The Biosynthesis, Accumulation of Phenolic Compounds and Antioxidant Response in Lactuca sativa L. Plants Inoculated with a Biofertilizer Based on Soil Yeast and Iron Nanoparticles
Abstract
1. Introduction
2. Results
2.1. Determination and Quantification of Phenolic Compounds via HPLC-ESI-QToF in Lettuce Leaves
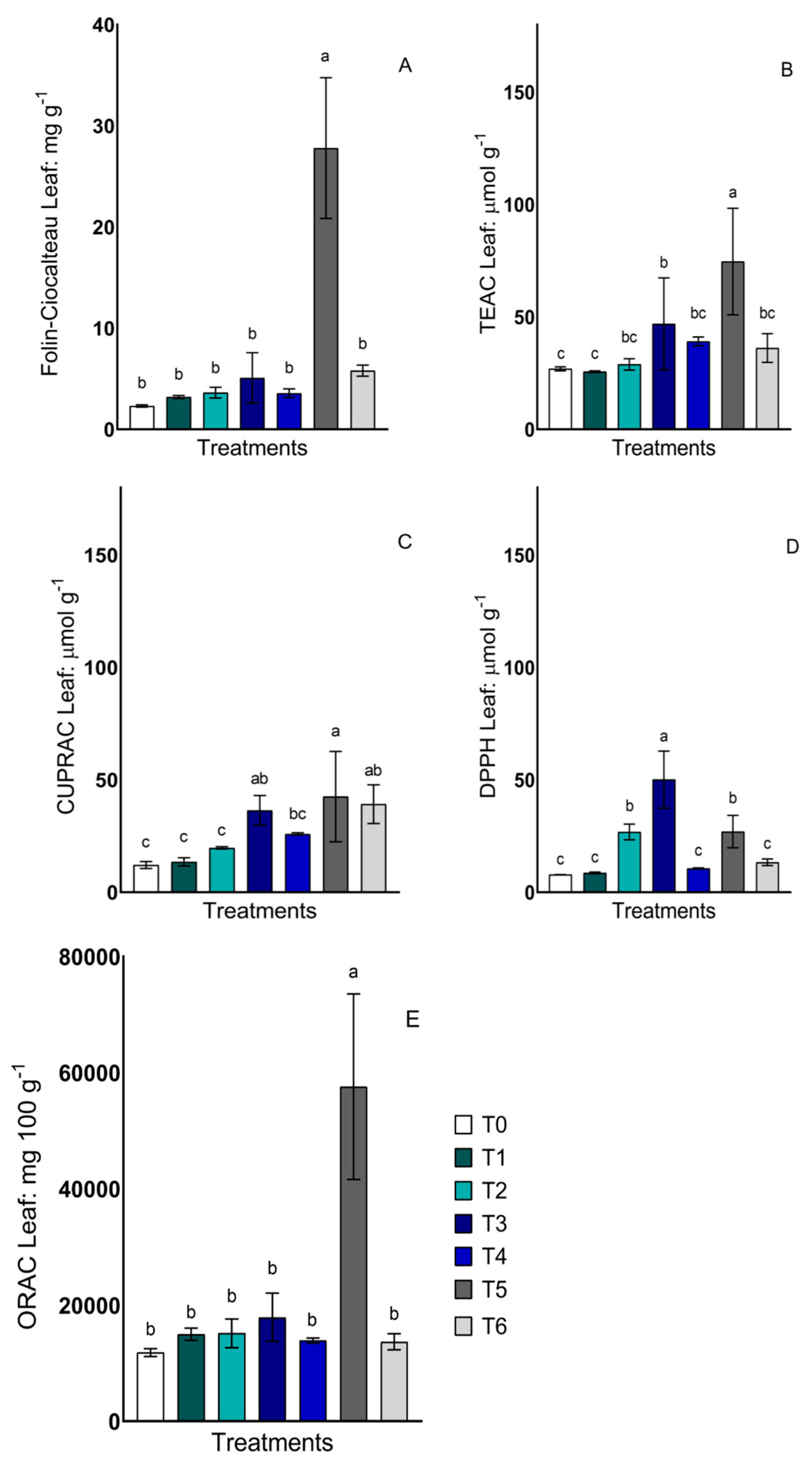
2.2. Total Phenols and Antioxidant Activity in Leaves
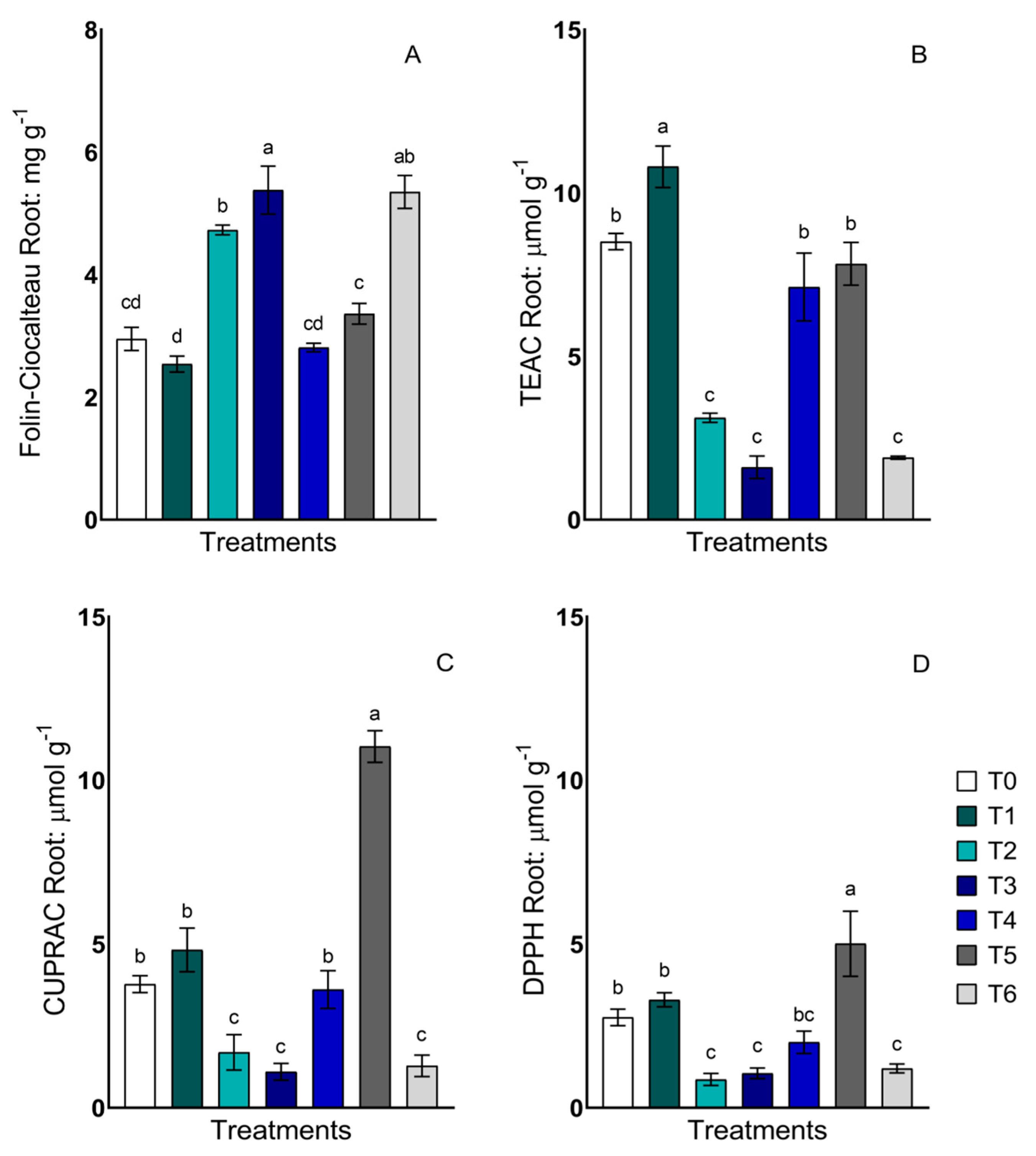
2.3. Total Phenols and Antioxidant Activity in Roots
2.4. Multivariate Analysis
3. Discussion
4. Materials and Methods
4.1. Experimental Design and Growing Conditions
4.2. Identification and Quantification of Phenolic Compounds and Hydroxycinnamic Acids (HCAD) in Leaves by Using HPLC-ESI-QToF
4.3. Total Phenols and Antioxidant Activity in Leaves and Roots
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Food and Agriculture Organization of the United Nations. FAOSTAT-Vegetables Production. 2020. Available online: https://www.fao.org/documents/card/en/c/cc3751en (accessed on 31 August 2023).
- Liu, C.; Chang, C.; Fei, Y.; Li, F.; Wang, Q.; Zhai, G.; Lei, J. Cadmium accumulation in edible flowering cabbages in the Pearl River Delta, China: Critical soil factors and enrichment models. Environ. Pollut. 2018, 233, 880–888. [Google Scholar] [CrossRef]
