GA3-Induced SlXTH19 Expression Enhances Cell Wall Remodeling and Plant Height in Tomatoes
Abstract
1. Introduction
2. Results
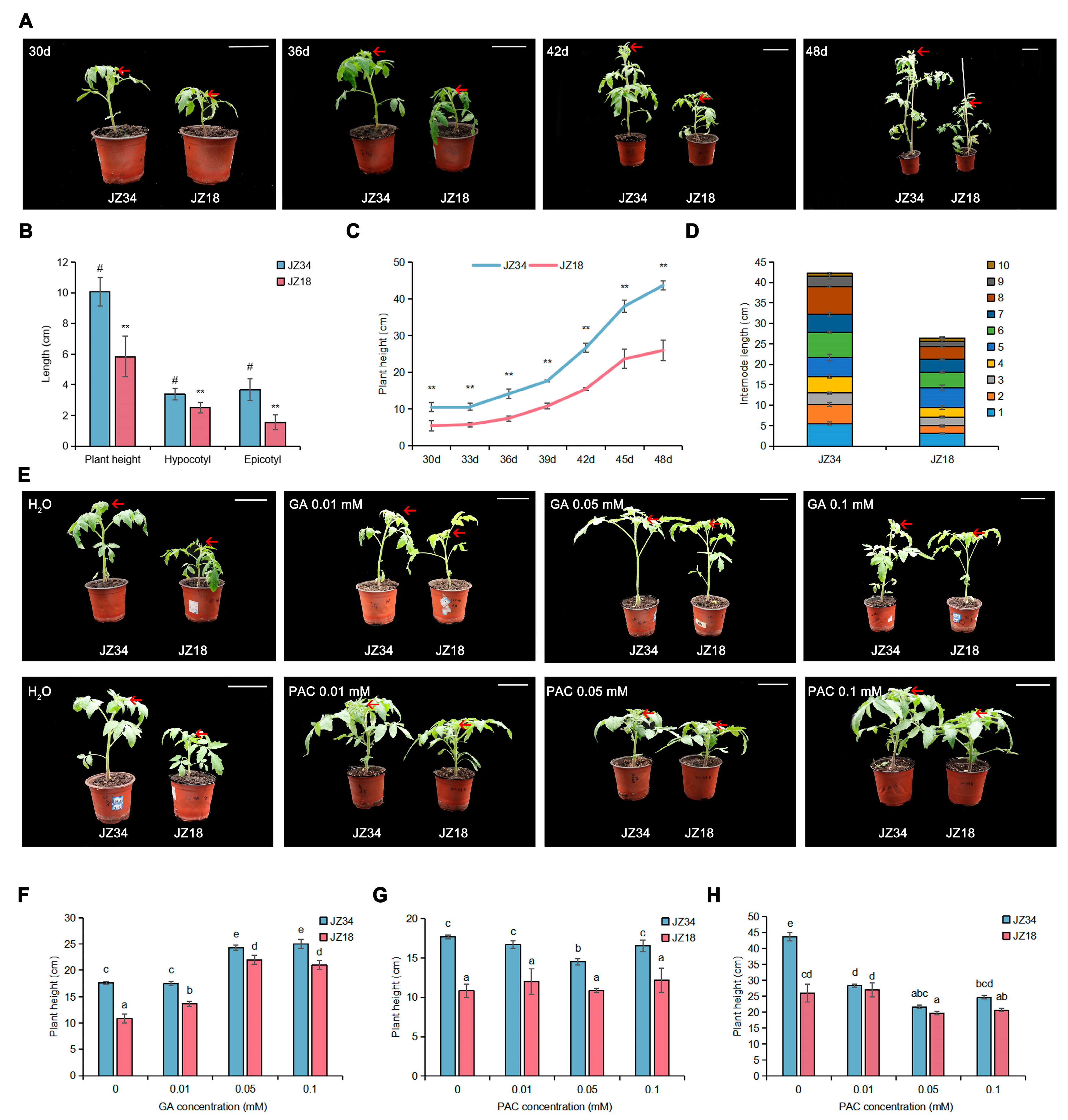
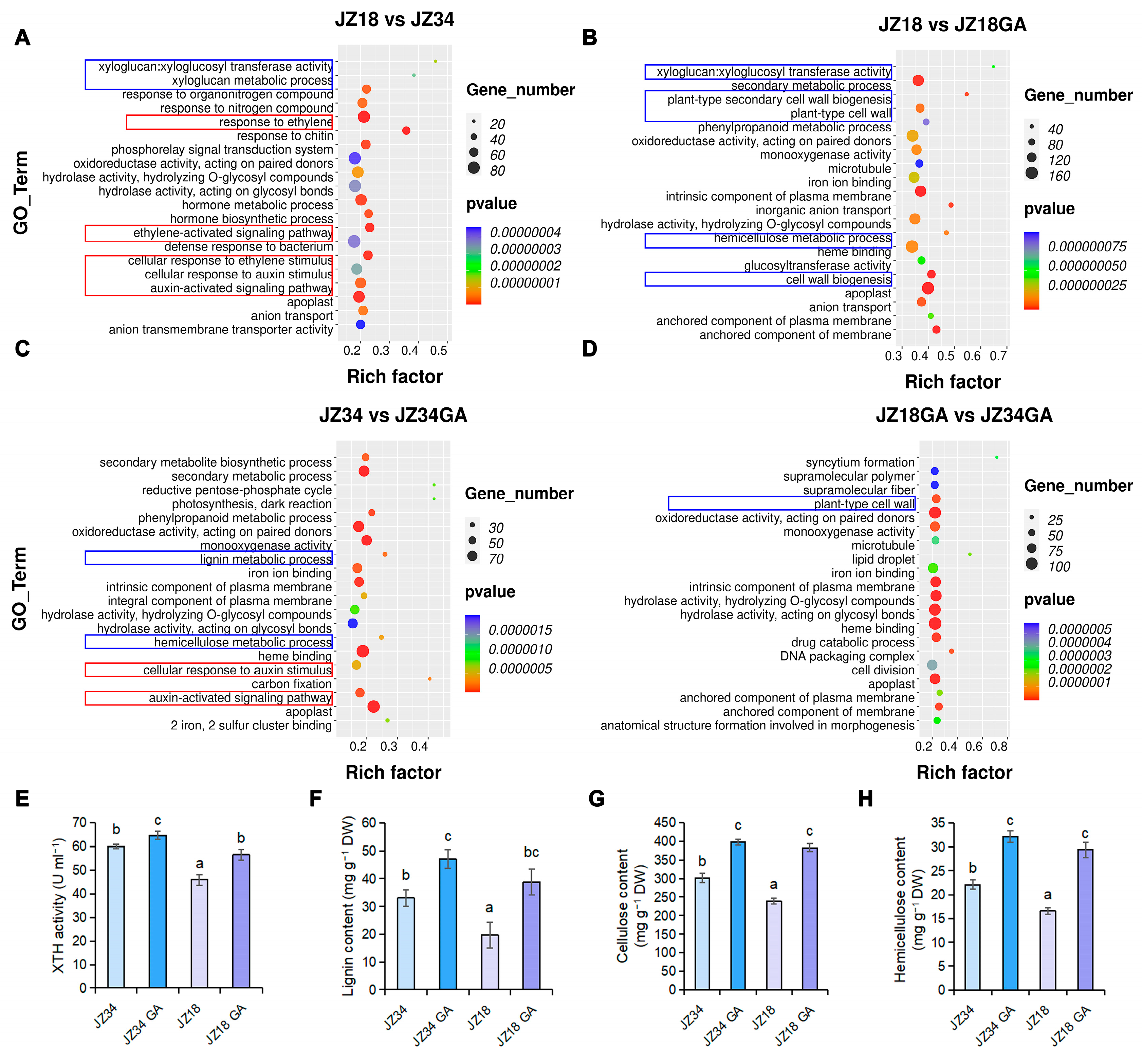
2.1. GA Treatments Promote Cell Wall Remodeling and Plant Height
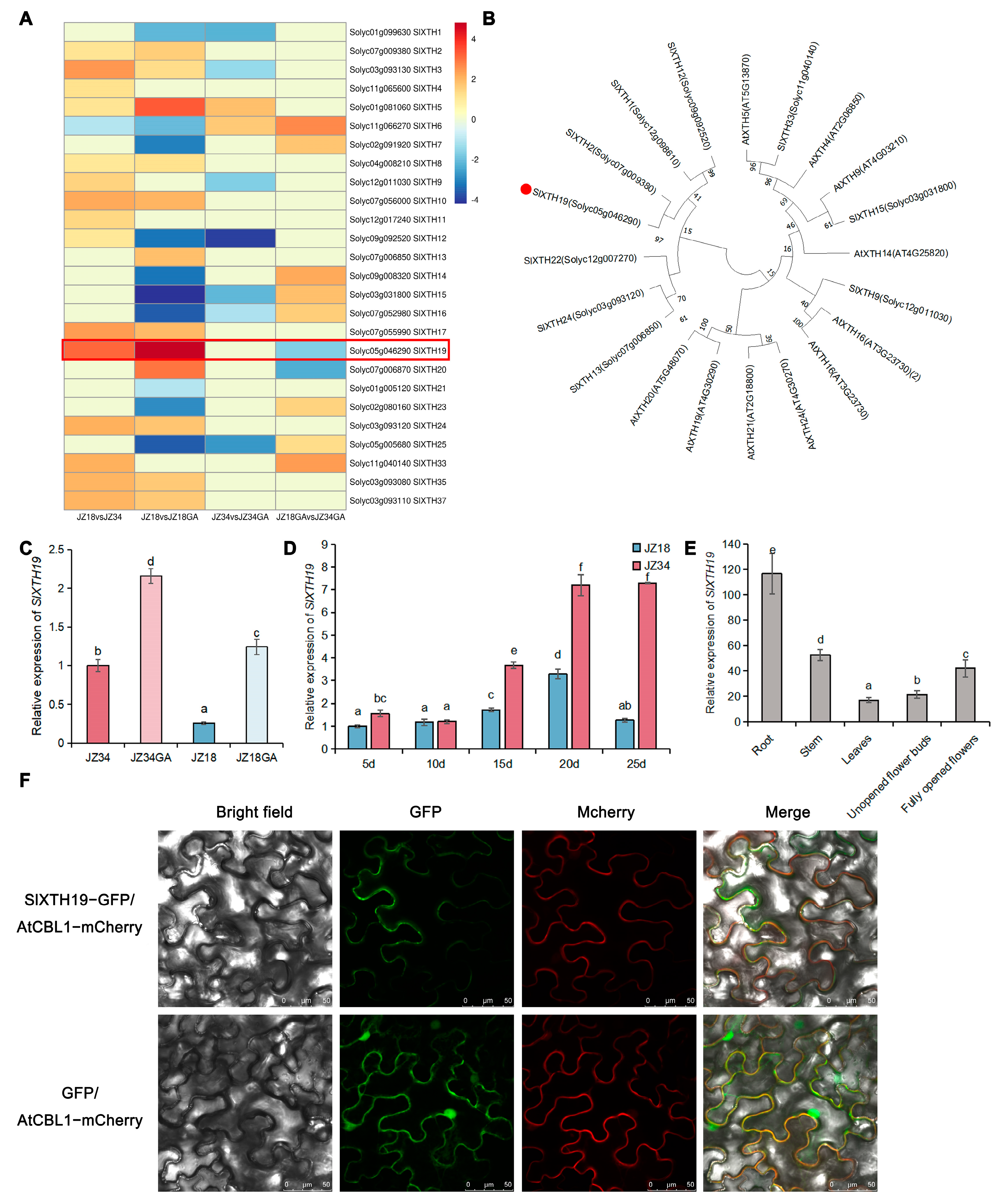
2.2. SlXTH19 Displayed Stable and High Expression Levels During Tomato Stem Development in GA Treatment
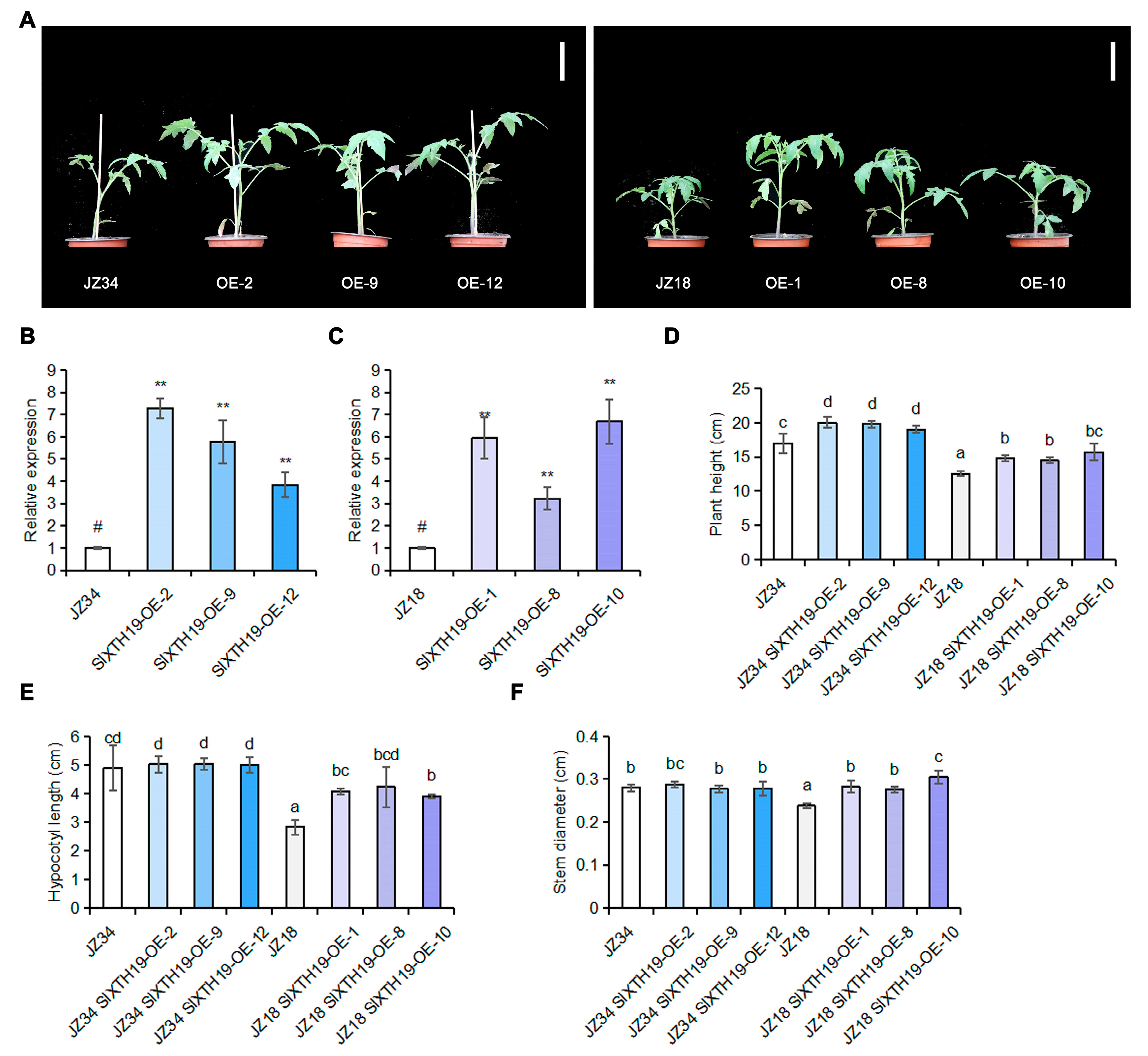
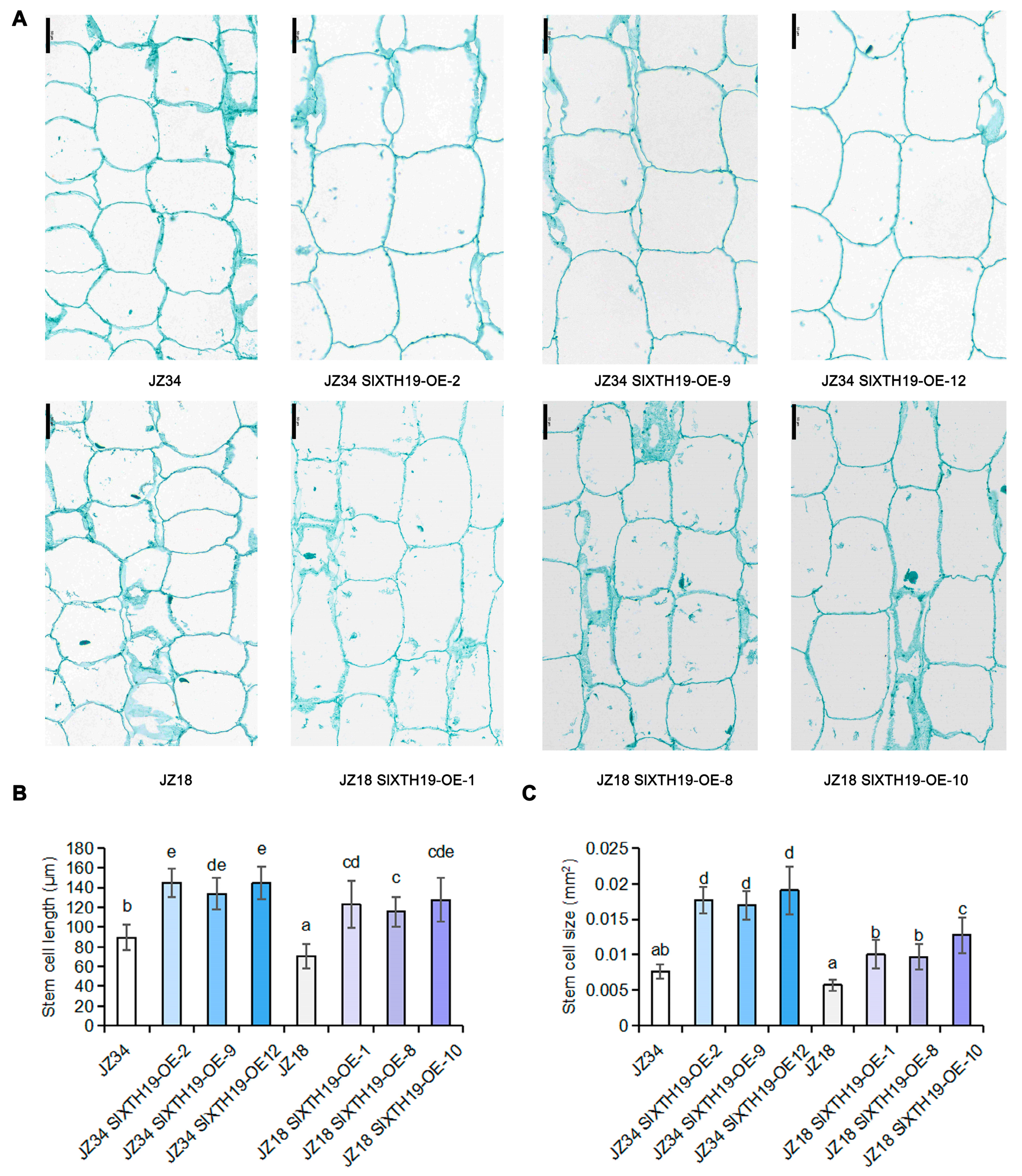
2.3. Phenotypic Analysis of SlXTH19
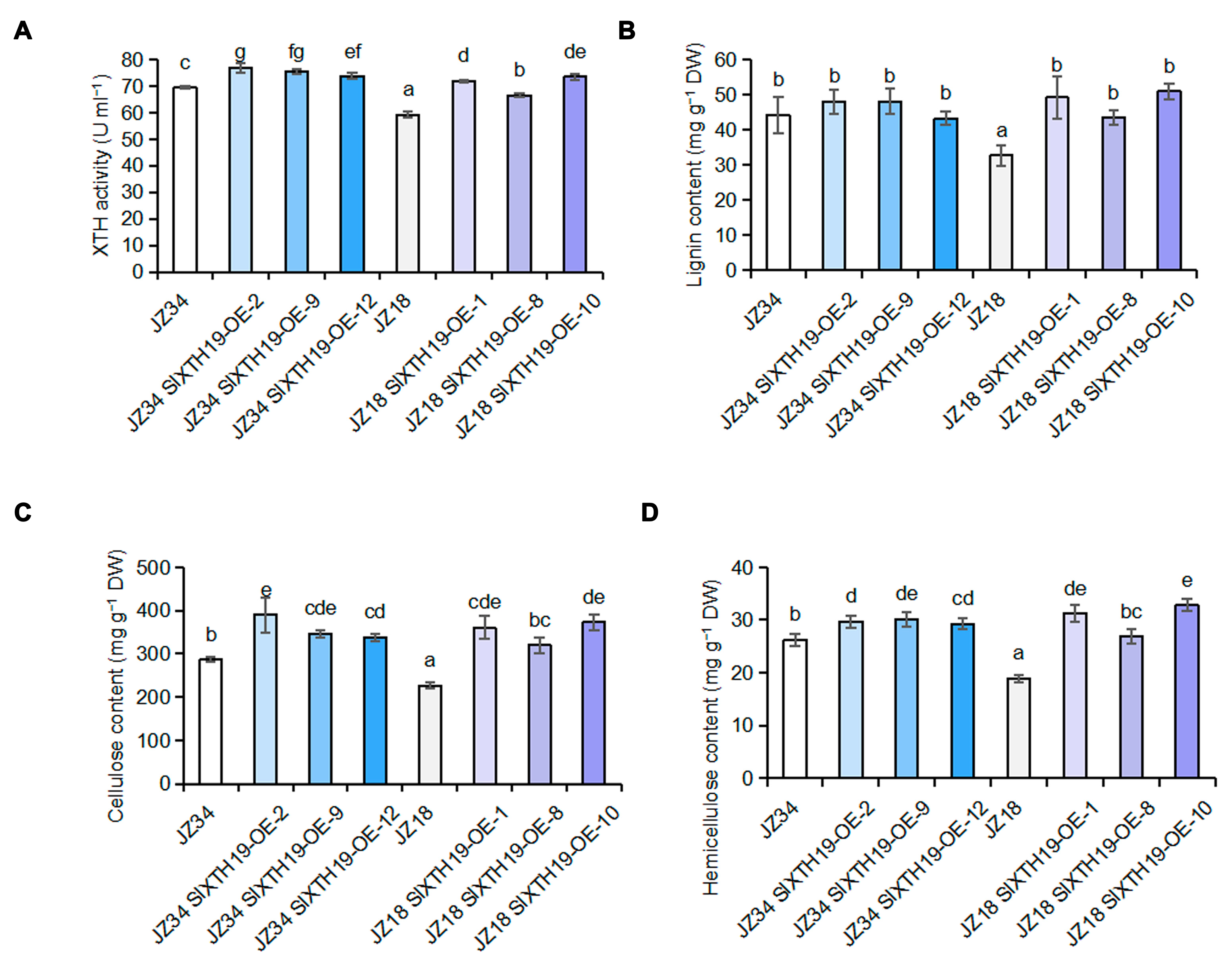
2.4. Comparison of XTH Activity and Lignin, Cellulose, and Hemicellulose Contents
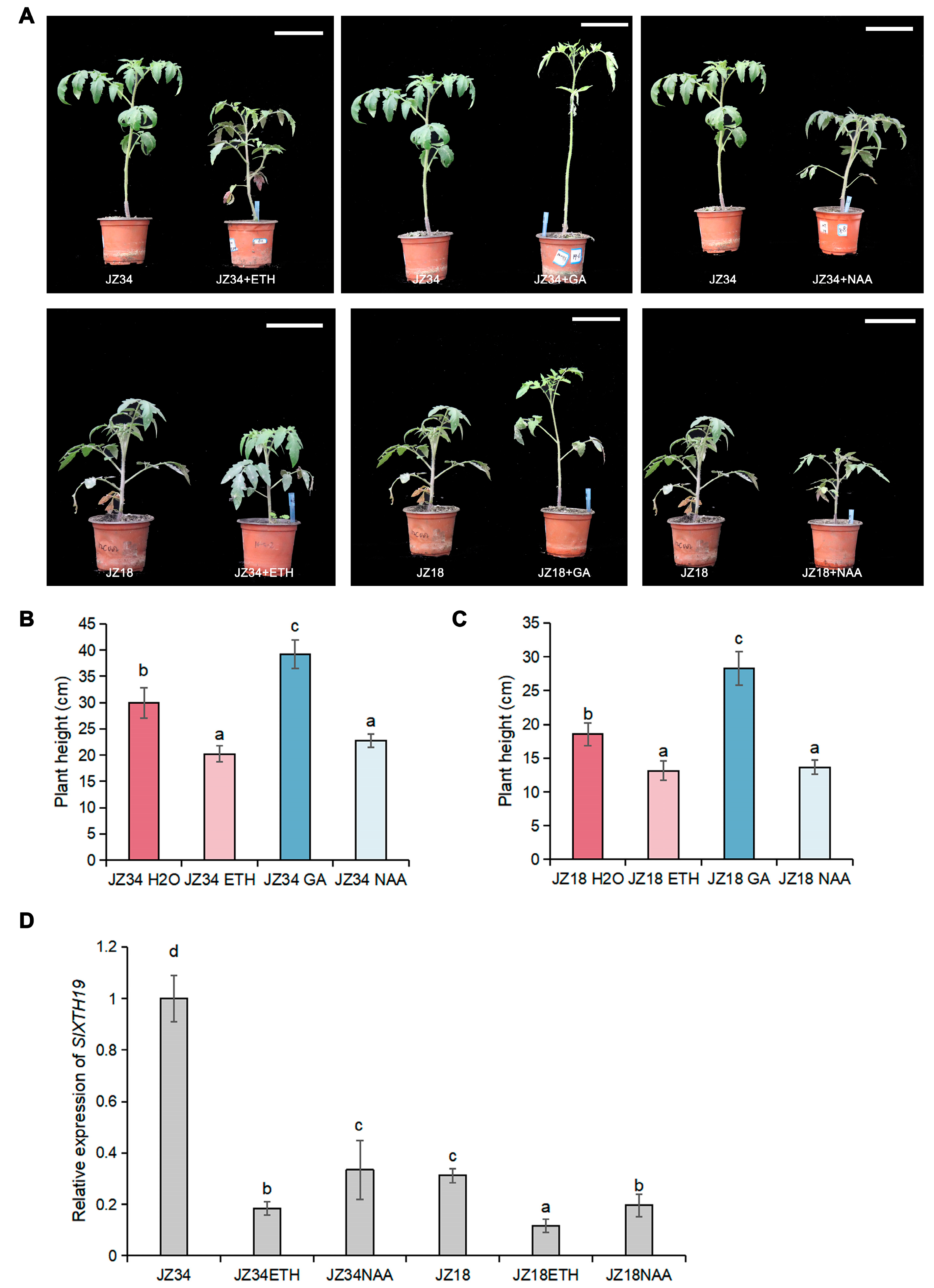
2.5. Response of Plant Height to Exogenous ETH and IAA Treatment in Tomatoes
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. Exogenous Hormone Treatment
4.3. Plasmid Construction and Plant Transformation
4.4. Phenotype Observation
4.5. Cellulose, Hemicellulose, and Lignin Content Measurements
4.6. Xyloglucan Endotransglucosyltransferase/Hydrolase (XTH) Activity Determination
4.7. Transcriptome Analysis
4.8. Subcellular Localization
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yang, X.C.; Hwa, C.M. Genetic modification of plant architecture and variety improvement in rice. Heredity 2008, 101, 396–404. [Google Scholar] [CrossRef]
- Liu, X.; Yang, W.; Wang, J.; Yang, M.; Wei, K.; Liu, X.; Qiu, Z.; van Giang, T.; Wang, X.; Guo, Y.; et al. SlGID1a Is a Putative Candidate Gene for qtph1.1, a Major-Effect Quantitative Trait Locus Controlling Tomato Plant Height. Front. Genet. 2020, 11, 881. [Google Scholar] [CrossRef]
