Centaurea benedicta—A Potential Source of Nutrients and Bioactive Components
Abstract
1. Introduction
2. Results and Discussion
Chemical Composition of C. benedicta Seeds
3. Materials and Methods
3.1. Materials
3.2. Chemical Composition
3.3. Physicochemical Properties
3.4. Lipid Composition
3.4.1. Fatty Acid Composition
3.4.2. Sterols
3.4.3. Tocopherols
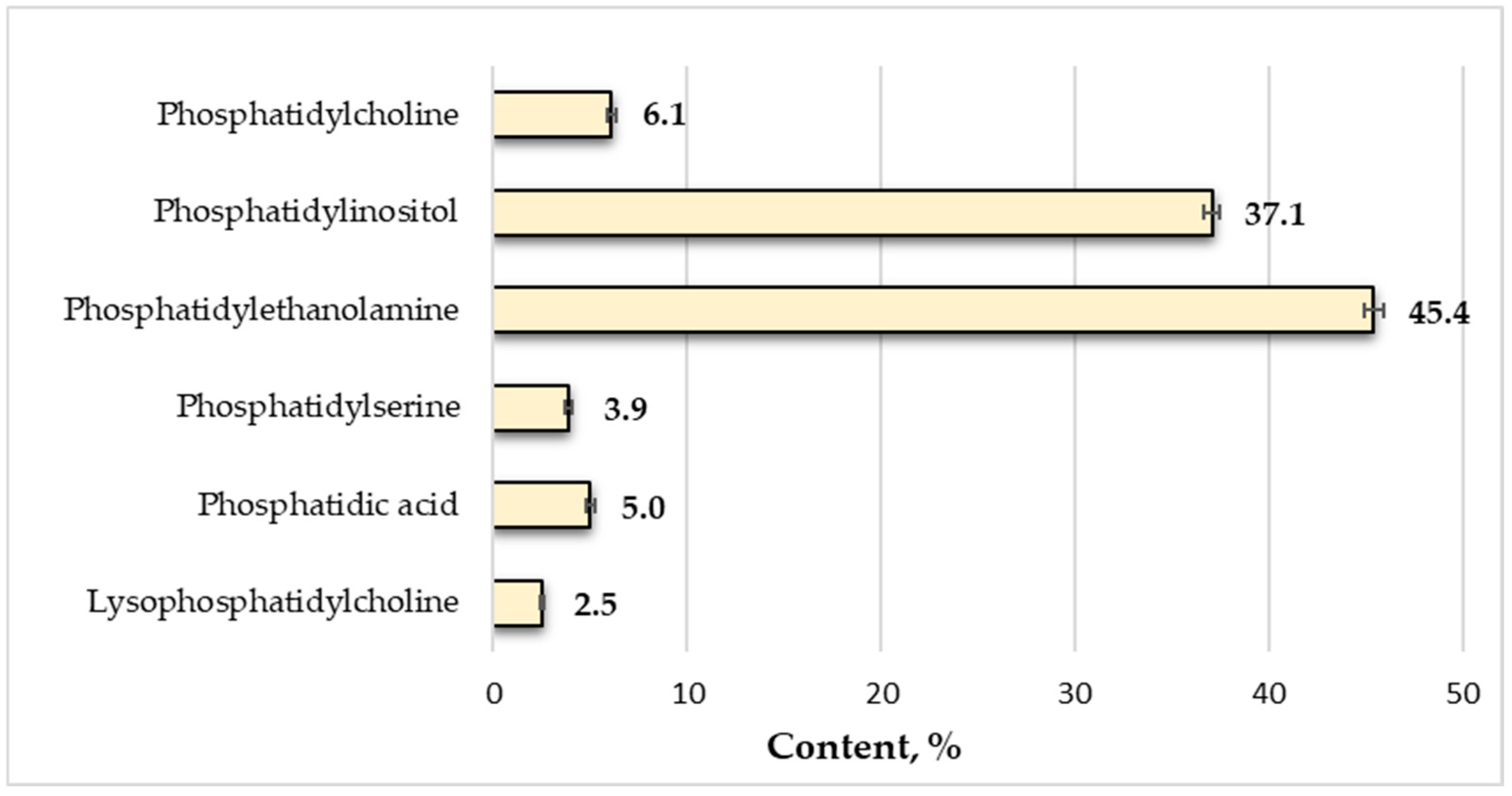
3.4.4. Phospholipids
3.5. Statistics
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Nikolić, M.; Stevović, S. Family Asteraceae as a sustainable planning tool in phytoremediation and its relevance in urban areas. Urban For. Urban Green. 2015, 14, 782–789. [Google Scholar] [CrossRef]
- Rolnik, A.; Olas, B. The plants of the Asteraceae family as agents in the protection of human health. Int. J. Mol. Sci. 2021, 22, 3009. [Google Scholar] [CrossRef] [PubMed]
- Keil, D. Centaurea benedicta. Jepson Flora Project. 2012. Available online: https://ucjeps.berkeley.edu/eflora/eflora_display.php?tid=2297 (accessed on 15 August 2024).
