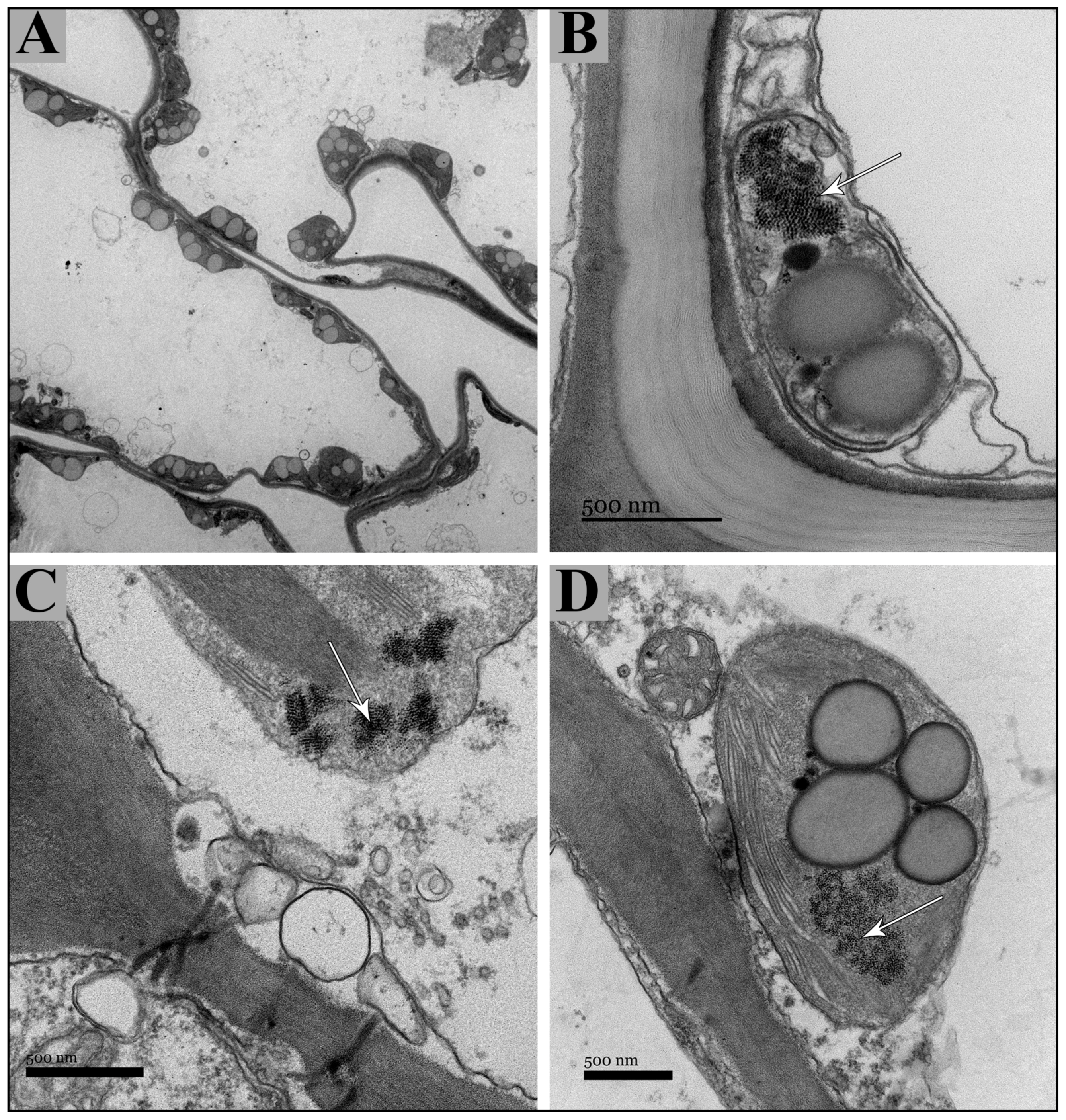
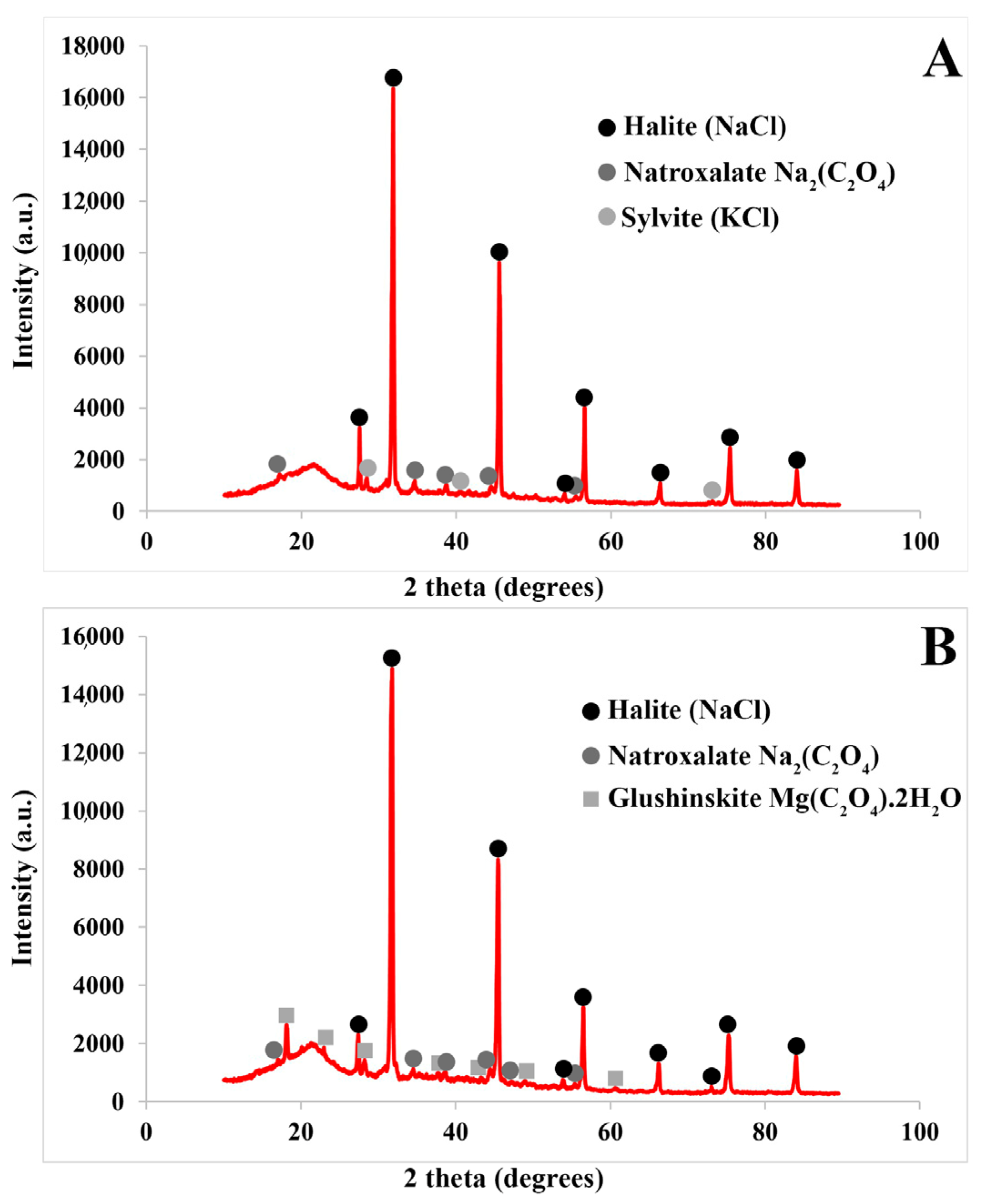
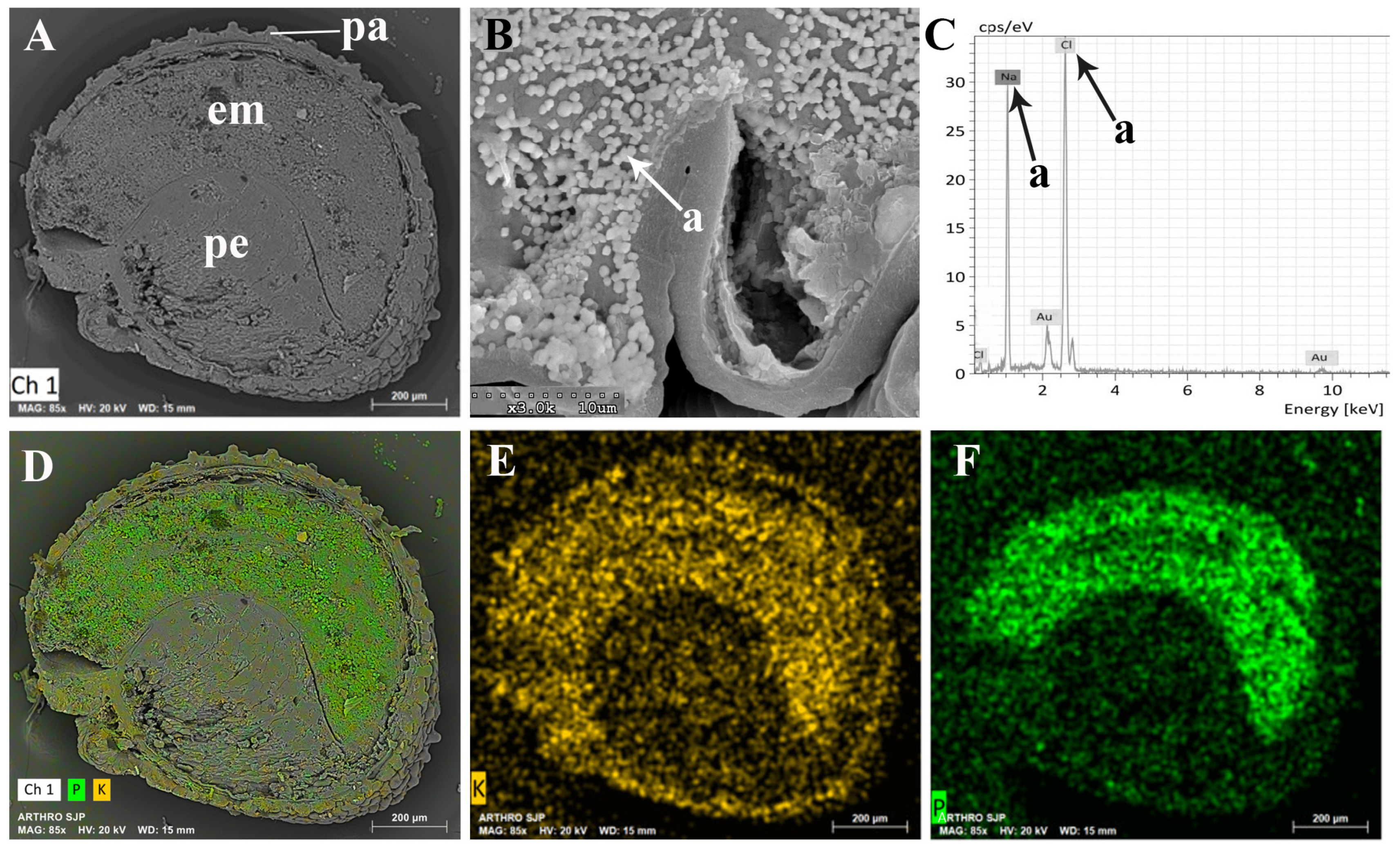


| ID | Na | Mg | K | Ca | Fe | Zn | B | Mn | Mo | Cu | Ni | Ba | Sr | Cr | As | Cd | Pb |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ST1 (SW) | 91,250.06 | 6286.93 | 23,498.67 | 3002.96 | 247.79 | 19.14 | 23.15 | 18.94 | 0.98 | 9.99 | 6.95 | 2.22 | 13.62 | 14.63 | 1.06 | 0.15 | 0.78 |
| ST 2 (SW) | 120,166.30 | 7088.59 | 33,717.76 | 5199.45 | 77.78 | 35.22 | 32.50 | 7.89 | 1.29 | 5.92 | 0.87 | 0.77 | 37.76 | 6.90 | 0.00 | 0.20 | 0.24 |
| ST 3 (SW) | 66,367.06 | 9042.43 | 15,197.18 | 8104.79 | 449.23 | 27.61 | 61.64 | 13.37 | 0.55 | 28.61 | 3.82 | 4.33 | 64.53 | 7.35 | 2.29 | 0.34 | 3.21 |
| ST 4 (SW) | 122,968.31 | 6328.36 | 27,103.31 | 6531.38 | 243.42 | 16.22 | 33.65 | 24.13 | 1.00 | 11.94 | 0.37 | 4.86 | 24.02 | 0.73 | 1.28 | 0.40 | 2.35 |
| ST 5 (SW) | 114,158.07 | 7750.63 | 6150.54 | 8162.98 | 670.65 | 37.34 | 47.95 | 20.38 | 1.46 | 34.64 | 14.71 | 8.76 | 27.60 | 27.97 | 3.59 | 1.65 | 7.59 |
| ST 6 (SW) | 77,774.43 | 6048.84 | 16,654.74 | 2884.32 | 209.29 | 24.18 | 35.07 | 7.58 | 1.76 | 35.51 | 1.09 | 2.66 | 35.21 | 3.67 | 0.53 | 0.06 | 1.60 |
