Application of Microwave-Assisted Water Extraction (MAWE) to Fully Realize Various Physiological Activities of Melaleuca quinquenervia Leaf Extract
Abstract
1. Introduction
2. Results and Discussion
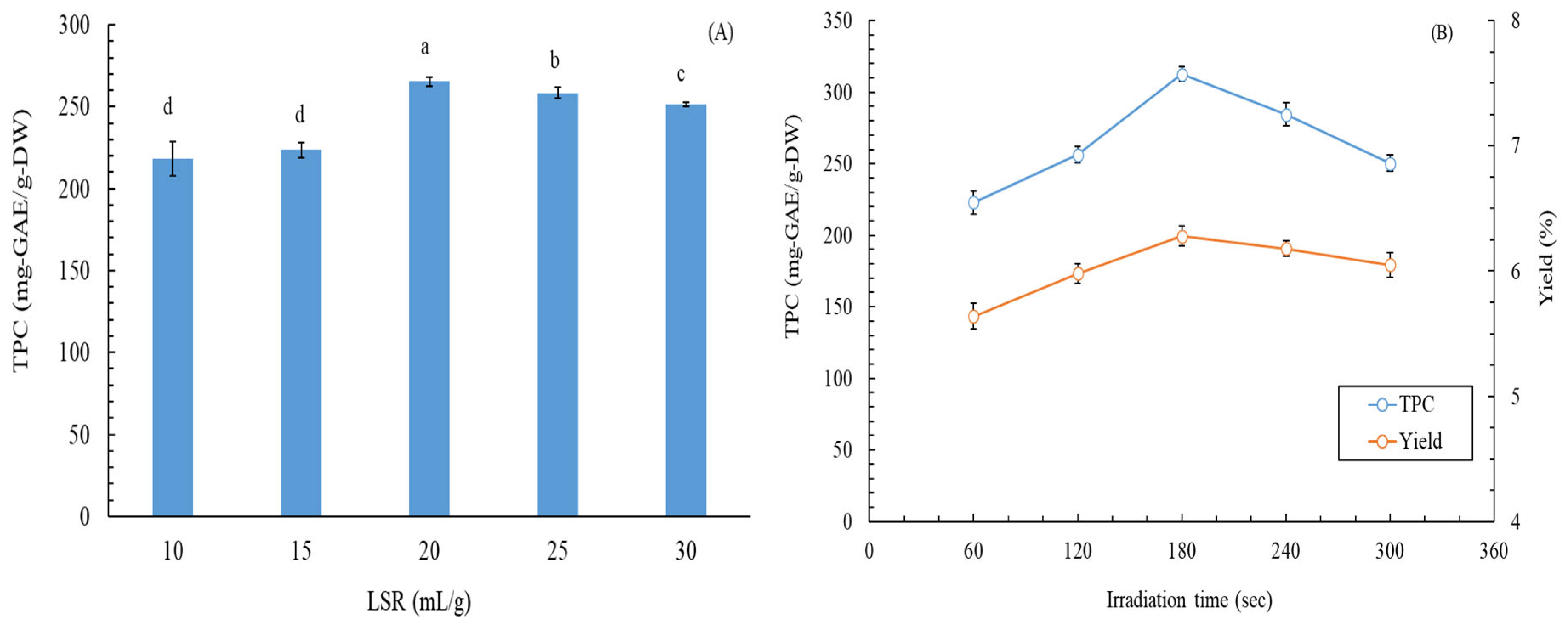
2.1. Optimization of MAE Conditions
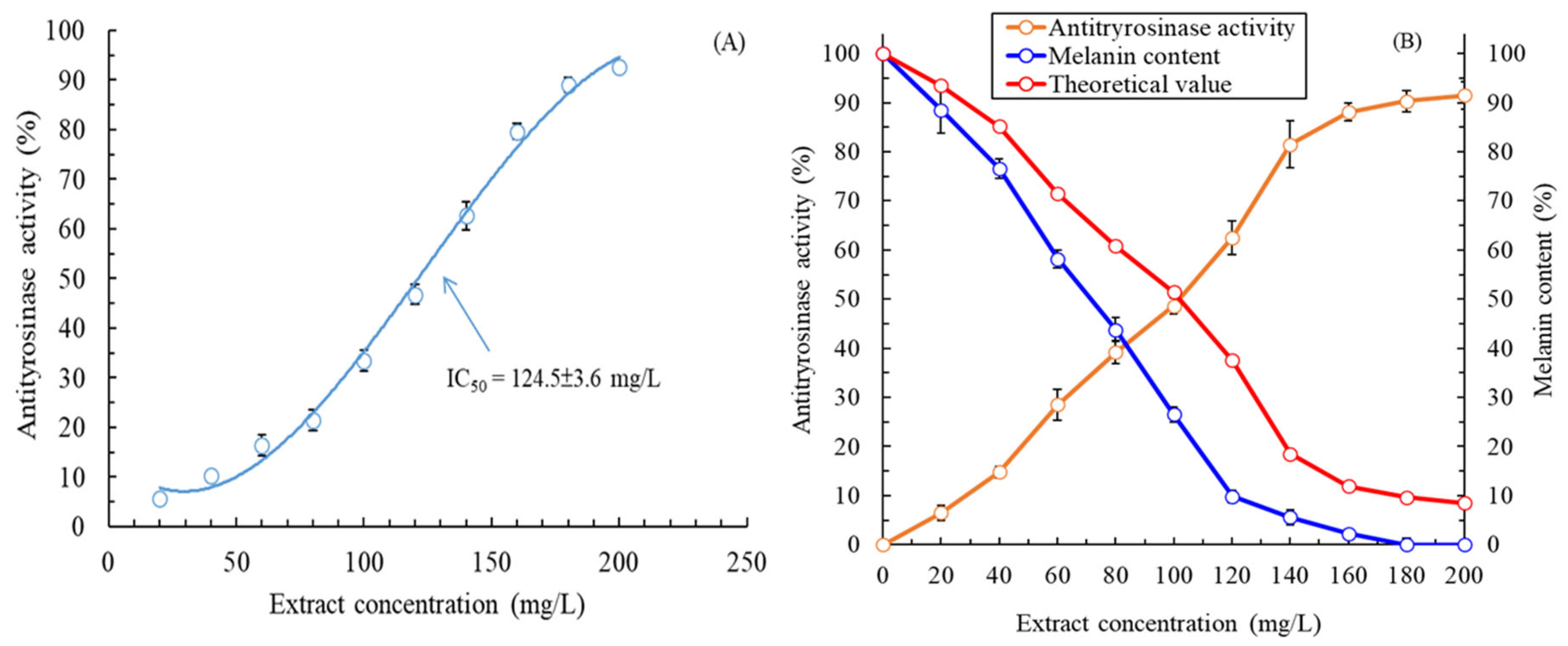
2.2. Extracellular and Intracellular Antityrosinase Activity
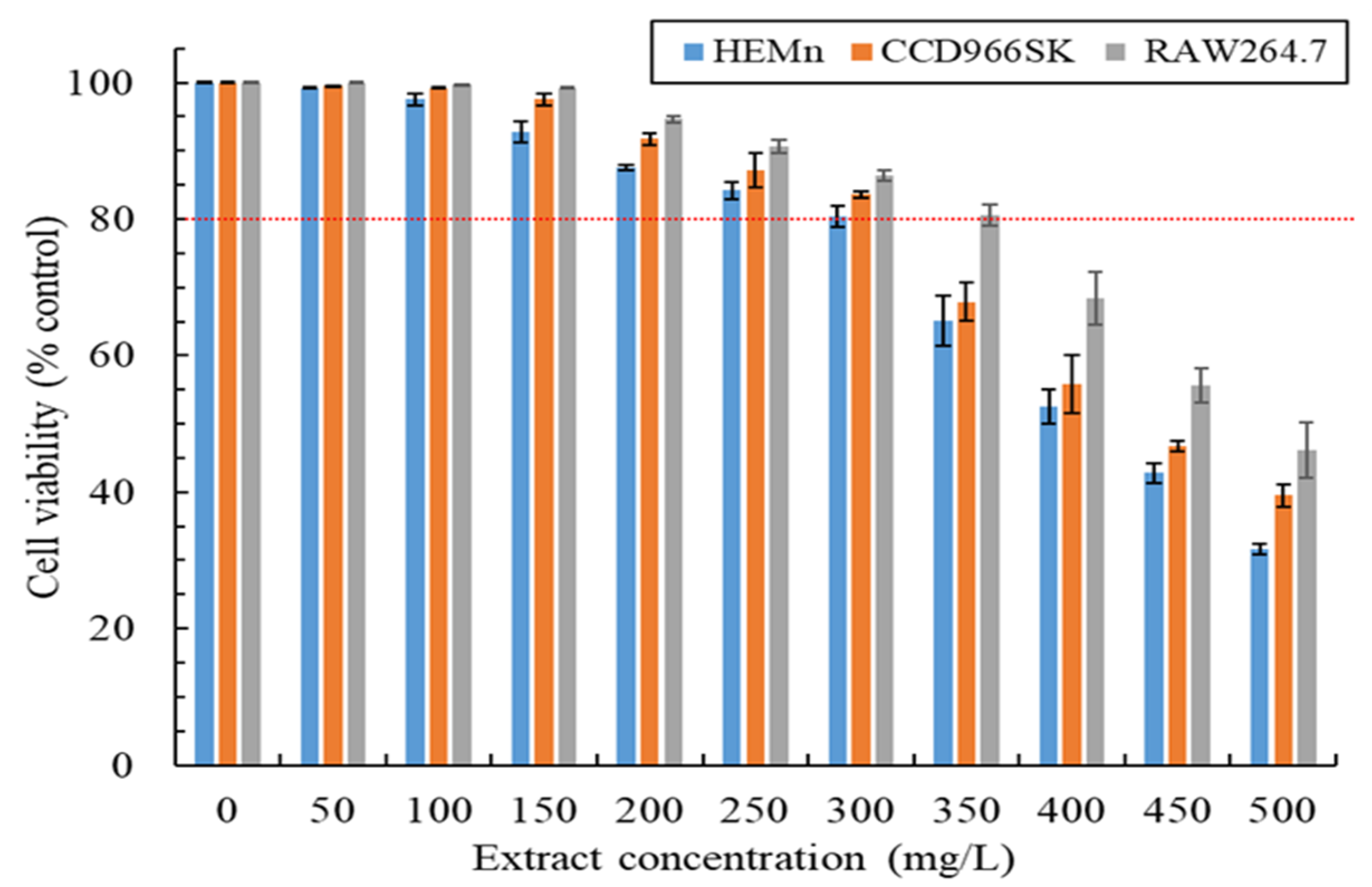
2.3. Cytotoxicity Assay
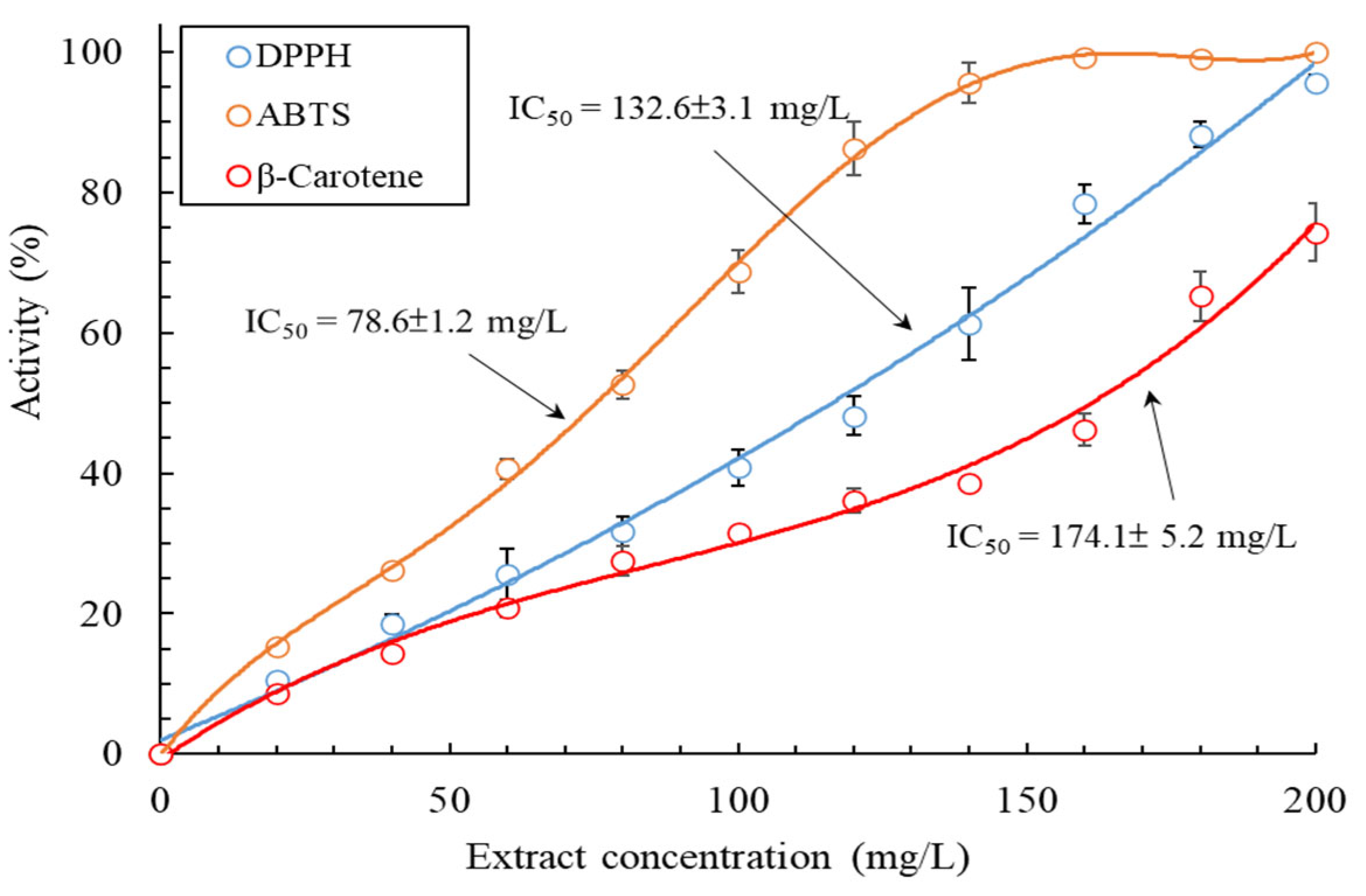
2.4. Evaluation of Antioxidant Activity
2.5. Evaluation of Antiaging Activity
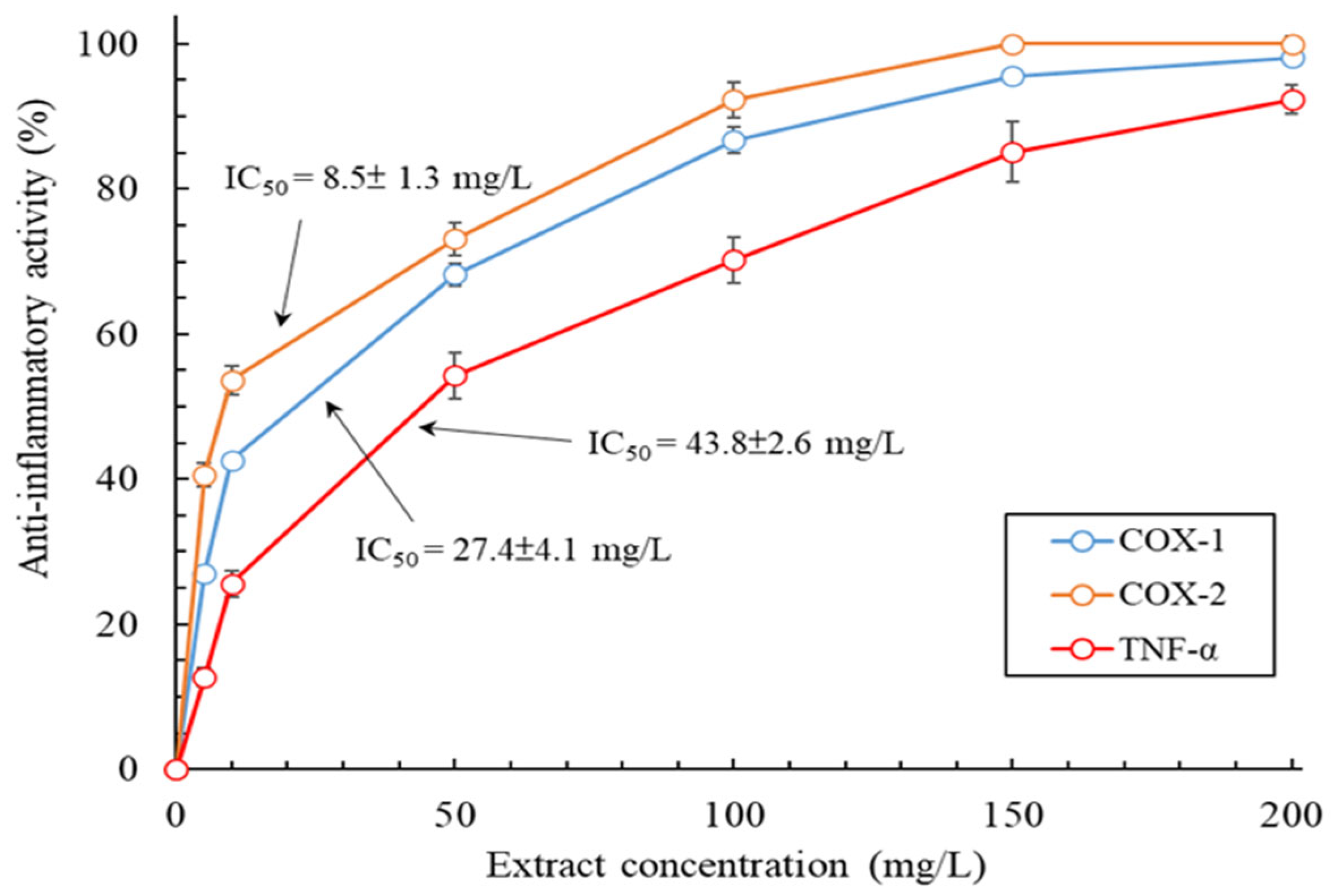
2.6. Evaluation of Anti-Inflammatory Activity
2.7. Evaluation of Antimicrobial Activity
2.8. Chemical Composition of MLE
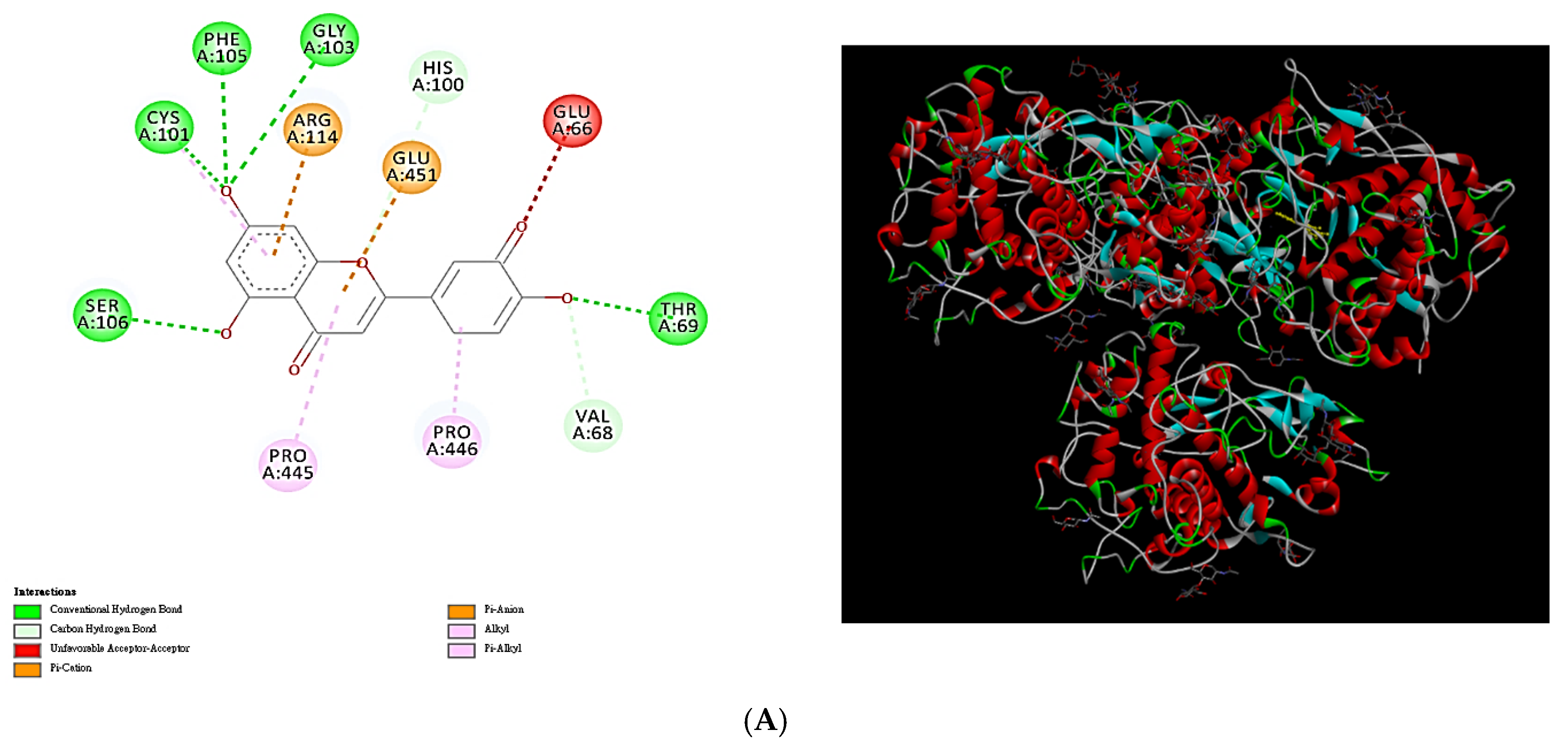
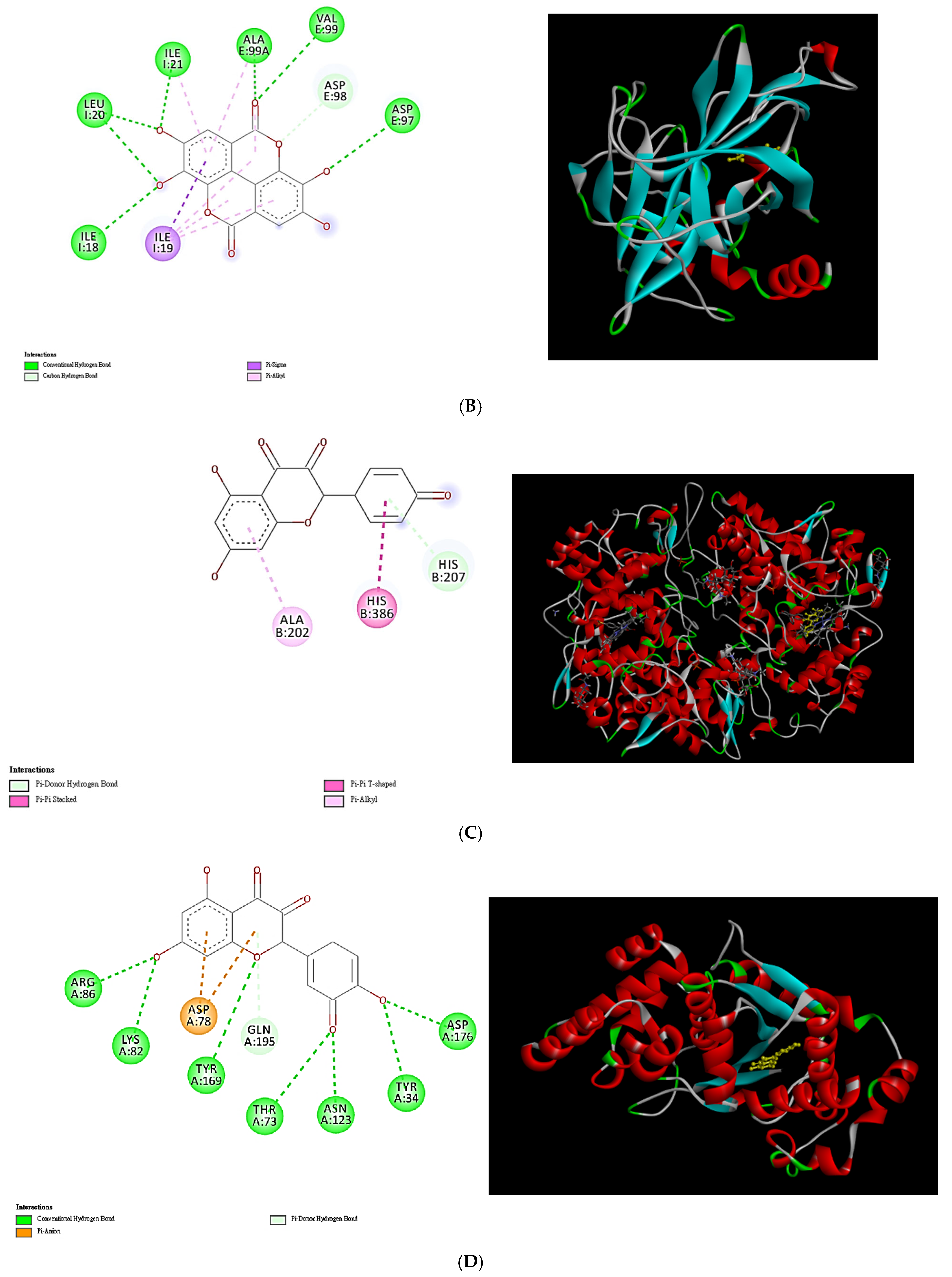
2.9. Molecular Docking Analysis
3. Materials and Methods
3.1. Plant Material and Extraction Procedure
3.2. Microbial Strains, Cells, and Reagents
3.3. Evaluation of Antioxidant Activity
3.4. Extracellular and Intracellular Antityrosinase Activity
3.5. Cytotoxicity Assay
3.6. Evaluation of Antiaging Activity
3.7. Evaluation of Anti-Inflammatory Activity
3.8. Evaluation of Antimicrobial Activity
3.9. Quantification of Chemical Compounds in MLE
3.10. Molecular Docking Study
3.11. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chao, W.W.; Su, C.C.; Peng, H.Y.; Chou, S.T. Melaleuca quinquenervia essential oil inhibits α-melanocyte-stimulating hormone-induced melanin production and oxidative stress in B16 melanoma cells. Phytomedicine 2017, 34, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Moharram, F.A.; Marzouk, M.S.; El-Toumy, S.A.A.; Ahmed, A.A.E.; Aboutabl, E.A. Polyphenols of Melaleuca quinquenervia leaves—Pharmacological studies of grandinin. Phytother. Res. 2003, 17, 767–773. [Google Scholar] [CrossRef] [PubMed]
- Shakeel, F.; Salem-Bekhit, M.M.; Haq, N.; Alshehri, S. Nanoemulsification improves the pharmaceutical properties and bioactivities of niaouli essential oil (Melaleuca quinquenervia L.). Molecules 2021, 26, 4750. [Google Scholar] [CrossRef] [PubMed]
- Valková, V.; Dúranová, H.; Vukovic, N.L.; Vukic, M.; Kluz, M.; Kačániová, M. Assessment of chemical composition and anti-Penicillium activity of vapours of essential oils from Abies alba and two Melaleuca species in food model systems. Molecules 2022, 27, 3101. [Google Scholar] [CrossRef]