- Zou, J.; Zhang, S.; Zhang, W.; Li, G.; Chen, Z.; Zhai, W.; Zhao, X.; Pan, X.; Xie, Q.; Zhu, L. The rice HIGH-TILLERING DWARF1 encoding an ortholog of Arabidopsis MAX3 is required for negative regulation of the outgrowth of axillary buds. Plant J. 2006, 48, 687–698. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Tian, F.; Tan, L.; Xie, D.; Sun, C. Characterization of a novel high-tillering dwarf 3 mutant in rice. J. Genet. Genomics 2011, 38, 411–418. [Google Scholar] [CrossRef]
- Manghwar, H.; Hussain, A.; Ali, Q.; Liu, F. Brassinosteroids (BRs) Role in Plant Development and Coping with Different Stresses. Int. J. Mol. Sci. 2022, 23, 1012. [Google Scholar] [CrossRef]
- Luo, J.; Tang, Y.; Chu, Z.; Peng, Y.; Chen, J.; Yu, H.; Shi, C.; Jafar, J.; Chen, R.; Tang, Y.; et al. SlZF3 regulates tomato plant height by directly repressing SlGA20ox4 in the gibberellic acid biosynthesis pathway. Hortic. Res. 2023, 10, uhad025. [Google Scholar] [CrossRef] [PubMed]
- Salas Fernandez, M.G.; Becraft, P.W.; Yin, Y.; Lübberstedt, T. From dwarves to giants? Plant height manipulation for biomass yield. Trends Plant Sci. 2009, 14, 454–461. [Google Scholar] [CrossRef]
- Tong, H.; Xiao, Y.; Liu, D.; Gao, S.; Liu, L.; Yin, Y.; Jin, Y.; Qian, Q.; Chu, C. Brassinosteroid regulates cell elongation by modulating gibberellin metabolism in ricec w open. Plant Cell 2014, 26, 4376–4393. [Google Scholar] [CrossRef] [PubMed]
