Nectar Characteristics and Honey Production Potential of Five Rapeseed Cultivars and Two Wildflower Species in South Korea
Abstract
1. Introduction
1.1. Ecological Significance and Economic Implications of Honey Plants
1.2. Research Plants: Utilization and Significance
2. Results
2.1. Comparison among B. napus Cultivars
2.1.1. Flowering and Growth Characteristics
2.1.2. Nectar Secretion and Sugar Composition
2.1.3. Amino Acid Content
2.1.4. Estimated Honey Production
2.2. Two Wildflowers
2.2.1. Flowering and Growth Characteristics
2.2.2. Nectar Secretion Characteristics
2.2.3. Sugar Content and Composition
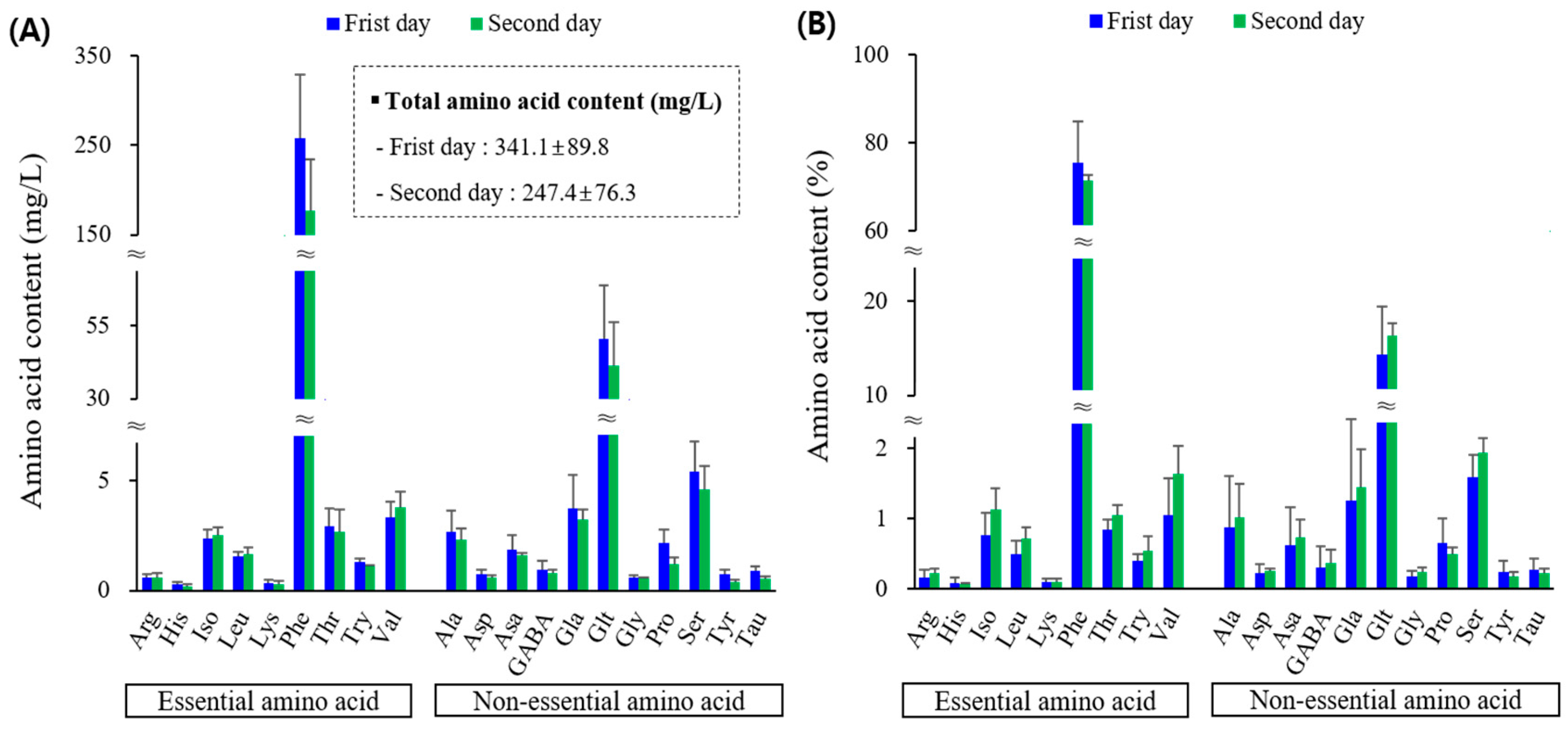
2.2.4. Amino Acid Content
2.2.5. Estimated Honey Production
3. Discussion
3.1. Estimation of Honey Production
3.2. Sugar Composition
3.3. Amino Acid Content
4. Materials and Methods
4.1. Plant Material
4.2. Measure of Nectar Volumes
4.3. Analysis of Sugar Contents
4.4. Analysis of Amino Acid Contents
4.5. Honey Production Potential
- 1 Nectar sugar content (mg/flower) = nectar volume (μL/flower) × free sugar content (μg/μL) × 0.001 (for unit conversion: μg to mg)
- 2 Honey potential = sugar content: honey = 85:100.
4.6. Statistics
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ioniță-Mîndrican, C.-B.; Mititelu, M.; Musuc, A.M.; Oprea, E.; Ziani, K.; Neacșu, S.M.; Grigore, N.D.; Negrei, C.; Dumitrescu, D.-E.; Mireșan, H.; et al. Honey and other Beekeeping products intake among the Romanian population and their therapeutic Use. Appl. Sci. 2022, 12, 9649. [Google Scholar] [CrossRef]
- Abd Rashid, N.; Mohammed, S.N.F.; Syed Abd Halim, S.A.; Ghafar, N.A.; Abdul Jalil, N.A. Therapeutic Potential of Honey and Propolis on Ocular Disease. Pharmaceuticals 2022, 15, 1419. [Google Scholar] [CrossRef]
- Bungau, S.G.; Popa, V.-C. Between religion and science: Some aspects: Concerning illness and healing in antiquity. Transylv. Rev. 2015, 26, 3–19. Available online: https://www.researchgate.net/publication/286442576 (accessed on 19 September 2023).
- Durazzo, A.; Lucarini, M.; Plutino, M.; Lucini, L.; Aromolo, R.; Martinelli, E.; Souto, E.B.; Santini, A.; Pignatti, G. Bee Products: A Representation of Biodiversity, Sustainability, and Health. Life 2021, 11, 970. [Google Scholar] [CrossRef]
- Morse, R.A.; Calderone, N.W. The value of honey bees as pollinators of US crops in 2000. Bee Cult. 2000, 128, 1–15. Available online: http://www.beeculture.com/beeculture/pollination2000 (accessed on 19 September 2023).
