Gynoxys reinaldii Cuatrec. and Gynoxys pulchella (Kunth) Cass.: Chemical and Enantioselective Analyses of Two Unprecedented Essential Oils from Ecuador
Abstract
1. Introduction
2. Results
2.1. Chemical Analyses of the EOs
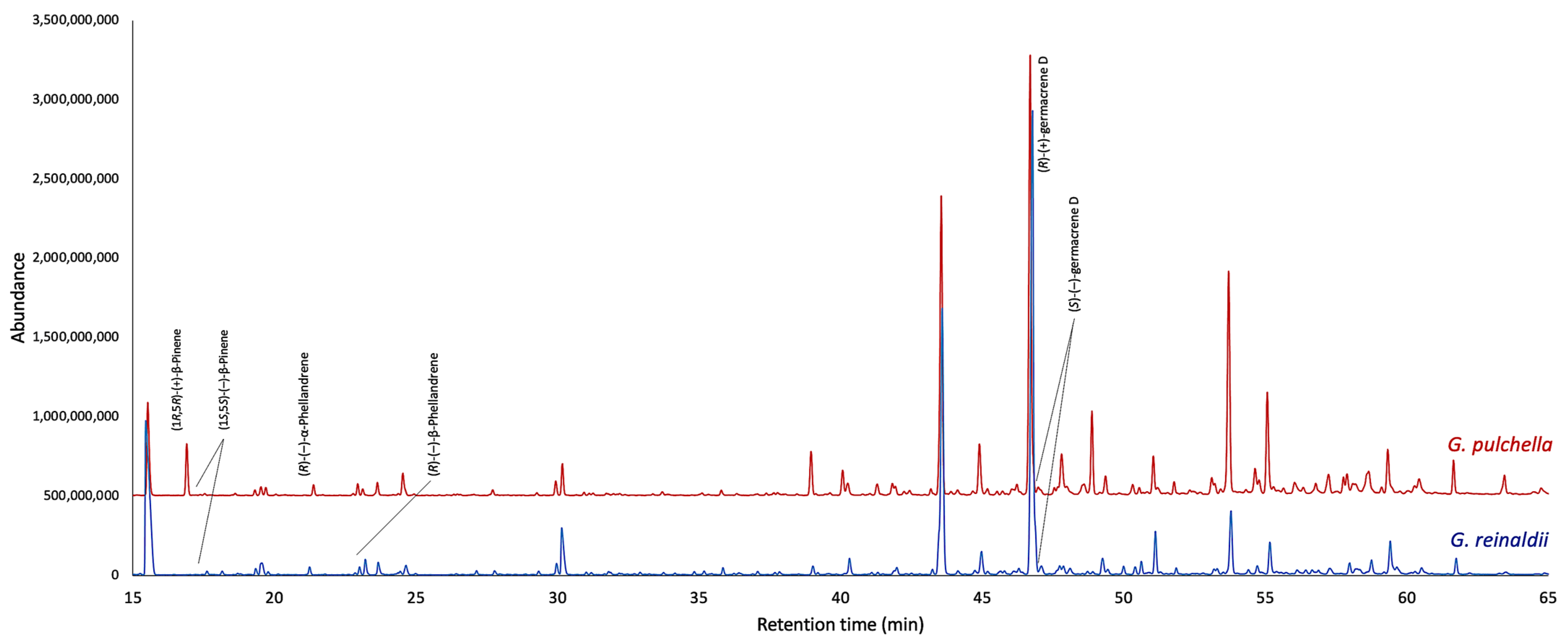
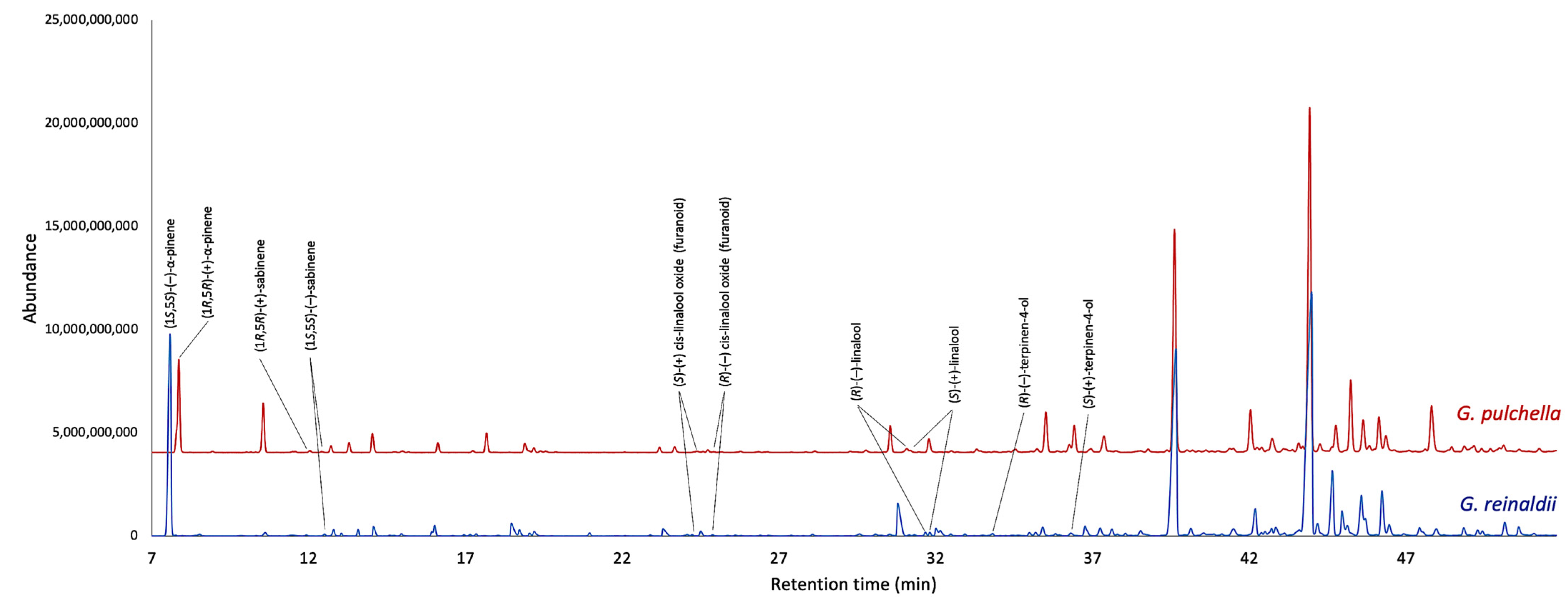
2.2. Enantioselective Analyses
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. EOs Distillation and Samples Preparation
4.3. Qualitative Analyses (GC-MS) of the EOs
4.4. Quantitative Analyses (GC-FID) of the EOs
4.5. Enantioselective Analyses
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huxtable, R.J.; Schwarz, S.K. The isolation of morphine—First principles in science and ethics. Mol. Interv. 2001, 1, 189–191. [Google Scholar] [PubMed]
- Megadiverse Countries, UNEP-WCMC. Available online: https://www.biodiversitya-z.org/content/megadiverse-countries (accessed on 12 June 2024).
- Malagón, O.; Ramírez, J.; Andrade, J.; Morocho, V.; Armijos, C.; Gilardoni, G. Phytochemistry and Ethnopharmacology of the Ecuadorian Flora. A Review. Nat. Prod. Commun. 2016, 11, 297. [Google Scholar] [CrossRef] [PubMed]
- Armijos, C.; Ramírez, J.; Salinas, M.; Vidari, G.; Suárez, A.I. Pharmacology and Phytochemistry of Ecuadorian Medicinal Plants: An Update and Perspectives. Pharmaceuticals 2021, 14, 1145. [Google Scholar] [CrossRef] [PubMed]
- Chiriboga, X.; Gilardoni, G.; Magnaghi, I.; Vita Finzi, P.; Zanoni, G.; Vidari, G. New Anthracene Derivatives from Coussarea macrophylla. J. Nat. Prod. 2003, 66, 905–909. [Google Scholar] [CrossRef] [PubMed]
- Gilardoni, G.; Tosi, S.; Mellerio, G.; Maldonado, M.E.; Chiriboga, X.; Vidari, G. Lipophilic Components from the Ecuadorian Plant Schistocarpha eupatorioides. Nat. Prod. Commun. 2011, 6, 767–772. [Google Scholar] [CrossRef]
- Gilardoni, G.; Chiriboga, X.; Finzi, P.V.; Vidari, G. New 3,4-Secocycloartane and 3,4-Secodammarane Triterpenes from the Ecuadorian Plant Coussarea macrophylla. Chem. Biodivers. 2015, 12, 946–954. [Google Scholar] [CrossRef]
- Maldonado, Y.E.; Montalván, M.; Cumbicus, N.; Gilardoni, G. Chemical and Enantioselective Analyses of an Unprecedented Essential Oil from Ecuadorian Aiouea montana: A Natural Source of S-Methyl-O-2-phenylethyl Carbonothioate. ACS Omega 2024, 9, 26495–26502. [Google Scholar] [CrossRef] [PubMed]
- Gilardoni, G.; Montalván, M.; Ortiz, M.; Vinueza, D.; Montesinos, J.V. The Flower Essential Oil of Dalea mutisii Kunth (Fabaceae) from Ecuador: Chemical, Enantioselective, and Olfactometric Analyses. Plants 2020, 9, 1403. [Google Scholar] [CrossRef]
- Espinosa, S.; Bec, N.; Larroque, C.; Ramírez, J.; Sgorbini, B.; Bicchi, C.; Gilardoni, G. Chemical, Enantioselective, and Sensory Analysis of a Cholinesterase Inhibitor Essential Oil from Coreopsis triloba S.F. Blake (Asteraceae). Plants 2019, 8, 448. [Google Scholar] [CrossRef] [PubMed]
- Plants of the World Online. Royal Botanic Gardens. Available online: https://powo.science.kew.org/ (accessed on 12 June 2024).