- Pei, Y.; Xue, Q.; Shu, P.; Xu, W.; Du, X.; Wu, M.; Liu, K.; Pirrello, J.; Bouzayen, M.; Hong, Y.; et al. Bifunctional transcription factors SlERF.H5 and H7 activate cell wall and repress gibberellin biosynthesis genes in tomato via a conserved motif. Dev. Cell 2024, 59, 1345–1359.e6. [Google Scholar] [CrossRef] [PubMed]
- Zhao, N.; Zhao, M.; Tian, Y.; Wang, Y.; Han, C.; Fan, M.; Guo, H.; Bai, M.Y. Interaction between BZR1 and EIN3 mediates signalling crosstalk between brassinosteroids and ethylene. New Phytol. 2021, 232, 2308–2323. [Google Scholar] [CrossRef]
- Zhong, C.; Patra, B.; Tang, Y.; Li, X.; Yuan, L.; Wang, X. A transcriptional hub integrating gibberellin-brassinosteroid signals to promote seed germination in Arabidopsis. J. Exp. Bot. 2021, 72, 4708–4720. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, Y.; He, Y.; Yan, Y.; Yu, X.; Ali, M.; Pan, C.; Lu, G. Cytokinin-inducible response regulator SlRR6 controls plant height through gibberellin and auxin pathways in tomato. J. Exp. Bot. 2023, 74, 4471–4488. [Google Scholar] [CrossRef] [PubMed]
- Le, L.; Guo, W.; Du, D.; Zhang, X.; Wang, W.; Yu, J.; Wang, H.; Qiao, H.; Zhang, C.; Pu, L. A spatiotemporal transcriptomic network dynamically modulates stalk development in maize. Plant Biotechnol. J. 2022, 20, 2313–2331. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ren, Z.; Xie, S.; Li, Z.; Zhou, Y.; Duan, L. Jasmonate mimic modulates cell elongation by regulating antagonistic bHLH transcription factors via brassinosteroid signaling. Plant Physiol. 2024, 195, 2712–2726. [Google Scholar] [CrossRef]