- Papa, G.; Maier, R.; Durazzo, A.; Lucarini, M.; Karabagias, I.K.; Plutino, M.; Bianchetto, E.; Aromolo, R.; Pignatti, G.; Ambrogio, A.; et al. The Honey Bee Apis mellifera: An Insect at the Interface between Human and Ecosystem Health. Biology 2022, 11, 233. [Google Scholar] [CrossRef] [PubMed]
- Isbell, F.; Adler, P.R.; Eisenhauer, N.; Fornara, D.; Kimmel, K.; Kremen, C.; Letourneau, D.K.; Liebman, M.; Polley, H.W.; Quijas, S.; et al. Benefits of increasing plant diversity in sustainable agroecosystems. J. Ecol. 2017, 105, 871–879. [Google Scholar] [CrossRef]
- Sutter, L.; Jeanneret, P.; Bartual, A.M.; Bocci, G.; Albrecht, M. Enhancing plant diversity in agricultural landscapes promotes both rare bees and dominant crop-pollinating bees through complementary increase in key floral resources. J. Appl. Ecol. 2017, 54, 1856–1864. [Google Scholar] [CrossRef]
- Futuyma, D.J.; Mitter, C. Insect-plant interactions: The evolution of component communities. Philos. Trans. R. Soc. Lond. B 1996, 351, 1361–1366. [Google Scholar] [CrossRef]
- Sharma, G.; Malthanar, P.A.; Mathur, V.; Notes, A. Insect-plant interactions: A multilayered relationship. Ann. Entomol. Soc. Am. 2021, 114, 1–16. [Google Scholar] [CrossRef]
- Baude, M.; Kunin, W.E.; Boatman, N.D.; Conyers, S.; Davies, N.; Gillespie, M.A.K.; Morton, R.D.; Smart, S.M.; Memmott, J. Historical nectar assessment reveals the fall and rise of floral resources in Britain. Nature 2016, 530, 85–88. [Google Scholar] [CrossRef]
- Reilly, J.R.; Artz, D.R.; Biddinger, D.; Bobiwash, K.; Boyle, N.K.; Brittain, C.; Brokaw, J.; Campbell, J.W.; Daniels, J.; Elle, E.; et al. Crop production in the USA is frequently limited by a lack of pollinators. Proc. R. Soc. B 2020, 287, 1931. [Google Scholar] [CrossRef]
- Neumann, P.; Carreck, N.L. Honey bee colony losses. J. Apic. Res. 2010, 49, 1–6. [Google Scholar] [CrossRef]
- Potts, S.G.; Roberts, S.P.M.; Dean, R.; Marris, G.; Brown, M.A.; Jones, R.; Neumann, P.; Settele, J. Declines of managed honey bees and beekeepers in Europe. J. Apic. Res. 2010, 49, 15–22. [Google Scholar] [CrossRef]
- Morawetz, L.; Köglberger, H.; Griesbacher, A.; Derakhshifar, I.; Crailsheim, K.; Brodschneider, R.; Moosbeckhofer, R. Health status of honey bee colonies (Apis mellifera) and disease-related risk factors for colony losses in Austria. PLoS ONE 2019, 14, e0219293. [Google Scholar] [CrossRef] [PubMed]
- López-Uribe, M.M.; Ricigliano, V.A.; Simone-Finstrom, M. Defining pollinator health: A holistic approach based on ecological, genetic, and physiological factors. Annu. Rev. Anim. Biosci. 2020, 8, 269–294. [Google Scholar] [CrossRef] [PubMed]
- Memmott, J.; Craze, P.G.; Waser, N.M.; Price, M.V. Global warming and the disruption of plant–pollinator interactions. Ecol. Lett. 2007, 10, 710–717. [Google Scholar] [CrossRef] [PubMed]
- Flores, J.M.; Gil-Lebrero, S.; Gámiz, V.; Rodríguez, M.I.; Ortiz, M.A.; Quiles, F.J. Effect of the climate change on honey bee colonies in a temperate Mediterranean zone assessed through remote hive weight monitoring system in conjunction with exhaustive colonies assessment. Sci. Total Environ. 2019, 653, 1111–1119. [Google Scholar] [CrossRef] [PubMed]
- Clermont, A.; Eickermann, M.; Kraus, F.; Hoffmann, L.; Beyer, M. Correlations between land covers and honey bee colony losses in a country with industrialized and rural regions. Sci. Total Environ. 2015, 532, 1–13. [Google Scholar] [CrossRef]
- Goulson, D.; Nicholls, E.; Botías, C.; Rotheray, E.L. Bee declines driven by combined stress from parasites, pesticides, and lack of flowers. Science 2015, 347, 1255957. [Google Scholar] [CrossRef]
- Potts, S.G.; Biesmeijer, J.C.; Kremen, C.; Neumann, P.; Schweiger, O.; Kunin, W.E. Global pollinator declines: Trends, impacts and drivers. Trends Ecol. Evol. 2010, 25, 345–353. [Google Scholar] [CrossRef]
- Favaro, R.; Bauer, L.M.; Rossi, M.; D’Ambrosio, L.; Bucher, E.; Angeli, S. Botanical origin of pesticide residues in pollen loads collected by honeybees during and after apple bloom. Front. Physiol. 2019, 10, 1069. [Google Scholar] [CrossRef] [PubMed]
- de Miranda, J.R.; Genersch, E. Deformed wing virus. J. Invertebr. Pathol. 2010, 103, S48–S61. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.M.; Loh, E.H.; Rostal, M.K.; Zambrana-Torrelio, C.M.; Mendiola, L.; Daszak, P. Pathogens, pests, and economics: Drivers of honey bee colony declines and losses. EcoHealth 2013, 10, 434–445. [Google Scholar] [CrossRef] [PubMed]
- Fairbrother, A.; Purdy, J.; Anderson, T.; Fell, R. Risks of neonicotinoid insecticides to honeybees. Environ. Toxicol. Chem. 2014, 33, 719–731. [Google Scholar] [CrossRef] [PubMed]
- Decourtye, A.; Mader, E.; Desneux, N. Landscape enhancement of floral resources for honey bees in agro-ecosystems. Apidologie 2010, 41, 264–277. [Google Scholar] [CrossRef]
- Wratten, S.D.; Gillespie, M.; Decourtye, A.; Mader, E.; Desneux, N. Pollinator habitat enhancement: Benefits to other ecosystem services. Agric. Ecosyst. Environ. 2012, 159, 112–122. [Google Scholar] [CrossRef]
- Proesmans, W.; Bonte, D.; Smagghe, G.; Meeus, I.; Decocq, G.; Spicher, F.; Kolb, A.; Lemke, I.; Diekmann, M.; Bruun, H.H.; et al. Small forest patches as pollinator habitat: Oases in an agricultural desert? Landsc. Ecol. 2019, 34, 487–501. [Google Scholar] [CrossRef]
- Other Livestock Statistics. Available online: https://www.mafra.go.kr/bbs/home/798/567228/artclView.do?layout=unknown (accessed on 18 December 2023).