- Guo, X.; Luo, J.; Zhang, R.; Gao, H.; Peng, L.; Liang, Y.; Li, T. Root cell wall remodeling mediates copper oxide nanoparticles phytotoxicity on lettuce (Lactuca sativa L.). Environ. Exp. Bot. 2022, 200, 104906. [Google Scholar] [CrossRef]
- Abd Rahim, M.; Hazrin-Chong, N.; Hazeera Harith, H.; Wan-Mohtar, W.; Sukor, R. Roles of fermented plant-, dairy- and meat-based foods in the modulation of allergic responses. Food Sci. Hum. Wellness 2023, 12, 691–701. [Google Scholar] [CrossRef]
- Kim, M.J.; Moon, Y.; Tou, J.C.; Mou, B.; Waterland, N.L. Nutritional value, bioactive compounds and health benefits of lettuce (Lactuca sativa L.). J. Food Compos. Anal. 2016, 49, 19–34. [Google Scholar] [CrossRef]
- Al-Karaki, G.; Altuntas, O. Growth, mineral content and antioxidant activity of romaine lettuce in relation to development stage in soilless system. Int. J. Agric. Sci. Technol. 2021, 1, 27–31. [Google Scholar] [CrossRef]
- Al-Karaki, G.N.; Othman, Y. Effect of foliar application of amino acid biostimulants on growth, macronutrient, total phenol contents and antioxidant activity of soilless grown lettuce cultivars. S. Afr. J. Bot. 2023, 154, 225–231. [Google Scholar] [CrossRef]
- Statistics, Food and Agriculture Organization of the United Nations. FAOSTAT-Lettuce Production. 2022. Available online: http://www.fao.org/faostat/es/#data/QC (accessed on 2 September 2023).