- Tenhaken, R. Cell wall remodeling under abiotic stress. Front. Plant Sci. 2015, 5, 771. [Google Scholar] [CrossRef]
- Rose, J.K.C.; Braam, J.; Fry, S.C.; Nishitani, K. The XTH family of enzymes involved in xyloglucan endotransglucosylation and endohydrolysis: Current perspectives and a new unifying nomenclature. Plant Cell Physiol. 2002, 43, 1421–1435. [Google Scholar] [CrossRef] [PubMed]
- Nishitani, K.; Vissenberg, K. Roles of the XTH Protein Family in the Expanding Cell; Springer: Berlin/Heidelberg, Germany, 2006; pp. 89–116. [Google Scholar]
- Li, Y.; Zheng, X.; Wang, C.; Hou, D.; Li, T.; Li, D.; Ma, C.; Sun, Z.; Tian, Y. Pear xyloglucan endotransglucosylase/hydrolases PcBRU1 promotes stem growth through regulating cell wall elongation. Plant Sci. 2021, 312, 111026. [Google Scholar] [CrossRef]
- Tang, Y.; Lu, L.; Huang, X.; Zhao, D.; Tao, J. The herbaceous peony transcription factor WRKY41a promotes secondary cell wall thickening to enhance stem strength. Plant Physiol. 2023, 191, 428–445. [Google Scholar] [CrossRef]
- Xu, P.; Fang, S.; Chen, H.; Cai, W. The brassinosteroid-responsive xyloglucan endotransglucosylase/hydrolase 19 (XTH19) and XTH23 genes are involved in lateral root development under salt stress in Arabidopsis. Plant J. 2020, 104, 59–75. [Google Scholar] [CrossRef]
- Sarmiento-López, L.G.; López-Espinoza, M.Y.; Juárez-Verdayes, M.A.; López-Meyer, M. Genome-wide characterization of the xyloglucan endotransglucosylase/hydrolase gene family in Solanum lycopersicum, L. and gene expression analysis in response to arbuscular mycorrhizal symbiosis. PeerJ 2023, 11, e15257. [Google Scholar] [CrossRef]
