Abstract
Cytokinins (CKs) are among the hormones that regulate plants’ growth and development, and the CKX and IPT genes, which are CK degradation and biosynthesis genes, respectively, play important roles in fine-tuning plants’ cytokinin levels. However, the current research on the function of IPT and CKX in cucumber’s growth, development, and response to abiotic stress is not specific enough, and their regulatory mechanisms are still unclear. In this study, we focused on the IPT and CKX genes in cucumber, analyzed the physiological and biochemical properties of their encoded proteins, and explored their expression patterns in different tissue parts and under low light, salt stress, and drought stress. Eight CsCKX and eight CsIPT genes were identified from the cucumber genome. We constructed a phylogenetic tree from the amino acid sequences and performed prediction analyses of the cis-acting elements of the CsCKX and CsIPT promoters to determine whether CsCKXs and CsIPTs are responsive to light, abiotic stress, and different hormones. We also performed expression analysis of these genes in different tissues, and we found that CsCKXs and CsIPTs were highly expressed in roots and male flowers. Thus, they are involved in the whole growth and development process of the plant. This paper provides a reference for further research on the biological functions of CsIPT and CsCKX in regulating the growth and development of cucumber and its response to abiotic stress.
1. Introduction
Cytokinins (CKs) play a crucial role in controlling the growth and development of plants, with a wide range of biological effects. They can be obtained in synthetic form or isolated from plants. As phytohormones, they play important regulatory roles in many plant development and abiotic stress responses [1,2,3]. Many reports have suggested that cytokinins exert stimulatory or inhibitory functions in different developmental processes, such as root growth and branching, control of root tip dominance, and chloroplast development [4]. Since cytokinins are mostly synthesized in the roots and stems of plant bodies, they also promote cell proliferation in stems, which includes the activity of apical and axillary meristematic tissues [5]. At the same time, they are important in the regulation of leaf phloem [6], the regulation of cell division and differentiation during photomorphogenesis in unfolding leaves [7], pistil development, female gametophyte development [8], and the development of the vascular formation layer [9,10]. Furthermore, these hormones also function in the distribution of biomass [11] and the reaction to environmental stimuli [12,13,14].
Isopentenyl transferases (IPTs) play a key role in the production of cytokinins in plants, utilizing both the methylerythritol phosphate (MEP) and mevalonate (MVP) pathways [12,15,16]. Further studies have shown that catalysis occurs both de novo and via tRNA degradation. CKs’ biosynthesis and degradation are catalyzed by IPTs and cytokinin oxidases/dehydrogenases (CKXs), respectively, which are responsible for regulating endogenous CK levels [17]. IPT is the first rate-limiting enzyme for CK signaling, which is a key function in plant development and abiotic stresses; it was first identified in Agrobacterium rhizogenes [18,19], where it can be expressed throughout the plant and exists in the form of ATP/ADP and tRNA IPTs, and IPT enzymes are involved in the first and critical step of cytokinin synthesis. In plants, IPTs mediate two types of CK synthesis pathways: the ATP/ADP pathway and the tRNA pathway. Cytokinin oxidases (CKXs) are responsible for the irreversible breakdown of cytokinins in plants [20]. This is encoded by a small family of genes, and the substrate specificity of IPTs has been shown to vary depending on origin and species [12]. In the same manner, CKX enzymes exhibit their own unique substrate specificity, with distinct patterns of expression both spatially and temporally [21]. Cytokinins have a significant function in plants’ ability to adjust to intricate variations in their environment [22], and the IPT and CKX gene families have been recognized and replicated in various plant species. In Arabidopsis thaliana, the atipt8 mutant and the atipt1, 3, 5, 7 quadruple mutant were found to enhance salt and drought tolerance in plants [23,24]. In tobacco, a stress-inducible promoter was found to drive the soil bacillus IPT gene, which was inserted into the tobacco genome, increasing the concentration of active cytokinins in the plant and enhancing drought tolerance [25]. Subsequently, similar phenomena were observed in transgenic rice and poplar, where stress-inducible promoters were found to drive the IPT gene [13,26]. Cold-inducible promoter-driven IPT gene expression was found to improve sugarcane’s tolerance to low temperatures [21]. Root-specific/constitutive promoters drive CKX gene expression, which reduces cytokinin contents and enhances drought tolerance in plants [27,28]. More importantly, stress can repress the activity of the root-specific promoter (WRKY6). Therefore, the root-specific promoter WRKY6 drives CKX gene expression, which reduces the cytokinin contents in roots, promotes root growth, and enhances drought tolerance. The IPT and CKX genes were also found to be involved in the resistance of maize and soybean to drought and salt stress. These findings indicate that the IPT and CKX genes have significant functions in controlling the ability of plants to adapt to challenging conditions. These investigations offer novel perspectives for the development of stress-tolerant plants through breeding.
Cucumber (Cucumis sativus L.) is a significant vegetable crop across the globe and is a vital part of meeting the global demand for vegetables. During their production process, cucumbers are often subjected to low temperatures, low light, salinity, drought, and other stresses, resulting in a decline in yield and quality. At present, there is insufficient research specifically examining how IPT and CKX contribute to the growth and development of cucumbers under stress conditions, and the underlying regulatory mechanisms remain unclear. To address this gap, our study focused on several key goals: identifying all IPT and CKX genes in cucumbers; analyzing the physiological and biochemical properties of proteins encoded by these genes; investigating the expression patterns of these genes in different cucumber tissues and their responses to low light, salt, and drought stresses; and examining how these mechanisms contribute to breeding efforts to overcome the effects of abiotic stress and exogenous phytohormones. Our aim is to provide a reference point for future research into the biological functions of CsIPT/CsCKX in regulating cucumber’s growth and development, and in responding to adverse conditions.
2. Experimental Materials and Methods
2.1. Plant Material and Growing Conditions
The experiment was conducted at Fujian University of Agriculture and Forestry, using Zhongnong 26 cucumber as the test material. Cucumber seeds were germinated at room temperature and sown in 32-hole trays with a substrate for seedling development. Upon reaching 2 leaves and 1 heart, uniformly grown cucumber seedlings were selected for hydroponic treatment by planting them in 3.5 L black plastic containers with Yamazaki’s nutrient solution (recorded as day 0). The greenhouse environment was maintained at a temperature of 26 ± 4 °C, with a 12 h/12 h photoperiod, approximately 55 ± 10% relative humidity, and light intensity of 200 µmol/m−2/s−1 PPFD with white LED lights (7:00–19:00). Treatments were carried out after 6 d of pre-cultivation, and 3 treatments were set up: T1: 65 µmol/m−2/s−1 PPFD far-red light (to simulate the effect of shade); T2: 20% PEG; T3: 200 mmol/L NaCl. Different tissue parts of the cucumbers were collected after 0, 1, 3, and 8 h of treatment, and the samples were placed in liquid nitrogen and immediately stored in a refrigerator at −80 °C. The experiment was replicated three times, with five plants from each treatment group.
