Application of Lanthanum at the Heading Stage Effectively Suppresses Cadmium Accumulation in Wheat Grains by Downregulating the Expression of TaZIP7 to Increase Cadmium Retention in Nodes
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Relative Expression Analysis of Genes
2.3. Element Analysis
2.4. Statistical Analysis
3. Results
3.1. Effects of La Application at Different Growth Stages on the Growth Performance of Wheat Under Cd Stress
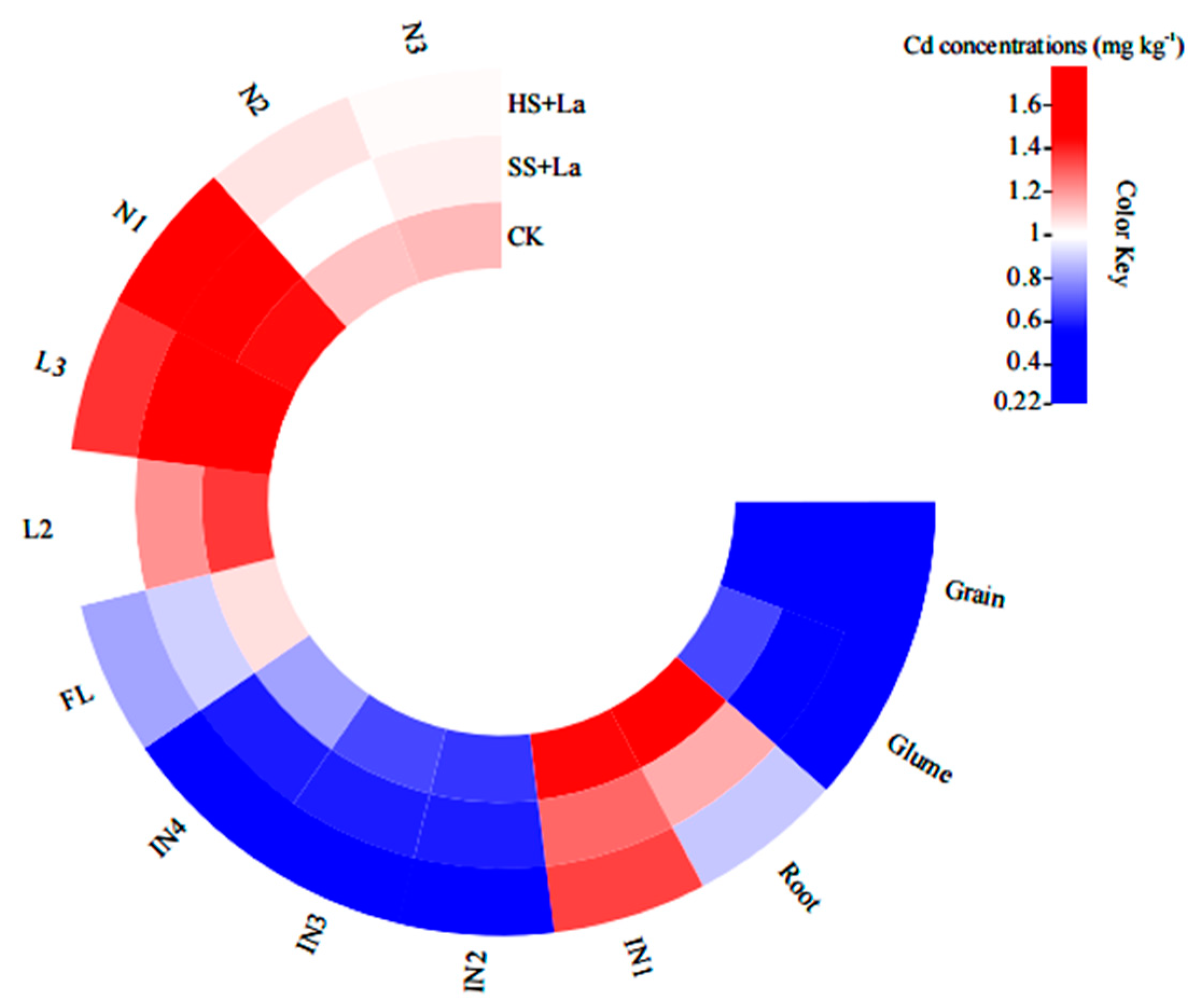
3.2. Effects of La Application at Different Growth Stages on Cd Accumulation in the Roots, Stems, Leaves, Glumes, and Grains of Wheat
3.3. Effects of La Application at Different Growth Stages on Cd Accumulation in the Functional Internodes, Leaves, and Nodes of Wheat
3.4. Distribution of Cd in Different Tissues of Wheat with La Treatment at Different Growth Stages
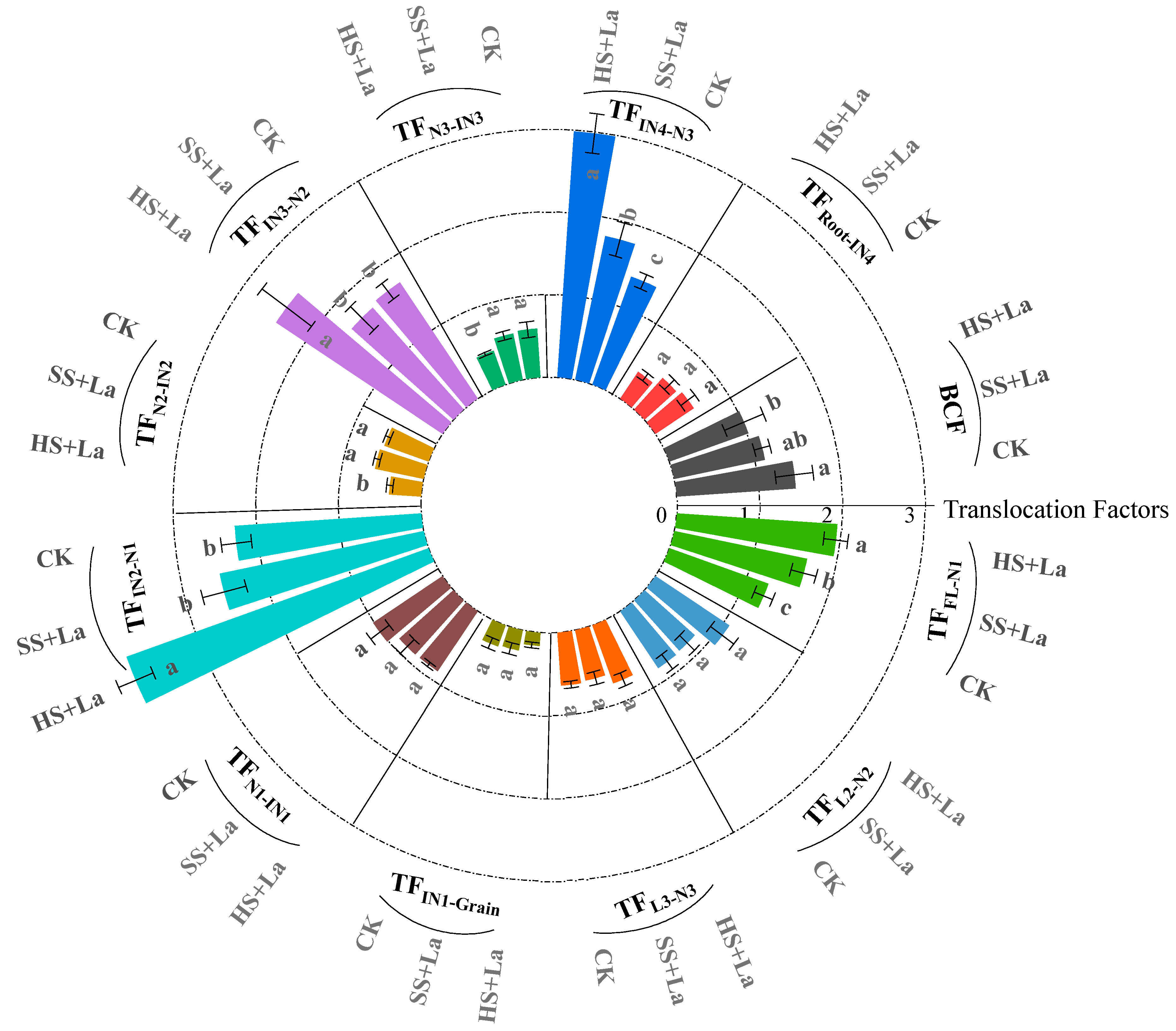
3.5. Effects of La Application at Different Growth Stages on the Transport Characteristics of Cd
3.6. Effects of La Application at the Heading Stage on the Expression of Genes in N1 of Wheat
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- IARC. Cadmium and cadmium compounds. Eval. Carcinog. Risks Hum. 2018, 100C, 121. [Google Scholar]
- Idrees, N.; Tabassum, B.; Abd_Allah, E.F.; Hashem, A.; Sarah, R.; Hashim, M. Groundwater contamination with cadmium concentrations in some West U.P. Regions, India. Saudi J. Biol. Sci. 2018, 25, 1365–1368. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Wang, P.; Zhao, F.J. Dietary cadmium exposure, risks to human health and mitigation strategies. Crit. Rev. Environ. Sci. Technol. 2022, 53, 939–963. [Google Scholar] [CrossRef]
- Kulsum, P.G.P.S.; Khanam, R.; Das, S.; Nayak, A.K.; Tack, F.M.G.; Meers, E.; Vithanage, M.; Shahid, M.; Kumar, A.; Chakraborty, S.; et al. A state-of-the-art review on cadmium uptake, toxicity, and tolerance in rice: From physiological response to remediation process. Environ. Res. 2023, 220, 115098. [Google Scholar] [CrossRef]
- China Ecological Environment State Bulletin. 2021. Available online: https://www.mee.gov.cn/ (accessed on 27 May 2022).
- Lian, J.P.; Cheng, L.P.; Zhai, X.; Wu, R.F.; Liu, W.T.; Pan, J.Q.; Shohag, M.J.I.; Xin, X.P.; He, Z.L.; Yang, X.E. Foliar spray of combined metal-oxide nanoparticles alters the accumulation, translocation and health risk of Cd in wheat (Triticum aestivum L.). J. Hazard. Mater. 2022, 440, 129857. [Google Scholar] [CrossRef]