- Wang, D.; Lu, Q.; Wang, X.; Ling, H.; Huang, N. Elucidating the role of SlXTH5 in tomato fruit softening. Hortic. Plant J. 2023, 9, 777–788. [Google Scholar] [CrossRef]
- Garg, R.; Mahato, H.; Choudhury, U.; Thakur, R.S.; Debnath, P.; Ansari, N.G.; Sane, V.A.; Sane, A.P. The tomato EAR-motif repressor, SlERF36, accelerates growth transitions and reduces plant life cycle by regulating GA levels and responses. Plant Biotechnol. J. 2024, 22, 848–862. [Google Scholar] [CrossRef] [PubMed]
- Voorend, W.; Nelissen, H.; Vanholme, R.; De Vliegher, A.; Van Breusegem, F.; Boerjan, W.; Roldán-Ruiz, I.; Muylle, H.; Inzé, D. Overexpression of GA20-OXIDASE1 impacts plant height, biomass allocation and saccharification efficiency in maize. Plant Biotechnol. J. 2016, 14, 997–1007. [Google Scholar] [CrossRef]
- McCann, M.C.; Roberts, K. Changes in cell wall architecture during cell elongation. J. Exp. Bot. 1994, 45, 1683–1691. [Google Scholar] [CrossRef]
- Li, Y.; Jones, L.; McQueen-Mason, S. Expansins and cell growth. Curr. Opin. Plant Biol. 2003, 6, 603–610. [Google Scholar] [CrossRef] [PubMed]
- Cosgrove, D.J. Plant cell wall extensibility: Connecting plant cell growth with cell wall structure, mechanics, and the action of wall-modifying enzymes. J. Exp. Bot. 2016, 67, 463–476. [Google Scholar] [CrossRef]