- Yaseen, A.; Takacs-Hajos, M. The effect of plant biostimulants on the macronutrient content and ion ratio of several lettuce (Lactuca sativa L.) cultivars grown in a plastic house. S. Afr. J. Bot. 2022, 147, 223–230. [Google Scholar] [CrossRef]
- Santander, C.; Vidal, G.; Ruiz, A.; Vidal, C.; Cornejo, P. Salinity Eustress Increases the Biosynthesis and Accumulation of Phenolic Compounds That Improve the Functional and Antioxidant Quality of Red Lettuce. Agronomy 2022, 12, 598. [Google Scholar] [CrossRef]
- Yang, X.; Lu, M.; Wang, Y.; Wang, Y.; Liu, Z.; Chen, S. Response Mechanism of Plants to Drought Stress. Horticulturae 2021, 7, 50. [Google Scholar] [CrossRef]
- Sawatdee, S.; Prommuak, C.; Jarunglumlert, T.; Pavasant, P.; Flood, A.E. Combined effects of cations in fertilizer solution on antioxidant content in red lettuce (Lactuca sativa L.). J. Sci. Food Agric. 2021, 101, 4632–4642. [Google Scholar] [CrossRef]
- Materska, M.; Olszówka, K.; Chilczuk, B.; Stochmal, A.; Pecio, T.; Sienicka, B.; Piacente, S.; Pizza, C.; Masullo, M. Polyphenolic profiles in lettuce (Lactuca sativa L.) after CaCl2 treatment and cold storage. Eur. Food Res. Technol. 2019, 245, 733–744. [Google Scholar] [CrossRef]
- Mohd Yusof, F.F.; Yaacob, J.S.; Osman, N.; Ibrahim, M.H.; Wan-Mohtar, W.A.A.Q.I.; Berahim, Z.; Mohd Zain, N.A. Shading. Effects on Leaf Gas Exchange, Leaf Pigments and Secondary Metabolites of Polygonum minus Huds., an Aromatic Medicinal Herb. Plants 2021, 10, 608. [Google Scholar] [CrossRef]
- Bhargava, S.; Sawant, K. Drought stress adaptation: Metabolic adjustment and regulation of gene expression. Plant Breed. 2013, 132, 21–32. [Google Scholar] [CrossRef]
- Govindasamy, V.; George, P.; Kumar, M.; Aher, L.; Raina, S.; Rane, J.; Annapurna, K.; Minhas, P. Multi-trait PGP rhizobacterial endophytes alleviate drought stress in a senescent genotype of sorghum (Sorghum bicolor (L.) Moench). 3 Biotech 2020, 10, 13. [Google Scholar] [CrossRef]
- Jacoby, R.; Peukert, M.; Succurro, A.; Koprivova, A.; Kopriva, S. The Role of Soil Microorganisms in Plant Mineral Nutrition—Current Knowledge and Future Directions. Front. Plant Sci. 2017, 8, 1617. [Google Scholar] [CrossRef] [PubMed]
- Silambarasan, S.; Logeswari, P.; Cornejo, P.; Abraham, J.; Valentine, A. Simultaneous mitigation of aluminum, salinity and drought stress in Lactuca sativa growth via formulated plant growth promoting Rhodotorula mucilaginosa CAM4. Ecotoxicol. Environ. Saf. 2019, 180, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Silambarasan, S.; Logeswari, P.; Cornejo, P.; Kannan, V. Evaluation of the production of exopolysaccharide by plant growth promoting yeast Rhodotorula sp. strain CAH2 under abiotic stress conditions. Int. J. Biol. Macromol. 2019, 121, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Alzandi, A.A.; Naguib, D.M. Effect of yeast application on soil health and root metabolic status of corn seedlings under drought stress. Arch. Microbiol. 2022, 204, 233. [Google Scholar] [CrossRef]
- Santander, C.; Ruiz, A.; García, S.; Aroca, R.; Cumming, J.; Cornejo, P. Efficiency of two arbuscular mycorrhizal fungal inocula to improve saline stress tolerance in lettuce plants by changes of antioxidant defense mechanisms. J. Sci. Food Agric. 2020, 100, 1577–1587. [Google Scholar] [CrossRef]
- Pérez, R.; Tapia, Y.; Antilén, M.; Casanova, M.; Vidal, C.; Silambarasan, S.; Cornejo, P. Rhizosphere Management for Phytoremediation of Copper Mine Tailings. J. Soil Sci. Plant Nutr. 2021, 21, 3091–3109. [Google Scholar] [CrossRef]