- Announcement of the National Bureau of Statistics on Grain Production Data in 2022. Available online: https://www.stats.gov.cn/ (accessed on 12 December 2022).
- Wang, P.; Chen, H.; Kopittke, P.M.; Zhao, F.-J. Cadmium contamination in agricultural soils of China and the impact on food safety. Environ. Pollut. 2019, 249, 1038–1048. [Google Scholar] [CrossRef]
- Zhang, L.X.; Gao, C.; Chen, C.; Zhang, W.W.; Huang, X.Y.; Zhao, F.J. Overexpression of Rice OsHMA3 in Wheat Greatly Decreases Cadmium Accumulation in Wheat Grains. Environ. Sci. Technol. 2020, 54, 10100–10108. [Google Scholar] [CrossRef]
- Sui, F.Q.; Chang, J.D.; Tang, Z.; Liu, W.J.; Huang, X.Y.; Zhao, F.J. Nramp5 expression and functionality likely explain higher cadmium uptake in rice than in wheat and maize. Plant Soil 2018, 433, 377–389. [Google Scholar] [CrossRef]
- Zhao, F.J.; Tang, Z.; Song, J.J.; Huang, X.Y.; Wang, P. Toxic metals and metalloids: Uptake, transport, detoxification, phytoremediation, and crop improvement for safer food. Mol. Plant 2022, 15, 27–44. [Google Scholar] [CrossRef]
- Adeel, M.; Lee, J.Y.; Zain, M.; Rizwan, M.; Nawab, A.; Ahmad, M.A.; Shafiq, M.; Yi, H.; Jilani, G.; Javed, R.; et al. Cryptic footprints of rare earth elements on natural resources and living organisms. Environ. Int. 2019, 127, 785–800. [Google Scholar] [CrossRef]
- Ozturk, M.; Metin, M.; Altay, V.; Prasad MN, V.; Gul, A.; Bhat, R.A.; Darvash, M.A.; Hasanuzzaman, M.; Nahar, K.; Unal, D.; et al. Role of Rare Earth Elements in Plants. Plant Mol. Biol. Report. 2023, 41, 345–368. [Google Scholar] [CrossRef]
- Thomas, P.J.; Carpenter, D.; Boutin, C.; Allison, J.E. Rare earth elements (REEs): Effects on germination and growth of selected crop and native plant species. Chemosphere 2014, 96, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Lyu, K.; Wang, X.; Wang, L.; Wang, G.X. Rare-earth element yttrium enhances the tolerance of curly-leaf pondweed (Potamogeton crispus) to acute nickel toxicity. Environ. Pollut. 2019, 248, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Cui, W.W.; Kamran, M.; Song, Q.H.; Zuo, B.Y.; Jia, Z.K.; Han, Q.F. Lanthanum chloride improves maize grain yield by promoting photosynthetic characteristics, antioxidants enzymes and endogenous hormone at reproductive stages. J. Rare Earths 2019, 37, 781–790. [Google Scholar] [CrossRef]