2.2. Identification and Physicochemical Properties of CsCKX and CsIPT Genes in the Cucumber Genome
Based on Arabidopsis IPT gene sequences and CKX gene sequences (see Attachment 1 for details), Blast searches were performed in the Cucumber Genome Database (http//cucumber.genomics.org.cn/page/cucumber/index.jsp, accessed on 4 July 2023), and combined with the retrieved gene annotations, 8 CsIPT and 8 CsCKX candidate genes were identified, with a critical expectation of 100. Cucumber (Cucumis sativus L.) CsCKX and CsIPT candidate proteins were searched by Blast with TBtools. Arabidopsis protein data were obtained from TAIR (https://www.arabidopsis.org/, accessed on 5 July 2023), and cucumber protein data were obtained from the Cucurbit database (http://cucurbitgenomics.org/, accessed on 5 July 2023). Hidden Markov model (HMM) files for CsIPT (PF01715)/CsCKX conserved structural domains (PF15628) were downloaded from the Pfam database (http://pfam.xfam.org/search, accessed on 5 July 2023), using PF01715/PF15628 as templates. The hmmsearch program of TBtools was used to search for proteins containing this conserved structural domain in the cucumber proteome. The search results of the above methods were de-duplicated and analyzed using the NCBI Conserved Domains Database (CDD; http://www.ncbi.nlm.nih.gov/cdd, accessed on 5 July 2023), and the cucumber protein data were obtained from the Cucurbit database (http://cucurbitgenomics.org/, accessed on 5 July 2023). These data were validated against the screened sequences, where the E-value was taken as 10−4. Protein identification and analysis tools on the ExPASy program (https://web.expasy.org/protparam/, accessed on 12 July 2022) were used to identify CsCKX and CsIPT proteins for physicochemical properties, including chemical properties, amino acid number (aa), protein size, isoelectric point (pI), molecular weight (MW), instability index, aliphaticity, and hydrophilicity prediction. The subcellular localization of CsCKX and CsIPT proteins was predicted using Plant-mPLoc (http://www.csbio.sjtu.edu.cn/bioinf/plant-multi/, accessed on 22 July 2022) [29].
2.3. Protein Structure Analysis of CsCKX and CsIPT
The ExPASy website (https://swissmodel.expasy.org/interactive, accessed on 11 July 2023) facilitated the analysis of the proteins’ secondary structure and the prediction of their tertiary structure. The necessary parameters were used to construct protein tertiary structure models.
2.4. Chromosome Localization and Collinearity Analysis
The Chinese long v3 gff3 file was downloaded from the Cucumber Genome Database to obtain the chromosome number, length, and the start and end positions of CsCKX and CsIPT gene family members on the chromosomes, and the chromosome distribution of the CsCKX and CsIPT genes was mapped by using TBtools (accessed on 3 June 2022). Homology searches were performed using the multicollinear scanning toolkit MCScanX to analyze gene duplication events using the default values. Using BLASTp, the cucumber genome’s protein-coding genes were compared to both its own genome and the Arabidopsis genome. The search threshold was set to an E-value less than 10−5, and no modifications were made to the default parameters for the other variables. Subsequently, TBtools (accessed on 29 July 2022) was used to highlight the collinear relationships within the identified CsIPT/CsCKX gene families and with genes homologous to these gene families in other species.
2.5. Phylogenetic Relationship Analysis
Phylogenetic trees were estimated using the MEGA 7.0 program, using the neighbor-joining (NJ) method with 1000 replicates for bootstrap analysis. PFAM structural domains and signal peptides of the IPT and CKX genes were obtained from the PFAM database. The exon/intron organization of the CsIPT and CsCKX genes was determined by comparing exon positions and gene lengths using the GSDS gene structure website (http://gsds.cbi.pku.edu.cn, accessed on 3 June 2022).
2.6. Analysis of Conserved Motifs and Gene Structure of Members of the CsCKX and CsIPT Families
We used the MEGA 7.0 method to construct phylogenetic trees for the IPT/CKX gene families of cucumber, Arabidopsis thaliana, melon, and non-bearing cabbage. The trees were built with 1000 bootstrap values and multiple sequence alignment. From these trees, we selected a stable minimum neighborhood tree to represent their evolutionary relationships. To enhance the visual presentation of the tree, we utilized iTOL (https://itol.embl.de/, accessed on 23 July 2022).
2.7. Analysis of Cis-Acting Elements
To analyze the cis-acting elements contained in the promoter regions of CsCKX and CsIPT family members, we obtained 2000 bp DNA sequences upstream of their start codons using TBtools software. The PlantCARE website (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/, accessed on 5 June 2022) was used to analyze the cis elements.
2.8. Expression Analysis of Gene Expression Levels under Multiple Stresses in Different Tissues and Organs
To investigate the gene-specific expression of CsCKX and CsIPT genes in different tissues of cucumber, firstly, we obtained their gene sequences from the Cucumber Genome Database (http://cucurbitgenomics.org/, accessed on 5 June 2022) and performed RNA extraction on different tissues (e.g., tendril, root, leaf, stem, flower, fruit) for quantitative analysis. The log2 function was employed to convert the data, and TBtools was utilized to create an expression heatmap for the CsCKX and CsIPT genes to further investigate the changes in gene expression levels under multiple stresses in different organs of cucumber.
2.9. RNA Extraction and Real-Time Fluorescence Quantitative qPCR Analysis
Quantitative analysis was performed using a kit (see Attachment 2 for details). The primers and internal reference genes used for qPCR amplification are listed in Table 1. Relative gene expression was calculated using the 2−ΔΔCT method. Sample analysis was carried out using three biological replicates.

Table 1.
The gene-specific primers used.
2.10. Data Analysis
Data were processed and plotted using SPSS (IBM®SPSS® Statistical Version 24) and GraphPadPrism8 (San Diego, CA, USA).
3. Results
3.1. Identification of CsCKX and CsIPT Genes in the Cucumber Genome and Characterization of Their Physicochemical Properties
Arabidopsis AtIPT and AtCKX proteins were used as query sequences [30,31] to identify CsIPT/CsCKX genes in the cucumber genome. After screening, eight CsIPT genes and eight CsCKX genes with confirmed conserved structural domains were identified and annotated (Table 2), and the genes were named according to their homologues in Arabidopsis. As shown in Table 2, the eight CsCKX genes have amino acids ranging from 516 to 699 aa, relative molecular weights ranging from 57.37 to 79.32 kD, and isoelectric points between 5.27 and 9.1, with CsCKX2, -5, -7, and -8 being acidic proteins. The stability coefficients were less than 40, except for CsCKX6, which was found to be a stable protein. In addition, all members are hydrophilic proteins except for CsCKX3, which is hydrophobic. Subcellular localization prediction of the proteins of CsCKXs revealed that, except for the proteins of CsCKX1, -3, and -4, which are located in vesicles, the rest are located in the cytoplasmic matrix. Subsequently, the physicochemical properties of the eight CsIPTs were analyzed, and it was found that they had 320 to 474 aa, relative molecular weights from 35.8 to 53.14 kD, and isoelectric points ranging from 5.71 to 9.0, with CsIPT4, -6, -7, and -8 being acidic proteins. Except for CsIPT1, -2, and -8, which were found to be unstable proteins, the rest have a stability factor less than 40, indicating that they are stabilizing proteins. In addition, all of these protein members are hydrophilic proteins. Prediction of subcellular localization of the CsIPT proteins revealed that the CsIPT1, -3, and -6 proteins are located in chloroplasts, the CsIPT2 and -5 proteins are located in chloroplasts and mitochondria, CsIPT4 is located in mitochondria, CsIPT7 is located in chloroplasts, mitochondria, and peroxisomes, and CsIPT8 is located in chloroplasts, cytoplasm, mitochondria, and peroxisomes.

Table 2.
The general information of CsCKX and CsIPT family members.
3.2. Protein Structure Analysis of CsCKX and CsIPT
The sequence and structure of a protein can reflect its function and provide the basis for its tertiary structure model. We utilized SOPMA online (https://npsa-prabi.ibcp.fr/cgi-bin/npsa_automat.pl?page=npsa%20_sopma.html, accessed on 1 June 2022)to predict the secondary structure of CsCKX and CsIPT proteins, aiming to gain a better understanding of their structural characteristics. As shown in Table 3, all CsCKX and CsIPT protein structures consisted of α-helices, β-folds, extended strands, and irregular coils, with the proportion of α-helices in CsCKXs ranging between 31.44% and 35.85%, the proportion of β-folds ranging between 4.94% and 7.13%, the proportion of extended strands ranging between 17.05% and 20.66%, and the proportion of irregular coils ranging between 40.77% and 45.52%. The proportion of α-helices in CsIPTs ranged between 43.33% and 56.35%, the proportion of β-folds ranged between 4.43% and 8.59%, the proportion of extended strands ranged between 10.34% and 13.33%, and the proportion of irregular coils ranged between 25.39% and 36.97%. An analysis of the secondary structures showed that there were differences between CsCKX and CsIPT proteins, but the differences were smaller for CsIPTs than for CsCKXs.