- Nasir Khan, M.; Mobin, M.; Abbas, Z.; AlMutairi, K.; Siddiqui, Z. Role of nanomaterials in plants under challenging environments. Plant Physiol. Biochem. 2017, 110, 194–209. [Google Scholar] [CrossRef] [PubMed]
- Feregrino-Perez, A.; Magaña-López, E.; Guzmán, C.; Esquivel, K. A general overview of the benefits and possible negative effects of the nanotechnology in horticulture. Sci. Hortic. 2018, 238, 126–137. [Google Scholar] [CrossRef]
- Rafique, R.; Zahra, Z.; Virk, N.; Shahid, M.; Pinelli, E.; Kallerhoff, J.; Park, T.J.; Arshad, M. Data on rhizosphere pH, phosphorus uptake and wheat growth responses upon TiO2 nanoparticles application. Data Brief 2018, 17, 890–896. [Google Scholar] [CrossRef] [PubMed]
- Fatima, F.; Hashim, A.; Anees, S. Efficacy of nanoparticles as nanofertilizer production: A review. Environ. Sci. Pollut. 2021, 28, 1292–1303. [Google Scholar] [CrossRef] [PubMed]
- Gomez, A.; Narayan, M.; Zhao, L.; Jia, X.; Bernal, R.; Lopez-Moreno, M.; Peralta-Videa, J. Effects of nano-enabled agricultural strategies on food quality: Current knowledge and future research needs. J. Hazard. Mater. 2021, 401, 123385. [Google Scholar] [CrossRef] [PubMed]
- Grillo, R.; Fraceto, L.; Amorim, M.; Scott-Fordsmand, J.; Schoonjans, R.; Chaudhry, Q. Ecotoxicological and regulatory aspects of environmental sustainability of nanopesticides. J. Hazard. Mater. 2021, 404, 124148. [Google Scholar] [CrossRef]
- Fincheira, P.; Hoffmann, N.; Tortella, G.; Ruiz, A.; Cornejo, P.; Diez, M.C.; Seabra, A.B.; Benavides-Mendoza, A.; Rubilar, O. Eco-Efficient Systems Based on Nanocarriers for the Controlled Release of Fertilizers and Pesticides: Toward Smart Agriculture. Nanomaterials 2023, 13, 1978. [Google Scholar] [CrossRef]
- Lavicoli, I.; Leso, V.; Beezhold, D.; Shvedova, A. Nanotechnology in agriculture: Opportunities, toxicological implications, and occupational risks. Toxicol. Appl. Pharmacol. 2017, 329, 96–111. [Google Scholar] [CrossRef]
- Al-Mamun, R.; Hasan, R.; Ahommed, S.; Bacchu, S.; Ali, R.; Hossain Khan, Z. Nanofertilizers towards sustainable agriculture and environment. Environ. Technol. Innov. 2021, 23, 101658. [Google Scholar] [CrossRef]
- Elanchezhian, R.; Kumar, D.; Ramesh, K.; Biswas, A.; Guhey, A.; Kumar Patra, A. Morpho-physiological and biochemical response of maize (Zea mays L.) plants fertilized with nano-iron (Fe3O4) micronutrient. J. Plant Nutr. 2017, 40, 1969–1977. [Google Scholar] [CrossRef]
- Lu, K.; Shen, D.; Liu, X.; Dong, S.; Jing, X.; Wu, W.; Tong, Y.; Gao, S.; Mao, L. Uptake of iron oxide nanoparticles inhibits the photosynthesis of the wheat after foliar exposure. Chemosphere 2020, 259, 127445. [Google Scholar] [CrossRef] [PubMed]
- Pariona, N.; Martinez, A.I.; Hdz-García, H.M.; Cruz, L.A.; Hernandez-Valdes, A. Effects of hematite and ferrihydrite nanoparticles on germination and growth of maize seedlings. Saudi J. Biol. Sci. 2017, 24, 1547–1554. [Google Scholar] [CrossRef] [PubMed]
- Du, W.; Yan, J.; Peng, Q.; Liang, X.; Mao, H. Comparison study of zinc nanoparticles and zinc sulphate on wheat growth: From toxicity and zinc biofortification. Chemosphere 2019, 227, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.; Rafique, S.; Zafar, A.; Loomba, S.; Khan, R.; Hassan, S.G.; Khan, M.W.; Zahra, S.; Zia, M.; Mustafa, G.; et al. Physiological and anti-oxidative response of biologically and chemically synthesized iron oxide: Zea mays a case study. Heliyon 2020, 6, e04595. [Google Scholar] [CrossRef] [PubMed]