- Si, Y.; Wang, L.H.; Zhou, Q.; Huang, X.H. Effects of lanthanum and silicon stress on bio-sequestration of lanthanum in phytoliths in rice seedlings. Environ. Sci. Pollut. Res. 2018, 25, 10752–10770. [Google Scholar] [CrossRef]
- Hong, F.S.; Ling, W.; Chao, L. Study of lanthanum on seed germination and growth of rice. Biol. Trace Elem. Res. 2003, 94, 273–286. [Google Scholar]
- Guo, B.; Xu, L.L.; Guan, Z.J.; Wei, Y.H. Effect of Lanthanum on Rooting of In Vitro Regenerated Shoots of Saussurea involucrata Kar. et Kir. Biol. Trace Elem. Res. 2012, 147, 334–340. [Google Scholar] [CrossRef]
- Chen, W.J.; Tao, Y.; Gu, Y.H.; Zhao, G.W. Effect of lanthanide chloride on photosynthesis and dry matter accumulation in tobacco seedlings. Biol. Trace Elem. Res. 2001, 79, 169–176. [Google Scholar] [CrossRef]
- Luo, H.W.; Chen, Y.L.; He, L.X.; Tang, X.R. Lanthanum (La) improves growth, yield formation and 2-acetyl-1-pyrroline biosynthesis in aromatic rice (Oryza sativa L.). BMC Plant Biol. 2021, 21, 233. [Google Scholar] [CrossRef]
- Yang, H.; Xiong, Z.; Xu, Z.; Liu, R. Interactive Effects of Lanthanum and Calcium on Cadmium Accumulation in Wheat with Special Reference to TaNramp5 Expression Regulated by Calmodulin. J. Agric. Food Chem. 2021, 69, 6870–6878. [Google Scholar] [CrossRef]
- Yang, H.; Xu, Z.R.; Liu, R.X.; Xiong, Z.T. Lanthanum reduces the cadmium accumulation by suppressing expression of transporter genes involved in cadmium uptake and translocation in wheat. Plant Soil 2019, 441, 235–252. [Google Scholar] [CrossRef]
- Dai, H.; Shan, C.; Zhao, H.; Jia, G.; Chen, D. Lanthanum improves the cadmium tolerance of Zea mays seedlings by the regulation of ascorbate and glutathione metabolism. Biol. Plant. 2017, 61, 551–556. [Google Scholar] [CrossRef]
- Xia, R.Z.; Zhou, J.; Cui, H.B.; Liang, J.N.; Liu, Q.Q.; Zhou, J. Nodes play a major role in cadmium (Cd) storage and redistribution in low-Cd-accumulating rice (Oryza sativa L.) cultivars. Sci. Total Environ. 2023, 859, 160436. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Liang, Y.; Hu, H.; Shaheen, S.M.; Zhong, H.; Tack, F.M.G.; Wu, M.; Li, Y.-F.; Gao, Y.; Rinklebe, J.; et al. Speciation, transportation, and pathways of cadmium in soil-rice systems: A review on the environmental implications and remediation approaches for food safety. Environ. Int. 2021, 156, 106749. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Dong, J.; Qu, M.; Lv, Q.; Zhang, L.; Peng, C.; Hu, Y.; Li, Y.; Ji, Z.; Mao, B.; et al. Knockout of OsNRAMP5 enhances rice tolerance to cadmium toxicity in response to varying external cadmium concentrations via distinct mechanisms. Sci. Total Environ. 2022, 832, 155006. [Google Scholar] [CrossRef]