- Aziz, Z.A.A.; Ahmad, A.; Setapar, S.H.M.; Karakucuk, A.; Azim, M.M.; Lokhat, D.; Rafatullah, M.; Ganash, M.; Kamal, M.A.; Ashraf, G.M. Essential oils: Extraction techniques, pharmaceutical and therapeutic potential-a review. Curr. Drug. Metab. 2018, 19, 1100–1110. [Google Scholar] [CrossRef]
- Wang, Y.; Yan, M.; Qin, R.; Gong, Y. Enzymolysis-microwave-assisted hydrodistillation for extraction of volatile oil from Atractylodes chinensis and its hypoglycemic activity in vitro. J. AOAC Int. 2021, 104, 1196–1205. [Google Scholar] [CrossRef]
- Yilmaz, E.; Güneşer, B.A. Cold pressed versus solvent extracted lemon (Citrus limon L.) seed oils: Yield and properties. J. Food Sci. Technol. 2017, 54, 1891–1900. [Google Scholar] [CrossRef]
- Paibon, W.; Yimnoi, C.A.; Tembab, N.; Boonlue, W.; Jampachaisri, K.; Nuengchamnong, N.; Waranuch, N.; Ingkaninan, K. Comparison and evaluation of volatile oils from three different extraction methods for some Thai fragrant flowers. Int. J. Cosmet. Sci. 2011, 33, 150–156. [Google Scholar] [CrossRef]
- Haro-González, J.N.; Castillo-Herrera, G.A.; Martínez-Velázquez, M.; Espinosa-Andrews, H. Clove essential oil (Syzygium aromaticum L. Myrtaceae): Extraction, chemical composition, food applications, and essential bioactivity for human health. Molecules 2021, 26, 6387. [Google Scholar] [CrossRef]
- Bagade, S.B.; Patil, M. Recent advances in microwave assisted extraction of bioactive compounds from complex herbal samples: A review. Crit. Rev. Anal. Chem. 2021, 51, 138–149. [Google Scholar] [CrossRef]
- Cock, I.E.; Winnett, V.; Sirdaarta, J.; Matthews, B. The potential of selected Australian medicinal plants with anti-Proteus activity for the treatment and prevention of rheumatoid arthritis. Pharmacogn Mag. 2015, 11 (Suppl. S1), S190–S208. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wu, X.; Huang, L. Correlation between antioxidant activities and phenolic contents of radix Angelicae sinensis (Danggui). Molecules 2009, 14, 5349–5361. [Google Scholar] [CrossRef] [PubMed]
- Al-Abd, N.M.; Mohamed Nor, Z.; Mansor, M.; Azhar, F.; Hasan, M.S.; Kassim, M. Antioxidant, antibacterial activity, and phytochemical characterization of Melaleuca cajuputi extract. BMC Complement. Altern. Med. 2015, 15, 385. [Google Scholar] [CrossRef] [PubMed]
- Hou, W.; Zhang, W.; Chen, G.; Luo, Y. Optimization of extraction conditions for maximal phenolic, flavonoid and antioxidant activity from Melaleuca bracteata leaves using the response surface methodology. PLoS ONE 2016, 11, e0162139. [Google Scholar] [CrossRef] [PubMed]