- Pallavi, C.M.M.; Srivastava, R.; Arora, S.; Sharma, A.K. Impact assessment of silver nanoparticles on plant growth and soil bacterial diversity. 3 Biotech 2016, 6, 254. [Google Scholar] [CrossRef] [PubMed]
- Nimsi, K.A.; Manjusha, K.; Kathiresan, K.; Arya, H. Plant growth-promoting yeasts (PGPY), the latest entrant for use in sustainable agriculture: A review. J. Appl. Microbiol. 2023, 134, lxac088. [Google Scholar] [CrossRef] [PubMed]
- Nassar, A.H.; El-Tarabily, K.A.; Sivasithamparam, K. Promotion of plant growth by an auxin producing isolate of the yeast Williopsis saturnus endophytic in maize (Zea mays L.) roots. Biol. Fertil. Soils 2005, 42, 97–108. [Google Scholar] [CrossRef]
- Nutaratat, P.; Srisuk, N.; Arunrattiyakorn, P.; Limtong, S. Plant growth-promoting traits of epiphytic and endophytic yeasts isolated from rice and sugar cane leaves in Thailand. Fungal Biol. 2014, 118, 683–694. [Google Scholar] [CrossRef]
- Amprayn, K.O.; Rose, M.T.; Kecskés, M.; Pereg, L.; Nguyen, H.T.; Kennedy, I.R. Plant growth promoting characteristics of soil yeast (Candida tropicalis HY) and its effectiveness for promoting rice growth. Appl. Soil Ecol. 2012, 61, 295–299. [Google Scholar] [CrossRef]
- Hesham, A.E.L.; Mohamed, H. Molecular genetic identification of yeast strains isolated from Egyptian soils for solubilization of inorganic phosphates and growth promotion of corn plants. J. Microbiol. Biotechnol. 2011, 21, 55–61. [Google Scholar] [CrossRef]
- Bright, J.P.; Karunanadham, K.; Maheshwari, H.S.; Karuppiah, E.A.A.; Thankappan, S.; Nataraj, R.; Pandian, D.; Ameen, F.; Poczai, P.; Sayyed, R.Z. Seed-Borne Probiotic Yeasts Foster Plant Growth and Elicit Health Protection in Black Gram (Vigna mungo L.). Sustainability 2022, 14, 4618. [Google Scholar] [CrossRef]
- Lonhienne, T.; Mason, M.G.; Ragan, M.A.; Hugenholtz, P.; Schmidt, S.; Paungfoo-Lonhienne, C. Yeasts as a biofertilizer alters plant growth and morphology. Crop Sci. 2014, 54, 785–790. [Google Scholar] [CrossRef]
- Yang, X.; Wei, S.; Liu, B.; Guo, D.; Zheng, B.; Feng, L.; Liu, Y.; Tomás-Barberán, F.; Luo, L.; Huang, D. A novel integrated non-targeted metabolomic analysis reveals significant metabolite variations between different lettuce (Lactuca sativa. L) varieties. Hortic. Res. 2018, 5, 33. [Google Scholar] [CrossRef] [PubMed]
- Hameed, M.K.; Umar, W.; Razzaq, A.; Wei, S.; Niu, Q.; Huang, D.; Chang, L. Quantification of total polyphenols, antioxidants, anthocyanins and secondary metabolites by UPLC VION IMS QTOF MS/MS analysis in green and red lettuce cultivars. Sci. Hortic. 2023, 315, 111994. [Google Scholar] [CrossRef]
- Llorach, R.; Martínez-Sánchez, A.; Tomás-Barberán, F.; Gil, M.; Ferreres, F. Characterisation of polyphenols and antioxidant properties of five lettuce varieties and escarole. Food Chem. 2008, 108, 1028–1038. [Google Scholar] [CrossRef] [PubMed]
- López, A.; García-Alonso, J.; Fenoll, J.; Hellín, P.; Flores, P. Chemical composition and antioxidant capacity of lettuce: Comparative study of regular-sized (Romaine) and baby-sized (Little Gem and Mini Romaine) types. J. Food Compos. Anal. 2014, 33, 39–48. [Google Scholar] [CrossRef]
- de Souza, A.S.N.; de Oliveira Schmidt, H.; Pagno, C.; Rodrigues, E.; da Silva, M.A.S.; Flôres, S.H.; de Oliveira Rios, A. Influence of cultivar and season on carotenoids and phenolic compounds from red lettuce influence of cultivar and season on lettuce. Food Res. Int. 2022, 155, 11110. [Google Scholar] [CrossRef]