- Tropicos.org. Missouri Botanical Garden. Available online: https://www.tropicos.org (accessed on 12 June 2024).
- Arias, R.; Espinosa-Ortega, N.; Revilla, I.; Ansaloni, R.; Tomasello, S. Gynoxys revolutifolia (Senecioneae, Asteraceae): A new species from southern Ecuador. Phytotaxa 2024, 644, 211. [Google Scholar] [CrossRef]
- Malagón, O.; Cartuche, P.; Montaño, A.; Cumbicus, N.; Gilardoni, G. A New Essential Oil from the Leaves of the Endemic Andean Species Gynoxys miniphylla Cuatrec. (Asteraceae): Chemical and Enantioselective Analyses. Plants 2022, 11, 398. [Google Scholar] [CrossRef]
- Maldonado, Y.E.; Malagón, O.; Cumbicus, N.; Gilardoni, G. A New Essential Oil from the Leaves of Gynoxys rugulosa Muschl. (Asteraceae) Growing in Southern Ecuador: Chemical and Enantioselective Analyses. Plants 2023, 12, 849. [Google Scholar] [CrossRef] [PubMed]
- Cumbicus, C.; Malagón, O.; Cumbicus, N.; Gilardoni, G. The Leaf Essential Oil of Gynoxys buxifolia (Kunth) Cass. (Asteraceae): A Good Source of Furanoeremophilane and Bakkenolide A. Plants 2023, 12, 1323. [Google Scholar] [CrossRef]
- Gilardoni, G.; Lara, L.R.; Cumbicus, N.; Malagón, O. A New Leaf Essential Oil from Endemic Gynoxys laurifolia (Kunth) Cass. of Southern Ecuador: Chemical and Enantioselective Analyses. Plants 2023, 12, 2878. [Google Scholar] [CrossRef] [PubMed]
- Maldonado, Y.E.; Betancourt, E.A.; León, E.S.; Malagón, O.; Cumbicus, N.; Gilardoni, G. New Essential Oils from Ecuadorian Gynoxys cuicochensis Cuatrec. and Gynoxys sancti-antonii Cuatrec. Chemical Compositions and Enantioselective Analyses. ACS Omega 2024, 9, 25902–25913. [Google Scholar] [CrossRef] [PubMed]
- Maldonado, Y.E.; Malagón, O.; Cumbicus, N.; Gilardoni, G. A New Leaf Essential Oil from the Andean Species Gynoxys szyszylowiczii Hieron. of Southern Ecuador: Chemical and Enantioselective Analyses. Sci. Rep. 2024, 14, 16360. [Google Scholar] [CrossRef]
- Jorgensen, P.; Leon-Yanez, S. Catalogue of the Vascular Plants of Ecuador; Missouri Botanical Garden Press: St. Louis, MO, USA, 1999; pp. 286–288. [Google Scholar]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured Publishing Corporation: Carol Stream, IL, USA, 2007; ISBN 10-193263321. [Google Scholar]
- Buttery, R.G.; Orts, W.J.; Takeoka, G.R.; Nam, Y. Volatile flavor components of rice cakes. J. Agric. Food Chem. 1999, 47, 4353–4356. [Google Scholar] [CrossRef]
- Fanciullino, A.L.; Gancel, A.L.; Froelicher, Y.; Luro, F.; Ollitrault, P.; Brillouet, J.M. Effects of Nucleo Cytoplasmic Interactions on Leaf Volatile Compounds from Citrus Somatic Diploid Hybrids. J. Agric. Food Chem. 2005, 53, 4517–4523. [Google Scholar] [CrossRef] [PubMed]
- Dugo, P.; Mondello, L.; Zappia, G.; Bonaccorsi, I.; Cotroneo, A.; Russo, M.-T. The composition of the volatile fraction and the enantiomeric distribution of five volatile components of faustrime oil (Monocitrus australatica x Fortunella sp. x Citrus aurantifolia). J. Essent. Oil Res. 2004, 16, 328–333. [Google Scholar] [CrossRef]
- Flamini, G.; Tebano, M.; Cioni, P.L.; Bagci, Y.; Dural, H.; Ertugrul, K.; Uysal, T.; Savran, A. A Multivariate Statistical Approach to Centaurea Classification Using Essential Oil Composition Data of Some Species from Turkey. Plant Syst. Evol. 2006, 261, 217–228. [Google Scholar] [CrossRef]
- Yu, E.J.; Kim, T.H.; Kim, K.H.; Lee, H.J. Aroma-active compounds of Pinus densiflora (red pine) needles. Flavour Fragr. J. 2004, 19, 532–537. [Google Scholar] [CrossRef]
- Bianchi, F.; Careri, M.; Mangia, A.; Musci, M. Retention indices in the analysis of food aroma volatile compounds in temperature programmed gas chromatography: Database creation and evaluation of precision and robustness. J. Sep. Sci. 2007, 39, 4. [Google Scholar] [CrossRef] [PubMed]
- Ruiz Perez-Cacho, P.; Mahattanatawee, K.; Smoot, J.M.; Rouseff, R. Identification of sulfur volatiles in canned orange juices lacking orange flavor. J. Agric. Food Chem. 2007, 55, 5761–5767. [Google Scholar] [CrossRef]
- Fernandez-Segovia, I.; Escriche, I.; Gomez-Sintes, M.; Fuentes, A.; Serra, J.-A. Influence of different preservation treatments on the volatile fraction of desalted cod. Food Chem. 2006, 98, 473–482. [Google Scholar] [CrossRef]
- Mahattanatawee, K.; Perez-Cacho, P.-R.; Davenport, T.; Rouseff, R. Comparison of three lychee cultivar odor profiles using gas chromatography-olfactometry and gas chromatography-sulfur detection. J. Agric. Food Chem. 2007, 55, 1939–1944. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-C.; Kuo, M.-C.; Liu, S.-E.; Wu, C.-M. Volatile components of salted and pickled prunes (Prunus mume Sieb. et Zucc.). J. Agric. Food Chem. 1986, 34, 140–144. [Google Scholar] [CrossRef]
- Shellie, R.; Mondello, L.; Marriott, P.; Dugo, G. Characterization of lavender essential oils by using gas chromatography-mass spectrometry with correlation of linear retention indices and comparison with comprehensive two-dimensional gas chromatography. J. Chromatogr. A 2002, 970, 225–234. [Google Scholar] [CrossRef]
- Kim, T.H.; Thuy, N.T.; Shin, J.H.; Baek, H.-H.; Lee, H.J. Aroma-active compounds of miniature beefsteakplant (Mosla dianthera Maxim.). J. Agric. Food Chem. 2000, 48, 2877–2881. [Google Scholar] [CrossRef]
- Bader, A.; Caponi, C.; Cioni, P.L.; Flamini, G.; Morelli, I. Acorenone in the essential oil of flowering aerial parts of Seseli tortuosum L. Flavour Fragr. J. 2003, 18, 57–58. [Google Scholar] [CrossRef]
- Wu, S.; Zorn, H.; Krings, U.; Berger, R.G. Characteristic Volatiles from Young and Aged Fruiting Bodies of Wild Polyporus sulfureus (Bull.:Fr.) Fr. J. Agric. Food Chem. 2005, 53, 4524–4528. [Google Scholar] [CrossRef] [PubMed]
- Cho, I.H.; Namgung, H.-J.; Choi, H.-K.; Kim, Y.-S. Volatiles and key odorants in the pileus and stipe of pine-mushroom (Tricholoma matsutake Sing.). Food Chem. 2008, 106, 71–76. [Google Scholar] [CrossRef]
- Saroglou, V.; Marin, P.D.; Rancic, A.; Veljic, M.; Skaltsa, H. Composition and antimicrobial activity of the essential oil of six Hypericum species from Serbia. Biochem. Syst. Ecol. 2007, 35, 146–152. [Google Scholar] [CrossRef]
- Da Porto, C.; Pizzale, L.; Bravin, M.; Conte, L.S. Analyses of orange spirit flavour by direct-injection gas chromatography–mass spectrometry and headspace solid-phase microextraction/GC–MC. Flavour Fragr. J. 2003, 18, 66–72. [Google Scholar] [CrossRef]
- Ka, M.H.; Choi, E.H.; Chun, H.S.; Lee, K.G. Antioxidative Activity of Volatile Extracts Isolated from Angelica tenuissimae Roots, Peppermint Leaves, Pine Needles, and Sweet Flag Leaves. J. Agric. Food Chem. 2005, 53, 4124–4129. [Google Scholar] [CrossRef] [PubMed]
- Riu-Aumatell, M.; Lopez-Tamames, E.; Buxaderas, S. Assessment of the Volatile Composition of Juices of Apricot, Peach, and Pear According to Two Pectolytic Treatments. J. Agric. Food Chem. 2005, 53, 7837–7843. [Google Scholar] [CrossRef]
- Kollmannsberger, H.; Nitz, S.; Drawert, F. Über die Aromastoffzusammensetzung von Hochdruckextrakten. I. Pfeffer (Piper nigrum, Var. muntok). Z. Für Lebensm. Unters. Und-Forsch. 1992, 194, 545–551. [Google Scholar] [CrossRef]
- Yu, E.J.; Kim, T.H.; Kim, K.H.; Lee, H.J. Characterization of aroma-active compounds of Abies nephrolepis (Khingan fir) needles using aroma extract dilution analysis. Flavour Fragr. J. 2004, 19, 74–79. [Google Scholar] [CrossRef]
- Tu, N.T.M.; Onishi, Y.; Choi, H.-S.; Kondo, Y.; Bassore, S.M.; Ukeda, H.; Sawamura, M. Characteristic odor components of Citrus sphaerocarpa Tanaka (Kabosu) cold-pressed peel oil. J. Agric. Food Chem. 2002, 50, 2908–2913. [Google Scholar]
