The Application of a Foliar Spray Containing Methylobacterium symbioticum Had a Limited Effect on Crop Yield and Nitrogen Recovery in Field and Pot-Grown Maize
Abstract
1. Introduction
2. Materials and Methods
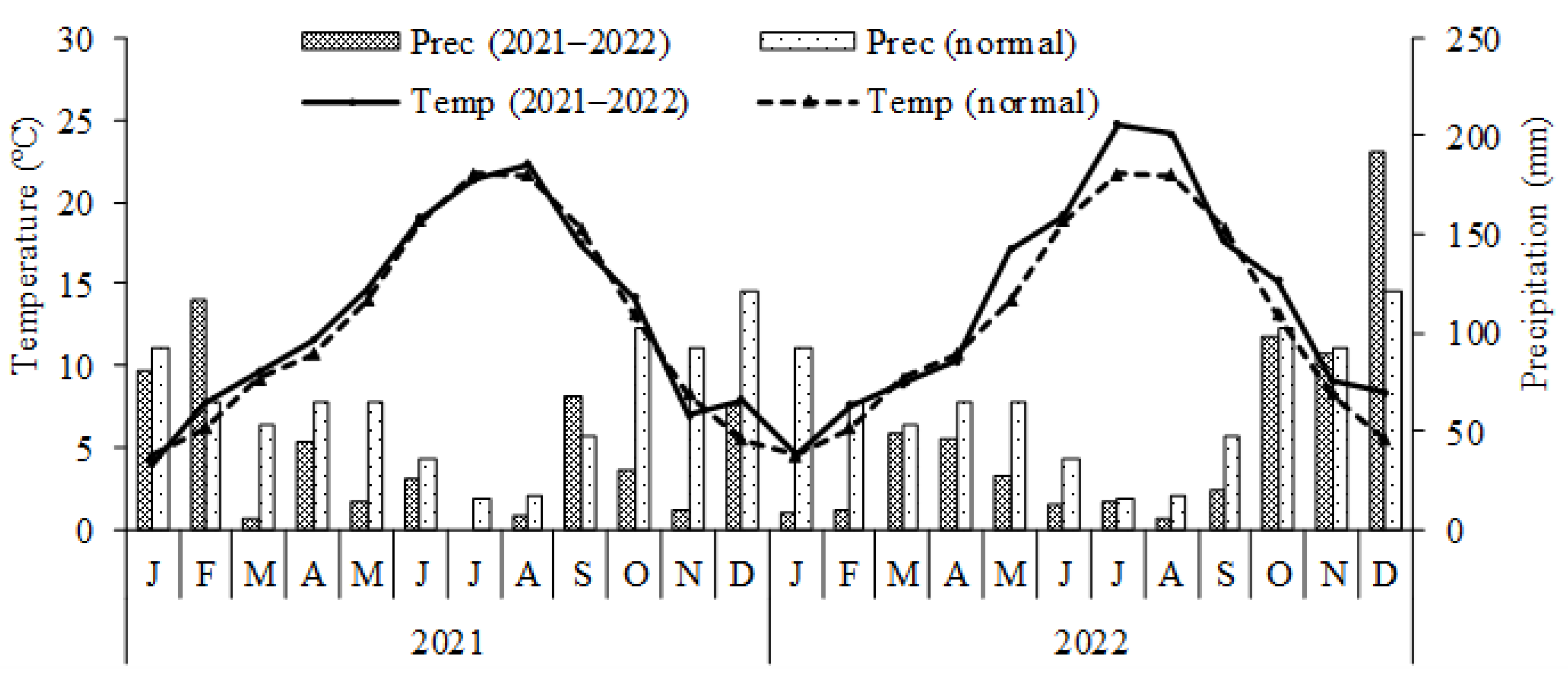
2.1. Experimental Conditions
2.2. Experimental Designs