- Bohm, B.A.; Stuessy, T.F. Flavonoids of the Sunflower Family (Asteraceae); Springer Science & Business Media: Wien, Austria, 2001; pp. 116–119. [Google Scholar]
- Paniagua-Zambrana, N.Y.; Bussmann, R.W.; Kikvidze, Z.; Khojimatov, O.K. Centaurea benedicta (L.) L. Centaurea cyanus L. Asteraceae. In Ethnobotany of the Mountain Regions of Eastern Europe: Carpathians; Springer International Publishing: Cham, Switzerland, 2024; pp. 1–13. [Google Scholar]
- Ulbricht, C.; Basch, E.; Dacey, C.; Hammemess, P.; Hashmi, S.; Seamon, E.; Vora, M.; Weissner, W. An evidence-based systematic review of blessed thistle (Cnicus benedictus) by the natural standard research collaboration. J. Diet. Suppl. 2008, 5, 422–437. [Google Scholar] [CrossRef]
- Koc, S.; Isgor, B.S.; Isgor, Y.G.; Shomali Moghaddam, N.; Yildirim, O. The potential medicinal value of plants from Asteraceae family with antioxidant defense enzymes as biological targets. Pharm. Biol. 2015, 53, 746–751. [Google Scholar] [CrossRef] [PubMed]
- Tiwana, G.; Fua, J.; Lu, L.; Cheesman, M.J.; Cock, I.E. A review of the traditional uses, medicinal properties and phytochemistry of Centaurea benedicta L. Pharmacogn. J. 2021, 13, 798–812. [Google Scholar] [CrossRef]
- Al-Snafi, A.E. The constituents and pharmacology of Cnicus benedictus—A review. Pharm. Chem. J. 2016, 3, 129–135. [Google Scholar]
- Can, Z.; Baltaş, N.; Keskin, S.; Yıldız, O.; Kolaylı, S. Properties of antioxidant and anti-inflammatory activity and phenolic profiles of Şevketi Bostan (Cnicus benedictus L.) cultivated in Aegean Region from Turkey. Turk. J. Agric.-Food Sci. Technol. 2017, 5, 308–314. [Google Scholar] [CrossRef]
- Ghiasy-Oskoee, M.; Agha Alikhani, M. Towards utilizing Asteraceae alternative oilseed species on marginal lands: Agronomic performance, fatty acid composition, oil biocompounds, and oil physicochemical properties of Asteraceae species. J. Agric. Food Res. 2023, 14, 100799. [Google Scholar] [CrossRef]
- Belitz, H.D.; Grosch, W.; Schieberle, P. Food Chemistry, 4th ed.; Springer: Berlin/Heidelberg, Germany, 2009. [Google Scholar]
- Vishwakarma, K.L.; Dubey, V. Nutritional analysis of indigenous wild edible herbs used in eastern Chhattisgarh, India. Emir. J. Food Agric. 2011, 23, 554–560. [Google Scholar]
- Tunçtürk, R.; Tunçtürk, M.; Nohutcu, L. Study on chemical composition of Centaurea karduchorum Boiss. species from endemic plants of Eastern Anatolia/Turkey. Curr. Perspect. Med. Aromat. Plants 2019, 2, 47–52. [Google Scholar] [CrossRef]
- CXS210-1999; Codex Alimentarius, International Food Standards. Standard for named Vegetable Oils. Food and Agriculture Organization of the United Nations and World Health Organization. Codex Alimentarius Commission: Rome, Italy, 1999; Adopted in 1999, Revised in 2001, 2003, 2009, 2017, 2019. Amended in 2005, 2011, 2013, 2015, 2019, 2021, 2022, 2023.