- Werkhoff, P.; Güntert, M. Identification of some ester compounds in bourbon vanilla beans. Lebensm. Wiss. Technol. 1997, 30, 429–431. [Google Scholar] [CrossRef]
- Tunalier, Z.; Candan, N.T.; Demirci, B.; Baser, K.H. The essential oil composition of Acroptilon repens (L.) DC. of Turkish origin. Flavour Fragr. J. 2006, 21, 462–464. [Google Scholar] [CrossRef]
- Pozo-Bayon, M.A.; Ruiz-Rodriguez, A.; Pernin, K.; Cayot, N. Influence of eggs on the aroma composition of a sponge cake and on the aroma release in model studies on flavored sponge cakes. J. Agric. Food Chem. 2007, 55, 1418–1426. [Google Scholar] [CrossRef]
- Kotseridis, Y.; Baumes, R. Identification of impact odorants in Bordeaux red grape juice, in the commercial yeast used for its fermentation, and in the produced wine. J. Agric. Food Chem. 2000, 48, 400–406. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, S.S.; Goddik, L.; Qian, M.C. Aroma Compounds in Sweet Whey Powder. J. Dairy Sci. 2004, 87, 4057–4063. [Google Scholar] [CrossRef]
- Flamini, G.; Cioni, P.L.; Morelli, I.; Maccioni, S.; Baldini, R. Phytochemical typologies in some populations of Myrtus communis L. on Caprione Promontory (East Liguria, Italy). Food Chem. 2004, 85, 599–604. [Google Scholar] [CrossRef]
- Varming, C.; Andersen, M.L.; Poll, L. Influence of thermal treatment on black currant (Ribes nigrum L. ) juice aroma. J. Agric. Food Chem. 2004, 52, 7628–7636. [Google Scholar] [CrossRef]
- Grujic-Jovanovic, S.; Skaltsa, H.D.; Marin, P.; Sokovic, M. Composition and antibacterial activity of the essential oil of six Stachys species from Serbia. Flavour Fragr. J. 2004, 19, 139–144. [Google Scholar] [CrossRef]
- Gauvin-Bialecki, A.; Marodon, C. Essential oil of Ayapana triplinervis from Reunion Island: A good natural source of thymohydroquinone dimethyl ether. Biochem. Syst. Ecol. 2009, 36, 853–858. [Google Scholar] [CrossRef]
- Shellie, R.; Marriott, P.; Zappia, G.; Mondello, L.; Dugo, G. Interactive use of linear retention indices on polar and apolar columns with an MS-Library for reliable characterization of Australian tea tree and other Melaleuca sp. Oils. J. Essent. Oil Res. 2003, 15, 305–312. [Google Scholar] [CrossRef]
- Ngassoum, M.B.; Yonkeu, S.; Jirovetz, L.; Buchbauer, G.; Schmaus, G.; Hammerschmidt, F.-J.H. Chemical composition of essential oils of Lantana camara leaves and flowers from Cameroon and Madagascar. Flavour Fragr. J. 1999, 14, 245–250. [Google Scholar] [CrossRef]
- Baser, K.H.C.; Ozek, G.; Ozek, T.; Duran, A. Composition of the essential oil of Centaurea huber-morathii Wagenitz isolated from seeds by microdistillation. Flavour Fragr. J. 2006, 21, 568–570. [Google Scholar] [CrossRef]
- Paniandy, J.-C.; Chane-Ming, J.; Pierbattesti, J.-C. Chemical Composition of the Essential Oil and Headspace Solid-Phase Microextraction of the Guava Fruit (Psidium guajava L.). J. Essent. Oil Res. 2000, 12, 153–158. [Google Scholar] [CrossRef]
- Cavalli, J.-F.; Tomi, F.; Bernardini, A.-F.; Casanova, J. Composition and chemical variability of the bark oil of Cedrelopsis grevei H. Baillon from Madagascar. Flavour Fragr. J. 2003, 18, 532–538. [Google Scholar] [CrossRef]
- Blazevic, I.; Mastelic, J. Free and bounded volatiles of rocket (Eruca sativa Mill.). Fravour Fragr. J. 2008, 23, 278–285. [Google Scholar] [CrossRef]
- Ferretti, G.; Maggi, F.; Tirillini, B. Essential oil composition of Hypericum richeri Vill. from Italy. Flavour Fragr. J. 2005, 20, 295–298. [Google Scholar] [CrossRef]
- Chen, C.-C.; Ho, C.-T. Gas chromatographic analysis of volatile components of ginger oil (Zingiber officinale Roscoe) extracted with liquid carbon dioxide. J. Agric. Food Chem. 1988, 36, 322–328. [Google Scholar] [CrossRef]
- Brat, P.; Rega, B.; Alter, P.; Reynes, M.; Brillouet, J.-M. Distribution of volatile compounds in the pulp, cloud, and serum of freshly squeezed orange juice. J. Agric. Food Chem. 2003, 51, 3442–3447. [Google Scholar] [CrossRef]
- Zheng, C.H.; Kim, K.H.; Kim, T.H.; Lee, H.J. Analysis and characterization of aroma-active compounds of Schizandra chinensis (omija) leaves. J. Sci. Food Agric. 2005, 85, 161–166. [Google Scholar] [CrossRef]
- Wu, S.; Zorn, H.; Krings, U.; Berger, R.G. Volatiles from submerged and surface-cultured beefsteak fungus, Fistulina hepatica. Flavour Fragr. J. 2007, 22, 53–60. [Google Scholar] [CrossRef]
- Karlsson, M.F.; Birgersson, G.; Prado, A.M.C.; Bosa, F.; Bengtsson, M.; Witzgall, P. Plant Odor Analysis of Potato: Response of Guatemalan Moth to Above- and Background Potato Volatiles. J. Agric. Food Chem. 2009, 57, 5903–5909. [Google Scholar] [CrossRef] [PubMed]
- Jerkovic, I.; Mastelic, J.; Milos, M.; Juteau, F.; Masotti, V.; Viano, J. Chemical variability of Artemisia vulgaris L. essential oils originated from the Mediterranean area of France and Croatia. Flavour Fragr. J. 2003, 18, 436–440. [Google Scholar] [CrossRef]
- Hachicha, S.F.; Skanji, T.; Barrek, S.; Ghrabi, Z.G.; Zarrouk, H. Composition of the essential oil of Teucrium ramosissimum Desf. (Lamiaceae) from Tunisia. Flavour Fragr. J. 2007, 22, 101–104. [Google Scholar] [CrossRef]
- Jirovetz, L.; Buchbauer, G.; Shafi, P.M.; Rosamma, M.K.; Geissler, M. Analysis of the composition and aroma of the essential leaf oil of Syzygium travancoricum from South India by GC-FID, GC-MS, and olfactometry. Seasonal changes of composition. Chromatographia 2001, 53, S372–S374. [Google Scholar] [CrossRef]
- Marques, F.A.; McElfresh, J.S.; Millar, J.G. Kováts retention indexes of monounsaturated C12, C14, and C16 alcohols, acetates and aldehydes commonly found in lepidopteran pheromone blends. J. Braz. Chem. Soc. 2000, 11, 592–599. [Google Scholar] [CrossRef]
- Paolini, J.; Tomi, P.; Bernardini, A.-F.; Bradesi, P.; Casanova, J.; Kaloustian, J. Detailed analysis of the essential oil from Cistus albidus L. by combination of GC/RI, GC/MS and 13C-NMR spectroscopy. Nat. Prod. Res. 2008, 22, 1270–1278. [Google Scholar] [CrossRef] [PubMed]
- Bendiabdellah, A.; El Amine Dib, M.; Djabou, N.; Allali, H.; Tabti, B.; Costa, J.; Myseli, A. Biological activities and volatile constituents of Daucus muricatus L. from Algeria. Chem. Cent. J. 2012, 6, 48. [Google Scholar] [CrossRef]
- Neves, A.; Rosa, S.; Goncalves, J.; Rufino, A.; Judas, F.; Salgueiro, L.; Lopes, M.C.; Cavaleiro, C.; Mendes, A.F. Screening of five essential oils for identification of potential inhibitors of IL-1-unduced Nf-kB activation and NO production in human chondrocytes: Characterization of the inhibitory activity of alpha-pinene. Planta Medica 2010, 76, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.-S. Character impact odorants of Citrus Hallabong [(C. unshiu Marcov × C. sinensis Osbeck) × C. reticulata Blanco] cold-pressed peel oil. J. Agric. Food Chem. 2003, 51, 2687–2692. [Google Scholar] [CrossRef]
- Ledauphin, J.; Basset, B.; Cohen, S.; Payot, T.; Barillier, D. Identification of trace volatile compounds in freshly distilled Calvados and Cognac: Carbonyl and sulphur compounds. J. Food Compos. Anal. 2006, 19, 28–40. [Google Scholar] [CrossRef]
- Pennarun, A.L.; Prost, C.; Demaimay, M. Identification and origin of the character-impact compounds of raw oyster Crassostrea gigas. J. Sci. Food Agric. 2002, 82, 1652–1660. [Google Scholar] [CrossRef]
- Vinogradov, B.A. Production, Composition, Properties and Application of Essential Oils. 2004. Available online: http://viness.narod.ru (accessed on 20 November 2024).