- Bianchini Silva, L.S.; Perasoli, F.B.; Carvalho, K.V.; Vieira, K.M.; Paz Lopes, M.T.; Bianco de Souza, G.H.; Henrique Dos Santos, O.D.; Freitas, K.M. Melaleuca leucadendron (L.) flower extract exhibits antioxidant and photoprotective activities in human keratinocytes exposed to ultraviolet B radiation. Free Radic. Biol. Med. 2020, 159, 54–65. [Google Scholar] [CrossRef] [PubMed]
- Surh, J.; Yun, J.M. Antioxidant and anti-inflammatory activities of butanol extract of Melaleuca leucadendron L. Prev. Nutr. Food Sci. 2012, 17, 22–28. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, H.; Jiang, D.; Yang, Y.; Tang, W.; Xu, K. The antifungal activity of essential oil from Melaleuca leucadendra (L.) L. grown in China and its synergistic effects with conventional antibiotics against Candida. Nat. Prod. Res. 2019, 33, 2545–2548. [Google Scholar] [CrossRef]
- Pino, J.A.; Regalado, E.L.; Rodríguez, J.L.; Fernández, M.D. Phytochemical analysis and in vitro free-radical-scavenging activities of the essential oils from leaf and fruit of Melaleuca leucadendra L. Chem. Biodivers. 2010, 7, 2281–2288. [Google Scholar] [CrossRef]
- An, N.T.G.; Huong, L.T.; Satyal, P.; Tai, T.A.; Dai, D.N.; Hung, N.H.; Ngoc, N.T.B.; Setzer, W.N. Mosquito larvicidal activity, antimicrobial activity, and chemical compositions of essential oils from four species of Myrtaceae from central Vietnam. Plants 2020, 9, 544. [Google Scholar] [CrossRef]
- Mady, M.S.; Elsayed, H.E.; El-Sayed, E.K.; Hussein, A.A.; Ebrahim, H.Y.; Moharram, F.A. Polyphenolic profile and ethno pharmacological activities of Callistemon subulatus (Cheel) Craven leaves cultivated in Egypt. J. Ethnopharmacol. 2022, 284, 114698. [Google Scholar] [CrossRef]
- Ebrahim, H.Y.; Mady, M.S.; Atya, H.B.; Ali, S.A.; Elsayed, H.E.; Moharram, F.A. Melaleuca rugulosa (Link) Craven Tannins: Appraisal of anti-inflammatory, radical scavenging activities, and molecular modeling studies. J. Ethnopharmacol. 2022, 298, 115596. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.H.; Lin, Y.M.; Kuo, J.T.; Lin, C.P.; Chang, C.F.; Hsieh, M.C.; Cheng, C.Y.; Chung, Y.C. Comparison of biofunctional activity of Asparagus cochinchinensis (Lour.) Merr. extract before and after fermentation with Aspergillus oryzae. J. Biosci. Bioeng. 2019, 127, 59–65. [Google Scholar] [CrossRef] [PubMed]