- Jan, A.; Yang, G.; Nakamura, H.; Ichikawa, H.; Kitano, H.; Matsuoka, M.; Matsumoto, H.; Komatsu, S. Characterization of a xyloglucan endotransglucosylase gene that is up-regulated by gibberellin in rice. Plant Physiol. 2004, 136, 3670–3681. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Liu, Y.; Qin, G.; Wu, P.; Zi, H.; Xu, Z.; Zhao, X.; Wang, Y.; Li, Y.; Yang, S.; et al. The TOR–EIN2 axis mediates nuclear signalling to modulate plant growth. Nature 2021, 591, 288–292. [Google Scholar] [CrossRef]
- Woeste, K.E.; Ye, C.; Kieber, J.J. Two Arabidopsis mutants that overproduce ethylene are affected in the posttranscriptional regulation of 1-aminocyclopropane-1-carboxylic acid synthase. Plant Physiol. 1999, 119, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Wessels, B.; Seyfferth, C.; Escamez, S.; Vain, T.; Antos, K.; Vahala, J.; Delhomme, N.; Kangasjärvi, J.; Eder, M.; Felten, J.; et al. An AP2/ERF transcription factor ERF139 coordinates xylem cell expansion and secondary cell wall deposition. New Phytol. 2019, 224, 1585–1599. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, X.; Zhao, Y.; Li, Y.; Zhang, G.; Peng, Z.; Zhang, J. Enhancing auxin accumulation in maize root tips improves root growth and dwarfs plant height. Plant Biotechnol. J. 2018, 16, 86–99. [Google Scholar] [CrossRef]
- Li, L.; Wonder, J.; Helming, T.; van Asselt, G.; Pantazopoulou, C.K.; van de Kaa, Y.; Kohlen, W.; Pierik, R.; Kajala, K. Evaluation of the roles of brassinosteroid, gibberellin and auxin for tomato internode elongation in response to low red:far-red light. Physiol. Plant 2024, 176, e14558. [Google Scholar] [CrossRef] [PubMed]