- Shiratsuchi, H.; Shimoda, M.; Imayoshi, K.; Noda, K.; Osajima, Y. Volatile flavor compounds in spray-dried skim milk powder. J. Agric. Food Chem. 1994, 42, 984–988. [Google Scholar] [CrossRef]
- Kirimer, N.; Tabanca, N.; Özek, T.; Tümen, G.; Baser, K.H.C. Essential oils of annual Sideritis species growing in Turkey. Pharm. Biol. 2000, 38, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Condurso, C.; Verzera, A.; Romeo, V.; Ziino, M.; Trozzi, A.; Ragusa, S. The leaf volatile constituents of Isatis tinctoria by solid-phase microextraction and gas chromatography/mass spectrometry. Planta Medica 2006, 72, 924–928. [Google Scholar] [CrossRef] [PubMed]
- Radulovic, N.; Blagojevic, P.; Palic, R. Comparative study of the leaf volatiles of Arctostaphylos uva-ursi (L. ) Spreng. and Vaccinium vitis-idaea L. (Ericaceae). Molecules 2010, 15, 6168–6185. [Google Scholar]
- Dewick, P.M. Medicinal Natural Products. A Biosynthetic Approach, 3rd ed.; John Wiley & Sons Ltd.: Chichester, UK, 2009; ISBN 9780470741672. [Google Scholar]
- Røstelien, T.; Borg-Karlson, A.K.; Fäldt, J.; Jacobsson, U.; Mustaparta, H. The Plant Sesquiterpene Germacrene D Specifically Activates a Major Type of Antennal Receptor Neuron of the Tobacco Budworm Moth Heliothis virescens. Chem. Senses 2000, 25, 141. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mozuraitis, R.; Stranden, M.; Ramirez, M.I.; Borg-Karlson, A.K.; Mustaparta, H. (-)-Germacrene D Increases Attraction and Oviposition by the Tobacco Budworm Moth Heliothis virescens. Chem. Senses 2002, 27, 505–509. [Google Scholar] [CrossRef] [PubMed]
- Stranden, M.; Liblikas, I.; Koenig, W.A.; Almaas, T.J.; Borg-Karlson, A.K.; Mustaparta, H. (-)-GermacreneD Receptor Neurones in Three Species of Heliothine Moths: Structure-activity Relationships. J. Comp. Physiol. A 2003, 189, 563–577. [Google Scholar] [CrossRef]
- Langenheim, J.H. Higher Plant Terpenoids: A Phytocentric Overview of Their Ecological Roles. J. Chem. Ecol. 1994, 20, 1223–1280. [Google Scholar] [CrossRef] [PubMed]
- Allenspach, M.; Steuer, C. α-Pinene: A Never-Ending Story. Phytochemistry 2021, 190, 112857. [Google Scholar] [CrossRef]
- Salehi, B.; Upadhyay, S.; Erdogan Orhan, I.; Kumar Jugran, A.; Jayaweera, S.L.D.; Dias, D.A.; Sharopov, F.; Taheri, Y.; Martins, N.; Baghalpour, N.; et al. Therapeutic Potential of α- and β-Pinene: A Miracle Gift of Nature. Biomolecules 2019, 9, 738. [Google Scholar] [CrossRef] [PubMed]
- Francomano, F.; Caruso, A.; Barbarossa, A.; Fazio, A.; La Torre, C.; Ceramella, J.; Mallamaci, R.; Saturnino, C.; Iacopetta, D.; Sinicropi, M.S. β-Caryophyllene: A Sesquiterpene with Countless Biological Properties. Appl. Sci. 2019, 9, 5420. [Google Scholar] [CrossRef]
- Scandiffio, R.; Geddo, F.; Cottone, E.; Querio, G.; Antoniotti, S.; Gallo, M.P.; Maffei, M.E.; Bovolin, P. Protective Effects of (E)-β-Caryophyllene (BCP) in Chronic Inflammation. Nutrients 2020, 12, 3273. [Google Scholar] [CrossRef] [PubMed]
- Fidyt, K.; Fiedorowicz, A.; Strządała, L.; Szumny, A. β-Caryophyllene and β-Caryophyllene Oxide—Natural Compounds of Anticancer and Analgesic Properties. Cancer Med. 2016, 5, 3007–3017. [Google Scholar] [CrossRef] [PubMed]
- do Nascimento, K.F.; Moreira, F.M.F.; Santos, J.A.; Kassuya, C.A.L.; Croda, J.H.R.; Cardoso, C.A.L.; Vieira, M.D.C.; Ruiz, A.L.T.G.; Foglio, M.A.; de Carvalho, J.E.; et al. Antioxidant, Anti-inflammatory, Antiproliferative and Antimycobacterial Activities of the Essential Oil of Psidium guineense Sw. and Spathulenol. J. Ethnopharmacol. 2018, 210, 351–358. [Google Scholar] [CrossRef]
- Van Den Dool, H.; Kratz, P.D. A Generalization of the Retention Index System Including Linear Temperature Programmed Gas—Liquid Partition Chromatography. J. Chromatogr. A 1963, 11, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Gilardoni, G.; Matute, Y.; Ramírez, J. Chemical and Enantioselective Analysis of the Leaf Essential Oil from Piper coruscans Kunth (Piperaceae), a Costal and Amazonian Native Species of Ecuador. Plants 2020, 9, 791. [Google Scholar] [CrossRef] [PubMed]