2.3. Preparation and Management of Field and Pot Experiments
2.4. Measurements During the Growing Season
2.5. Sample Collection and Preparation for Laboratory Analysis
2.6. Soil and Plant Analysis
2.7. Data Analysis
3. Results
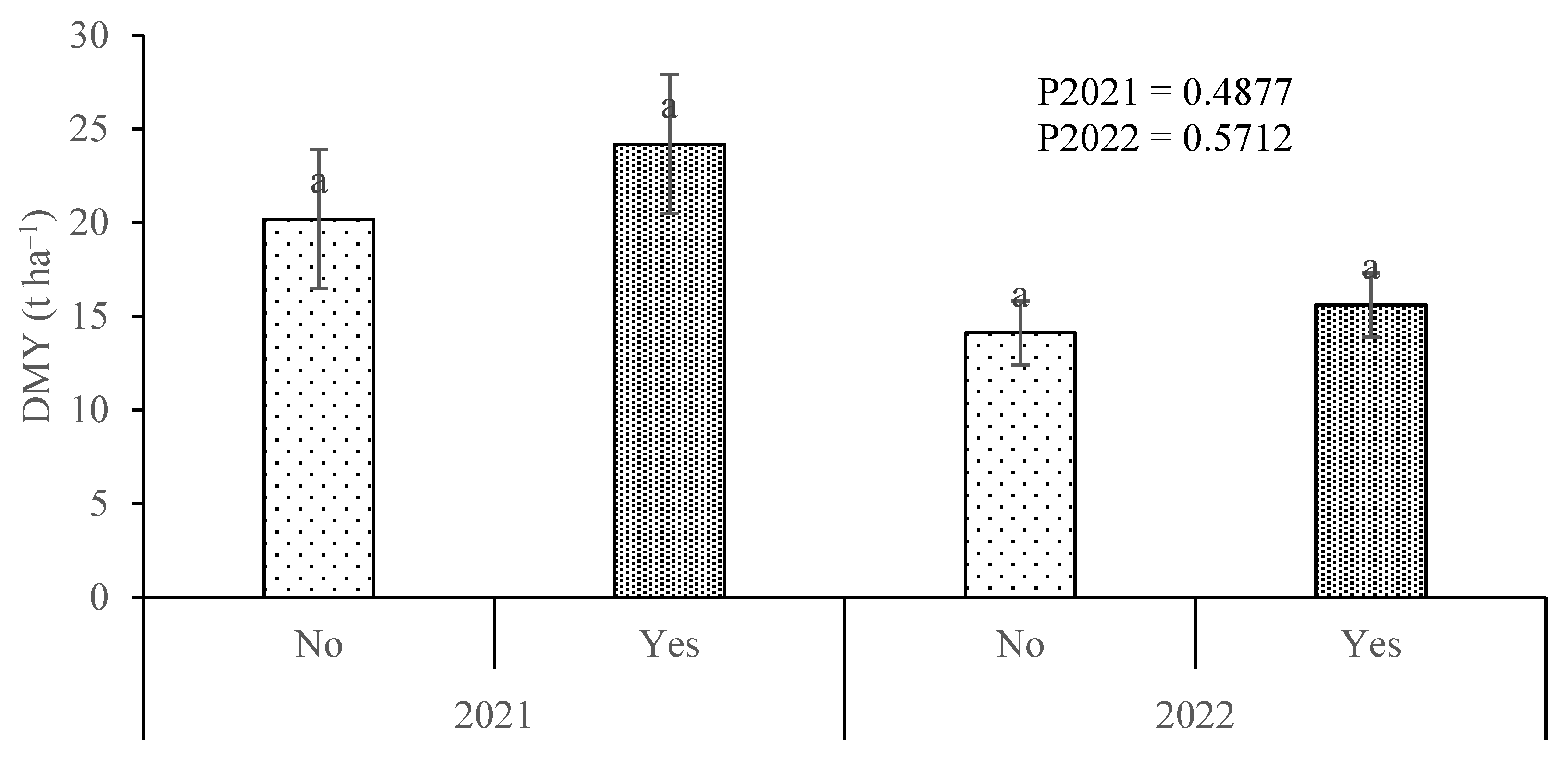
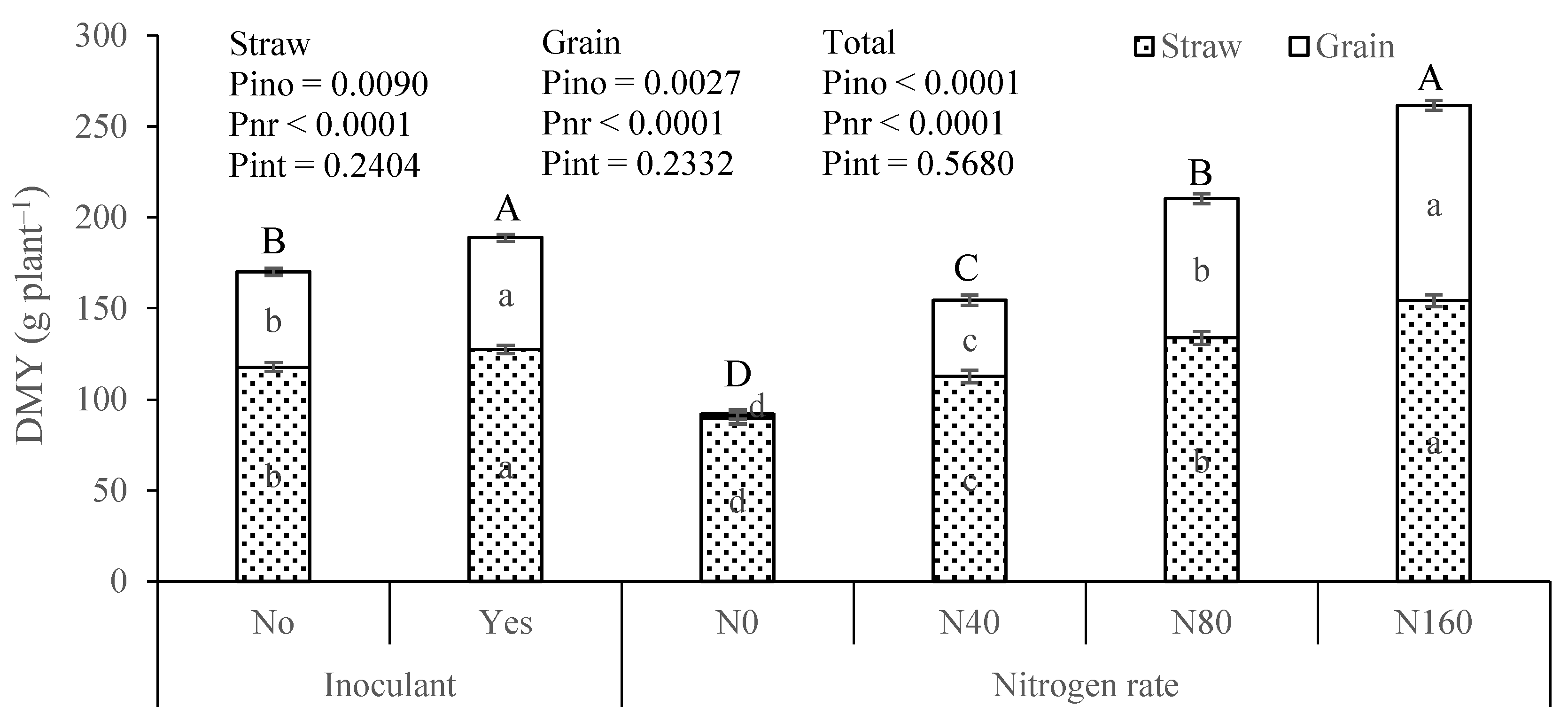
3.1. Maize Growth Performance