- Adgaba, N.; Al-Ghamdi, A.; Tadesse, Y.; Getachew, A.; Awad, A.M.; Ansari, M.J.; Owayss, A.A.; Mohammed, S.E.A.; Alqarni, A.S. Nectar secretion dynamics and honey production potentials of some major honey plants in Saudi Arabia. Saudi J. Biol. Sci. 2017, 24, 180–191. [Google Scholar] [CrossRef]
- Bareke, T.; Addi, A.; Wakjira, K.; Kumsa, T. Dynamics of nectar secretion, honey production potential and colony carrying capacity of Coffea arabica L., Rubiaceae. J. Agric. Environ. Int. 2021, 115, 125–138. [Google Scholar] [CrossRef]
- Kim, Y.K. A Study on the Valuation of Honey Plants by Investigating the Nectar Characteristics. Ph.D. Thesis, Kangwon National University, Kangwon, Republic of Korea, 2022. [Google Scholar]
- Simcock, N.K.; Gray, H.E.; Wright, G.A. Single amino acids in sucrose rewards modulate feeding and associative learning in the honeybee. J. Insect Physiol. 2014, 69, 41–48. [Google Scholar] [CrossRef]
- Paoli, P.P.; Donley, D.; Stabler, D.; Saseendranath, A.; Nicolson, S.W.; Simpson, S.J.; Wright, G.A. Nutritional balance of essential amino acids and carbohydrates of the adult worker honeybee depends on age. Amino Acids 2014, 46, 1449–1458. [Google Scholar] [CrossRef] [PubMed]
- Herrera, C.M.; Pérez, R.; Alonso, C. Extreme intraplant variation in nectar sugar composition in an insect-pollinated perennial herb. Am. J. Bot. 2006, 93, 575–581. [Google Scholar] [CrossRef] [PubMed]
- Antoñ, S.; Denisow, B. Nectar production and carbohydrate composition across floral sexual phases: Contrasting patterns in two protandrous Aconitum species (Delphinieae, Ranunculaceae). Flora 2014, 209, 464–470. [Google Scholar] [CrossRef]
- Lu, N.-N.; Li, X.-H.; Li, L.; Zhao, Z.-G. Variation of nectar production in relation to plant characteristics in protandrous Aconitum gymnandrum. J. Plant Ecol. 2015, 8, 122–129. [Google Scholar] [CrossRef]
- Waddington, K.D. Honey bee foraging profitability and round dance correlates. J. Comp. Physiol. 1982, 148, 297–301. [Google Scholar] [CrossRef]
- Grüter, C.; Ratnieks, F.L.W. Flower constancy in insect pollinators: Adaptive foraging behaviour or cognitive limitation? Commun. Integr. Biol. 2011, 4, 633–636. [Google Scholar] [CrossRef] [PubMed]
- Baker, H.G.; Baker, I. The occurrence and significance of amino acids in floral nectar. Plant Syst. Evol. 1986, 151, 175–186. [Google Scholar] [CrossRef]
- Petanidou, T.; Van Laere, A.; Ellis, W.N.; Smets, E. What shapes amino acid and sugar composition in Mediterranean floral nectars? Oikos 2006, 115, 155–169. [Google Scholar] [CrossRef]
- Nicolson, S.W.; Thornburg, R.W. Nectar chemistry. In Nectaries and Nectar; Nicolson, S.W., Nepi, M., Pacini, E., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 215–264. [Google Scholar]
- Nepi, M. Beyond nectar sweetness: The hidden ecological role of non-protein amino acids in nectar. J. Ecol. 2014, 102, 108–115. [Google Scholar] [CrossRef]
- Haydak, M.H. Honey bee nutrition. Annu. Rev. Entomol. 1970, 15, 143–156. [Google Scholar] [CrossRef]
- Shiraishi, A.; Kuwabara, M. The effects of amino acids on the labellar hair chemosensory cells of the fly. J. Gen. Physiol. 1970, 56, 768–782. [Google Scholar] [CrossRef] [PubMed]
- Dadd, R.H. Insect nutrition: Current developments and metabolic implications. Annu. Rev. Entomol. 1973, 18, 381–420. [Google Scholar] [CrossRef] [PubMed]
- Poulis, B.A.D.; O’Leary, S.J.B.; Haddow, J.D.; von Aderkas, P. Identification of proteins present in the Douglas fir ovular secretion: An insight into conifer pollen selection and development. Int. J. Plant Sci. 2005, 166, 733–739. [Google Scholar] [CrossRef]
- Gardener, M.C.; Gillman, M.P. The taste of nectar—A neglected area of pollination ecology. Oikos 2002, 98, 552–557. [Google Scholar] [CrossRef]
- Tamm, S.; Gass, C.L. Energy intake rates and nectar concentration preferences by hummingbirds. Oecologia 1986, 70, 20–23. [Google Scholar] [CrossRef]
- Jang, J.W. A Study on Honey Plants in Korea—The Kind of Honey Plants in Korea and Around a Former Scanning Electron Microscope Form Structure of the Pollen. Ph.D. Thesis, Daegu University, Daegu, Republic of Korea, 2008. [Google Scholar]
- Škorić, D.; Jocić, S.; Sakac, Z.; Lećić, N. Genetic possibilities for altering sunflower oil quality to obtain novel oils. Can. J. Physiol. Pharmacol. 2008, 86, 215–221. [Google Scholar] [CrossRef]
- Enkegaard, A.; Kryger, P.; Boelt, B. Determinants of nectar production in oilseed rape. J. Apic. Res. 2016, 55, 89–99. [Google Scholar] [CrossRef]
- Wang, B.; Wu, Z.; Li, Z.; Zhang, Q.; Hu, J.; Xiao, Y.; Cai, D.; Wu, J.; King, G.J.; Li, H.; et al. Dissection of the genetic architecture of three seed-quality traits and consequences for breeding in Brassica napus. Plant Biotechnol. J. 2018, 16, 1336–1348. [Google Scholar] [CrossRef]
- Marles, M.S.; Gruber, M.Y. Histochemical characterisation of unextractable seed coat pigments and quantification of extractable lignin in the Brassicaceae. J. Sci. Food Agric. 2004, 84, 251–262. [Google Scholar] [CrossRef]
- Farkas, Á.; Zajácz, E. Nectar production for the Hungarian honey industry. Eur. J. Plant Sci. Biotechnol. 2007, 1, 125–151. Available online: https://www.researchgate.net/publication/228856362 (accessed on 21 September 2023).
- Shubharani, R.; Roopa, P.; Sivaram, V. Pollen morphology of selected bee forage plants. Glob. J. Bio-Sci. Biotechnol. 2013, 2, 82–90. Available online: https://www.academia.edu/download/31906096/GJBB_-_Shubha_et_al.pdf (accessed on 21 September 2023).