- Fincheira, P.; Espinoza, J.; Vera, J.; Berrios, D.; Nahuelcura, J.; Ruiz, A.; Quiroz, A.; Bustamante, L.; Cornejo, P.; Tortella, G. The Impact of 2-Ketones Released from Solid Lipid Nanoparticles on Growth Modulation and Antioxidant System of Lactuca sativa. Plants 2023, 12, 3094. [Google Scholar] [CrossRef]
- González, F.; Santander, C.; Ruiz, A.; Pérez, R.; Moreira, J.; Vidal, G.; Aroca, R.; Santos, C.; Cornejo, P. Inoculation with Actinobacteria spp. Isolated from a Hyper-Arid Environment Enhances Tolerance to Salinity in Lettuce Plants (Lactuca sativa L.). Plants 2023, 12, 2018. [Google Scholar] [CrossRef]
- Mahmoudi, H.; Huang, J.; Gruber, M.; Kaddour, R.; Lachaâl, M.; Ouerghi, Z.; Hannoufa, A. The Impact of Genotype and Salinity on Physiological Function, Secondary Metabolite Accumulation, and Antioxidative Responses in Lettuce. J. Agric. Food Chem. 2010, 58, 5122–5130. [Google Scholar] [CrossRef]
- Rebelo, M.J.; Rego, R.; Ferreira, M.; Oliveira, M.C. Comparative study of the antioxidant capacity and polyphenol content of Douro wines by chemical and electrochemical methods. Food Chem. 2013, 141, 566–573. [Google Scholar] [CrossRef] [PubMed]
- Cavalcanti, V.; Smail Aazza, S.; Bertolucci, S.; Pereira, M.; Cavalcanti, P.; Buttrós, V.; Oliveira e Silva, A.; Pasqual, M.; Dória, J. Plant, pathogen and biocontrol agent interaction effects on bioactive compounds and antioxidant activity in garlic. Physiol. Mol. Plant Pathol. 2020, 112, 101550. [Google Scholar] [CrossRef]
- Zárate-Martínez, W.; González-Morales, S.; Ramírez-Godina, F.; Robledo-Olivo, A.; Juárez-Maldonado, A. Effect of phenolic acids on the antioxidant system of tomato plants (Solanum lycopersicum Mill.). Agron. Mesoam. 2021, 32, 854–868. [Google Scholar] [CrossRef]
- Avio, L.; Sbrana, C.; Giovannetti, M.; Frassinetti, S. Arbuscular mycorrhizal fungi affect total phenolics content and antioxidant activity in leaves of oak leaf lettuce varieties. Sci. Hortic. 2017, 224, 265–271. [Google Scholar] [CrossRef]
- Khan, Z.S.; Rizwan, M.; Hafeez, M.; Ali, S.; Adrees, M.; Qayyum, M.F.; Khalid, S.; Rehman, M.Z.U.; Sarwar, M.A. Effects of silicon nanoparticles on growth and physiology of wheat in cadmium contaminated soil under different soil moisture levels. Environ. Sci. Pollut. 2020, 27, 4958–4968. [Google Scholar] [CrossRef] [PubMed]
- García-López, J.I.; Niño-Medina, G.; Olivares-Sáenz, E.; Lira-Saldivar, R.H.; Barriga-Castro, E.D.; Vázquez-Alvarado, R.; Rodríguez-Salinas, P.A.; Zavala-García, F. Foliar application of zinc oxide nanoparticles and zinc sulfate boosts the content of bioactive compounds in habanero peppers. Plants 2019, 8, 254. [Google Scholar] [CrossRef] [PubMed]
- García-Saucedo, C.; Field, J.; Otero-Gonzalez, L.; Sierra-Álvarez, R. Low toxicity of HfO2, SiO2, Al2O3 and CeO2 nanoparticles to the yeast, Saccharomyces cerevisiae. J. Hazard. Mater. 2011, 192, 1572–1579. [Google Scholar] [CrossRef]
- Otero-González, L.; García-Saucedo, C.; Field, J.; Sierra-Álvarez, R. Toxicity of TiO2, ZrO2, Fe0, Fe2O3, and Mn2O3 nanoparticles to the yeast, Saccharomyces cerevisiae. Chemosphere 2013, 93, 1201–1206. [Google Scholar] [CrossRef]
- Ameen, F.; Alsamhary, K.; Alabdullatif, J.; ALNadhari, S. A review on metal-based nanoparticles and their toxicity to beneficial soil bacteria and fungi. Ecotoxicol. Environ. Saf. 2021, 213, 112027. [Google Scholar] [CrossRef]