- Wiggenhauser, M.; Aucour, A.-M.; Bureau, S.; Campillo, S.; Telouk, P.; Romani, M.; Ma, J.F.; Landrot, G.; Sarret, G. Cadmium transfer in contaminated soil-rice systems: Insights from solid-state speciation analysis and stable isotope fractionation. Environ. Pollut. 2021, 269, 155006. [Google Scholar] [CrossRef]
- Takahashi, R.; Ishimaru, Y.; Shimo, H.; Ogo, Y.; Senoura, T.; Nishizawa, N.K.; Nakanishi, H. The OsHMA2 transporter is involved in root-to-shoot translocation of Zn and Cd in rice. Plant Cell Environ. 2012, 35, 1948–1957. [Google Scholar] [CrossRef]
- Shao, J.F.; Xia, J.; Yamaji, N.; Shen, R.F.; Ma, J.F. Effective reduction of cadmium accumulation in rice grain by expressing OsHMA3 under the control of the OsHMA2 promoter. J. Exp. Bot. 2018, 69, 2743–2752. [Google Scholar] [CrossRef]
- Zhou, J.; Gao, M.; Cui, H.B.; Li, D.M.; Xia, R.Z.; Wang, T.; Zhou, J. Influence of Silicon and Selenium and Contribution of the Node to Cadmium Allocation and Toxicity in Rice. ACS Agric. Sci. Technol. 2021, 1, 550–557. [Google Scholar] [CrossRef]
- Guo, J.; Li, K.; Zhang, X.; Huang, H.; Huang, F.; Zhang, L.; Wang, Y.; Li, T.; Yu, H. Genetic properties of cadmium translocation from straw to brown rice in low-grain cadmium rice (Oryza sativa L.) line. Ecotoxicol. Environ. Saf. 2019, 182, 109422. [Google Scholar] [CrossRef]
- Tan, L.T.; Zhu, Y.X.; Fan, T.; Peng, C.; Wang, J.R.; Sun, L.; Chen, C.Y. OsZIP7 functions in xylem loading in roots and inter-vascular transfer in nodes to deliver Zn/Cd to grain in rice. Biochem. Biophys. Res. Commun. 2019, 512, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Rodda, M.S.; Li, G.; Reid, R.J. The timing of grain Cd accumulation in rice plants: The relative importance of remobilisation within the plant and root Cd uptake post-flowering. Plant Soil 2011, 347, 105–114. [Google Scholar] [CrossRef]
- Uraguchi, S.; Fujiwara, T. Rice breaks ground for cadmium-free cereals. Curr. Opin. Plant Biol. 2013, 16, 328–334. [Google Scholar] [CrossRef] [PubMed]
- Uraguchi, S.; Kamiya, T.; Sakamoto, T.; Kasai, K.; Sato, Y.; Nagamura, Y.; Yoshida, A.; Kyozuka, J.; Ishikawa, S.; Fujiwara, T. Low-affinity cation transporter (OsLCT1) regulates cadmium transport into rice grains. Proc. Natl. Acad. Sci. USA 2011, 108, 20959–20964. [Google Scholar] [CrossRef]