- López-García, J.; Lehocký, M.; Humpolíček, P.; Sáha, P. HaCaT keratinocytes response on antimicrobial atelocollagen substrates: Extent of cytotoxicity, cell viability and proliferation. J. Funct. Biomater. 2014, 5, 43–57. [Google Scholar] [CrossRef] [PubMed]
- Hashim, A.N.; Swilam, N.F.; Moustafa, E.S.; Bakry, S.M.; Labib, R.M.; Barakat, H.H.; Singab, A.B.; Linscheid, M.W.; Nawwar, M.A. A cytotoxic flavonol glycoside from Melaleuca leucadendra leaves extract with immunostimulant activity. Pharmazie. 2018, 73, 61–64. [Google Scholar]
- Capanoglu, E.; Cekic, S.D.; Baskan, K.S.; Avan, A.N.; Uzunboy, S.; Apak, R. Antioxidant activity and capacity measurement. In Plant Antioxidants and Health; Reference Series in Phytochemistry; Ekiert, H.M., Ramawat, K.G., Arora, J., Eds.; Springer: Cham, Switzerland, 2022; pp. 709–773. [Google Scholar]
- Bautista-Silva, J.P.; Seibert, J.B.; Amparo, T.R.; Rodrigues, I.V.; Teixeira, L.F.M.; Souza, G.H.B.; Dos Santos, O.D.H. Melaleuca leucadendra essential oil promotes loss of cell membrane and wall integrity and inhibits bacterial growth: An in silico and in vitro approach. Curr. Microbiol. 2020, 77, 2181–2191. [Google Scholar] [CrossRef]
- Monzote, L.; Scherbakov, A.M.; Scull, R.; Satyal, P.; Cos, P.; Shchekotikhin, A.E.; Gille, L.; Setzer, W.N. Essential oil from Melaleuca leucadendra: Antimicrobial, antikinetoplastid, antiproliferative and cytotoxic assessment. Molecules 2020, 25, 5514. [Google Scholar] [CrossRef]
- Van, N.T.B.; Vi, O.T.; Yen, N.T.P.; Nhung, N.T.; Cuong, N.V.; Kiet, B.T.; Hoang, N.V.; Hien, V.B.; Thwaites, G.; Campell, J.; et al. Minimum inhibitory concentrations of commercial essential oils against common chicken pathogenic bacteria and their relationship with antibiotic resistance. J. Appl. Microbiol. 2022, 132, 1025–1035. [Google Scholar] [CrossRef]
- Vázquez, A.; Tabanca, N.; Kendra, P.E. HPTLC analysis and chemical composition of selected Melaleuca essential oils. Molecules 2023, 28, 3925. [Google Scholar] [CrossRef]
- Hu, W.; Sarengaowa; Guan, Y.; Feng, K. Biosynthe sis of phenolic compounds and antioxidant activity in fresh-cut fruits and vegetables. Front. Microbiol. 2022, 13, 906069. [Google Scholar]
- Saikia, S.; Bordoloi, M. Molecular docking: Challenges, advances and its use in drug discovery perspective. Curr. Drug Targets 2019, 20, 501–521. [Google Scholar] [CrossRef]
- Manzoor, M.F.; Ahmad, N.; Ahmed, Z.; Siddique, R.; Zeng, X.A.; Rahaman, A.; Muhammad Aadil, R.; Wahab, A. Novel extraction techniques and pharmaceutical activities of luteolin and its derivatives. J. Food Biochem. 2019, 43, e12974. [Google Scholar] [CrossRef] [PubMed]