- Pérez, R.; Tapia, Y.; Antilén, M.; Ruiz, A.; Pimentel, P.; Santander, C.; Aponte, H.; González, F.; Cornejo, P. Beneficial Interactive Effects Provided by an Arbuscular Mycorrhizal Fungi and Yeast on the Growth of Oenothera picensis Established on Cu Mine Tailings. Plants 2023, 12, 4012. [Google Scholar] [CrossRef]
- Dasgan, H.Y.; Yilmaz, D.; Zikaria, K.; Ikiz, B.; Gruda, N.S. Enhancing the Yield, Quality and Antioxidant Content of Lettuce through Innovative and Eco-Friendly Biofertilizer Practices in Hydroponics. Horticulturae 2023, 9, 1274. [Google Scholar] [CrossRef]
- Harsela, C.N. Growth and yields of bima brebes shallot variety planted using a floating hydroponics system. Eduvest-J. Univers. Stud. 2023, 3, 1381–1388. [Google Scholar] [CrossRef]
- Kah, M.; Kookana, R.S.; Gogos, A.; Bucheli, T.D. A critical evaluation of nanopesticides and nanofertilizers against their conventional analogues. Nat. Nanotechnol. 2018, 13, 677–684. [Google Scholar] [CrossRef]
- Lopes, M.J.S.; Dias-Filho, M.B.; Gurgel, E.S.C. Successful Plant Growth-Promoting Microbes: Inoculation Methods and Abiotic. Front. Sustain. Food Syst. 2021, 5, 606454. [Google Scholar] [CrossRef]
- Niksad, N.; Parastar, H. Evaluation of the effect of organic pollutants exposure on the antioxidant activity, total phenolic and total flavonoid content of lettuce (Lactuca sativa L.) using UV–Vis spectrophotometry and chemometrics. Microchem. J. 2021, 170, 106632. [Google Scholar] [CrossRef]
- Favre, G.; González-Neves, G.; Piccardo, D.; Gómez-Alonso, S.; José Pérez-Navarro, J.; Hermosín-Gutiérrez, I. New acylated flavonols identified in Vitis vinifera grapes and wines. Food Res. Int. 2018, 112, 98–107. [Google Scholar] [CrossRef]
- Ou, B.; Chang, T.; Huang, D.; Prior, R.L. Determination of Total Antioxidant Capacity by Oxygen Radical Absorbance Capacity (ORAC) Using Fluorescein as the Fluorescence Probe: First Action. J. AOAC Int. 2013, 96, 1372–1376. [Google Scholar] [CrossRef]


| Peak | Rt (min) | Identification | Λmax (nm) | [M − H]− | Productions |
|---|---|---|---|---|---|
| 1 | 26.1 | Caftaric acid | 330 | 311 | 135.0, 149.0, 179.0, 112.0 |
| 2 | 31.5 | 5-Caffeoylquinic acid | 325 | 295 | 191.0, 163.0 |
| 3 | 35.3 | Caffeic acid derivate | 330 | 135 | 135.0, 112.0 |
| 4 | 36.8 | Coumaroylquinic acid derivative | 325 | 337 | - |
| 5 | 38.4 | Caffeic acid derivate | 330 | 591 | 133.0, 179.0, 295.0 |
| 6 | 39.5 | Chlorogenic acid derivative | 332 | 337 | 163.0, 191.0, 206.0 |
| 7 | 40.4 | Chicoric acid isomer | 334 | 473 | 135.0, 149.0, 179.0, 293.0, 311.0 |
| 8 | 43.3 | No identified | - | - | - |
| 9 | 46.1 | Quercetin 3-glucuronide | 359 | 477 | 301.0 |
| 10 | 47.2 | Quercetin acetyl hexoside derivative | 360 | 505 | 301.0 |
| Method | Standard | Equation | R2 | DL | QL | LR | CV % | |
|---|---|---|---|---|---|---|---|---|
| Folin | Gallic acid | Y = 0.0007 x + 0.0332 | 0.9964 | 3.747 µM | 12.491 µM | 12.491–500 µM | 87.65 | |
| TEAC | Trolox | Y = 0.4157 x + 0.0183 | 0.9980 | 0.008 µM | 0.027 µM | 0.027–0.7 µM | 48.67 | |
| Leaves | CUPRAC | Trolox | Y = 3.2264 x + 0.1414 | 0.9975 | 0.031 µM | 0.105 µM | 0.105–0.7 µM | 98.30 |
| DPPH | Trolox | Y = 0.5678 x − 0.0034 | 0.9905 | 0.023 µM | 0.076 µM | 0.076–0.7 µM | 81.24 | |
| ORAC | Trolox | Y = 0.4155 x + 6.2634 | 0.9922 | 8.42 µM | 28.06 µM | 28–80 µM | 87.07 | |
| HPLC-ESI-QToF | Caftaric acid | Y = 1394.1 x − 245.61 | 0.9995 | 0.57 mg L−1 | 1.91 mg L−1 | 1.9–20 mg L−1 | 61.18 | |
| HPLC-ESI-QToF | Chlorogenic acid | Y = 1738.2 x − 438.63 | 0.9991 | 0.75 mg L−1 | 2.53 mg L−1 | 2.5–20 mg L−1 | 60.59 | |