Table 3.
Secondary structure of CsCKX and CsIPT proteins.
The function of a gene is linked to the structure of the protein that it encodes. Homology modeling is a technique that relies on the amino acid sequence of a protein to create a 3D structure for a specific protein of interest by utilizing the 3D structure of a closely related protein. Using the homology modeling method, we successfully predicted the 3D structures of the eight proteins of CsIPT/CsCKX (Figure 1). The modeling results reveal that the GMQE values of the CsIPT/CsCKX protein sequences were all in close proximity to 1, demonstrating a strong uniformity among the proteins and a significant level of certainty in the predictions.
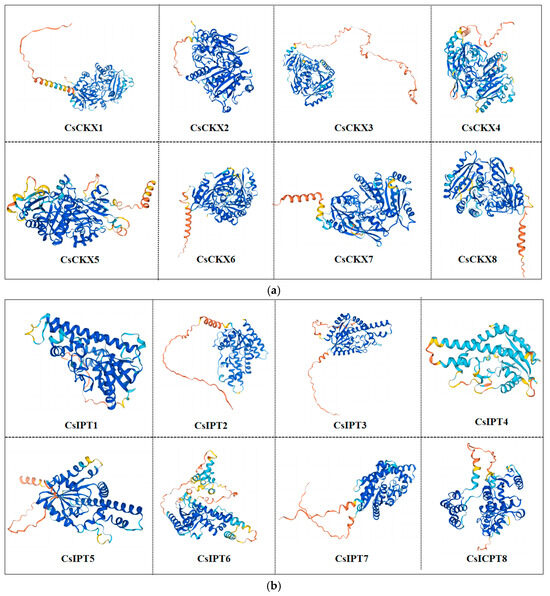
Figure 1.
Tertiary structure of CsCKX (a) and CsIPT (b) proteins.
3.3. Chromosomal Placement and Collinearity Analysis of CsCKXs and CsIPTs
The locations of the CsCKX and CsIPT gene families on chromosomes in relation to covariance within species are shown in Figure 2. According to the cucumber genome annotation file, we identified eight members of the CsCKX gene family and eight members of the CsIPT gene family. These genes are located on different chromosomes: the CsCKX genes are found on chromosomes 1–5, and the CsIPT genes are found on chromosomes 1, 3, 4, 5, 6, and 7 (Figure 2). Among the eight CsIPT family members, only CsIPT3 and -4 are located in regions far from the telomeric region.
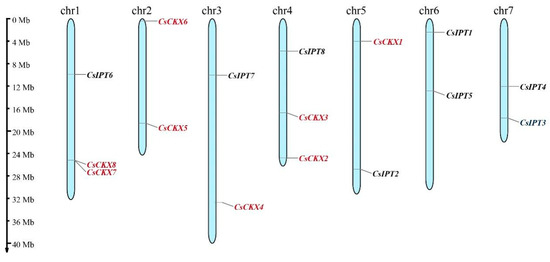
Figure 2.
Location of CsCKX and CsIPT gene family members on chromosomes. The scale bar on the left was used to estimate the length of the chromosomes. CsCKX and CsIPT gene family members are numbered in order from 1 to 8, with CsCKXs marked in red and CsIPTs in black.
To further infer the phylogenetic mechanisms of the CsCKX and CsIPT families and identify the collinear relationships between the eight genes of these families and those of other species, we performed collinearity analysis of the CsCKX and CsIPT family members of Arabidopsis thaliana, melon, and cucumber. As shown in Figure 3, a strong collinear region was observed between the dicotyledonous and Cucurbitaceae plants. Among these three plants, cucumbers and melons are the most closely related due to their genetic background as members of the Cucurbitaceae family. The analysis of genetic relationships and gene functions of various species can be facilitated by comparing the families of CsCKX and CsIPT with those of other species, thus offering a valuable research reference.
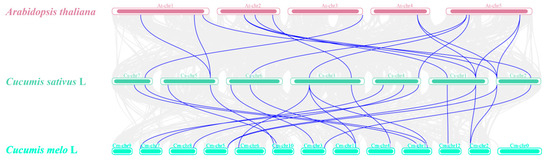
Figure 3.
Collinearity between CsCKX and CsIPT gene family members was analyzed in cucumber, Arabidopsis thaliana, melon, and other species. The chromosomes of these plants are marked with different colors, and the chromosome is indicated above each chromosome. Collinear relationships between CsCKX and CsIPT gene family members in different species are connected by lines. Homologous CsCKX and CsIPT gene pairs are represented by blue lines.
3.4. Analysis of Phylogenetic Relationships
In order to examine how CsCKX and CsIPT genes are related to other similar genes, we built a phylogenetic tree using the amino acid sequences of several identified genes. This included 13 AtCKX genes and 12 AtIPT genes, 10 CmCKX genes and 8 CmIPT genes, 10 BrCKX genes and 12 BrIPT genes, and 8 CsCKX genes and 8 CsIPT genes (Figure 4). By analyzing the phylogeny of homologous genes in each species, it was found that cucumber and melon showed a close evolutionary relationship for both CKX and IPT genes, which might be related to their belonging to the cucurbit family. Considering the frequency of members of the same subfamily performing similar tasks enhances our understanding of the potential biological functions of the CsCKX and CsIPT families.
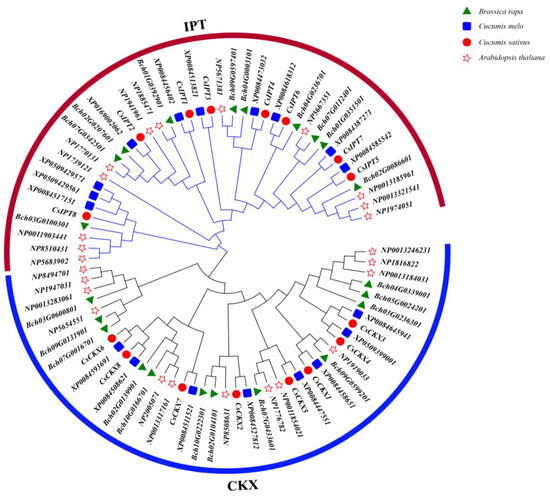
Figure 4.
Phylogenetic tree: different species are labeled with different shapes. Circles represent cucumber, pentagrams represent Arabidopsis thaliana, squares represent melon, triangles represent Brassica rapa, blue lines represent CKX, and red lines represent IPT.
3.5. Conserved Motif and Gene Structure Analyses of Cucumber CsCKX and CsIPT Family Members
We used MEGA-7 to construct a phylogenetic tree consisting exclusively of genes belonging to the CsCKX and CsIPT families, and the results were consistent with the previous evolutionary tree. The gene members of the CsCKX and CsIPT families were then analyzed for conserved motifs and gene structures based on this tree (Figure 5).
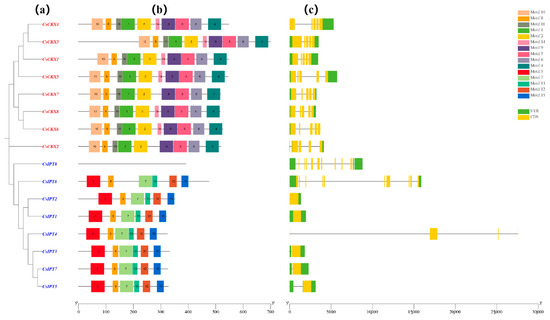
Figure 5.