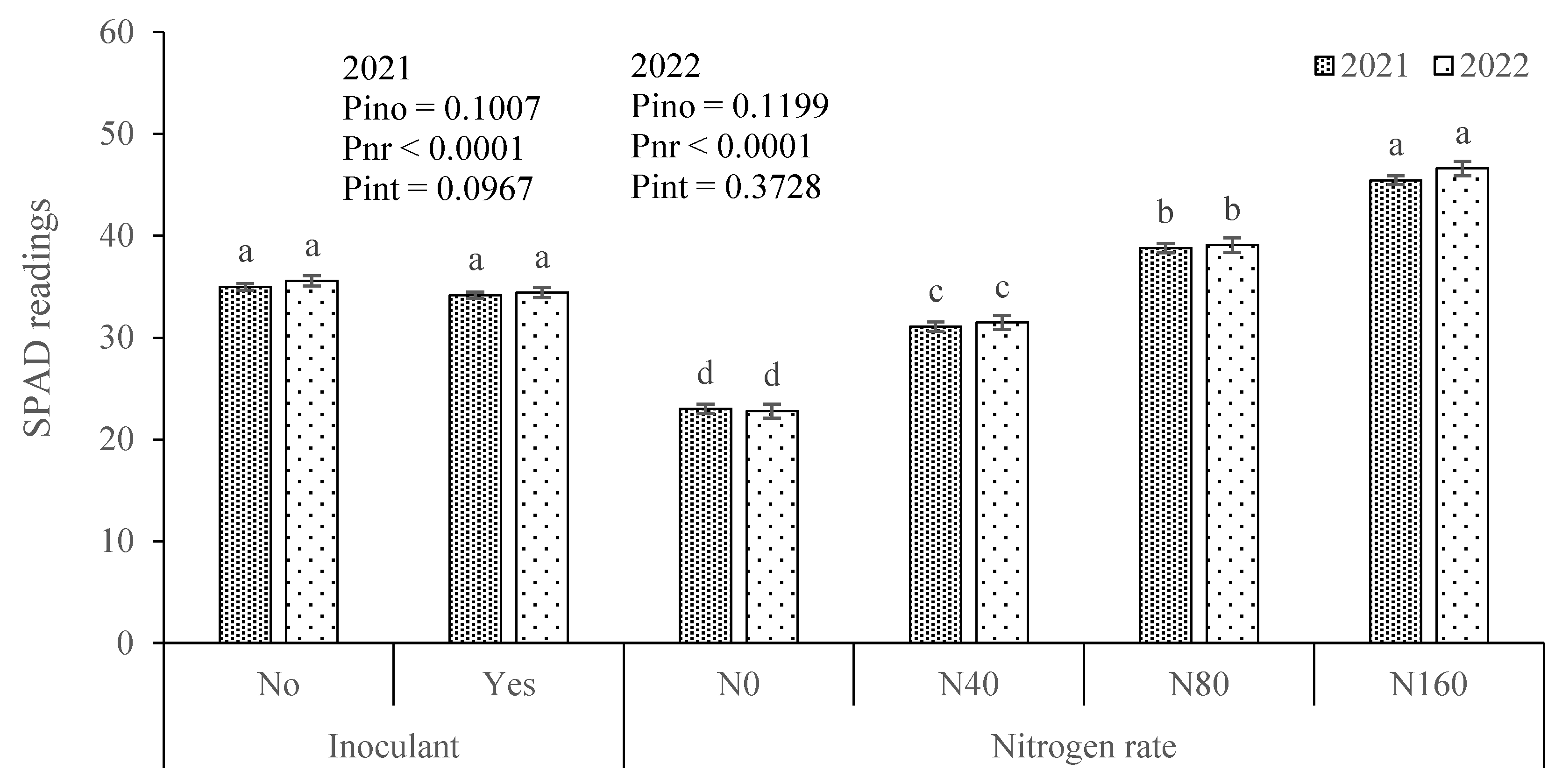
3.2. Plant N Nutritional Status, N Recovery and Apparent N Fixation
4. Discussion
4.1. Dry Matter Yield Increased Significantly with Soil-Applied N and to a Much Lesser Extent with the Inoculant Application
4.2. Indicators of N Nutritional Status Increased with Soil-Applied N but Did Not Change Significantly with the Inoculant Application
4.3. The Inoculant Application Was Inconsistent in N Fixation
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- FAOSTAT. Production: Crops and Livestock Products. 2024. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 4 August 2024).