| ST 7 (SW) | 58,089.68 | 6031.30 | 10,351.36 | 3458.96 | 317.03 | 19.28 | 45.98 | 73.68 | 1.33 | 52.85 | 4.57 | 2.76 | 38.97 | 9.27 | 1.09 | 0.08 | 3.46 |
| ST 8 (SW) | 57,747.82 | 6358.98 | 11,933.12 | 4346.96 | 266.93 | 23.29 | 36.93 | 23.44 | 1.65 | 50.01 | 2.53 | 2.54 | 28.31 | 4.26 | 0.27 | 0.05 | 3.52 |
| ST 9 (SW) | 76,702.87 | 7757.82 | 13,588.86 | 4265.36 | 2135.60 | 107.03 | 76.47 | 69.84 | 11.58 | 72.38 | 5.78 | 8.52 | 41.19 | 12.27 | 13.03 | 0.13 | 38.65 |
| ST 10 (SW) | 146,528.62 | 5398.36 | 21,465.90 | 4029.47 | 358.47 | 19.66 | 39.26 | 34.00 | 1.15 | 54.03 | 8.04 | 5.16 | 19.32 | 17.86 | 0.90 | 0.58 | 1.87 |
| ST 11 (SW) | 62,742.67 | 4739.91 | 16,343.87 | 1829.82 | 319.68 | 26.10 | 27.30 | 6.25 | 1.24 | 71.40 | 1.93 | 5.11 | 21.64 | 4.29 | 1.11 | 0.00 | 4.30 |
| ST 12 (C) | 311,083.18 | 41,491.40 | 45,374.40 | 5122.27 | 418.10 | 79.74 | 397.30 | 173.69 | 10.34 | 45.56 | 14.33 | 2.29 | 39.86 | 9.11 | 0.41 | 0.24 | 0.21 |
| ST 13 (C) | 239,613.72 | 41,993.52 | 64,870.74 | 11,817.14 | 400.32 | 40.58 | 4.76 | 77.80 | 3.48 | 25.43 | 9.11 | 6.71 | 93.72 | 10.54 | 0.28 | 0.98 | 0.45 |