- De Saint Laumer, J.Y.; Cicchetti, E.; Merle, P.; Egger, J.; Chaintreau, A. Quantification in Gas Chromatography: Prediction of Flame Ionization Detector Response Factors from Combustion Enthalpies and Molecular Structures. Anal. Chem. 2010, 82, 6457–6462. [Google Scholar] [CrossRef] [PubMed]
- Tissot, E.; Rochat, S.; Debonneville, C.; Chaintreau, A. Rapid GC-FID quantification technique without authentic samples using predicted response factors. Flavour Fragr. J. 2012, 27, 290–296. [Google Scholar] [CrossRef]
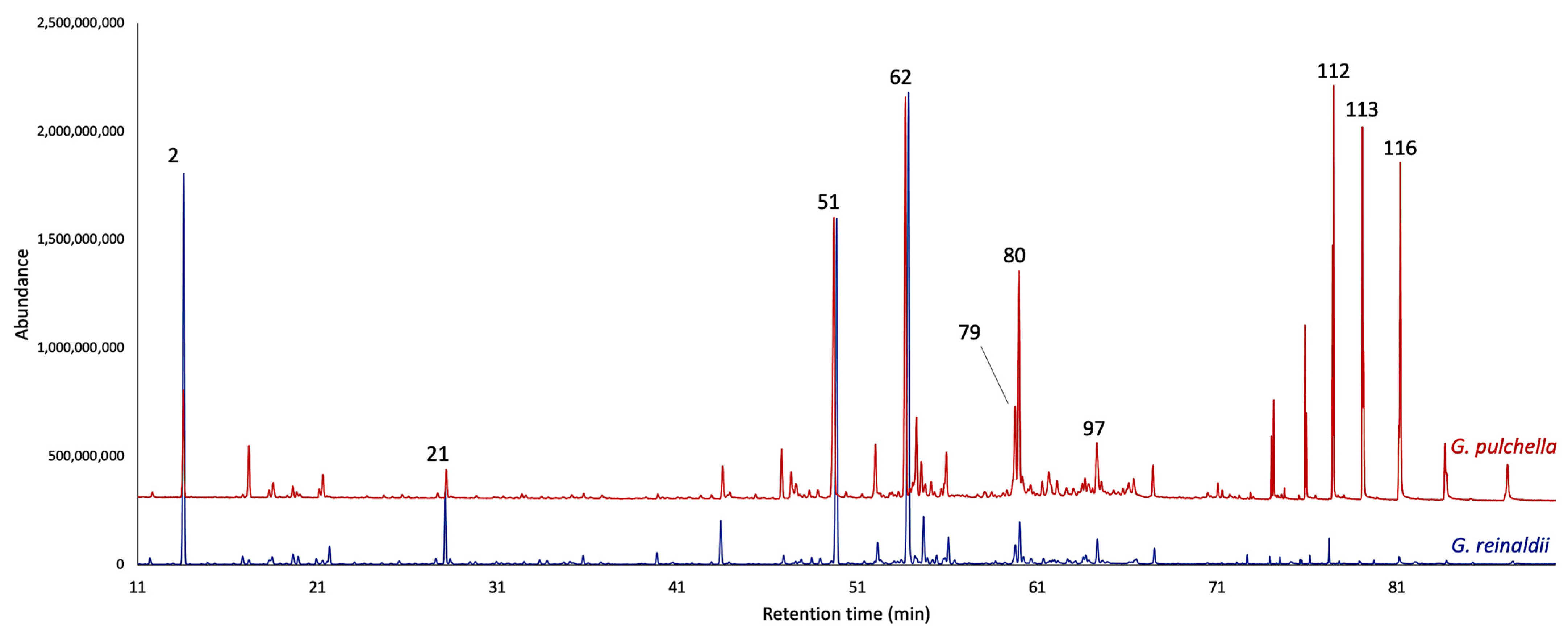
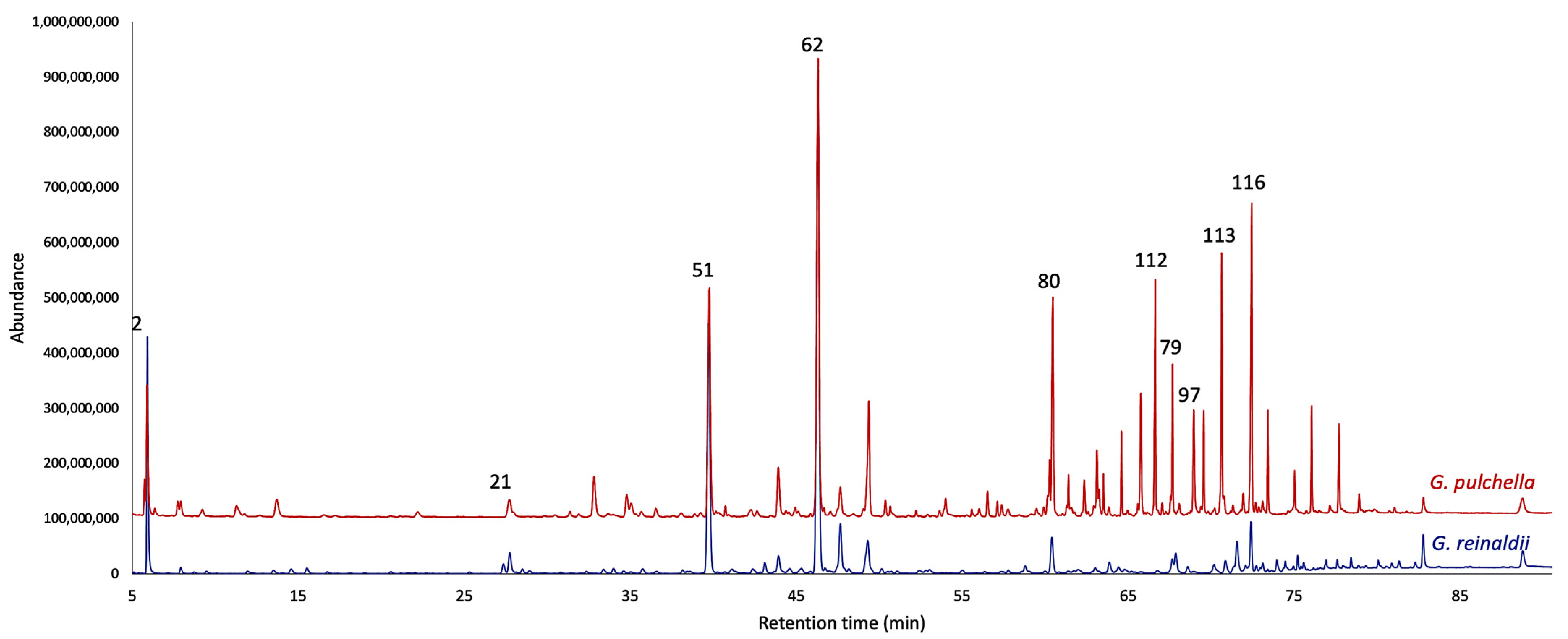
| N° | Compounds | 5% Phenyl-Methylpolysiloxane | Polyethylene Glycol | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LRI | G. reinaldii | G. pulchella | LRI | G. reinaldii | G. pulchella | Lit. | ||||||||
| Calc. | Ref. [21] | % | σ | % | σ | Calc. | Ref. | % | σ | % | σ | |||
| 1 | heptanal | 911 | 901 | 0.3 | 0.10 | - | - | 1180 | 1180 | 0.4 | 0.08 | - | - | [22] |
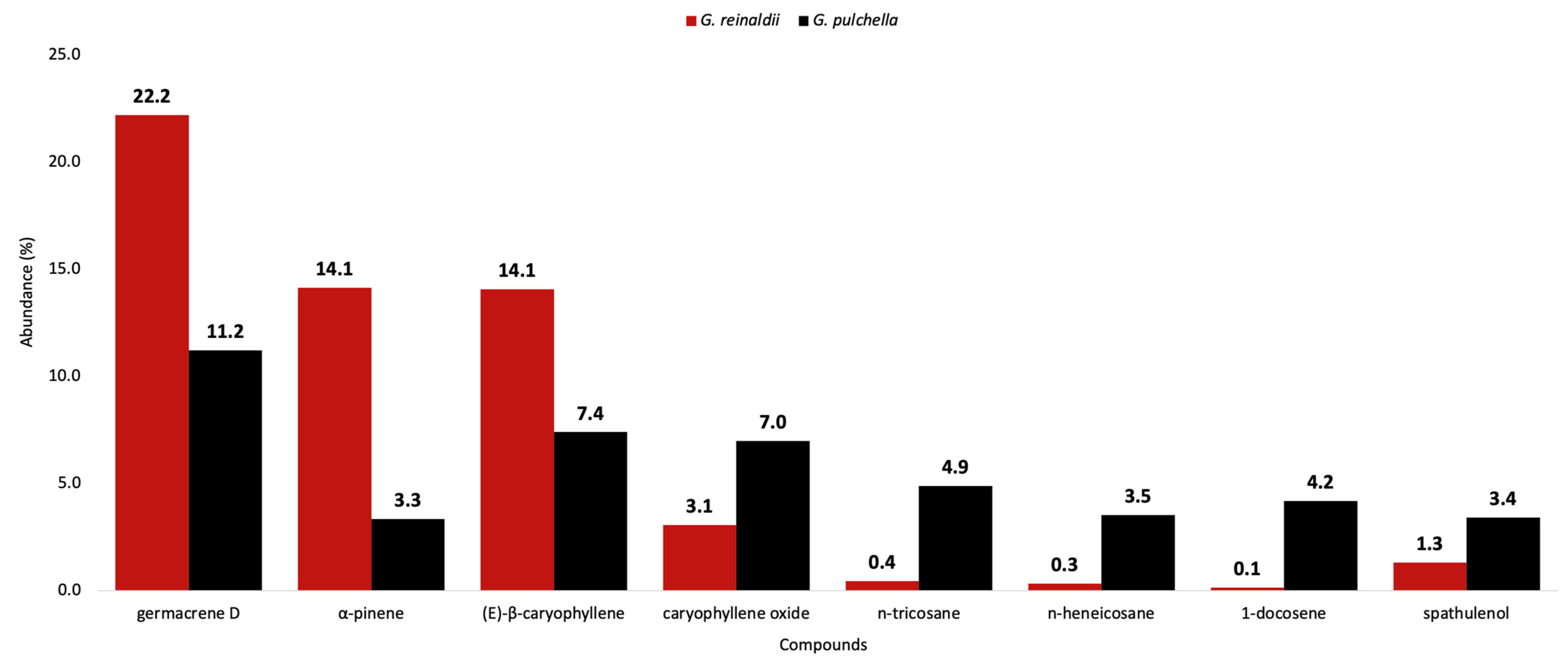
| 2 | α-pinene | 934 | 932 | 14.2 | 3.81 | 3.7 | 0.77 | 1015 | 1015 | 14.1 | 3.57 | 3.0 | 0.55 | [23] |
| 3 | α-fenchene | 950 | 945 | 0.1 | 0.02 | - | - | 1053 | 1048 | trace | - | - | - | [24] |
| 4 | thuja-2,4(10)-diene | 955 | 953 | 0.1 | 0.00 | - | - | 1116 | 1116 | 0.3 | 0.08 | - | - | [25] |
| 5 | sabinene | 974 | 969 | 0.3 | 0.08 | 0.1 | 0.02 | 1113 | 1114 | trace | - | 0.1 | 0.03 | [26] |
| 6 | β-pinene | 978 | 974 | 0.2 | 0.05 | 2.0 | 0.46 | 1101 | 1102 | 0.2 | 0.05 | 1.7 | 0.34 | [27] |
| 7 | myrcene | 992 | 988 | 0.1 | 0.03 | 0.2 | 0.05 | 1159 | 1159 | 0.1 | 0.03 | 0.2 | 0.04 | [28] |
| 8 | 2-pentyl furan | 994 | 984 | 0.6 | 0.15 | 0.6 | 0.10 | 1228 | 1229 | 0.5 | 0.08 | 0.2 | 0.10 | [29] |
| 9 | n-decane | 1000 | 1000 | 0.2 | 0.10 | - | - | 1000 | 1000 | trace | - | - | - | - |
| 10 | α-phellandrene | 1008 | 1002 | 0.3 | 0.06 | 0.6 | 0.08 | 1154 | 1153 | 0.3 | 0.09 | 0.2 | 0.04 | [23] |
| 11 | n-octanal | 1012 | 1017 | 0.4 | 0.13 | 0.4 | 0.10 | 1295 | 1295 | 0.2 | 0.04 | trace | - | [30] |
| 12 | (2E,4E)-heptadienal | 1024 | 1005 | 0.5 | 0.08 | 0.1 | 0.02 | 1482 | 1481 | 0.9 | 0.11 | 0.1 | 0.01 | [31] |
| 13 | p-cymene | 1029 | 1022 | 0.3 | 0.16 | 0.7 | 0.14 | 1260 | 1254 | 0.1 | 0.02 | 0.5 | 0.11 | [32] |
| 14 | limonene | 1031 | 1024 | 0.1 | 0.05 | - | - | 1188 | 1189 | 0.1 | 0.01 | - | - | [33] |
| 15 | β-phellandrene | 1033 | 1025 | 0.8 | 0.20 | - | - | 1197 | 1197 | 0.6 | 0.17 | - | - | [34] |
| 16 | (E)-β-ocimene | 1050 | 1044 | 0.1 | 0.03 | - | - | 1248 | 1246 | trace | - | - | - | [33] |
| 17 | cis-linalool oxide (furanoid) | 1075 | 1067 | 0.2 | 0.02 | 0.1 | 0.01 | 1461 | 1465 | trace | - | trace | - | [26] |
| 18 | n-octanol | 1081 | 1063 | 0.2 | 0.03 | - | - | 1555 | 1555 | 0.2 | 0.02 | - | - | [35] |
| 19 | cis-vertocitral C | 1088 | 1076 | - | - | 0.1 | 0.01 | 1206 | - | - | - | trace | - | - |
| 20 | linalool | 1106 | 1095 | 0.3 | 0.02 | 0.2 | 0.02 | 1548 | 1547 | 0.3 | 0.02 | 0.4 | 0.26 | [36] |
| 21 | n-nonanal | 1113 | 1100 | 3.0 | 0.59 | 1.0 | 0.09 | 1386 | 1387 | 2.3 | 0.38 | 0.7 | 0.12 | [37] |
| 22 | cis-β-terpineol | 1131 | 1140 | 0.1 | 0.02 | - | - | 1590 | 1639 | 0.1 | 0.01 | - | - | [38] |
| 23 | α-campholenal | 1135 | 1122 | 0.1 | 0.04 | 0.1 | 0.03 | 1475 | 1472 | 0.2 | 0.05 | trace | - | [39] |
| 24 | citronellal | 1150 | 1148 | 0.2 | 0.06 | - | - | - | - | - | - | - | - | - |
| 25 | verbenol | 1154 | 1140 | 0.2 | 0.04 | - | - | - | - | - | - | - | - | - |
| 26 | isomer of compound 29 | 1159 | 1166 | 0.1 | 0.05 | - | - | 1653 | - | 0.3 | 0.03 | - | - | - |
| 27 | ethyl benzoate | 1167 | 1169 | 0.1 | 0.03 | - | - | - | - | - | - | - | - | - |
| 28 | (2E)-nonen-1-al | 1171 | 1157 | 0.2 | 0.03 | - | - | 1523 | 1524 | 0.2 | 0.02 | - | - | [40] |
| 29 | p-mentha-1,5-dien-8-ol | 1182 | 1185 | 0.3 | 0.05 | - | - | 1718 | 1719 | 0.3 | 0.06 | - | - | [41] |
| 30 | terpinen-4-ol | 1187 | 1174 | 0.2 | 0.03 | trace | - | 1589 | 1589 | 0.1 | 0.01 | trace | - | [42] |
| 31 | n-dodecane | 1200 | 1200 | 0.1 | 0.01 | 0.1 | 0.17 | 1200 | 1200 | trace | - | 0.2 | 0.09 | |