- Ma, C.; Xie, P.; Zhang, K.; Yang, J.X.; Li, X.Z.; Liu, F.Y.; Lin, L.; Zhang, H.Z. Contribution of the flag leaf to lead absorption in wheat grain at the grain-filling stage. Ecotoxicol. Environ. Saf. 2021, 225, 112722. [Google Scholar] [CrossRef]
- Huang, B.Y.; Zhao, F.J.; Wang, P. The relative contributions of root uptake and remobilization to the loading of Cd and As into rice grains: Implications in simultaneously controlling grain Cd and As accumulation using a segmented water management strategy. Environ. Pollut. 2022, 293, 118497. [Google Scholar] [CrossRef]
- Huang, S.; Ma, J.F. Silicon suppresses zinc uptake through down-regulating zinc transporter gene in rice. Physiol. Plant. 2020, 170, 580. [Google Scholar] [CrossRef]
- Liu, J.G.; Qu, P.; Zhang, W.; Dong, Y.; Li, L.; Wang, M.X. Variations among rice cultivars in subcellular distribution of Cd: The relationship between translocation and grain accumulation. Environ. Exp. Bot. 2014, 107, 25–31. [Google Scholar] [CrossRef]
- Wang, Y.L.; Xu, Y.M.; Liang, X.F.; Li, L.P.; Huang, Q.Q. Soil addition of MnSO4 reduces wheat Cd accumulation by simultaneously increasing labile Mn and decreasing labile Cd concentrations in calcareous soil: A two-year pot study. Chemosphere 2023, 317, 137900. [Google Scholar] [CrossRef]
- Zhong, Y.Q.; Chen, J.J. Ameliorative effects of Lanthanum(III) on Copper(II) stressed rice (Oryza sativa) and its molecular mechanism revealed by transcriptome profiling. Plant Physiol. Biochem. 2020, 152, 184–193. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, J.M. Lanthanum Promotes Bahiagrass (Paspalum notatum) Roots Growth by Improving Root Activity, Photosynthesis and Respiration. Plants 2022, 11, 382. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.W.; Lin, Y.S.; Wang, X. Effects of lanthanum on growth, element uptake, and oxidative stress in rice seedlings. J. Plant Nutr. Soil Sci. 2012, 175, 907–911. [Google Scholar] [CrossRef]
- Liu, D.; Wang, X.; Zhang, X.; Gao, Z. Effects of lanthanum on growth and accumulation in roots of rice seedlings. Plant Soil Environ. 2013, 59, 196–200. [Google Scholar] [CrossRef]
- Wang, C.R.; Wang, Q.Y.; Tian, Y.; Zhang, J.F.; Li, Z.X.; Cao, P.; Zhu, M.; Li, T.T. Lanthanum ions intervened in enzymatic production and elimination of reactive oxygen species in leaves of rice seedlings under cadmium stress. Environ. Toxicol. Chem. 2014, 33, 1656–1664. [Google Scholar] [CrossRef]