- Moon, N.R.; Kang, S.; Park, S. Consumption of ellagic acid and dihydromyricetin synergistically protects against UV-B induced photoaging, possibly by activating both TGF-beta1 and wnt signaling pathways. J. Photochem. Photobiol. B 2018, 178, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Baradaran Rahimi, V.; Ghadiri, M.; Ramezani, M.; Askari, V.R. Antiinflammatory and anti-cancer activities of pomegranate and its constituent, ellagic acid: Evidence from cellular, animal, and clinical studies. Phytother. Res. 2020, 34, 685–720. [Google Scholar] [CrossRef] [PubMed]
- Pozos-Nonato, S.; Domínguez-Delgado, C.L.; Campos-Santander, K.A.; Benavides, A.A.; Pacheco-Ortin, S.M.; Higuera-Piedrahita, R.I.; Resendiz-González, G.; Molina-Trinidad, E.M. Novel nanotechnological strategies for skin anti-aging. Curr. Pharm. Biotechnol. 2023, 24, 1397–1419. [Google Scholar]
- Qi, W.; Qi, W.; Xiong, D.; Long, M. Quercetin: Its antioxidant mechanism, antibacterial properties and potential application in prevention and control of toxipathy. Molecules 2022, 27, 6545. [Google Scholar] [CrossRef]
- Chagas, M.D.S.S.; Behrens, M.D.; Moragas-Tellis, C.J.; Penedo, G.X.M.; Silva, A.R.; Gonçalves-de-Albuquerque, C.F. Flavonols and flavones as potential anti-inflammatory, antioxidant, and antibacterial compounds. Oxid. Med. Cell. Longev. 2022, 2022, 9966750. [Google Scholar] [CrossRef]
- Tchuente Tchuenmogne, M.A.; Kammalac, T.N.; Gohlke, S.; Kouipou, R.M.T.; Aslan, A.; Kuzu, M.; Comakli, V.; Demirdag, R.; Ngouela, S.A.; Tsamo, E.; et al. Compounds from Terminalia mantaly L. (Combretaceae) stem bark exhibit potent inhibition against some pathogenic yeasts and enzymes of metabolic significance. Medicines 2017, 4, 6. [Google Scholar] [CrossRef]
- Kujala, T.S.; Loponen, J.M.; Klika, K.D.; Pihlaja, K. Phenolics and betacyanins in red beetroot (Beta vulgaris) root: Distribution and effect of cold storage on the content of total phenolics and three individual compounds. J. Agric. Food Chem. 2000, 48, 5338–5342. [Google Scholar] [CrossRef]
- Pourmorad, F.; Hosseinimehr, S.J.; Shahabimajd, N. Antioxidant activity, phenol and flavonoid contents of some selected Iranian medicinal plants. Afr. J. Biotechnol. 2006, 5, 1142–1145. [Google Scholar]
- Wu, L.; Chen, C.Y.; Cheng, C.Y.; Dai, H.; Ai, Y.; Lin, C.H.; Chung, C.Y. Evaluation of tyrosinase inhibitory, antioxidant, antimicrobial, and antiaging activities of Magnolia officinalis extracts after Aspergillus niger fermentation. BioMed Res. Int. 2018, 2018, 5201786. [Google Scholar] [CrossRef]