The members of the CsCKX and CsIPT gene families exhibit a conserved gene structure and motifs. The gene family members are presented in a phylogenetic tree (a) showing the relationships between CsCKXs and CsIPTs. The distribution order of the conserved motifs in CsCKXs and CsIPTs is shown in (b), with different motifs indicated by different colored boxes (c). Additionally, the distribution of UTR and CDS in CsCKXs and CsIPTs is represented by green and yellow colors, respectively. The scale bar at the bottom allows for the comparison of gene and protein lengths.
In the gene structure analysis, the eight CsCKX genes contained five exons each, and all of them contained two introns except for CsCKX1 and -2, which contained one intron. Among the CsIPT gene family members, CsIPT1, -3, and -7 contained one exon and two introns, CsIPT2 contained one exon and one intron, CsIPT4 contained only two introns, CsIPT5 contained one exon and three introns, CsIPT6 contained ten exons and two introns, and CsIPT8 contained eleven exons and three introns. During the investigation of CsCKX conserved motifs, we discovered and assigned names to 10 conserved motifs: motifs 1, 2, 3, 4, 6, 8, 9, 10, 11, and 14. The analysis revealed that all genes in the group contained these 10 conserved motifs arranged in an identical sequence, except for CsCKX2 and -7, where motif 14 was absent. Examining CsIPT, six conserved motifs were identified, except for CsIPT8, which did not have any motifs; they were named motifs 5, 7, 8, 12, 13, and 15.
3.6. Analysis of Cucumber CsCKX and CsIPT Family Members to Predict Their Cis-Acting Elements
In this study, we utilized the sequences located 2000 bp before each gene to forecast cis-acting elements within the eight genes from the CsCKX and CsIPT families, emphasizing their significance in gene transcription (Figure 6). Figure 6a,b show the enrichment and location of cis-acting elements in the promoters of the CsCKX and CsIPT gene families. The findings indicate that both gene families contain significant numbers of cis-acting elements, primarily related to the responses to abiotic stress (58), light (178), growth and development (23), and phytohormones (66). Notably, numerous elements associated with light response, such as G-box, Box-4, and GT1-motif, are prevalent in most CsCKX and CsIPT genes, suggesting that light may play a role in inducing or repressing the expression of these genes.
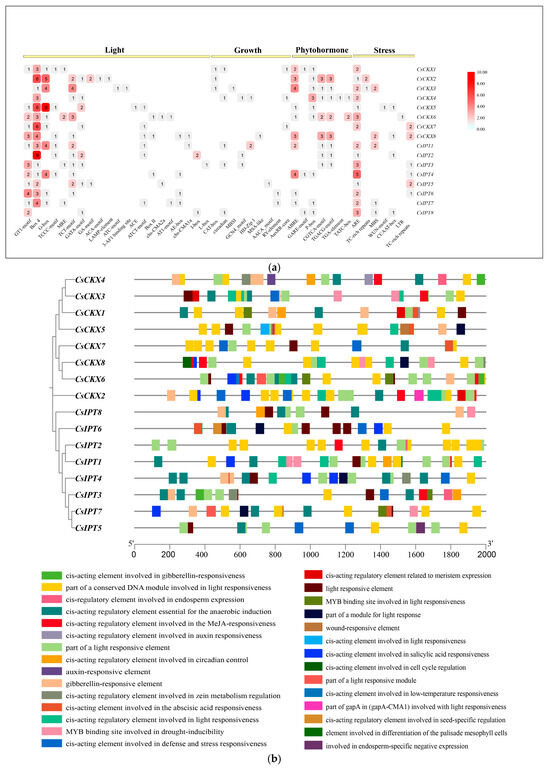
Figure 6.
Analysis of promoters of CsCKXs and CsIPTs to predict the presence of cis-acting elements: (a) For each promoter, the number of detected cis-acting elements was counted, and they were classified into four types. (b) TBtools was used to visualize response elements in promoters of CsCKXs and CsIPTs, showing their location and type.
All CsCKX family members contain Box-4, and all family members except for CsCKX8 contain ARE. Similarly, all CsIPT family members contain ARE, and all members except for CsIPT8 contain Box-4. The largest proportion of cis-acting elements in the promoter regions of CsCKX and CsIPT family members consists of light-responsive elements, totaling 178. The smallest proportion of elements consists of those associated with growth and development, with 23. CsCKX6 and -7, along with CsIPT4, lack any elements related to growth and development.
3.7. Analysis of CsCKX and CsIPT Gene Expression in Different Tissues and Organs of Cucumber
We determined the expression of each of the eight genes of CsCKX and CsIPT—i.e., CsCKX1–CsCKX8 and CsIPT1-CsIPT8—in different tissues, and the results are shown in Figure 7. Among the eight CsCKX genes, they were expressed at high levels in the root system and male flowers, and CsCKX8 was expressed at the highest level, which may be related to the expression of cytokinins in the root system and may also be involved in the opening of the flowers. Among the eight CsIPT genes, the trend of expression was similar, except for CsIPT8. It was found through the data that, except for the high level of the expression of the higher expression of the CsIPT 1, 2, 3, 5, and -7 genes may be related to the expression of cytokinin in the root system, indicating that these genes are involved in the whole growth and development process of the plant.
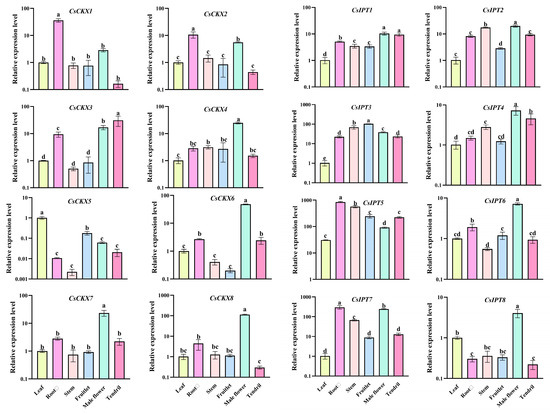
Figure 7.
Analysis of CsCKX and CsIPT gene expression in different tissues and organs of cucumber. The data are expressed as the mean ± SD (n = 3 biological replicates) and are referenced to the mean value of actin gene expression, which is the average of 2−ΔΔCT, The letters stand for significance, p-value < 0.05.
3.8. Expression Pattern of CKX/IPT Genes in Cucumber under Abiotic Stresses
In order to investigate the potential involvement of CKX/IPT genes from cucumber in various stress responses, we employed qRT-PCR to examine their expression levels. As shown in Figure 8a, the expression of all CsCKX members reached the highest levels after 1 h of FR treatment, with the root system showing the highest levels. However, as time progressed, expression among the members started to decline. The trend of CsIPT members under FR treatment was broadly similar to that of CsCKX members, except for CsIPT6, whose expression in leaves reached a peak at 3 h. The expression of the remaining members in leaves showed a decreasing trend. As shown in Figure 8b, following the application of NaCl, there was a gradual decrease in the expression of CsCKX members in leaves over time, except for CsCKX4 and -8, which decreased in leaves and were elevated in the root system. Conversely, the expression in stems increased after NaCl treatment, with CsIPT4 displaying the highest expression level, reaching a peak at the 1 h mark. Surprisingly, following PEG treatment (Figure 8c), the expression levels of CsCKX members in the root system peaked after 8 h. In the leaves, the expression of CsCKX1, -2, -3, -5, and -8 initially increased and then decreased over time, reaching a peak at 3 h. Additionally, the expression of CsIPT members in stems reached a maximum after 8 h, with a similar pattern seen among the members.