- Khan, K.A.; Ghramh, H.A. Pollen source preferences and pollination efficacy of honey bee, Apis mellifera (Apidae: Hymenoptera) on Brassica napus crop. J. King Saud Univ. Sci. 2021, 33, 101487. [Google Scholar] [CrossRef]
- Davis, A.R.; Sawhney, V.K.; Fowke, L.C.; Low, N.H. Floral nectar secretion and ploidy in Brassica rapa and B. napus (Brassicaceae). I. Nectary size and nectar carbohydrate production and composition. Apidologie 1994, 25, 602–614. [Google Scholar] [CrossRef]
- Free, J.B.; Nuttall, P.M. The pollination of oilseed rape (Brassica napus) and the behaviour of bees on the crop. J. Agric. Sci. 1968, 71, 91–94. [Google Scholar] [CrossRef]
- Albach, D.C.; Chase, M.W. Paraphyly of Veronica (Veroniceae; Scrophulariaceae): Evidence from the internal transcribed spacer (ITS) sequences of nuclear ribosomal DNA. J. Plant Res. 2001, 114, 9–18. [Google Scholar] [CrossRef]
- Luo, J.; Gong, Q.; Zhou, M.; Liu, Q.; Cheng, R.; Ge, Y. Complete chloroplast genome of the medicinal herb Veronicastrum axillare (Sieb. et Zucc.) Yamazaki and the phylogenetic relationship analysis within the tribe Veroniceae. Mitochondrial DNA Part B 2022, 7, 783–785. [Google Scholar] [CrossRef]
- Ding, S.X.; Jiang, H.; Tian, J.; Ren, J.; Mutie, F.M.; Waswa, E.N.; Hu, G.W.; Wang, Q.F. Veronicastrum Wulingense (Plantaginaceae), a New Species from Southwestern Hubei, China. Bot. Stud. 2023, 64, 3. [Google Scholar] [CrossRef]
- Albach, D.C.; Meudt, H.M.; Oxelman, B. Piecing together the “new” Plantaginaceae. Am. J. Bot. 2005, 92, 297–315. [Google Scholar] [CrossRef]
- Hawke, R.G. Comparative studies of Veronica and Veronicastrum. Plant Eval. Notes 2010, 33, 1–8. Available online: https://citeseerx.ist.psu.edu/document?repid=rep1&type=pdf&doi=3473917bbc02e2e9afd4964f54dd5ebddf38ef97 (accessed on 21 September 2023).
- Gao, W.; Zhang, R.; Jia, W.; Zhang, J.; Takaishi, Y.; Duan, H. Immunosuppressive diterpenes from Veronicastrum sibiricum. Chem. Pharm. Bull. 2004, 52, 136–137. [Google Scholar] [CrossRef][Green Version]
- Kim, M.I.; Kim, C.Y. Four new acylated iridoid glycosides from the aerial part of Veronicastrum sibiricum and their antioxidant response element-inducing activity. Chem. Biodivers. 2018, 15, e1700447. [Google Scholar] [CrossRef]
- Wang, Z.; Liang, L.; Jie, M.; Wei, G. Anti-inflammation, analgesic and hemostatic effects of Veronicastrum sibiricum. Herald Med. 2017, 12, 489–492. Available online: https://pesquisa.bvsalud.org/portal/resource/pt/wpr-512344 (accessed on 21 September 2023).
- Yin, L.; Han, H.; Zheng, X.; Wang, G.; Li, Y.; Wang, W. Flavonoids analysis and antioxidant, antimicrobial, and anti-inflammatory activities of crude and purified extracts from Veronicastrum latifolium. Ind. Crops Prod. 2019, 137, 652–661. [Google Scholar] [CrossRef]
- Peng, C. Chinese Genuine Medicinal Materials; Chinese Press of Traditional Chinese Medicine: Beijing, China, 2011; pp. 4187–4188. [Google Scholar]
- Peng, F.; Xiong, L.; Zhao, X.M. A bicyclic diterpenoid with a new 15,16-dinorlabdane carbon skeleton from Leonurus japonicus and its coagulant bioactivity. Molecules 2013, 18, 13904–13909. [Google Scholar] [CrossRef] [PubMed]
- Xiong, L.; Peng, C.; Zhou, Q.M.; Wan, F.; Xie, X.F.; Guo, L.; Li, X.H.; He, C.J.; Dai, O. Chemical composition and antibacterial activity of essential oils from different parts of Leonurus japonicus Houtt. Molecules 2013, 18, 963–973. [Google Scholar] [CrossRef] [PubMed]
- Xiong, L.; Zhou, Q.M.; Zou, Y.K.; Chen, M.H.; Guo, L.; Hu, G.Y.; Liu, Z.H.; Peng, C. Leonuketal, a spiroketal diterpenoid from Leonurus japonicus. Org. Lett. 2015, 17, 6238–6241. [Google Scholar] [CrossRef]
- Yang, H.; Zhou, Q.M.; Peng, C.; Liu, L.S.; Xie, X.F.; Xiong, L.; Liu, Z.H. Coumarins from Leonurus japonicus and their anti-platelet aggregative activity. China J. Chin. Mater. Med. 2014, 39, 4356–4359. [Google Scholar]
- Zhou, Q.M.; Peng, C.; Yang, H.; Liu, L.S.; Yang, Y.T.; Xie, X.F.; Guo, L.; Liu, Z.H.; Xiong, L. Steroids from the aerial parts of Leonurus japonicus. Phytochem. Lett. 2015, 12, 287–290. [Google Scholar] [CrossRef]
- Peng, Y.; Zheng, C.; Wang, Y.N.; Dai, O. Novel labdane diterpenoids from the aerial parts of Leonurus japonicus. Phytochem. Lett. 2017, 20, 45–48. [Google Scholar] [CrossRef]
- Xiong, L.; Zhou, Q.M.; Peng, C.; Xie, X.F.; Liu, L.S.; Guo, L.; He, Y.C.; Yang, L.; Liu, Z.H. Bis-spirolabdane diterpenoids from Leonurus japonicus and their anti-platelet aggregative activity. Fitoterapia 2015, 100, 1–6. [Google Scholar] [CrossRef] [PubMed]
- He, Y.L.; Shi, J.Y.; Peng, C.; Hu, L.J.; Liu, J.; Zhou, Q.M.; Guo, L.; Xiong, L. Angiogenic effect of motherwort (Leonurus japonicus) alkaloids and toxicity of motherwort essential oil on zebrafish embryos. Fitoterapia 2018, 128, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Peng, C.; Zhou, Q.M.; Guo, L.; Liu, Z.H.; Xiong, L. Alkaloids and flavonoid glycosides from the aerial parts of Leonurus japonicus and their opposite effects on uterine smooth muscle. Phytochemistry 2018, 145, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Bertazzini, M.; Forlani, G. Intraspecific variability of floral nectar volume and composition in rapeseed (Brassica napus L. var. oleifera). Front. Plant Sci. 2016, 7, 288. [Google Scholar] [CrossRef] [PubMed]