| 32 | γ-terpineol | 1204 | 1199 | 0.2 | 0.09 | - | - | 1709 | - | trace | - | - | - | - |
| 33 | isomer of compound 29 | 1207 | - | 0.1 | 0.02 | - | - | 1771 | - | trace | - | - | - | - |
| 34 | n-decanal | 1215 | 1201 | 0.4 | 0.05 | 0.1 | 0.02 | 1491 | 1493 | 0.4 | 0.03 | 0.5 | 0.14 | [43] |
| 35 | trans-piperitol | 1218 | 1207 | 0.1 | 0.05 | - | - | 1736 | 1738 | 0.1 | 0.04 | - | - | |
| 36 | pulegone | 1228 | 1233 | 0.2 | 0.03 | - | - | - | - | - | - | - | - | - |
| 37 | exo-fenchyl acetate | 1230 | 1229 | - | - | 0.1 | 0.02 | 1457 | 1458 | - | - | 0.6 | 0.15 | [44] |
| 38 | (2E)-decenal | 1272 | 1260 | 0.7 | 0.09 | 0.2 | 0.02 | 1630 | 1630 | 0.6 | 0.08 | 0.4 | 0.04 | [36] |
| 39 | 1-tridecene | 1292 | 1290 | 0.1 | 0.02 | - | - | 1351 | 1352 | 0.1 | 0.01 | - | - | [45] |
| 40 | (2E,4Z)-decadienal | 1306 | 1292 | 0.1 | 0.01 | - | - | 1753 | 1793 | 0.3 | 0.08 | - | - | [46] |
| 41 | p-vinyl guaiacol | 1324 | 1309 | 2.0 | 0.17 | 1.4 | 0.14 | 2186 | 2187 | 2.2 | 0.15 | 0.8 | 0.35 | [47] |
| 42 | (2E,4E)-decadienal | 1331 | 1315 | 0.2 | 0.04 | 0.4 | 0.02 | 1794 | 1795 | 0.4 | 0.03 | 0.7 | 0.03 | [48] |
| 43 | α-cubebene | 1347 | 1348 | 0.1 | 0.02 | - | - | 1521 | 1521 | 0.1 | 0.05 | - | - | [49] |
| 44 | α-ylangene | 1376 | 1373 | 0.4 | 0.01 | 1.4 | 0.14 | 1472 | 1472 | 0.3 | 0.02 | 1.4 | 0.19 | [23] |
| 45 | β-bourbonene | 1384 | 1387 | - | - | 1.2 | 0.28 | 1487 | 1491 | - | - | 0.8 | 0.05 | [26] |
| 46 | (E)-β-damascenone | 1386 | 1383 | 0.2 | 0.04 | 0.2 | 0.04 | 1802 | 1802 | 0.1 | 0.05 | 0.4 | 0.11 | [50] |
| 47 | β-cubebene | 1389 | 1387 | - | - | trace | - | 1469 | 1468 | - | - | 0.2 | 0.04 | [51] |
| 48 | β-Elemene | 1391 | 1389 | 0.5 | 0.04 | - | - | 1597 | 1596 | s.p. 123 | - | - | - | [52] |
| 49 | n-tetradecane | 1400 | 1400 | 0.2 | 0.01 | 0.4 | 0.04 | 1400 | 1400 | 0.3 | 0.03 | 0.1 | 0.02 | |
| 50 | α-gurjunene | 1407 | 1409 | 0.3 | 0.02 | - | - | 1508 | 1507 | trace | - | - | - | [53] |
| 51 | (E)-β-caryophyllene | 1422 | 1417 | 13.6 | 1.42 | 7.0 | 0.77 | 1574 | 1575 | 14.5 | 1.1 | 7.8 | 1.56 | [33] |
| 52 | β-copaene | 1432 | 1430 | 0.1 | 0.00 | 0.2 | 0.02 | 1521 | 1522 | 0.1 | 0.02 | 0.0 | 0.01 | [23] |
| 53 | sesquisabinene | 1456 | 1457 | 0.1 | 0.02 | - | - | 1661 | 1648 | s.p. 65 | - | - | - | [54] |
| 54 | unidentified (MW = 204) | 1458 | 1452 | - | - | 1.8 | 0.22 | 1271 | - | - | - | 1.9 | 0.57 | - |
| 55 | α-humulene | 1458 | 1452 | 1.1 | 0.07 | - | - | 1644 | 1644 | 1.1 | 0.11 | - | - | [42] |
| 56 | allo-aromadendrene | 1461 | 1458 | 0.1 | 0.01 | 0.1 | 0.02 | 1655 | 1655 | 0.2 | 0.02 | 0.1 | 0.03 | [55] |
| 57 | trans-cadina-1(6),4-diene | 1465 | 1475 | - | - | 0.1 | 0.02 | 1505 | - | - | - | trace | - | - |
| 58 | 9-epi-(E)-caryophyllene | 1466 | 1464 | 0.2 | 0.01 | - | - | 1568 | 1572 | 0.1 | 0.01 | - | - | [56] |
| 59 | 4,5-di-epi-aristolochene | 1473 | 1471 | 0.2 | 0.02 | 0.2 | 0.14 | 1657 | 1665 | s.p. 57 | - | 0.1 | 0.03 | [57] |
| 60 | β-chamigrene | 1476 | 1476 | 0.2 | 0.04 | - | - | - | - | - | - | - | - | - |
| 61 | γ-gurjunene | 1478 | 1475 | - | - | 0.4 | 0.09 | - | - | - | - | - | - | - |
| 62 | germacrene D | 1485 | 1480 | 22.3 | 2.86 | 9.5 | 1.02 | 1685 | 1685 | 22.1 | 2.82 | 12.9 | 2.10 | [42] |
| 63 | (E)-β-ionone | 1488 | 1487 | - | - | 1.3 | 0.18 | 1883 | 1889 | - | - | 0.8 | 0.04 | [58] |
| 64 | cis-β-guaiene | 1491 | 1492 | 0.4 | 0.12 | - | - | 1669 | 1667 | 0.3 | 0.03 | - | - | [59] |
| 65 | widdra-2,4(14)-diene | 1491 | 1481 | - | - | 0.4 | 0.12 | 1554 | - | - | - | 0.6 | 0.10 | - |
| 66 | α-zingiberene | 1494 | 1493 | - | - | 1.2 | 0.12 | 1694 | 1696 | - | - | 2.4 | 0.57 | [60] |
| 67 | γ-amorphene | 1496 | 1495 | 0.1 | 0.02 | - | - | - | - | - | - | - | - | - |
| 68 | bicyclogermacrene | 1499 | 1500 | 2.6 | 0.34 | 1.0 | 0.19 | 1709 | 1707 | 2.3 | 0.32 | 1.2 | 0.25 | [61] |
| 69 | α-muurolene | 1503 | 1500 | 1705 | 1700 | [62] | ||||||||
| 70 | (E,E)-α-farnesene | 1508 | 1505 | 0.4 | 0.05 | 0.4 | 0.03 | 1743 | 1743 | 0.3 | 0.06 | 0.2 | 0.03 | [33] |
| 71 | β-bisabolene | 1511 | 1505 | 0.3 | 0.03 | - | - | 1713 | 1710 | s.p. 69 | - | - | - | [49] |