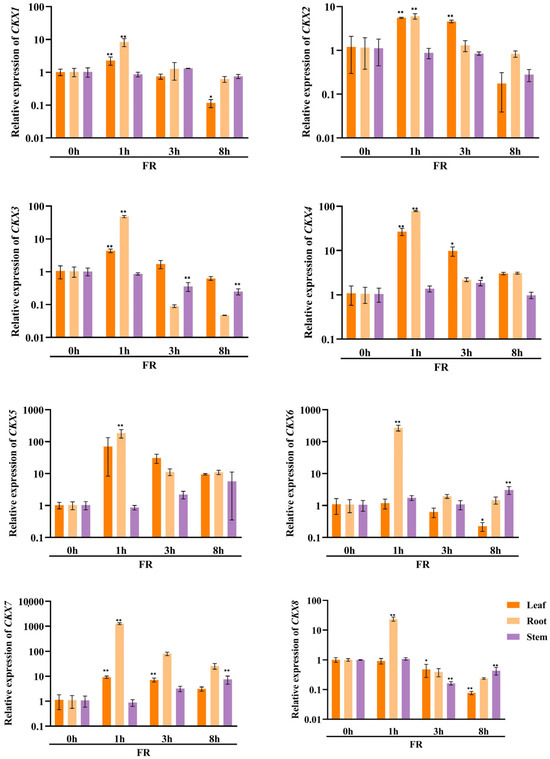
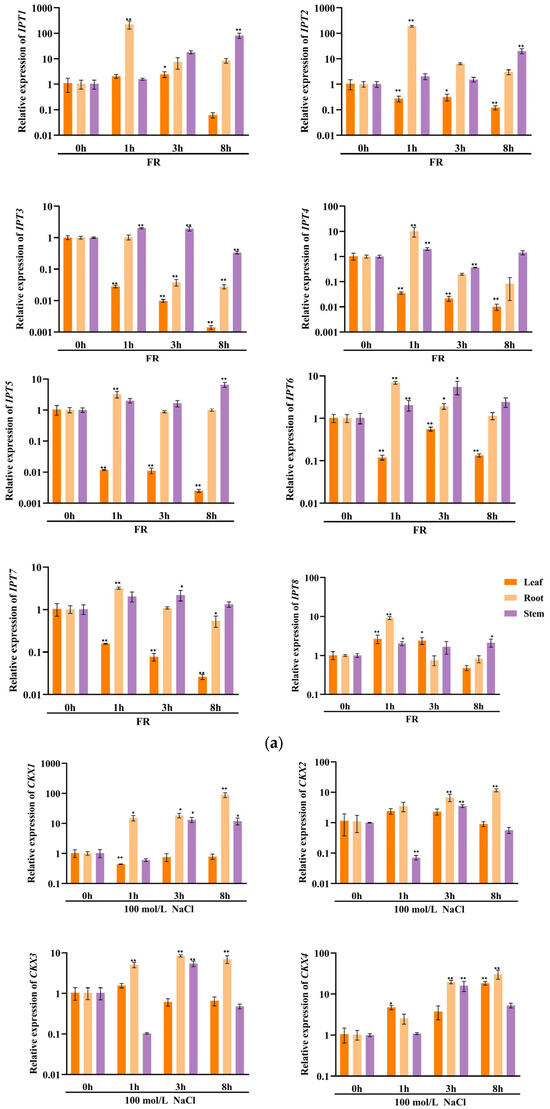
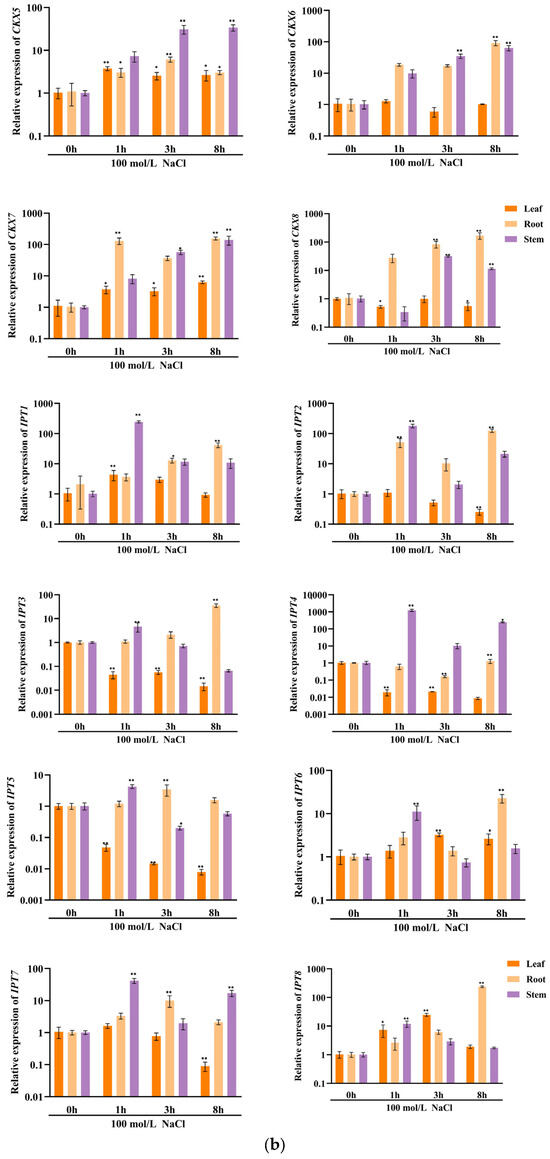
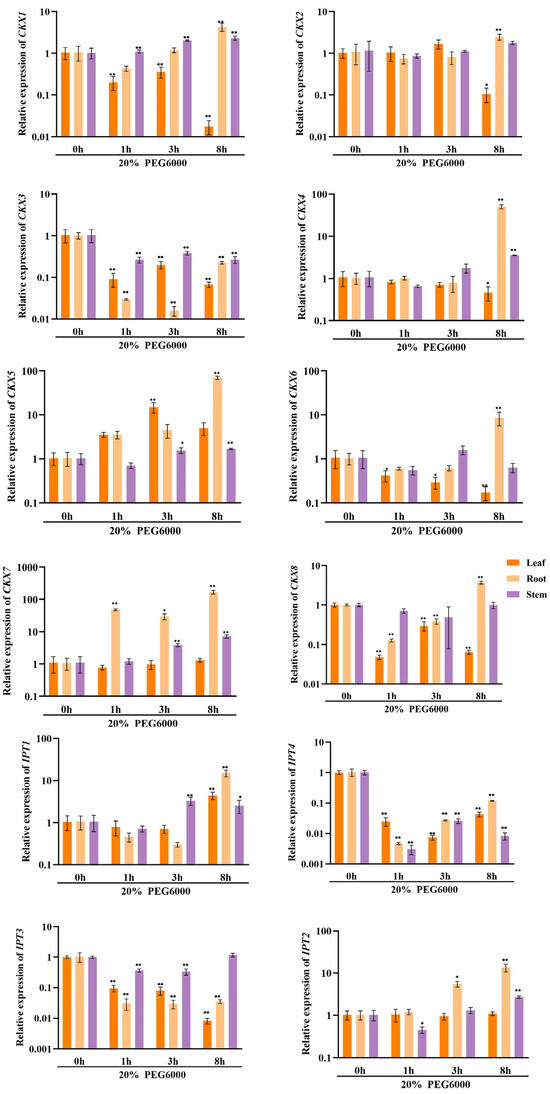
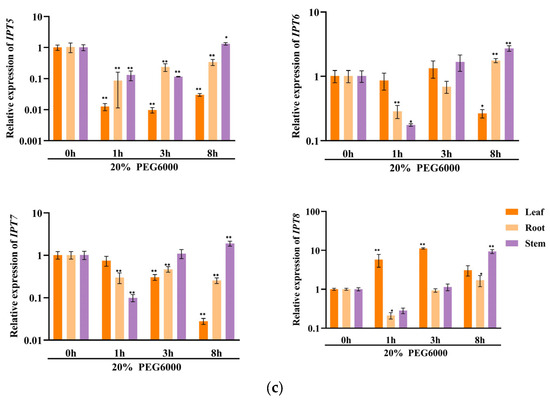
Figure 8.