| HPLC-ESI-QToF | Quercetin-3-glucoronide | Y = 1104.5 x −148.01 | 0.9981 | 0.76 mg L−1 | 2.52 mg L−1 | 2.5–20 mg L−1 | 70.71 | |
| Folin | Trolox | Y = 0.0008 x + 0.0158 | 0.9963 | 7.349 µM | 24.49 µM | 24.49–500 µM | 31.40 | |
| TEAC | Trolox | Y = 0.4149 x + 0.0371 | 0.9933 | 0.020 µM | 0.067 µM | 0.067–0.7 µM | 60.21 | |
| Roots | CUPRAC | Trolox | Y = 2.6455 x + 0.1776 | 0.9892 | 0.014 µM | 0.048 µM | 0.048–0.7 µM | 85.74 |
| DPPH | Trolox | Y = 0.5761 x + 0.0138 | 0.9972 | 0.016 µM | 0.055 µM | 0.055–0.7 µM | 67.13 |
| Treatments | Peak 1 | Peak 2 | Peak 3 | Peak 4 | Peak 5 | Peak 6 | Peak 7 | Peak 8 | Peak 9 | Peak 10 |
|---|---|---|---|---|---|---|---|---|---|---|
| T0 | 25.98 ± 2.69 b | 22.61 ± 3.73 d | 6.85 ± 1.83 cd | 7.78 ± 0.53 cd | 6.93 ± 0.42 c | 3.86 ± 0.10 cd | 31.45 ± 15.64 c | 10.95 ± 0.36 b | 24.95 ± 0.48 de | 27.63 ± 2.87 cd |
| T1 | 58.45 ± 6.73 a | 53.64 ± 3.15 bc | 48.82 ± 8.02 a | 9.51 ± 0.41 bcd | 18.18 ± 0.63 bc | 4.12 ± 0.90 cd | 135.13 ± 23.20 ab | 34.23 ± 5.7 a | 46.22 ± 7.25 cd | 60.68 ± 4.49 bc |
| T2 | 39.76 ± 5.68 b | 96.80 ± 14.47 a | 11.18 ± 1.71 c | 17.79 ± 2.40 b | 26.46 ± 2.62 b | 13.78 ± 3.33 b | 153.59 ± 33.66 a | 32.91 ± 8.89 a | 96.04 ± 18.66 a | 135.14 ± 22.06 a |
| T3 | 31.52 ± 13.45 b | 68.02 ± 19.32 b | 19.86 ± 3.92 b | 35.11 ± 14.40 a | 25.42 ± 8.49 b | 25.34 ± 12.25 ª | 92.16 ± 40.96 b | 30.71 ± 8.89 a | 89.65 ± 25.10 ab | 102.56 ± 21.28 ab |
| T4 | 6.49 ± 0.56 c | 5.33 ± 0.27 d | 2.67 ± 0.13 d | 1.79 ± 0.04 d | 5.83 ± 0.81 c | 2.65 ± 0.13 d | 1.41 ± 0.01 c | 2.24 ± 1.37 b | 4.54 ± 0.38 e | 7.19 ± 0.43 d |
| T5 | 22.94 ± 3.02 bc | 29.52. ± 13.58 cd | 10.28 ± 3.71 c | 3.45 ± 0.91 cd | 44.25 ± 18.34 a | 7.91 ± 2.12 bcd | 2.84 ± 0.91 c | 0.00 ± 0.00 b | 19.42 ± 6.76 de | 29.60 ± 12.56 cd |
| T6 | 70.79 ± 19.89 a | 58.57 ± 24.55 b | 13.48 ± 4.60 bc | 12.77 ± 1.98 bc | 16.16 ± 3.31 bc | 11.74 ± 1.45 bc | 113.94 ± 50.34 ab | 40.51 ± 16.92 a | 67.54 ± 25.01 abc | 136.62 ± 70.60 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berríos, D.; Nahuelcura, J.; González, F.; Peña, F.; Cornejo, P.; Pérez-Navarro, J.; Gómez-Alonso, S.; Ruiz, A. The Biosynthesis, Accumulation of Phenolic Compounds and Antioxidant Response in Lactuca sativa L. Plants Inoculated with a Biofertilizer Based on Soil Yeast and Iron Nanoparticles. Plants 2024, 13, 388. https://doi.org/10.3390/plants13030388
Berríos D, Nahuelcura J, González F, Peña F, Cornejo P, Pérez-Navarro J, Gómez-Alonso S, Ruiz A. The Biosynthesis, Accumulation of Phenolic Compounds and Antioxidant Response in Lactuca sativa L. Plants Inoculated with a Biofertilizer Based on Soil Yeast and Iron Nanoparticles. Plants. 2024; 13(3):388. https://doi.org/10.3390/plants13030388
Chicago/Turabian StyleBerríos, Daniela, Javiera Nahuelcura, Felipe González, Fabiola Peña, Pablo Cornejo, José Pérez-Navarro, Sergio Gómez-Alonso, and Antonieta Ruiz. 2024. "The Biosynthesis, Accumulation of Phenolic Compounds and Antioxidant Response in Lactuca sativa L. Plants Inoculated with a Biofertilizer Based on Soil Yeast and Iron Nanoparticles" Plants 13, no. 3: 388. https://doi.org/10.3390/plants13030388
APA StyleBerríos, D., Nahuelcura, J., González, F., Peña, F., Cornejo, P., Pérez-Navarro, J., Gómez-Alonso, S., & Ruiz, A. (2024). The Biosynthesis, Accumulation of Phenolic Compounds and Antioxidant Response in Lactuca sativa L. Plants Inoculated with a Biofertilizer Based on Soil Yeast and Iron Nanoparticles. Plants, 13(3), 388. https://doi.org/10.3390/plants13030388