| ST 14 (E) | 142,924.73 | 13,760.08 | 15,337.49 | 8136.56 | 485.43 | 18.86 | 266.07 | 36.06 | 4.56 | 6.06 | 13.56 | 5.41 | 91.41 | 26.61 | 0.28 | 0.27 | 0.72 |
| ST 15 (E) | 193,966.51 | 7110.62 | 28,936.80 | 7263.91 | 400.82 | 41.50 | 0.00 | 13.82 | 1.46 | 15.79 | 2.89 | 7.31 | 57.10 | 1.10 | 0.31 | 0.16 | 1.01 |
| ST 16 (E) | 130,930.10 | 21,258.66 | 15,266.57 | 26,030.18 | 1004.92 | 34.76 | 0.00 | 97.61 | 2.50 | 70.60 | 2.04 | 15.32 | 146.85 | 2.54 | 0.68 | 0.46 | 3.46 |
| ST 17 (E) | 180,198.04 | 10,452.45 | 21,346.67 | 6911.34 | 133.91 | 17.01 | 0.00 | 25.30 | 1.87 | 6.08 | 1.10 | 1.89 | 52.67 | 0.99 | 0.13 | 0.06 | 0.23 |
| ID | Na | Mg | K | Ca | Fe | Zn | B | Mn | Mo | Cu | Ni | Ba | Sr | Cr | As | Cd | Pb |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ST18 | 95,912.62 | 20,554.81 | 13,271.65 | 25,407.54 | 1833.34 | 19.10 | 0.00 | 89.51 | 3.75 | 5.20 | 15.46 | 20.54 | 438.38 | 33.24 | 0.63 | 0.70 | 1.17 |
| ST19 | 49,275.46 | 21,597.93 | 4972.64 | 11,762.08 | 543.45 | 61.41 | 0.00 | 35.70 | 1.28 | 10.55 | 8.19 | 11.25 | 162.15 | 15.89 | 0.35 | 0.90 | 1.01 |
| ST20 | 218,830.71 | 13,095.79 | 36,941.86 | 10,772.48 | 247.93 | 44.58 | 0.00 | 14.55 | 3.86 | 4.60 | 4.99 | 2.93 | 66.22 | 5.93 | 0.25 | 0.12 | 0.31 |