| 72 | germacrene A | 1511 | 1508 | - | - | 0.7 | 0.33 | - | - | - | - | - | - | - |
| 73 | γ-cadinene | 1518 | 1513 | 0.3 | 0.03 | 0.2 | 0.11 | 1738 | 1738 | 0.4 | 0.05 | 0.1 | 0.04 | [42] |
| 74 | n-tridecanal | 1519 | 1509 | 0.3 | 0.04 | - | - | 1806 | 1805 | 0.2 | 0.04 | - | - | [43] |
| 75 | δ-cadinene | 1522 | 1522 | 1.4 | 0.15 | 1.4 | 0.18 | 1738 | 1737 | 1.5 | 0.17 | 1.1 | 0.25 | [26] |
| 76 | trans-cadina-1,4-diene | 1538 | 1533 | 0.1 | 0.02 | - | - | - | - | - | - | - | - | - |
| 77 | (E)-nerolidol | 1567 | 1561 | 0.2 | 0.02 | - | - | 2036 | 2033 | 0.2 | 0.01 | - | - | [63] |
| 78 | germacrene D-4-ol | 1584 | 1574 | 1.4 | 0.17 | - | - | 2042 | 2044 | 0.1 | 0.01 | - | - | [64] |
| 79 | spathulenol | 1585 | 1577 | 3.6 | 0.66 | 2110 | 2106 | 1.3 | 0.26 | 3.2 | 0.19 | [26] | ||
| 80 | caryophyllene oxide | 1589 | 1582 | 3.0 | 0.44 | 7.2 | 1.29 | 1948 | 1944 | 3.1 | 0.32 | 6.7 | 0.75 | [65] |
| 81 | n-hexadecane | 1600 | 1600 | 0.3 | 0.03 | 0.5 | 0.04 | 1600 | 1600 | 0.3 | 0.02 | 0.2 | 0.05 | |
| 82 | ledol | 1612 | 1602 | 0.2 | 0.03 | - | - | 2000 | 2007 | 0.2 | 0.02 | - | - | [66] |
| 83 | unidentified (MW = 222) | 1613 | - | - | - | 0.8 | 0.02 | 2181 | - | - | - | 0.9 | 0.05 | - |
| 84 | β-oplopenone | 1616 | 1607 | 0.2 | 0.01 | - | - | 2039 | 2051 | 0.1 | 0.02 | - | - | [66] |
| 85 | humulene epoxide II | 1619 | 1608 | - | - | 1.3 | 0.04 | 1964 | 1972 | - | - | 0.8 | 0.12 | [67] |
| 86 | n-tetradecanal | 1621 | 1611 | 0.4 | 0.13 | - | - | 1911 | 1910 | trace | - | - | - | [68] |
| 87 | allo-aromadendrene epoxide | 1627 | 1639 | 0.4 | 0.03 | 0.4 | 0.02 | 2093 | 2095 | 0.2 | 0.02 | 0.2 | 0.05 | [69] |
| 88 | unidentified (MW = 220) | 1628 | - | - | - | 0.7 | 0.07 | 1940 | - | - | - | 0.8 | 0.07 | - |
| 89 | junenol | 1630 | 1618 | 0.2 | 0.03 | - | - | - | - | - | - | - | - | - |
| 90 | 1-epi-Cubenol | 1636 | 1627 | 0.3 | 0.04 | 0.4 | 0.02 | 2052 | 2046 | 0.3 | 0.06 | - | - | [70] |
| 91 | cis-cadin-4-en-7-ol | 1644 | 1635 | 0.2 | 0.03 | - | - | 2090 | - | 0.2 | 0.04 | - | - | - |
| 92 | epi-α-cadinol | 1652 | 1638 | 0.5 | 0.24 | 0.7 | 0.06 | 2153 | 2154 | 0.8 | 0.13 | 0.6 | 0.09 | [71] |
| 93 | epi-α-muurolol | 1654 | 1640 | 0.4 | 0.30 | 0.6 | 0.05 | 2170 | 2171 | 0.7 | 0.11 | 0.5 | 0.20 | [59] |
| 94 | α-muurolol (=torreyol) | 1658 | 1645 | - | - | 0.7 | 0.37 | 2150 | 2150 | - | - | 0.5 | 0.20 | [67] |
| 95 | 7-epi-α-eudesmol | 1657 | 1662 | 0.3 | 0.04 | - | - | - | - | - | - | - | - | - |
| 96 | unidentified (MW = 220) | 1660 | - | - | - | 0.7 | 0.05 | 2243 | - | - | - | 0.5 | 0.12 | - |
| 97 | α-cadinol | 1666 | 1652 | 1.6 | 0.22 | 2.1 | 0.13 | 2211 | 2211 | 2.0 | 0.27 | 3.0 | 0.29 | [72] |
| 98 | α-amyl cinnamyl alcohol | 1672 | 1682 | - | - | 0.8 | 0.15 | 2010 | - | - | - | 0.2 | 0.02 | - |
| 99 | ar-turmerone | 1673 | 1668 | 0.1 | 0.02 | - | - | - | - | - | - | - | - | - |
| 100 | n-heptadecane | 1700 | 1700 | 0.3 | 0.03 | 0.6 | 0.07 | - | - | - | - | 0.2 | 0.03 | - |
| 101 | amorpha-4,9-dien-2-ol | 1703 | 1700 | 0.7 | 0.07 | 1.2 | 0.24 | 2343 | - | 0.6 | 0.03 | 1.2 | 0.90 | - |
| 102 | n-pentadecanal | 1725 | 1724 | 0.7 | 0.06 | 1.0 | 0.05 | 2031 | 2024 | 0.7 | 0.19 | 0.4 | 0.09 | [73] |
| 103 | 1-octadecene | 1795 | 1789 | - | - | 0.5 | 0.02 | 1831 | 1823 | - | - | 0.2 | 0.02 | [74] |
| 104 | n-octadecane | 1800 | 1800 | - | - | 0.5 | 0.06 | 1800 | 1800 | - | - | 0.2 | 0.01 | - |
| 105 | cyclopentadecanolide | 1831 | 1832 | 0.1 | 0.02 | - | - | 2255 | 2255 | 0.1 | 0.01 | - | - | [75] |
| 106 | 1-nonadecene | 1895 | 1895 | - | - | 0.7 | 0.03 | 1934 | 1938 | - | - | 0.8 | 0.08 | [76] |
| 107 | n-nonadecane | 1900 | 1900 | 0.1 | 0.05 | 0.8 | 0.05 | 1900 | 1900 | 0.1 | 0.01 | 0.8 | 0.06 | - |
| 108 | (5E,9E)-farnesyl acetone | 1921 | 1913 | 0.1 | 0.06 | - | - | 2368 | 2364 | 0.2 | 0.03 | - | - | [77] |
| 109 | 1-eicosene | 1996 | 1987 | 0.2 | 0.15 | 1.4 | 0.10 | 2057 | 2047 | 0.1 | 0.01 | 1.3 | 0.12 | [78] |
| 110 | n-eicosane | 2000 | 2000 | 0.1 | 0.10 | 0.6 | 0.05 | 2000 | 2000 | trace | - | 2.2 | 0.10 | - |
| 111 | unidentified (MW = 270) | 2095 | - | - | - | 2.4 | 0.24 | 2136 | - | - | - | 2.3 | 0.26 | - |