- Merchán Arenas, D.R.; Muñoz Acevedo, A.; Vargas Méndez, L.Y.; Kouznetsov, V.V. Scavenger activity evaluation of the clove bud essential oil (Eugenia caryophyllus) and eugenol derivatives employing ABTS+• decolorization. Sci. Pharm. 2011, 79, 779–792. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.C.; Mahmud, R.; Pillai, S.; Perumal, S.; Ismail, S. Antioxidant activities of essential oil of Psidium guajava L. leaves. APCBEE Proc. 2012, 2, 86–91. [Google Scholar] [CrossRef]
- Zheng, Z.P.; Tan, H.Y.; Wang, M. Tyrosinase inhibition constituents from the roots of Morus australis. Fitoterapia 2012, 83, 1008–1013. [Google Scholar] [CrossRef] [PubMed]
- Sittisart, P.; Chitsomboon, B. Intracellular ROS scavenging activity and downregulation of inflammatory mediators in RAW264.7 macrophage by fresh leaf extracts of Pseuderanthemum palatiferum. Evid. Based Complement. Alternat. Med. 2014, 2014, 309095. [Google Scholar] [CrossRef]
- Chen, C.Y.; Hu, C.Y.; Chen, Y.H.; Li, Y.T.; Chung, Y.C. Submerged fermentation with Lactobacillus brevis significantly improved the physiological activities of Citrus aurantium flower extract. Heliyon 2022, 8, e10498. [Google Scholar] [CrossRef]
- Li, H.; Dasilva, N.A.; Liu, W.; Xu, J.; Dombi, G.W.; Dain, J.A.; Li, D.; Chamcheu, J.C.; Seeram, N.P.; Ma, H. Thymocid®, a standardized black cumin (Nigella sativa) seed extract, modulates collagen cross-linking, collagenase and elastase activities, and melanogenesis in murine B16F10 melanoma cells. Nutrients 2020, 12, 2146. [Google Scholar] [CrossRef]
- Sumantran, V.N.; Kulkarni, A.A.; Harsulkar, A.; Wele, A.; Koppikar, S.J.; Chandwaskar, R.; Gaire, V.; Dalvi, M.; Wagh, U.V. Hyaluronidase and collagenase inhibitory activities of the herbal formulation Triphala guggulu. J. Biosci. 2007, 32, 755–761. [Google Scholar] [CrossRef]
- Rahman, M.A.; Imran, T.B.; Islam, S. Antioxidative, antimicrobial and cytotoxic effects of the phenolics of Leea indica leaf extract. Saudi J. Biol. Sci. 2013, 20, 213–225. [Google Scholar] [CrossRef]
- Trabelsi, N.; Waffo-Teguo, P.; Snoussi, M.; Ksouri, R.; Merillon, J.M.; Smaoui, A.; Abdelly, C. Variability of phenolic composition and biological activities of two Tunisian halophyte species from contrasted regions. Acta Physiol. Plant 2013, 35, 749–761. [Google Scholar] [CrossRef]