- Pierre, J.; Mesquida, J.; Marilleau, R.; Pham-Delègue, M.H.; Renard, M. Nectar secretion in winter oilseed rape, Brassica napus—Quantitative and qualitative variability among 71 genotypes. Plant Breed. 1999, 118, 471–476. [Google Scholar] [CrossRef]
- Mohr, N.A.; Jay, S.C. Nectar production of selected cultivars of Brassica campestris L. and Brassica napus L. J. Apic. Res. 1990, 29, 95–100. [Google Scholar] [CrossRef]
- Jakobsen, H.B.; Kristjansson, K. Influence of temperature and floret age on nectar secretion in Trifolium repens L. Ann. Bot. 1994, 74, 327–334. [Google Scholar] [CrossRef]
- Búrquez, A.; Corbet, S.A. Dynamics of production and exploitation of nectar: Lessons from Impatiens glandulifera Royle. In Nectary Biology; Dattsons: Nagpur, India, 1998; pp. 130–152. [Google Scholar]
- Li, Y.; Chen, Z.; Feng, Z.; Yang, Y.; Jiang, J.; Zhang, P. Hepatoprotective glycosides from Leonurus japonicus Houtt. Carbohydr. Res. 2012, 348, 42–46. [Google Scholar] [CrossRef]
- Wolff, D. Nectar sugar composition and volumes of 47 species of Gentianales from a southern Ecuadorian montane forest. Ann. Bot. 2006, 97, 767–777. [Google Scholar] [CrossRef]
- Petanidou, T. Sugars in Mediterranean floral nectars: An ecological and evolutionary approach. J. Chem. Ecol. 2005, 31, 1065–1088. [Google Scholar] [CrossRef]
- Chalcoff, V.R.; Aizen, M.A.; Galetto, L. Nectar concentration and composition of 26 species from the temperate forest of South America. Ann. Bot. 2006, 97, 413–421. [Google Scholar] [CrossRef]
- Abrol, D.P.; Shankar, U. Pollination in oil crops: Recent advances and future strategies. In Technological Innovations in Major World Oil Crops; Springer: New York, NY, USA, 2012; Volume 2, pp. 221–267. [Google Scholar] [CrossRef]
- Nepi, M. Nectary structure and ultrastructure. In Nectaries and Nectar; Nicolson, S.W., Nepi, M., Pacini, E., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 129–166. [Google Scholar]
- Kevan, P.G.; Lee, H.; Shuel, R.W. Sugar ratios in nectars of varieties of canola (Brassica napus). J. Apic. Res. 1991, 30, 99–102. [Google Scholar] [CrossRef]
- Westcott, L.; Nelson, D. Canola pollination: An update. Bee World 2001, 82, 115–129. [Google Scholar] [CrossRef]
- Ruhlmann, J.M.; Kram, B.W.; Carter, C.J. CELL WALL INVERTASE 4 is required for nectar production in Arabidopsis. J. Exp. Bot. 2010, 61, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Lohaus, G.; Schwerdtfeger, M. Comparison of sugars, iridoid glycosides and amino acids in nectar and phloem sap of Maurandya barclayana, Lophospermum erubescens, and Brassica napus. PLoS ONE 2014, 9, e87689. [Google Scholar] [CrossRef] [PubMed]
- Heil, M. Nectar: Generation, regulation and ecological functions. Trends Plant Sci. 2011, 16, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Afik, O.; Dag, A.; Kerem, Z.; Shafir, S. Analyses of avocado (Persea americana) nectar properties and their perception by honey bees (Apis mellifera). J. Chem. Ecol. 2006, 32, 1949–1963. [Google Scholar] [CrossRef]
- Schmidt, K.; Orosz-Kovács, Z.S.; Farkas, Á. The effect of blossom structure and nectar composition of some raspberry and blackberry cultivars on the behaviour of pollinators. J. Plant Reprod. Biol. 2008, 1, 1–6. Available online: https://scholar.google.com/scholar_lookup?title=The+effect+of+blossom+structure+and+nectar+composition+of+some+raspberry+and+blackberry+cultivars+on+the+behaviour+of+pollinators&author=Schmidt,+K.&author=Orosz-Kov%C3%A1cs,+Z.S.&author=Farkas,+%C3%81.&publication_year=2008&journal=J.+Plant+Reprod.+Biol.&volume=1&pages=1%E2%80%936 (accessed on 21 September 2023).
- Wykes, G.R. The preferences of honey bees for solutions of various sugars which occur in nectar. J. Exp. Biol. 1952, 29, 511–519. [Google Scholar] [CrossRef]
- Baker, H.G.; Baker, I. Floral nectar sugars constituents in relation to pollinator type. In Handbook of Experimental Pollination Biology; Jones, C.E., Little, R.J., Eds.; Van Nostrand Reinhold Co.: New York, NY, USA, 1983; pp. 117–141. [Google Scholar]
- Elisens, W.J.; Freeman, C.E. Floral nectar sugar composition and pollinator type among new world genera in tribe Antirrhineae (Scrophulariaceae). Am. J. Bot. 1988, 75, 971–978. [Google Scholar] [CrossRef]
- van Wyk, B.E. Nectar sugar composition in southern African Papilionoideae (Fabaceae). Biochem. Syst. Ecol. 1993, 21, 271–277. [Google Scholar] [CrossRef]
- Baker, H.G.; Baker, I.; Hodges, S.A. Sugar composition of nectars and fruits consumed by birds and bats in the tropics deciduous forests. Biotropica 1998, 30, 559–586. [Google Scholar] [CrossRef]
- Galetto, L.; Bernardello, G. Sugar nectar composition in angiosperms from Chaco and Patagonia (Argentina): An animal visitor’s matter? Plant Syst. Evol. 2003, 238, 69–86. [Google Scholar] [CrossRef]
- Nicolson, S.W.; Fleming, P.A. Nectar as food for birds: The physiological consequences of drinking dilute sugar solutions. Plant Syst. Evol. 2003, 238, 139–153. [Google Scholar] [CrossRef]
- Dupont, Y.L.; Hansen, D.M.; Rasmussen, J.T.; Olesen, J.M. Evolutionary changes in nectar sugar composition associated with switches between bird and insect pollination: The Canarian bird-flower element revisited. Funct. Ecol. 2004, 18, 670–676. [Google Scholar] [CrossRef]