- Ibrahim, W.; Iverson, J.; Firestone, D. Safflower Oil: Physical and Chemical Properties, and Fatty Acid Composition. J. AOAC Int. 1964, 47, 776–780. [Google Scholar] [CrossRef][Green Version]
- Popov, A.; Ilinov, P. Chemistry of Lipids; Science and Art: Sofia, Bulgaria, 1986; p. 290. [Google Scholar]
- Peker, S.; Baştürk, A. Volatile compounds, fatty acid composition and antioxidant activity of Centaurea albonitens and Centaurea balsamita seeds growing in Van, Turkey. Eur. J. Nutr. Food Saf. 2020, 11, 187–199. [Google Scholar] [CrossRef]
- Teneva, O.; Petkova, Z.; Antova, G.; Angelova-Romova, M.; Stoyanov, P.; Todorov, K.; Mladenova, T.; Radoukova, T.; Mladenov, R.; Petkov, V.; et al. Chemical Composition and Lipid Bioactive Components of Centaurea thracica dwelling in Bulgaria. Molecules 2024, 29, 3282. [Google Scholar] [CrossRef] [PubMed]
- Yildirim, N.; Sunar, S.; Agar, G.; Bozari, S.; Aksakal, O. Biochemical and molecular characterization of some Centaurea species growing in the Eastern Anatolia region of Turkey. Biochem. Genet. 2009, 47, 850–859. [Google Scholar] [CrossRef] [PubMed]
- Erdogan, T.; Gonenc, T.; Cakilcioglu, U.; Kivcak, B. Fatty acid composition of the aerial parts of some Centaurea species in Elazig, Turkey. Trop. J. Pharm. Res. 2014, 13, 613–616. [Google Scholar] [CrossRef]
- Tekeli, Y.; Zengin, G.; Aktumsek, A.; Sezgin, M. Comparison of the fatty acid compositions of Six Centaurea species. Chem. Nat. Compd. 2013, 49, 496–498. [Google Scholar] [CrossRef]
- Fayed, M.; Al-Wahaibi, L.; Bakr, R.; Nour, M.; Basudan, O.; Parvez, M.; Al-Dosari, M.; Abdel-Mageed, W. Sterols from Centaurea pumilio L. with cell proliferative activity: In vitro and in silico studies. Open Chem. 2023, 21, 20220316. [Google Scholar] [CrossRef]
- ISO 659:2014; Oilseeds. Determination of oil content (Reference method). ISO: Geneva, Switzerland, 2014.
- AOAC—Association of Official Analytical Chemist. Official Methods of Analysis, 20th ed.; AOAC: Arlington, VA, USA, 2016; Method 976.06. [Google Scholar]
- FAO. Food Energy—Methods of Analysis and Conversion Factors; Report of a Technical Workshop, FAO Food and Nutrition Paper 77; FAO: Rome, Italy, 2003. [Google Scholar]
- AOCS (American Oil Chemists Society). Official Methods and Recommended Practices of the American Oil Chemists Society, Calculated Iodine Value, 5th ed.; AOCS Press: Champaign, IL, USA, 1999; p. Cd 1c-8. [Google Scholar]
- ISO 3960:2007; Animal and Vegetable Fats and Oils. Determination of Peroxide Value: Iodometric (Visual) Endpoint Determination. ISO: Geneva, Switzerland, 2007.
- ISO 6320:2000; Animal and Vegetable Fats and Oils. Determination of Refractive Index. ISO: Geneva, Switzerland, 2000.
- ISO 6886:2006; Animal and vegetable fats and oils. Determination of Oxidative Stability (Accelerated Oxidation Test). ISO: Geneva, Switzerland, 2006.
- ISO 12966-1:2014; Animal and Vegetable Fats and Oils. Gas Chromatography of Fatty Acid Methyl Esters—Part 1: Guidelines on Modern Gas Chromatography of Fatty Acid Methyl Esters. ISO: Geneva, Switzerland, 2014.
- ISO 12966-2:2017; Animal and Vegetable Fat and Oils. Gas Chromatography of Fatty Acid Methyl Esters—Part 2: Preparation of Methyl Esters of Fatty Acids. ISO: Geneva, Switzerland, 2017.
- ISO 18609:2000; Animal and Vegetable Fats and Oils. Determination of Unsaponifiable Matter. Method Using Hexane Extraction. ISO: Geneva, Switzerland, 2000.
- Ivanov, S.; Bitcheva, P.; Konova, B. Méthode de détermination chromatographyque et colorimétrique des phytosterols dans les huiles végétales et les concentres steroliques. Rev. Fr. Corps Gras 1972, 19, 177–180. [Google Scholar]
- ISO 12228-1:2014; Animal and Vegetable Fats and Oils. Determination of Individual and Total Sterols Contents. Gas Chromatographic Method. ISO: Geneva, Switzerland, 2014.
- ISO 9936:2016; Animal and Vegetable Fats and Oils. Determination of Tocopherol and Tocotrienol Contents by High-Performance Liquid Chromatography. ISO: Geneva, Switzerland, 2016.