| Tested Sample | MMP-1 Activity | Collagenase Activity | Elastase Activity | Hyaluronidase Activity |
|---|---|---|---|---|
| M. quinquenervia leaf extract | 114.8 ± 8.1 | 187.2 ± 5.4 | 73.4 ± 2.4 | 60.4 ± 3.1 |
| EGCG | 42.3 ± 3.1 | 113.7 ± 9.1 | 93.5 ± 7.2 | 382 ± 12.6 |
| Gallic acid | – | 126.8 ± 3.7 | – | – |
| Oleanolic acid | – | – | 78.2 ± 1.8 | 98.6 ± 5.2 |
| Tested Sample | MIC | MFC | ||||
|---|---|---|---|---|---|---|
| S. aureus | E. coli | P. aeruginosa | C. acnes | C. albicans | A. brasiliensis | |
| M. quinquenervia leaf extract | 64 | 64 | 128 | 128 | 256 | 128 |
| Streptomycin | 64 | 32 | 32 | – | – | – |
| Erythromycin | – | – | – | 8 | – | – |
| Nystatin | – | – | – | – | 32 | 16 |
| No. | RI | Chemical Compounds | Categories | Relative Content (%) |
|---|---|---|---|---|
| 1 | 934 | α-pinene | monoterpene | 10.61 |
| 2 | 952 | camphene | monoterpene | 0.62 |
| 3 | 961 | benzaldehyde | hydrocarbons | 0.74 |
| 4 | 978 | β-pinene | monoterpene | 7.52 |
| 5 | 990 | myrcene | monoterpene | 0.52 |
| 6 | 1025 | p-cymene | aromatic compounds | 2.45 |
| 7 | 1029 | o-cymene | monoterpene | 0.75 |
| 8 | 1034 | limonene | monoterpene | 7.94 |
| 9 | 1042 | 1,8-cineole | monoterpene | 16.71 |
| 10 | 1062 | γ-terpinene | monoterpene | 1.52 |
| 11 | 1092 | terpinolene | monoterpene | 2.14 |
| 12 | 1102 | linalool | monoterpene | 0.67 |
| 13 | 1178 | 4-terpineol | monoterpene | 1.55 |
| 14 | 1205 | α-terpineol | monoterpene | 10.12 |
| 15 | 1352 | α-terpinyl acetate | monoterpene | 2.51 |
| 16 | 1428 | caryophyllene | sesquiterpene | 2.17 |
| 17 | 1465 | α-humulene | sesquiterpene | 1.05 |
| 18 | 1469 | β-humulene | sesquiterpene | 0.61 |
| 19 | 1501 | ledene | sesquiterpene | 8.52 |
| 20 | 1507 | α-selinene | sesquiterpene | 0.74 |
| 21 | 1567 | nerolidol | sesquiterpene | 1.81 |
| 22 | 1596 | viridiflorol | sesquiterpene | 13.27 |
| 23 | 1610 | ledol | sesquiterpene | 1.23 |
| 24 | 1615 | globulol | sesquiterpene | 2.67 |
| Chemical Compounds | Contents |
|---|---|
| Phenolic acids (mg GAE/g DW) | |
| gallic acid | 104.2 |
| vanillic acid | 10.2 |
| caffeic acid | 28.6 |
| ferulic acid | 8.1 |
| rosmarinic acid | 6.3 |
| ellagic acid | 88.1 |
| 3-O-methyl ellagic acid | 58.6 |
| Flavonoids (mg RE/g DW) | |
| rutin | 1.7 |
| luteolin | 23.1 |
| catechin | 3.8 |
| quercetin-3-O-glucuronopyranoside | 2.8 |
| kaempferol-3-O-glucoside | 3.2 |
| quercetin | 13.8 |
| apigenin | 2.4 |
| naringin | 1.2 |
| kaempferol | 16.3 |
| hesperidin | 1.8 |