The relative expression levels of CsCKX and CsIPT genes at different timepoints (0, 1, 3, and 8 h) under abiotic stress. The bar graphs represent the relative expression of CKX/IPT genes in cucumber subjected to far-red light (a), salt stress (b), and drought (c). The data are presented as the mean and standard deviation (n = 3 biological replicates), and the mean expression of the actin gene was used as a reference. The value was calculated as the mean of 2−ΔΔCT; * indicates a significant difference (p-value < 0.05), and ** indicates a highly significant difference (p-value < 0.01).
4. Discussion
IPT family genes have been shown to induce unisexual fruiting in tomatoes [32], and only the CsIPT1 gene has been isolated from cucumber, but its specific gene function has not been analyzed and identified [33]. The ability to sequence the cucumber genome and create a database [34] has created an opportunity for comprehensive investigation into the molecular mechanisms behind unisexual nodulation in cucumbers. Gene family analysis has become an effective method for better understanding genes’ structure, function, and evolution.
Cytokinin dehydrogenases have the ability to control cytokinin levels and are crucial for maintaining a balance between the synthesis and breakdown of cytokinins in plants [35,36]. Researchers have extensively studied the IPT and CKX gene families to understand their role and evolutionary history in various plant species. For instance, Arabidopsis thaliana has 7 AtCKX genes and 9 AtIPT genes; rice has 11 OsCKX and 10 OsIPT genes; soybean has 17 GmCKX and 14 GmIPT genes; cabbage has 12 BrCKX and 13 BrIPT genes; and maize has 13 ZmCKX and 8 ZmIPT genes [37,38,39,40,41,42,43]. Currently, the studies on IPT/CKX in cucumber’s growth, development, and responses to stress are not specific enough and have not clarified their regulatory mechanism(s). The primary goals of this research were to identify all IPT and CKX genes in cucumber, examine the physiochemical characteristics of the associated proteins, investigate the gene expression patterns in various cucumber tissues, and determine their responses to low light, salt, and drought stresses. Additionally, we aimed to uncover the mechanisms by which these genes cope with abiotic stresses and external phytohormones in order to facilitate breeding efforts. Ultimately, our findings will help in establishing a foundation for future investigations into the biological functions of CsIPT/CsCKX in cucumber’s growth, development, and responses to stressful conditions.
A phylogenetic tree was created in this research by using the amino acid sequences of 8 CsCKX and 8 CsIPT genes, 13 AtCKX and 12 AtIPT genes, 10 CmCKX and 8 CmIPT genes, and 10 BrCKX and 12 BrIPT genes that were detected in the cucumber genome (Figure 4). Phylogenetic analysis using protein sequences from multiple species revealed that cucumber and melon exhibited a significant evolutionary connection in terms of both CKX and IPT genes, suggesting a potential relationship within the cucurbit plant family. By considering the frequency of members of the same subfamily performing similar tasks, the CsCKX and CsIPT families can provide us with a deeper understanding of their potential biological activities.
The examination of the gene structure and conserved structural domains of CsCKX and CsIPT genes revealed that the eight members of the CsCKX gene family included two introns and five exons, with the exception of CsCKX1 and -2, which had a single intron. The gene structure of the CsIPT gene family members was more complex and variable, and it was unstable compared with CsCKX. From the results, it can be seen that except for CsCKX2 and -7, which lacked motif 14, all of the remaining member genes possessed all 10 conserved motifs, and the motifs were organized in an identical sequence. During the examination of the conserved motifs in CsIPT, with the exception of CsIPT8, which lacked any motifs, six conserved motifs were discovered and designated as motifs 5, 7, 8, 12, 13, and 15, respectively.
The growth and expression of CsCKX and CsIPT genes are also regulated by gene promoter activity [30]. The results of cis-acting element prediction analysis of CsCKX and CsIPT promoters showed that both gene families may be responsive to light, abiotic stresses, and different hormones. The analysis focused on their expression in various tissues and organs of cucumber (Figure 7). Upon analysis of the data, it was found that the expression of CsCKX and CsIPT genes was significantly higher in the root system and male flowers. It was concluded that the roots are the main site of cytokinin biosynthesis, and cytokinins synthesized by the roots not only regulate their own differentiation but are also transported to other parts of the plant to affect growth and development [44,45]. Therefore, their involvement extends throughout the entire growth and development of the plant. When the CKX1 gene is overexpressed in regular maize plants, it can result in male sterility [46]. It is hypothesized that the high expression of CsCKX4, CsCKX6, CsCKX7, CsCKX8, CsIPT1, CsIPT2, CsIPT4, CsIPT6, CsIPT7, and CsIPT8 genes in male flowers of cucumber ensures the normal growth and development of male flowers. The role of cytokinins in regulating leaf morphogenesis has also been demonstrated. In cucumber, CsCKX5, CsIPT2, CsIPT3, CsIPT4, and CsIPT5 had the highest expression levels in both leaves and stems, and these two components are potentially significant in the local synthesis of cytokinins within the leaves and stems.
The results of qRT-PCR expression analysis of CKX and IPT family genes under FR, drought, and salt stress treatments (Figure 8) show that cucumber seedlings exhibited a similar expression pattern for most members under light quality treatment. All members of the CsCKX group reached their highest expression levels after 1 h of FR treatment, with the root system showing the highest levels. As time passed, the expression levels started to decline. The trend of all CsIPT members under FR treatment was broadly similar to that of CsCKX members, except for CsIPT6, for which the peak expression in leaves occurred at 3 h, after which the expression of all members gradually decreased. As shown in Figure 8b, after NaCl treatment, the expression of CsCKX members in leaves showed a decreasing trend over time, except for CsCKX4 and CsCKX8, for which there was a decline in the trend and a significant increase in expression in the root system. The expression in stems was also upregulated following treatment with NaCl, and CsIPT4 had the highest expression, which peaked at 1 h. Interestingly, after PEG treatment (Figure 8c), at 8 h, the members of CsCKX had their highest expression levels in the root system. The expression of CsCKX1, -2, -3, -5, and -8 in leaves showed an initial increase followed by a decrease over time, with the peak occurring at 3 h. Interestingly, the expression of CsIPT members in the stems reached its highest values at 8 h, with a similar trend among the members.
In order to gain a deeper understanding of how the CsCKX and CsIPT families are phylogenetically related, and to determine the collinear relationships between the eight genes in each family and genes from other species, we conducted further analyses. We performed collinearity analysis of CsCKX and CsIPT family members in Arabidopsis, melon, and cucumber. As shown in Figure 3, a strong collinear relationship exists between dicots and melons. Of the three species, cucumber and melon are members of Cucurbitaceae and, thus, are the most closely related. A comparison of the CsCKX and CsIPT families with other species is useful in order to analyze genetic relationships and gene functions in multiple species.