- Erenstein, O.; Jaleta, M.; Sonder, K.; Mottaleb, K.; Prasanna, B.M. Global maize production, consumption and trade: Trends and R&D implications. Food Sec. 2022, 14, 1295–1319. [Google Scholar] [CrossRef]
- Heuzé, V.; Tran, G.; Edouard, N.; Lebas, F. Maize Green Forage. INRAE, CIRAD, AFZ and FAO, 2017a. Available online: https://www.feedipedia.org/node/358 (accessed on 15 August 2024).
- Heuzé, V.; Tran, G.; Edouard, N.; Lebas, F. Maize Silage. INRAE, CIRAD, AFZ and FAO, 2017b. Available online: https://www.feedipedia.org/node/13883 (accessed on 15 August 2024).
- Grassini, P.; Cassman, K.G. High-yield maize with large net energy yield and small global warming intensity. Proc. Natl. Acad. Sci. USA 2012, 109, 1074–1079. Available online: www.pnas.org/cgi/doi/10.1073/pnas.1116364109 (accessed on 15 August 2024). [CrossRef]
- Nasielski, J.; Grant, B.; Smith, W.; Niemeyer, C.; Janovicek, K.; Deen, B. Effect of nitrogen source, placement and timing on the environmental performance of economically optimum nitrogen rates in maize. Field Crop. Res. 2020, 246, 107608. [Google Scholar] [CrossRef]
- Arrobas, M.; Chiochetta, J.C.; Damo, L.; Júlio, A.C.; Hendges, I.P.; Wagner, A.; Godoy, W.I.; Cassol, L.C.; Rodrigues, M.A. Controlled-release and stabilized fertilizers are equivalent options to split application of ammonium nitrate in a double maize-oats cropping system. J. Plant Nutr. 2022, 46, 996–1008. [Google Scholar] [CrossRef]
- Jiang, Y.; Nyiraneza, J.; Khakbazan, M.; Geng, X.; Murray, B.J. Nitrate leaching and potato yield under varying plow timing and nitrogen rate. Agrosyst. Geosci. Environ. 2019, 2, 190032. [Google Scholar] [CrossRef]
- Hina, N.S. Global meta-analysis of nitrate leaching vulnerability in synthetic and organic fertilizers over the past four decades. Water 2024, 16, 457. [Google Scholar] [CrossRef]
- Tian, D.; Zhang, Y.; Mu, Y.; Liu, J.; He, K. Effect of N fertilizer types on N2O and NO emissions under drip fertigation from an agricultural field in the North China Plain. Sci. Total Environ. 2020, 715, 136903. [Google Scholar] [CrossRef]
- Pan, S.-Y.; He, K.-H.; Lin, K.-T.; Fan, C.; Chang, C.-T. Addressing nitrogenous gases from croplands toward low-emission agriculture. NPJ Clim. Atmos. Sci. 2022, 5, 43. [Google Scholar] [CrossRef]
- Smreczak, B.; Ukalska-Jaruga, A. Dissolved organic matter in agricultural soils. Soil Sci. Annu. 2021, 72, 132234. [Google Scholar] [CrossRef]
- Findlay, S.E.G.; Parr, T.B. Dissolved organic matter. In Methods in Stream Ecology, Ecosystem Function; Lamberti, A.G., Hauer, F.R., Eds.; Academic Press: London, UK, 2017; Volume 2, pp. 21–35. [Google Scholar]
- Xenopoulos, M.A.; Barnes, R.T.; Boodoo, K.S.; Butman, D.; Catalán, N.; D’Amario, S.C.; Fasching, C.; Kothawala, D.N.; Pisani, O.; Solomon, C.T.; et al. How humans alter dissolved organic matter composition in freshwater: Relevance for the Earth’s biogeochemistry. Biogeochemistry 2021, 154, 323–348. [Google Scholar] [CrossRef]