- van Wyk, B.E.; Whitehead, C.S.; Glen, H.F.; Hardy, D.S.; van Jaarsveld, E.J.; Smith, G.F. Nectar sugar composition in the subfamily Alooideae (Asphodelaceae). Biochem. Syst. Ecol. 1993, 21, 249–253. [Google Scholar] [CrossRef]
- Galetto, L.; Bernardello, G.; Sosa, C.A. The relationship between floral nectar composition and visitors in Lycium (Solanaceae) from Argentina and Chile: What does it reflect? Flora 1998, 193, 303–314. [Google Scholar] [CrossRef]
- Kingston, A.B.; Mc Quillan, P.B. Are pollination syndromes useful predictors of floral visitors in Tasmania? Austral Ecol. 2000, 25, 600–609. [Google Scholar] [CrossRef]
- Perret, M.; Chautems, A.; Spichiger, R.; Peixoto, M.; Savolainen, V. Nectar sugar composition in relation to pollination syndromes in Sinningieae (Gesneriaceae). Ann. Bot. 2001, 87, 267–273. [Google Scholar] [CrossRef]
- Wolff, D.; Witt, T.; Jürgens, A.; Gottsberger, G. Nectar dynamics and reproductive success in Saponaria officinalis (Caryophyllaceae) in southern Germany. Flora 2006, 201, 353–364. [Google Scholar] [CrossRef]
- Cavalcante, M.C.; Galetto, L.; Maués, M.M.; Pacheco Filho, A.J.S.; Bomfim, I.G.A.; Freitas, B.M. Nectar production dynamics and daily pattern of pollinator visits in Brazil nut (Bertholletia excelsa Bonpl.) plantations in Central Amazon: Implications for fruit production. Apidologie 2018, 49, 505–516. [Google Scholar] [CrossRef]
- Broyles, S.B.; Stoj, K.R. Patterns of nectar production in Asclepias curassavica (Apocynaceae). J. Poll. Ecol. 2019, 25, 78–88. [Google Scholar] [CrossRef]
- Irwin, R.E.; Adler, L.S. Nectar secondary compounds affect self-pollen transfer: Implications for female and male reproduction. Ecology 2008, 89, 2207–2217. [Google Scholar] [CrossRef] [PubMed]
- Kessler, D.; Bhattacharya, S.; Diezel, C.; Rothe, E.; Gase, K.; Schöttner, M.; Baldwin, I.T. Unpredictability of nectar nicotine promotes outcrossing by hummingbirds in Nicotiana attenuata. Plant J. 2012, 71, 529–538. [Google Scholar] [CrossRef]
- Leonhardt, S.D.; Blüthgen, N. The same, but different: Pollen foraging in honeybee and bumblebee colonies. Apidologie 2012, 43, 449–464. [Google Scholar] [CrossRef]
- Stephenson, A.G. Iridoid glycosides in the nectar of Catalpa speciosa are unpalatable to nectar thieves. J. Chem. Ecol. 1982, 8, 1025–1034. [Google Scholar] [CrossRef]
- Adler, L.S. The ecological significance of toxic nectar. Oikos 2000, 91, 409–420. [Google Scholar] [CrossRef]
- Pacini, E.; Nepi, M. Nectar production and presentation. In Nectaries and Nectar; Springer: Dordrecht, The Netherlands, 2007; pp. 167–214. [Google Scholar]
- Gottsberger, G.; Schrauwen, J.; Linskens, H.F. Amino acids and sugars in nectar, and their putative evolutionary significance. Plant Syst. Evol. 1984, 145, 55–77. [Google Scholar] [CrossRef]
- Gardener, M.C.; Gillman, M.P. Analyzing variability in nectar amino acids: Composition is less variable than concentration. J. Chem. Ecol. 2001, 27, 2545–2558. [Google Scholar] [CrossRef]
- Mevi-Schütz, J.; Erhardt, A. Amino acids in nectar enhance butterfly fecundity: A long-awaited link. Am. Nat. 2005, 165, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Chalisova, N.I.; Kamyshev, N.G.; Lopatina, N.G.; Kontsevaya, E.A.; Urtieva, S.A.; Urtieva, T.A. Effect of encoded amino acids on associative learning of honey bee: Apis mellifera. J. Evol. Biochem. Physiol. 2011, 47, 607–610. [Google Scholar] [CrossRef]
- de Groot, A.P. Protein and amino acid requirements of the honey bee (Apis mellifera L.). Physiol. Comp. Oecol. 1953, 3, 197–285. Available online: https://lccn.loc.gov/54030108 (accessed on 5 October 2023).
- Somerville, D.C. Nutritional Value of Bee Collected Pollens; RIRDC Publication No. 01/047; New South Wales Agriculture: Canberra, Australia, 2001.
- McGregor, S.E. Insect Pollination of Cultivated Crop Plants; Agricultural Research Service; U.S. Government Publishing Office: Washington, DC, USA, 1976; 411p.
- Bertazzini, M.; Medrzycki, P.; Bortolotti, L.; Maistrello, L.; Forlani, G. Amino acid content and nectar choice by forager honeybees (Apis mellifera L.). Amino Acids 2010, 39, 315–318. [Google Scholar] [CrossRef] [PubMed]
- Hendriksma, H.P.; Oxman, K.L.; Shafir, S. Amino acid and carbohydrate tradeoffs by honey bee nectar foragers and their implications for plant–pollinator interactions. J. Insect Physiol. 2014, 69, 56–64. [Google Scholar] [CrossRef]
- Biancucci, M.; Mattioli, R.; Forlani, G.; Funck, D.; Costantino, P.; Trovato, M. Role of proline and GABA in sexual reproduction of angiosperms. Front. Plant Sci. 2015, 6, 680. [Google Scholar] [CrossRef]
- Hansen, K.; Wacht, S.; Seebauer, H.; Schnuch, M. New aspects of chemoreception in flies. Ann. N. Y. Acad. Sci. 1998, 855, 143–147. [Google Scholar] [CrossRef]
- Wacht, S.; Lunau, K.; Hansen, K. Chemosensory control of pollen ingestion in the hoverfly Eristalis tenax by labellar taste hairs. J. Comp. Physiol. A 2000, 186, 193–203. [Google Scholar] [CrossRef]
- Micheu, S.; Crailsheim, K.; Leonhard, B. Importance of proline and other amino acids during honeybee flight (Apis mellifera carnica POLLMANN). Amino Acids 2000, 18, 157–175. [Google Scholar] [CrossRef]