- Huang, X.H.; Zhou, Q. Alleviation Effect of Lanthanum on Cadmium Stress in Seedling Hydroponic Culture of Kidney Bean and Corn. J. Rare Earths 2006, 24, 248–252. [Google Scholar] [CrossRef]
- Ruan, Y.L. Sucrose Metabolism: Gateway to Diverse Carbon Use and Sugar Signaling. Annu. Rev. Plant Biol. 2014, 65, 33–67. [Google Scholar] [CrossRef]
- Řezanka, T.; Kaineder, K.; Mezricky, D.; Řezanka, M.; Bišová, K.; Zachleder, V.; Vítová, M. The effect of lanthanides on photosynthesis, growth, and chlorophyll profile of the green alga Desmodesmus quadricauda. Photosynth. Res. 2016, 130, 335–346. [Google Scholar] [CrossRef]
- Edington, S.C.; Gonzalez, A.; Middendorf, T.R.; Halling, D.B.; Aldrich, R.W.; Baiz, C.R. Coordination to lanthanide ions distorts binding site conformation in calmodulin. Proc. Natl. Acad. Sci. USA 2018, 115, E3126–E3134. [Google Scholar] [CrossRef]
- Huang, G.Q.; Wang, D.F. Effects of lanthanum on the cadmium uptake of pacific oyster Crassostrea gigas. Indian J. Geo-Mar. Sci. 2016, 45, 653–657. [Google Scholar]
- Xiong, S.L.; Xiong, Z.T.; Chen, Y.C.; Huang, H. Interactive Effects of Lanthanum and Cadmium on Plant Growth and Mineral Element Uptake in Crisped-Leaf Mustard Under Hydroponic Conditions. J. Plant Nutr. 2006, 29, 1889–1902. [Google Scholar] [CrossRef]
- Huang, G.X.; Ding, C.F.; Guo, F.Y.; Li, X.G.; Zhou, Z.G.; Zhang, T.L.; Wang, X.X. The Role of Node Restriction on Cadmium Accumulation in the Brown Rice of 12 Chinese Rice (Oryza sativa L.) Cultivars. J. Agric. Food Chem. 2017, 65, 10157–10164. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.G.; Zhang, C.; Du, B.Y.; Lu, B.X.; Zhou, D.M.; Zhou, J.; Zhou, J. Effects of node restriction on cadmium accumulation in eight Chinese wheat (Triticum turgidum) cultivars. Sci. Total Environ. 2020, 725, 138358. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zeng, X.N.; Song, Q.L.; Sun, Y.; Feng, Y.J.; Lai, Y.C. Identification of key genes and modules in response to Cadmium stress in different rice varieties and stem nodes by weighted gene co-expression network analysis. Sci. Rep. 2020, 10, 9525. [Google Scholar] [CrossRef] [PubMed]
- Yan, B.F.; Nguyen, C.; Pokrovsky, O.S.; Candaudap, F.; Coriou, C.; Bussière, S.; Robert, T.; Cornu, J.Y. Contribution of remobilization to the loading of cadmium in durum wheat grains: Impact of post-anthesis nitrogen supply. Plant Soil 2018, 424, 591–606. [Google Scholar] [CrossRef]