- Cagnola, J.I.; Ploschuk, E.; Benech-Arnold, T.; Finlayson, S.A.; Casal, J.J. Stem transcriptome reveals mechanisms to reduce the energetic cost of shade-avoidance responses in tomato. Plant Physiol. 2012, 160, 1110–1119. [Google Scholar] [CrossRef] [PubMed]
- Xiong, H.; Lu, D.; Li, Z.; Wu, J.; Ning, X.; Lin, W.; Bai, Z.; Zheng, C.; Sun, Y.; Chi, W.; et al. The DELLA-ABI4-HY5 module integrates light and gibberellin signals to regulate hypocotyl elongation. Plant Commun. 2023, 4, 100597. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, Q.; Luo, J.; Liu, X.; Jiang, J. Major-effect quantitative trait locus qLKR4.1 encodes a phospholipase Dδ protein associated with low-K+ stress tolerance by promoting root length. Theor. Appl. Genet. 2023, 136, 88. [Google Scholar] [CrossRef]
- Nascimento, V.L.; Pereira, A.M.; Siqueira, J.A.; Pereira, A.S.; Silva, V.F.; Costa, L.C.; Ribeiro, D.M.; Zsögön, A.; Nunes-Nesi, A.; Araújo, W.L. Exogenous ethylene reduces growth via alterations in central metabolism and cell wall composition in tomato (Solanum lycopersicum). J. Plant Physiol. 2021, 263, 153460. [Google Scholar] [CrossRef]
- Chaabouni, S.; Jones, B.; Delalande, C.; Wang, H.; Li, Z.; Mila, I.; Frasse, P.; Latché, A.; Pech, J.C.; Bouzayen, M. Sl-IAA3, a tomato Aux/IAA at the crossroads of auxin and ethylene signalling involved in differential growth. J. Exp. Bot. 2009, 60, 1349–1362. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Zhang, W.; Ding, Y.; Zhang, J.; Cambula, E.D.; Weng, F.; Liu, Z.; Ding, C.; Tang, S.; Chen, L.; et al. Shading contributes to the reduction of stem mechanical strength by decreasing cell wall synthesis in japonica rice (Oryza sativa L.). Front. Plant Sci. 2017, 8, 881. [Google Scholar] [CrossRef]