- Zhou, P.; Tian, L.; Graham, N.; Song, S.; Zhao, R.; Siddique, M.S.; Hu, Y.; Cao, X.; Lu, Y.; Elimelech, M.; et al. Spatial patterns and environmental functions of dissolved organic matter in grassland soils of China. Nat. Commun. 2024, 15, 6356. [Google Scholar] [CrossRef]
- Ray, P.P.; Jarrett, J.; Knowlton, K.F. Effect of dietary phytate on phosphorus digestibility in dairy cows. J. Dairy Sci. 2013, 96, 1156–1163. [Google Scholar] [CrossRef]
- Sharma, S.; AnandKumar, L.H.D.; Tyagi, A.; Muthumilarasan, M.; Kumar, K.; Gaikwad, K. An insight into phytic acid biosynthesis and its reduction strategies to improve mineral bioavailability. Nucleus 2022, 65, 255–267. [Google Scholar] [CrossRef]
- Kokulan, V.; Ige, D.; Akinremi, O.O. Agri-environmental implications of N- and P-based manure application to perennial and annual cropping systems. Nutr. Cycl. Agroecosyst. 2022, 122, 205–218. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, S.; Peng, C.; Chi, G.; Chen, X.; Huang, B.; Lu, C.; Li, J.; Xu, L. Quantifying Phosphorus Leaching Loss from Mollisol with Organic Amendments. Agronomy 2022, 12, 2490. [Google Scholar] [CrossRef]
- Steinman, A.D.; Duhamel, S. Phosphorus Limitation, Uptake, and Turnover in Benthic Stream Algae. In Methods in Stream Ecology, Ecosystem Function; Lamberti, A.G., Hauer, F.R., Eds.; Academic Press: London, UK, 2017; Volume 2, pp. 197–218. [Google Scholar]
- Russelle, M.P. Biological dinitrogen fixation in agriculture. In Nitrogen in Agricultural Systems; Schepers, J.S., Raun, W.R., Eds.; Agronomy Monograph No. 49; ASA, CSSA, SSSA: Madison, WI, USA, 2008; pp. 281–359. [Google Scholar]
- Ohyama, T. The role of legume-rhizobium symbiosis in sustainable agriculture. In Legume Nitrogen Fixation in Soils with Low Phosphorus Availability Adaptation and Regulatory Implication; Sulieman, S., Tran, L.-S.P., Eds.; Springer: Cham, Switzerland, 2017; pp. 1–20. [Google Scholar] [CrossRef]
- Hungria, M.; Nogueira, M.A. Nitrogen fixation. In Marschner’s Mineral Nutrition of Plants, 4th ed.; Rengel, Z., Cakmak, I., White, P.J., Eds.; Elsevier, Ltd.: Chennai, India, 2023; pp. 615–650. [Google Scholar] [CrossRef]
- Bhuvaneshwari, K.; Singh, P.K. Response of nitrogen-fixing water fern Azolla biofertilization to rice crop. Biotech 2015, 5, 523–529. [Google Scholar] [CrossRef]
- Buragohain, S.; Sharma, B.; Nath, J.D.; Gogaoi, N.; Meena, R.S.; Lal, R. Effect of 10 years of biofertiliser use on soil quality and rice yield on an Inceptisol in Assam, India. Soil. Res. 2018, 56, 49–58. [Google Scholar] [CrossRef]