5. Conclusions
During the course of this research, we successfully identified eight members from the CsCKX gene family, found on five different chromosomes, and eight members from the CsIPT gene family, distributed across six chromosomes, in cucumber. These genes play key roles in cytokinin biosynthesis and catabolic metabolic pathways. We performed a thorough and organized investigation of the CsCKX and CsIPT families, encompassing various aspects, including phylogenetic analysis, conserved structural domains, chromosomal localization, gene structure, and gene expression. In order to gain a deeper understanding of the underlying phylogenetic mechanisms, we performed a collinearity analysis of CsCKX and CsIPT family members from Arabidopsis, melon, and cucumber, the most closely related members of the Cucurbitaceae family. The results show that there was a highly significant correlation between the expression of CsCKX and CsIPT genes and promoter cis-elements under conditions of light, stress, and hormone treatments. In conclusion, this study’s findings offer new insights for further examination of the function of CKXs and IPTs, and for the development of the regulatory network in cucumbers. This provides a reference for related studies in other crops.
Author Contributions
Conceptualization, Y.X. and S.L.; methodology, Y.X., J.L. and W.H.; software, Y.X.; validation, Y.X.; formal analysis, S.R.; investigation, J.Z. and M.H.; funding acquisition, F.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by a grant from the Fujian Modern Agricultural Vegetable Industry System Construction Project to Fenglin Zhong (grant number 2019-897).
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Hai, N.N.; Chuong, N.N.; Tu, N.H.C.; Kisiala, A.; Hoang, X.L.T.; Thao, N.P. Role and Regulation of Cytokinins in Plant Response to Drought Stress. Plants 2020, 9, 422. [Google Scholar] [CrossRef]
- Peleg, Z.; Blumwald, E. Hormone balance and abiotic stress tolerance in crop plants. Curr. Opin. Plant Biol. 2011, 14, 290–295. [Google Scholar] [CrossRef]
- Verslues, P.E. ABA and cytokinins: Challenge and opportunity for plant stress research. Plant Mol. Biol. 2016, 91, 629–640. [Google Scholar] [CrossRef] [PubMed]
- Yoshihisa, I.; David, Z.; Ivona, K.; Michaela, K.; Wolfram, G.B.; Mitsuhiro, A. Interpreting Cytokinin Action as Anterograde Signaling and Beyond. Front. Plant Sci. 2021, 12, 641257. [Google Scholar]
- Katharina, H.; Alberto, M.A.; Lluis, F.; Lucia, L.E. The multifaceted role of cell cycle regulators in the coordination of growth and metabolism. FEBS J. 2020, 288, 3813–3833. [Google Scholar]
- Besnard, F.; Refahi, Y.; Morin, V.; Marteaux, B.; Brunoud, G.; Chambrier, P.; Rozier, F.; Mirabet, V.; Legrand, J.; Lainé, S. Cytokinin signalling inhibitory fields provide robustness to phyllotaxis. Nature 2014, 505, 417–421. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.P.; Li, J.Z.; Yang, L.; Chan, Z.L. Melatonin Antagonizes Cytokinin Responses to Stimulate Root Growth in Arabidopsis. J. Plant Growth Regul. 2022, 42, 1833–1845. [Google Scholar] [CrossRef]
- Cheng, C.Y.; Mathews, D.E.; Schaller, G.E.; Kieber, J.J. Cytokinin-dependent specification of the functional megaspore in the Arabidopsis female gametophyte. Plant J. 2013, 73, 929–940. [Google Scholar] [CrossRef] [PubMed]
- Schaller, G.E.; Bishopp, A.; Kieber, J.J. The yin-yang of hormones: Cytokinin and auxin interactions in plant development. Plant Cell 2015, 27, 44–63. [Google Scholar] [CrossRef]
- Kieber, J.J.; Schaller, G.E. Cytokinin signaling in plant development. Development 2018, 145, dev149344. [Google Scholar] [CrossRef]
- Dello, I.R.; Galinha, C.; Fletcher, A.G.; Grigg, S.P.; Molnar, A.; Willemsen, V.; Scheres, B.; Sabatini, S.; Baulcombe, D.; Maini, P.K.; et al. A PHABULOSA/cytokinin feedback loop controls root growth in Arabidopsis. Curr. Biol. 2012, 22, 1699–1704. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.N.; Nguyen, T.Q.; Kisiala, A.B.; Emery, R.J.N. Beyond transport: Cytokinin ribosides are translocated and active in regulating the development and environmental responses of plants. Planta 2021, 254, 45. [Google Scholar] [CrossRef] [PubMed]
- Werner, T.; Nehnevajova, E.; Köllmer, I.; Novák, O.; Strnad, M.; Krämer, U.; Schmülling, T. Root-Specific Reduction of Cytokin in Causes Enhanced Root Growth, Drought Tolerance, and Leaf Mineral Enrichment in Arabidopsis and Tobacco. Plant Cell 2010, 22, 3905–3920. [Google Scholar] [CrossRef] [PubMed]
- Anthony, B.; Hanna, H.; Sedeer, E.S.; Dolf, W.; Ben, S.; Jiří, F.; Eva, B.; Ari, P.M.; Ykä, H. A mutually inhibitory interaction between auxin and cytokinin specifies vascular pattern in roots. Curr. Biol. 2011, 21, 917–926. [Google Scholar]
- Villaécija-Aguilar, J.A.; Hamon-Josse, M.; Carbonnel, S.; Kretschmar, A.; Schmidt, C.; Dawid, C.; Bennett, T.; Gutjahr, C. SMAX1/SMXL2 regulate root and root hair development downstream of KAI2-mediated signalling in Arabidopsis. PLoS Genet. 2019, 15, e1008327. [Google Scholar] [CrossRef]
- Li, L.J.; Zheng, Q.F.; Jiang, W.; Xiao, N.Y.; Zeng, F.R.; Chen, G.; Mak, M.; Chen, Z.H.; Deng, F.L. Molecular Regulation and Evolution of Cytokinin Signaling in Plant Abiotic Stresses. Plant Cell Physiol. 2022, 63, 1787–1805. [Google Scholar] [CrossRef] [PubMed]
- Ding, Q.Q.; Wang, F.; Xue, J.; Yang, X.X.; Fan, J.M.; Chen, H.; Li, Y.; Wu, H. Identification and Expression Analysis of Hormone Biosynthetic and Metabolism Genes in the 2OGD Family for Identifying Genes That May Be Involved in Tomato Fruit Ripening. Int. J. Mol. Sci. 2020, 21, 5344. [Google Scholar] [CrossRef]
- Peng, C.; Pan, Q.W.; Xue, F.; Xu, S.; Gu, T.T. DNA methylation and histone modification patterns around Agrobacterium T-DNA integrations in woodland strawberry Fragaria vesca. Sci. Hortic. 2023, 326, 112760. [Google Scholar] [CrossRef]
- Frebort, I.; Kowalska, M.; Hluska, T.; Frebortova, J.; Galuszka, P. Evolution of cytokinin biosyntiiesis and degradation. J. Exp. Bot. 2011, 62, 2431–2452. [Google Scholar] [CrossRef] [PubMed]
- Zwack, P.J.; Rashotte, A.M. Interactions betten cytokinin signalling and abiotic stress responses. J. Exp. Bot. 2015, 66, 4863–4871. [Google Scholar] [CrossRef] [PubMed]
- Nishiyama, R.; Watanabe, Y.; Fujita, Y.; Le, D.T.; Kojima, M.; Werner, T.; Vankova, R.; Yamaguchi-Shinozaki, K.; Kakimoto, T.; Sakakibara, H.; et al. Analysis of cytokinin mutants and regulation of cytokinin metabolic genes reveals important regulatory roles of cytokinins in drought, salt and abscisic acid responses, and abscisic acid biosynthesis. Plant Cell 2011, 23, 2169–2183. [Google Scholar] [CrossRef]