- Yang, R.; Wu, Z.; Bai, C.; Sun, Z.; Wang, M.; Huo, Y.; Zhang, H.; Wang, Y.; Zhou, H.; Dai, S.; et al. Overexpression of PvWOX3a in switchgrass promotes stem development and increases plant height. Hortic. Res. 2021, 8, 252. [Google Scholar] [CrossRef]
- Batistič, O.; Sorek, N.; Schültke, S.; Yalovsky, S.; Kudla, J. Dual fatty acyl modification determines the localization and plasma membrane targeting of CBL/CIPK Ca21 signaling complexes in Arabidopsis. Plant Cell 2008, 20, 1346–1362. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, J.; Wang, X.; Pang, W.; Jiang, J. GA3-Induced SlXTH19 Expression Enhances Cell Wall Remodeling and Plant Height in Tomatoes. Plants 2024, 13, 3578. https://doi.org/10.3390/plants13243578
Luo J, Wang X, Pang W, Jiang J. GA3-Induced SlXTH19 Expression Enhances Cell Wall Remodeling and Plant Height in Tomatoes. Plants. 2024; 13(24):3578. https://doi.org/10.3390/plants13243578
Chicago/Turabian StyleLuo, Junfeng, Xi Wang, Wenxing Pang, and Jing Jiang. 2024. "GA3-Induced SlXTH19 Expression Enhances Cell Wall Remodeling and Plant Height in Tomatoes" Plants 13, no. 24: 3578. https://doi.org/10.3390/plants13243578
APA StyleLuo, J., Wang, X., Pang, W., & Jiang, J. (2024). GA3-Induced SlXTH19 Expression Enhances Cell Wall Remodeling and Plant Height in Tomatoes. Plants, 13(24), 3578. https://doi.org/10.3390/plants13243578