- Carter, C.; Shafir, S.; Yehonatan, L.; Palmer, R.G.; Thornburg, R. A novel role for proline in plant floral nectars. Naturwissenschaften 2006, 93, 72–79. [Google Scholar] [CrossRef]
- Alm, J.; Ohnmeiss, T.E.; Lanza, J.; Vriesenga, L. Preference of cabbage white butterflies and honey bees for nectar that contains amino acids. Oecologia 1990, 84, 53–57. [Google Scholar] [CrossRef]
- Verbruggen, N.; Hua, X.J.; May, M.; Van Montagu, M. Environmental and developmental signals modulate proline homeostasis: Evidence for a negative transcriptional regulator. Proc. Natl. Acad. Sci. USA 1996, 93, 8787–8791. [Google Scholar] [CrossRef]
- Kaczorowski, R.L.; Gardener, M.C.; Holtsford, T.P. Nectar traits in Nicotiana section alatae (Solanaceae) in relation to floral traits, pollinators, and mating system. Am. J. Bot. 2005, 92, 1270–1283. [Google Scholar] [CrossRef] [PubMed]
- Nicolson, S.W.; Nepi, M.; Pacini, E. Nectar consumers. In Nectaries and Nectar; Nicolson, S.W., Ed.; Springer: Dordrecht, The Netherlands, 2007; pp. 312–322. [Google Scholar]
- Nepi, M.; von Aderkas, P.; Wagner, R.; Mugnaini, S.; Coulter, A.; Pacini, E. Nectar and pollination drops: How different are they? Ann. Bot. 2009, 104, 205–219. [Google Scholar] [CrossRef]
- Otvos, L.; Insug, O.; Rogers, M.E.; Consolvo, P.J.; Condie, B.A.; Lovas, S.; Bulet, P.; Blaszczyk-Thurin, M. Interaction between heat shock proteins and antimicrobial peptides. Biochemistry 2000, 39, 14150–14159. [Google Scholar] [CrossRef]
- Rolland, J.L.; Abdelouahab, M.; Dupont, J.; Lefevre, F.; Bachère, E.; Romestand, B. Stylicins, a new family of antimicrobial peptides from the Pacific blue shrimp Litopenaeus stylirostris. Mol. Immunol. 2010, 47, 1269–1277. [Google Scholar] [CrossRef]
- Avitabile, C.; D’Andrea, L.D.; Romanelli, A. Circular dichroism studies on the interactions of antimicrobial peptides with bacterial cells. Sci. Rep. 2014, 4, 4293. [Google Scholar] [CrossRef] [PubMed]
- De Souza Cândido, E.; e Silva Cardoso, M.H.; Sousa, D.A.; Viana, J.C.; de Oliveira-Júnior, N.G.; Miranda, V.; Franco, O.L. The use of versatile plant antimicrobial peptides in agribusiness and human health. Peptides 2014, 55, 65–78. [Google Scholar] [CrossRef] [PubMed]
- Ligoxygakis, P.; Pelte, N.; Hoffmann, J.A.; Reichhart, J.M. Activation of Drosophila Toll during fungal infection by a blood serine protease. Science 2002, 297, 114–116. [Google Scholar] [CrossRef] [PubMed]
- Vannette, R.L.; Mohamed, A.; Johnson, B.R. Forager bees (Apis mellifera) highly express immune and detoxification genes in tissues associated with nectar processing. Sci. Rep. 2015, 5, 16224. [Google Scholar] [CrossRef] [PubMed]
- Hrassnigg, N.; Leonhard, B.; Crailsheim, K. Free amino acids in the haemolymph of honey bee queens (Apis mellifera L.). Amino Acids 2003, 24, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.M.; Sun, Y.Y.; Ye, X.Y.; Li, Z.G. Signaling role of glutamate in plants. Front. Plant Sci. 2020, 10, 1743. [Google Scholar] [CrossRef] [PubMed]
- Lanza, J.; Smith, G.C.; Sack, S.; Cash, A. Variation in nectar volume and composition of Impatiens capensis at the individual, plant, and population levels. Oecologia 1995, 102, 113–119. [Google Scholar] [CrossRef]
- Chapman, R.F. The Insects: Structure and Function; Cambridge University Press: New York, NY, USA, 2012. [Google Scholar]
- Anraku, M.; Shintomo, R.; Taguchi, K.; Kragh-Hansen, U.; Kai, T.; Maruyama, T.; Otagiri, M. Amino acids of importance for the antioxidant activity of human serum albumin as revealed by recombinant mutants and genetic variants. Life Sci. 2015, 134, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; Yin, J.; Ren, W.; Liu, T.; Cui, Z.; Huang, X.; Wu, L.; Kim, S.W.; Liu, G.; Wu, X.; et al. Dietary supplementation with L-glutamate and L-aspartate alleviates oxidative stress in weaned piglets challenged with hydrogen peroxide. Amino Acids 2016, 48, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Chatt, E.C.; Von Aderkas, P.; Carter, C.J.; Smith, D.; Elliott, M.; Nikolau, B.J. Sex-dependent variation of pumpkin (Cucurbita maxima cv. Big Max) nectar and nectaries as determined by proteomics and metabolomics. Front. Plant Sci. 2018, 9, 860. [Google Scholar] [CrossRef]
- Inouye, D.W.; Waller, G.D. Responses of honeybees (Apis mellifera) to amino acid solutions mimicking floral nectars. Ecology 1984, 65, 618–625. [Google Scholar] [CrossRef]
- Gardener, M.C.; Gillman, M.P. The effects of soil fertilizer on amino acids in the floral nectar of corncockle, Agrostemma githago (Caryophyllaceae). Oikos 2021, 92, 101–106. [Google Scholar] [CrossRef]
- Nepi, M.; Soligo, C.; Nocentini, D.; Abate, M.; Guarnieri, M.; Cai, G.; Bini, L.; Puglia, M.; Bianchi, L.; Pacini, E. Amino acids and protein profile in floral nectar: Much more than a simple reward. Flora 2012, 207, 475–481. [Google Scholar] [CrossRef]
- Nocentini, D.; Pacini, E.; Guarnieri, M.; Nepi, M. Flower morphology, nectar traits and pollinators of Cerinthe major (Boraginaceae-Lithospermeae). Flora 2012, 207, 186–196. [Google Scholar] [CrossRef]
- Baker, H.G. Non-sugar chemical constituents of nectar. Apidologie 1977, 8, 349–356. Available online: https://www.apidologie.org/articles/apido/pdf/1977/04/Apidologie_0044-8435_1977_8_4_ART0005.pdf (accessed on 27 January 2024). [CrossRef]