- Schneiter, R.; Daum, G. Analysis of yeast lipids. In Methods in Molecular Biology, 2nd ed.; Xiao, W., Ed.; Humana Press Inc.: Totowa, NJ, USA, 2006; pp. 75–84. [Google Scholar] [CrossRef]
- Christie, W.W. Lipid Analysis, 3rd ed.; The Oily Press: Bridgwater, UK, 2003. [Google Scholar]
- ISO 10540-1:2014; Animal and Vegetable Fats and Oils. Determination of Phosphorus Content. Part 1: Colorimetric Method. ISO: Geneva, Switzerland, 2014.
| Compound | Content in 100 g of Seeds | Content in 1000 Seeds |
|---|---|---|
| Oil content, % (d.w.) | 11.0 ± 0.2 | 3.8 ± 0.1 |
| Total proteins, % (d.w.) | 16.4 ± 0.2 | 5.7 ± 0.1 |
| Total carbohydrates, % (d.w.) | 68.5 ± 0.5 | 23.8 ± 0.2 |
| Fibers, % (d.w.) | 32.2 ± 0.3 | 11.2 ± 0.1 |
| Ash, % (d.w.) | 4.1 ± 0.1 | 1.4 ± 0.0 |
| Energy value, kcal/100 g (d.w.) | 439 | 152 |
| Physicochemical Characteristic | Content |
|---|---|
| Peroxide value, meqO2/kg | 2.8 ± 0.2 |
| Iodine value, g I2/100 g | 147.3 ± 0.2 |
| Refractive index | 1.4748 ± 0.0004 |
| Biologically Active Component | Content |
|---|---|
| Unsaponifiable matter, % | 14.5 ± 0.4 |
| Sterols, % | 0.9 ± 0.1 |
| Phospholipids, % | 1.9 ± 0.1 |
| Tocopherols, mg/kg | 492 ± 82 |
| α-Tocopherol, mg/kg | 472 ± 78 |
| β-Tocopherol, mg/kg | 20 ± 4 |
| Fatty Acid, % | Content, % |
|---|---|
| C 6:0—Caproic acid | 0.1 ± 0.0 |
| C 8:0—Caprylic acid | 0.1 ± 0.0 |
| C 13:0—Tridecanoic acid | 0.1 ± 0.0 |
| C 14:0—Myristic acid | 0.1 ± 0.0 |
| C 15:1—Pentadecenoic acid | 0.1 ± 0.0 |
| C 16:0—Palmitic acid | 5.9 ± 0.1 |
| C 16:1—Palmitoleic acid C 17:0—Margaric acid | 0.1 ± 0.0 0.1 ± 0.0 |
| C 17:1—Heptadecenoic acid C 18:0—Stearic acid | 0.1 ± 0.0 2.6 ± 0.1 |
| C 18:1—Oleic acid | 18.1 ± 0.2 |
| C 18:2 (n-6)—Linoleic acid | 72.1 ± 0.4 |
| C 18:3 (n-3)—α-Linolenic acid | 0.2 ± 0.0 |
| C 20:1—Eicosenoic acid | 0.2 ± 0.0 |
| C 22:0—Behenic acid | 0.1 ± 0.0 |
| C 22:1—Erucic acid | 0.1 ± 0.0 |
| C 22:6 (n-3)—Docosahexaenoic acid | 0.1 ± 0.0 |
| Saturated fatty acids | 9.0 |
| Monounsaturated fatty acids | 18.0 |
| Polyunsaturated fatty acids | 73.0 |
| Sterol | Content, % |
|---|---|
| Campesterol | 7.5 ± 0.3 d |
| Stigmasterol | 19.4 ± 0.2 b |
| β—Sitosterol | 59.5 ± 0.5 a |
| Δ5—Avenasterol | 13.5 ± 0.3 c |
| Δ7—Stigmasterol | 0.1 ± 0.0 e |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teneva, O.; Petkova, Z.; Dobreva, A.; Dzhurmanski, A.; Stoyanova, L.; Angelova-Romova, M. Centaurea benedicta—A Potential Source of Nutrients and Bioactive Components. Plants 2024, 13, 3579. https://doi.org/10.3390/plants13243579
Teneva O, Petkova Z, Dobreva A, Dzhurmanski A, Stoyanova L, Angelova-Romova M. Centaurea benedicta—A Potential Source of Nutrients and Bioactive Components. Plants. 2024; 13(24):3579. https://doi.org/10.3390/plants13243579
Chicago/Turabian StyleTeneva, Olga, Zhana Petkova, Ana Dobreva, Anatoli Dzhurmanski, Liliya Stoyanova, and Maria Angelova-Romova. 2024. "Centaurea benedicta—A Potential Source of Nutrients and Bioactive Components" Plants 13, no. 24: 3579. https://doi.org/10.3390/plants13243579
APA StyleTeneva, O., Petkova, Z., Dobreva, A., Dzhurmanski, A., Stoyanova, L., & Angelova-Romova, M. (2024). Centaurea benedicta—A Potential Source of Nutrients and Bioactive Components. Plants, 13(24), 3579. https://doi.org/10.3390/plants13243579