| ID | Especie | Muestra | Na | Mg | K | Ca | Fe | Zn | B | Mn | Mo | Cu | Ni | Ba | Sr | Cr | As | Cd | Pb |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S1 | A. macrostachyum | Seeds | 7542.48 | 2421.98 | 6561.01 | 1196.26 | 93.43 | 65.60 | 31.24 | 18.86 | 0.04 | 12.90 | 0.00 | 0.17 | 6.84 | 0.00 | 0.00 | 0.00 | 0.00 |
| S2 | A. macrostachyum | Seeds | 3840.42 | 2730.72 | 7509.57 | 1722.64 | 173.54 | 47.13 | 49.29 | 83.76 | 0.45 | 13.56 | 0.47 | 0.31 | 8.64 | 0.45 | 0.29 | 0.00 | 0.10 |
| S3 | A. macrostachyum | Seeds | 3431.19 | 5780.67 | 7314.83 | 2049.41 | 100.85 | 428.68 | 74.37 | 30.85 | 0.75 | 13.59 | 2.02 | 0.00 | 24.12 | 0.00 | 0.23 | 0.00 | 0.19 |
| S4 | A. macrostachyum | Seed with pericarp | 25,498.87 | 3510.64 | 7421.10 | 4538.10 | 400.77 | 35.62 | 0.00 | 50.32 | 0.53 | 88.00 | 6.73 | 7.34 | 184.45 | 1.37 | 1.91 | 0.25 | 3.88 |
| S5 | A. macrostachyum | Seed with pericarp | 9952.39 | 5320.39 | 2526.52 | 85.00 | 757.14 | 73.77 | 103.42 | 29.99 | 2.66 | 243.78 | 1.22 | 7.76 | 218.31 | 1.81 | 2.79 | 0.20 | 15.26 |
| S6 | A. meridionale | Seeds | 4305.88 | 4071.00 | 4727.96 | 9717.41 | 781.12 | 62.34 | 45.08 | 55.54 | 0.00 | 13.40 | 2.63 | 7.46 | 77.20 | 0.00 | 0.00 | 0.01 | 0.00 |