- Kim, K.S.; Jang, Y.S.; Lee, Y.H.; Kim, C.W.; Choi, K.H.; Kang, D.S.; Kim, S.T.; Choi, I.H. A rapeseed intermediate parent ‘Jungmo 7001’ with wide adaptable and large flower. Korean J. Breed. Sci. 2014, 46, 302–306. [Google Scholar] [CrossRef]
- Kim, K.S.; Jang, Y.S.; Lee, Y.H.; Choi, K.H.; Shin, J.H.; Lee, K.B. A new rapeseed variety ‘Jungmo 7003’ with white flower, early flowering and disease resistance. Korean J. Breed. Sci. 2016, 48, 339–343. [Google Scholar] [CrossRef]
- Swanson, C.A.; Shuel, R.W. The centrifuge method for measuring nectar yield. Plant Physiol. 1950, 25, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, D.P.; Paton, D.C. Methods for measuring amounts of energy available from banksia inflorescences. Austral Ecol. 1990, 15, 291–297. [Google Scholar] [CrossRef]
- Petanidou, T. Introducing plants for bee-keeping at any cost?—Assessment of Phacelia tanacetifolia as nectar source plant under xeric Mediterranean conditions. Plant Syst. Evol. 2023, 238, 155–168. [Google Scholar] [CrossRef]





| Cultivars | Flowering Period | Height (cm) | No. of Flowers (ea/plant) | Plant Density (m2/plant) | |
|---|---|---|---|---|---|
| Mean | Range | ||||
| ‘JM7003’ | 5/25–6/13 | 73.1 ± 13.7 a | 65.3 ± 8.7 a | 49–77 | 148.8 ± 39.9 n.s. |
| ‘YS’ | 5/27–6/13 | 53.4 ± 7.9 d | 31.8 ± 7.2 c | 21–42 | 161.0 ± 31.2 |
| ‘TM’ | 5/30–6/16 | 54.7 ± 10.3 cd | 27.2 ± 5.3 c | 16–34 | 142.0 ± 27.4 |
| ‘TL’ | 5/30–6/16 | 60.7 ± 9.4 bc | 33.2 ± 10.2 c | 23–49 | 145.3 ± 9.2 |
| ‘JM7001’ | 5/30–6/20 | 65.3 ± 11.0 b | 43.7 ± 5.9 b | 36–56 | 158.7 ± 20.1 |
| p-value | - | <0.0001 | <0.0001 | - | 0.8829 |
| Cultivars | Nectar Volume (μL/flower) | Free Sugar Content (μg/μL) | |||
|---|---|---|---|---|---|
| Sucrose | Glucose | Fructose | Total | ||
| ‘JM7003’ | 1.54 ± 0.33 a | 10.1 ± 2.3 c | 307.5 ± 58.9 c | 325.3 ± 60.7 c | 641.0 ± 118.7 c |
| ‘YS’ | 1.08 ± 0.21 b | 19.3 ± 6.6 ab | 564.3 ± 122.0 ab | 563.2 ± 122.0 ab | 1146.8 ± 248.6 ab |
| ‘TM’ | 0.73 ± 0.18 b | 25.0 ± 5.0 a | 656.8 ± 200.6 a | 667.7 ± 181.0 a | 1349.4 ± 381.3 a |
| ‘TL’ | 0.81 ± 0.23 b | 13.5 ± 2.3 bc | 575.2 ± 132.2 ab | 585.7 ± 135.5 a | 1174.4 ± 269.8 ab |
| ‘JM7001’ | 1.04 ± 0.18 b | 12.3 ± 4.2 c | 388.8 ± 133.1 bc | 384.1 ± 132.2 bc | 785.25 ± 269.4 bc |
| p-value | 0.0005 | 0.0004 | 0.0062 | 0.0046 | 0.0048 |
| Honey Potential | ‘JM7003’ | ‘YS’ | ‘TM’ | ‘TL’ | ‘JM7001’ |
|---|---|---|---|---|---|
| Nectar sugar content 1 | 0.96 ± 0.02 | 1.24 ± 0.32 | 0.96 ± 0.24 | 0.95 ± 0.31 | 0.80 ± 0.20 |
| Honey production per plant 2 | 72.0 (54–85) | 45.3 (30–60) | 29.9 (18–37) | 36.3 (25–54) | 40.2 (33–52) |
| Honey yield per hectare 3 | 107.1 (81–141) | 73.0 (54–89) | 42.4 (36–54) | 52.7 (51–57) | 63.7 (56–72) |
| Characteristic | P. rotundum var. subintegrum | L. japonicus |
|---|---|---|
| Flowering period | 19 July–30 August | 2 August–2 September |
| Plant height (cm) | 83.0 ± 13.6 | 124.8 ± 27.8 |
| Number of flowers (ea/plant) | 3422 ± 370 (2064–5490) | 2894 ± 318 (1320–4490) |
| Planting density (m2/plant) | 17.5; 30 × 25 cm | 17.5; 30 × 25 cm |
| Nectar Characteristics | Flowering Time | t-Test | |
|---|---|---|---|
| First Day | Second Day | ||
| P. rotundum var. subintegrum | |||
| Nectar volume (μL/flower) | 0.07 ± 0.01 | 0.30 ± 0.09 | p = 0.0114 |
| Free sugar content (μg/μL) | 828.7 ± 266.2 | 767.9 ± 206.8 | n.s. |
| - Sucrose | 407.9 ± 116.5 | 310.2 ± 103.0 | n.s. |
| - Glucose | 197.7 ± 86.0 | 211.2 ± 45.8 | n.s. |
| - Fructose | 232.2 ± 93.8 | 246.5 ± 59.9 | n.s. |
| Nectar sugar content (mg/flower) * | 0.06 ± 0.03 | 0.22 ± 0.02 | p = 0.0015 |
| L. japonicus | |||
| Nectar volume (μL/flower) | 0.16 ± 0.05 | 0.33 ± 0.01 | p = 0.0057 |
| Free sugar content (μg/μL) | 775.4 ± 88.6 | 816.3 ± 139.4 | n.s. |
| - Sucrose | 624.7 ± 73.8 | 631.1 ± 95.1 | n.s. |
| - Glucose | 71.8 ± 8.5 | 88.9 ± 21.3 | n.s. |
| - Fructose | 78.9 ± 7.8 | 96.3 ± 23.5 | n.s. |
| Nectar sugar content (mg/flower) * | 0.12 ± 0.04 | 0.26 ± 0.03 | p = 0.0106 |
| Honey Potential | P. rotundum var. subintegrum | L. japonicus |
|---|---|---|
| Honey production per plant 1 | 870.6 (525–1397) | 864.6 (394–1341) |
| Honey yield per hectare 2 | 152.4 (92–244) | 151.3 (69–235) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Na, S.-J.; Kim, Y.-K.; Park, J.-M. Nectar Characteristics and Honey Production Potential of Five Rapeseed Cultivars and Two Wildflower Species in South Korea. Plants 2024, 13, 419. https://doi.org/10.3390/plants13030419
Na S-J, Kim Y-K, Park J-M. Nectar Characteristics and Honey Production Potential of Five Rapeseed Cultivars and Two Wildflower Species in South Korea. Plants. 2024; 13(3):419. https://doi.org/10.3390/plants13030419
Chicago/Turabian StyleNa, Sung-Joon, Young-Ki Kim, and Ji-Min Park. 2024. "Nectar Characteristics and Honey Production Potential of Five Rapeseed Cultivars and Two Wildflower Species in South Korea" Plants 13, no. 3: 419. https://doi.org/10.3390/plants13030419
APA StyleNa, S.-J., Kim, Y.-K., & Park, J.-M. (2024). Nectar Characteristics and Honey Production Potential of Five Rapeseed Cultivars and Two Wildflower Species in South Korea. Plants, 13(3), 419. https://doi.org/10.3390/plants13030419