- Tan, J.J.; Wang, J.W.; Chai, T.Y.; Zhang, Y.X.; Feng, S.S.; Li, Y.; Zhao, H.J.; Liu, H.M.; Chai, X.P. Functional analyses of TaHMA2, a P1B-type ATPase in wheat. Plant Biotechnol. J. 2013, 11, 420–431. [Google Scholar] [CrossRef]
- Clemens, S.; Antosiewicz, D.M.; Ward, J.M.; Schachtman, D.P.; Schroeder, J.I. The plant cDNA LCT1 mediates the uptake of calcium and cadmium in yeast. Proc. Natl. Acad. Sci. USA 1998, 95, 12043–12048. [Google Scholar] [CrossRef]
- Schachtman, D.P.; Kumar, R.; Schroeder, J.I.; Marsh, E. Molecular and functional characterization of a novel low-affinity cation transporter (LCT1) in higher plants. Proc. Natl. Acad. Sci. USA 1997, 94, 11079–11084. [Google Scholar] [CrossRef]
- Ye, C.; Chen, Z.; Peng, O. Effects of cadmium stress on growth and cadmium accumulation in rice at different growth stages. Acta Sci. Circumstantiae 2017, 37, 3201–3206. [Google Scholar]
- Yamaji, N.; Xia, J.; Mitani-Ueno, N.; Yokosho, K.; Ma, J.F. Preferential Delivery of Zinc to Developing Tissues in Rice Is Mediated by P-Type Heavy Metal ATPase OsHMA2. Plant Physiol. 2013, 162, 927–939. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, C.; Yang, H.; Chen, X.; Li, J.; Cai, X.; Long, J. Application of Lanthanum at the Heading Stage Effectively Suppresses Cadmium Accumulation in Wheat Grains by Downregulating the Expression of TaZIP7 to Increase Cadmium Retention in Nodes. Plants 2024, 13, 2921. https://doi.org/10.3390/plants13202921
Xiao C, Yang H, Chen X, Li J, Cai X, Long J. Application of Lanthanum at the Heading Stage Effectively Suppresses Cadmium Accumulation in Wheat Grains by Downregulating the Expression of TaZIP7 to Increase Cadmium Retention in Nodes. Plants. 2024; 13(20):2921. https://doi.org/10.3390/plants13202921
Chicago/Turabian StyleXiao, Caixia, Hua Yang, Xingwang Chen, Jie Li, Xiongfei Cai, and Jian Long. 2024. "Application of Lanthanum at the Heading Stage Effectively Suppresses Cadmium Accumulation in Wheat Grains by Downregulating the Expression of TaZIP7 to Increase Cadmium Retention in Nodes" Plants 13, no. 20: 2921. https://doi.org/10.3390/plants13202921
APA StyleXiao, C., Yang, H., Chen, X., Li, J., Cai, X., & Long, J. (2024). Application of Lanthanum at the Heading Stage Effectively Suppresses Cadmium Accumulation in Wheat Grains by Downregulating the Expression of TaZIP7 to Increase Cadmium Retention in Nodes. Plants, 13(20), 2921. https://doi.org/10.3390/plants13202921