| S7 | A. meridionale | Seeds | 11,608.57 | 3943.86 | 16,244.86 | 3570.68 | 236.02 | 95.53 | 66.38 | 65.69 | 0.51 | 31.55 | 2.37 | 1.43 | 76.51 | 0.46 | 0.00 | 0.00 | 0.34 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramírez, E.; Rodríguez, N.; de la Fuente, V. Arthrocnemum Moq.: Unlocking Opportunities for Biosaline Agriculture and Improved Human Nutrition. Plants 2024, 13, 496. https://doi.org/10.3390/plants13040496
Ramírez E, Rodríguez N, de la Fuente V. Arthrocnemum Moq.: Unlocking Opportunities for Biosaline Agriculture and Improved Human Nutrition. Plants. 2024; 13(4):496. https://doi.org/10.3390/plants13040496
Chicago/Turabian StyleRamírez, Esteban, Nuria Rodríguez, and Vicenta de la Fuente. 2024. "Arthrocnemum Moq.: Unlocking Opportunities for Biosaline Agriculture and Improved Human Nutrition" Plants 13, no. 4: 496. https://doi.org/10.3390/plants13040496
APA StyleRamírez, E., Rodríguez, N., & de la Fuente, V. (2024). Arthrocnemum Moq.: Unlocking Opportunities for Biosaline Agriculture and Improved Human Nutrition. Plants, 13(4), 496. https://doi.org/10.3390/plants13040496