| Gallic Acid | Ellagic Acid | 3-O- Methylellagic Acid | Luteolin | Quercetin | Kaempferol | α-Pinene | 1,8-Cineole | α-Terpineol | Viridiflorol | |
|---|---|---|---|---|---|---|---|---|---|---|
| Total binding energy (kcal/mol) | ||||||||||
| tyrosinase | −101.6 | −92.3 | −94.2 | −106.9 | −98.2 | −96.2 | −49.7 | −52.3 | −62.9 | −83.2 |
| elastase | −80.7 | −96.5 | −83.3 | −88.9 | −87.4 | −91.8 | −55.5 | −62.3 | −61.3 | −64.7 |
| collagenase | −78.4 | −91.8 | −92.6 | −94.2 | −98.8 | −95.1 | −56.6 | −60.7 | −69.8 | −73.6 |
| hyaluronidase | −78.0 | −102.5 | −96.2 | −90.1 | −99.9 | −95.6 | −55.2 | −57.9 | −59.2 | −64.6 |
| MMP-1 | −93.3 | −95.6 | −87.9 | −98.6 | −109.2 | −103.6 | −56.3 | −55.7 | −70.1 | −72.6 |
| COX-1 | −85.8 | −104.8 | −100.2 | −92.3 | −96.8 | −93.8 | −57.6 | −60.5 | −66.5 | −77.5 |
| COX-2 | −81.7 | −103.4 | −104.1 | −102.1 | −101.7 | −109.4 | −55.6 | −57.4 | −70.4 | −72.8 |
| TNF-α | −80.3 | −100.7 | −102.6 | −90.6 | −90.4 | −113.8 | −51.2 | −60.9 | −65.6 | −77.3 |
| tyrosyl-tRNA synthetase | −82.0 | −110.3 | −108.0 | −95.5 | −118.2 | −88.9 | −49.2 | −54.8 | −57.5 | −66.7 |
| sterol 14α-demethylase | −88.1 | −95.8 | −105.3 | −98.4 | −98.1 | −90.2 | −50.3 | −54.1 | −62.3 | −67.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, T.-K.; Leu, J.-Y.; Lai, Y.-L.; Chang, Y.-C.; Chung, Y.-C.; Liu, H.-W. Application of Microwave-Assisted Water Extraction (MAWE) to Fully Realize Various Physiological Activities of Melaleuca quinquenervia Leaf Extract. Plants 2024, 13, 3362. https://doi.org/10.3390/plants13233362
Lin T-K, Leu J-Y, Lai Y-L, Chang Y-C, Chung Y-C, Liu H-W. Application of Microwave-Assisted Water Extraction (MAWE) to Fully Realize Various Physiological Activities of Melaleuca quinquenervia Leaf Extract. Plants. 2024; 13(23):3362. https://doi.org/10.3390/plants13233362
Chicago/Turabian StyleLin, Ting-Kang, Jyh-Yih Leu, Yi-Lin Lai, Yu-Chi Chang, Ying-Chien Chung, and Hsia-Wei Liu. 2024. "Application of Microwave-Assisted Water Extraction (MAWE) to Fully Realize Various Physiological Activities of Melaleuca quinquenervia Leaf Extract" Plants 13, no. 23: 3362. https://doi.org/10.3390/plants13233362
APA StyleLin, T.-K., Leu, J.-Y., Lai, Y.-L., Chang, Y.-C., Chung, Y.-C., & Liu, H.-W. (2024). Application of Microwave-Assisted Water Extraction (MAWE) to Fully Realize Various Physiological Activities of Melaleuca quinquenervia Leaf Extract. Plants, 13(23), 3362. https://doi.org/10.3390/plants13233362