- Jutta, L.M. Auxins in the right space and time regulate pea fruit development. J. Exp. Bot. 2022, 73, 3831–3835. [Google Scholar]
- Zhao, Y.; Chan, Z.L.; Gao, J.H.; Xing, L.; Cao, M.J.; Yu, C.M.; Hu, Y.L.; You, J.; Shi, H.T.; Zhu, Y.F.; et al. ABA receptor PYL9 promotes drought resistance and leaf senescence. Plant Biol. 2016, 113, 1949–1954. [Google Scholar] [CrossRef]
- Peleg, Z.; Reguera, M.; Tumimbang, E.; Walia, H.; Blumwald, E. Cytokinin-mediated source/sink modifications improve drought tolerance and increase grain yield in rice under water-stress. Plant Biotechnol. J. 2011, 9, 747–758. [Google Scholar] [CrossRef]
- Qin, H.; Gu, Q.; Zhang, J.L.; Sun, L.; Kuppu, S.; Zhang, Y.Z.; Burow, M.; Payton, P.; Blumwald, E.; Zhang, H. Regulated Expression of an isopentenyltransferase gene (IP7) in peanutsignificantly improves drought tolerance and increases yield under field conditions. Plant Cell Physiol. 2011, 52, 1904–1914. [Google Scholar] [CrossRef] [PubMed]
- Belintani, N.G.; Guerzoni, J.T.; Moreira, R.M.P.; Vieira, L.G.E. Improving low-temperature tolerance in sugarcane by expressing the ipt gene under a cold inducible promoter. Biol. Plant. 2012, 56, 5671–5677. [Google Scholar] [CrossRef]
- Mackova, H.; Hronkova, M.; Dobra, J.; Tureckova, V.; Novak, O.; Lubovska, Z.; Motyka, V.H.; Hajek, T.; Prasil, L.T.; Gaudinova, A.; et al. Enhanced drought and heat stress tolerance of tobacco plants with ectopically enhanced cytokinin oxidase/dehydrogenase gene expression. J. Exp. Bot. 2013, 64, 2805–2815. [Google Scholar] [CrossRef] [PubMed]
- Penev, P.I.; McCann, H.M.; Meade, C.D.; Alvarez, C.C.; Maddala, A.; Bernier, C.R.; Chivukula, V.L.; Ahmad, M.; Gulen, B.; Sharma, A.; et al. ProteoVision: Web server for advanced visualization of ribosomal proteins. Nucleic Acids Res. 2021, 49, W578–W588. [Google Scholar] [CrossRef] [PubMed]
- Takei, K.; Sakakibara, H.; Sugiyama, T. Identification of genes encoding adenylate isopentenyltransferase, a cytokinin biosynthesis enzyme, in Arabidopsis thaliana. J. Biol. Chem. 2001, 276, 26405–26410. [Google Scholar] [CrossRef]
- Werner, T.; Motyka, V.; Laucou, V.; Smets, R.; Van-Onckelen, H.; Schmulling, T. Cytokinin-deficient transgenic Arabidopsis plants show multiple developmental alterations indicating opposite functions of cytokinins in the regulation of shoot and root meristem activity. Plant Cell 2003, 15, 2532–2550. [Google Scholar] [CrossRef] [PubMed]
- Mao, Z.C.; Yu, Q.J.; Zhen, W.; Guo, J.; Hu, Y.L.; Gao, Y.; Lin, Z.P. Expression of ipt gene driven by tomato fruit specific promoter and its effects on fruit development of tomato. Chin. Sci. Bull. 2002, 47, 928–933. [Google Scholar] [CrossRef]
- Zhang, W.W.; Pan, J.S.; Liu, C.Z.; He, H.L.; Cai, R. Cloning and sequence analysis of ispentenyltransferase gene in cucumber. J. Shanghai Jiaotong Univ. Sci. 2010, 28, 487–491. [Google Scholar]
- Huang, S.W.; Li, R.Q.; Zhang, Z.H.; Li, L.; Gu, X.F.; Fan, W.; Lucas, W.J.; Wang, X.W.; Xie, B.Y.; Ni, P.X.; et al. The genome of the cucumber, Cucumis sativus L. Nat. Genet. 2009, 41, 1275–1281. [Google Scholar] [CrossRef] [PubMed]
- Dash, P.K.; Rai, R. Green revolution to grain revolution: Florigen in the frontiers. J. Biotechnol. 2022, 343, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Durán-Medina, Y.; Díaz-Ramírez, D.; Marsch-Martínez, N. Cytokinins on the Move. Front. Plant Sci. 2017, 8, 146. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.N.; Kambhampati, S.; Kisiala, A.; Seegobin, M.; Emery, R. The soybean (Glycine max L.) cytokinin oxidase/dehydrogenase multigene family; Identification of natural variations for altered cytokinin content and seed yield. Plant Direct. 2021, 5, e308. [Google Scholar] [CrossRef]
- Wang, C.; Wang, H.; Zhu, H.; Ji, W.; Hou, Y.; Meng, Y.; Wen, J.; Mysore, K.S.; Li, X.; Lin, H. Genome-wide identification and characterization of cytokinin oxidase/dehydrogenase family genes in Medicago truncatula. J. Plant Physiol. 2021, 256, 153308. [Google Scholar] [CrossRef] [PubMed]
- Galuszka, P.; Frebortova, J.; Werner, T.; Yamada, M.; Strnad, M.; Schmulling, T.; Frebort, I. Cytokinin oxidase/dehydrogenase genes in barley and wheat: Cloning and heterologous expression. Eur. J. Biochem. 2004, 271, 3990–4002. [Google Scholar] [CrossRef]
- Mi, X.; Wang, X.; Wu, H.; Gan, L.; Ding, J.; Li, Y. Characterization and expression analysis of cytokinin biosynthesis genes in Fragaria vesca. Plant Growth Regul. 2017, 82, 139–149. [Google Scholar] [CrossRef]
- Brugiere, N.; Humbert, S.; Rizzo, N.; Bohn, J.; Habben, J.E. A member of the maize isopentenyl transferase gene family, Zea mays isopentenyl transferase 2 (ZmIPT2), encodes a cytokinin biosynthetic enzyme expressed during kernel development. Cytokinin biosynthesis in maize. Plant Mol. Biol. 2008, 67, 215–229. [Google Scholar] [CrossRef]
- Gu, R.; Fu, J.; Guo, S.; Duan, F.; Wang, Z.; Mi, G.; Yuan, L. Comparative expression and phylogenetic analysis of maize cytokinin dehydrogenase/oxidase (CKX) gene family. J. Plant Growth Regul. 2010, 29, 428–440. [Google Scholar] [CrossRef]
- Liu, Z.; Lv, Y.; Zhang, M.; Liu, Y.; Kong, L.; Zou, M.; Lu, G.; Cao, J.; Yu, X. Identification, expression, and comparative genomic analysis of the IPT and CKX gene families in Chinese cabbage (Brassica rapa ssp. pekinensis). BMC Genom. 2013, 14, 594. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, C.J. Multifaceted regulations of gateway enzyme phenylalanine ammonia-lyase in the biosynthesis of phenylpropanoids. Mol. Plant 2015, 8, 17–27. [Google Scholar] [CrossRef]
- Hirose, N.; Takei, K.; Kuroha, T.; Kamada-Nobusada, T.; Hayashi, H.; Sakakibara, H. Regulation of cytokinin biosynthesis, compartmentalization and translocation. J. Exp. Bot. 2008, 59, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Cerny, R.E.; Qi, Y.; Bhat, D.; Aydt, C.M.; Hanson, D.D.; Malloy, K.P.; Ness, L.A. Transgenic studies on the involvement of cytokinin and gibberellin in male development. Plant Physiol. 2003, 131, 1270–1282. [Google Scholar] [CrossRef] [PubMed]
- Shani, E.; Ben-Gera, H.; Shleizer-Burko, S.; Burko, Y.; Weiss, D.; Ori, N. Cytokinin regulates compound leaf development in tomato. Plant Cell 2010, 22, 3206–3217. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).