| 112 | n-heneicosane | 2100 | 2100 | 0.3 | 0.10 | 3.6 | 0.59 | 2100 | 2100 | 0.3 | 0.06 | 3.5 | 0.30 | |
| 113 | 1-docosene | 2196 | 2189 | 0.1 | 0.03 | 4.0 | 0.46 | 2234 | - | 0.2 | 0.08 | 4.3 | 0.95 | - |
| 114 | n-docosane | 2200 | 2200 | - | - | 1.3 | 0.21 | 2200 | 2200 | - | - | 1.6 | 0.18 | |
| 115 | 1-tricosene | 2297 | 2289 | - | - | 1.3 | 0.29 | 2235 | - | - | - | 1.6 | 0.21 | - |
| 116 | n-tricosane | 2300 | 2300 | 0.5 | 0.17 | 4.9 | 0.94 | 2300 | 2300 | 0.4 | 0.01 | 4.9 | 1.46 | |
| 117 | 1-teracosene | 2397 | - | - | - | 1.4 | 0.32 | 2439 | - | - | - | 1.8 | 0.20 | - |
| 118 | n-tetracosane | 2400 | 2400 | 0.1 | 0.01 | 0.5 | 0.12 | 2400 | 2400 | 0.2 | 0.06 | 0.6 | 0.15 | |
| 119 | 1-pentacosene | 2497 | 2486 | - | - | 0.4 | 0.09 | 2547 | - | - | - | 0.6 | 0.13 | - |
| 120 | n-pentacosane | 2500 | 2500 | - | - | 1.2 | 0.41 | 2500 | 2500 | - | - | 0.7 | 0.56 | |
| 121 | 1-hexacosene | 2597 | 2596 * | 0.3 | 0.04 | - | - | 2655 | - | 0.3 | 0.01 | 0.6 | 0.15 | - |
| 122 | n-hexacosane | 2600 | 2600 | 0.2 | 0.02 | trace | - | 2600 | 2600 | 0.2 | 0.02 | 0.6 | 0.20 | |
| 123 | n-tetracosanal | 2637 | 2650 | 0.1 | 0.01 | - | - | 2778 | - | 0.1 | 0.04 | - | - | - |
| monoterpenes | 16.4 | 7.4 | 15.8 | 5.7 | ||||||||||
| oxygenated monoterpenoids | 2.3 | 0.7 | 1.4 | 1.0 | ||||||||||
| sesquiterpenes | 44.8 | 27.5 | 43.4 | 31.3 | ||||||||||
| oxygenated sesquiterpenoids | 9.9 | 20.4 | 10.0 | 18.9 | ||||||||||
| others | 13.0 | 34.2 | 12.2 | 34.3 | ||||||||||
| total | 86.3 | 90.2 | 82.8 | 91.2 | ||||||||||
| Enantiomers | LRI | G. reinaldii | G. pulchella | ||
|---|---|---|---|---|---|
| Composition | ee (%) | Composition | ee (%) | ||
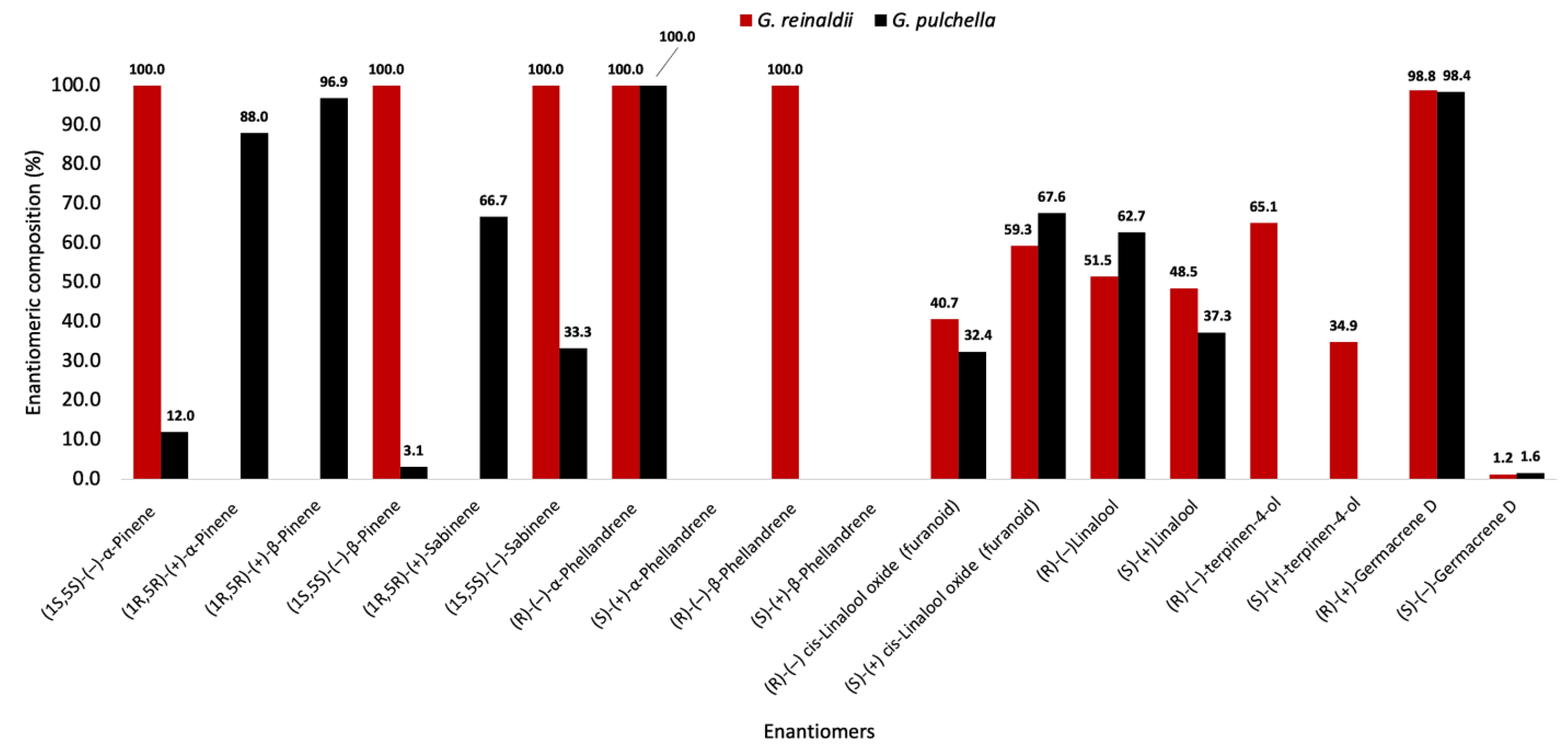
| (1S,5S)-(−)-α-pinene | 926 a | 100.0 | 100.0 | 12.0 * | 76.0 |
| (1R,5R)-(+)-α-pinene | 928 a | - | 88.0 * | ||
| (1R,5R)-(+)-β-pinene | 949 b | - | 100.0 | 96.9 | 93.8 |
| (1S,5S)-(−)-β-pinene | 959 b | 100.0 | 3.1 | ||
| (1R,5R)-(+)-sabinene | 1008 a | - | 100.0 | 66.7 | 33.4 |
| (1S,5S)-(−)-sabinene | 1014 a | 100.0 | 33.3 | ||
| (R)-(−)-α-phellandrene | 1019 b | 100.0 | 100.0 | 100.0 | 100.0 |
| (S)-(+)-α-phellandrene | 1024 b | - | - | ||
| (R)-(−)-β-phellandrene | 1051 b | 100.0 | 100.0 | - | - |
| (S)-(+)-β-phellandrene | 1058 b | - | - | ||
| (R)-(−)-cis-linalool oxide (furanoid) | 1209 a | 40.7 | 18.6 | 32.4 | 35.2 |
| (S)-(+)-cis-linalool oxide (furanoid) | 1197 a | 59.3 | 67.6 | ||
| (R)-(−)-linalool | 1300 a | 51.5 | 3.0 | 62.7 | 25.4 |
| (S)-(+)-linalool | 1301 a | 48.5 | 37.3 | ||
| (R)-(−)-terpinen-4-ol | 1338 a | 65.1 | 30.2 | - | - |
| (S)-(+)-terpinen-4-ol | 1379 a | 34.9 | - | ||
| (R)-(+)-germacrene D | 1466 b | 98.8 | 97.6 | 98.4 | 96.8 |
| (S)-(−)-germacrene D | 1471 b | 1.2 | 1.6 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maldonado, Y.E.; Rodríguez, M.d.C.; Calvopiña, K.; Malagón, O.; Cumbicus, N.; Gilardoni, G. Gynoxys reinaldii Cuatrec. and Gynoxys pulchella (Kunth) Cass.: Chemical and Enantioselective Analyses of Two Unprecedented Essential Oils from Ecuador. Plants 2024, 13, 3543. https://doi.org/10.3390/plants13243543
Maldonado YE, Rodríguez MdC, Calvopiña K, Malagón O, Cumbicus N, Gilardoni G. Gynoxys reinaldii Cuatrec. and Gynoxys pulchella (Kunth) Cass.: Chemical and Enantioselective Analyses of Two Unprecedented Essential Oils from Ecuador. Plants. 2024; 13(24):3543. https://doi.org/10.3390/plants13243543
Chicago/Turabian StyleMaldonado, Yessenia E., María del Carmen Rodríguez, Karyna Calvopiña, Omar Malagón, Nixon Cumbicus, and Gianluca Gilardoni. 2024. "Gynoxys reinaldii Cuatrec. and Gynoxys pulchella (Kunth) Cass.: Chemical and Enantioselective Analyses of Two Unprecedented Essential Oils from Ecuador" Plants 13, no. 24: 3543. https://doi.org/10.3390/plants13243543
APA StyleMaldonado, Y. E., Rodríguez, M. d. C., Calvopiña, K., Malagón, O., Cumbicus, N., & Gilardoni, G. (2024). Gynoxys reinaldii Cuatrec. and Gynoxys pulchella (Kunth) Cass.: Chemical and Enantioselective Analyses of Two Unprecedented Essential Oils from Ecuador. Plants, 13(24), 3543. https://doi.org/10.3390/plants13243543