- Ohyama, T.; Momose, A.; Ohtake, N.; Sueyoshi, K.; Sato, T.; Nakanishi, Y.; Asis, C.A., Jr.; Ruamsungsri, S.; Ando, S. Nitrogen fixation in sugarcane. In Advances in Biology and Ecology of Nitrogen Fixation; Ohyama, T., Ed.; AvE4EvA MuViMix Records, Intechopen: London, UK, 2014; pp. 49–70. [Google Scholar] [CrossRef]
- Dwivedi, M. Gluconobacter. In Beneficial Microbes in Agro-Ecology: Bacteria and Fungi; Amaresan, N., Kumar, M.S., Annapurna, K., Kumar, K., Sankaranarayanan, A., Eds.; Academic Press: London, UK; Elsevier: Amsterdam, The Netherlands, 2020; pp. 521–544. [Google Scholar]
- Hoffmann, M.P.; Swanepoel, C.M.; Nelson, W.C.D.; Beukes, D.J.; van der Laan, M.; Hargreaves, J.N.G.; Rötter, R.P. Simulating medium-term effects of cropping system diversification on soil fertility and crop productivity in southern Africa. Eur. J. Agron. 2020, 119, 126089. [Google Scholar] [CrossRef]
- Ton, A. Advantages of grain legume-cereal intercropping in sustainable agriculture. Turk. J. Agric. Food Sci. Technol. 2021, 9, 1560–1566. [Google Scholar] [CrossRef]
- Dimande, P.; Arrobas, M.; Rodrigues, M.Â. Intercropped maize and cowpea increased the land equivalent ratio and enhanced crop access to more nitrogen and phosphorus compared to cultivation as sole crops. Sustainability 2024, 16, 1440. [Google Scholar] [CrossRef]
- Chebotar, V.K.; Malfanova, N.V.; Shcherbakov, A.V.; Ahtemova, G.A.; Borisov, A.Y.; Lugtenberg, B.; Tikhonovich, I.A. Endophytic bacteria in microbial preparations that improve plant development (review). Appl. Biochem. Microbiol. 2015, 51, 271–277. [Google Scholar] [CrossRef]
- Matteoli, F.P.; Olivares, F.L.; Venancio, T.M.; Rocha, L.O.; Irineu, L.E.S.S.; Canellas, L.P. Herbaspirillum. In Beneficial Microbes in Agro-Ecology: Bacteria and Fungi; Amaresan, N., Kumar, M.S., Annapurna, K., Kumar, K., Sankaranarayanan, A., Eds.; Academic Press: London, UK; Elsevier: Amsterdam, The Netherlands, 2020; pp. 493–508. [Google Scholar]
- Monteiro, R.A.; Balsanelli, E.; Wassem, R.; Marin, A.M.; Brusamarello-Santos, L.C.C.; Schmidt, M.A.; Tadra-Sfeir, M.Z.; Pankievicz, V.C.S.; Cruz, L.M.; Chubatsu, L.S.; et al. Herbaspirillum-plant interactions: Microscopical, histological and molecular aspects. Plant Soil 2012, 356, 175–196. [Google Scholar] [CrossRef]
- Alves, G.C.; dos Santos, C.L.R.; Zilli, J.E.; dos Reis Junior, F.B.; Marriel, I.E.; Breda, F.A.F.; Boddey, R.M.; Reis, V.M. Agronomic evaluation of Herbaspirillum seropedicae strain ZAE94 as an inoculant to improve maize yield in Brazil. Pedosphere 2021, 31, 583–595. [Google Scholar] [CrossRef]
- Pascual, J.A.; Ros, M.; Martínez, J.; Carmona, F.; Bernabé, A.; Torres, R.; Lucena, T.; Aznar, R.; Arahal, D.R.; Fernández, F. Methylobacterium symbioticum sp. nov., a new species isolated from spores of Glomus iranicum var. tenuihypharum. Curr. Microbiol. 2020, 77, 2031–2041. [Google Scholar] [CrossRef]
- Leducq, J.-B.; Sneddon, D.; Santos, M.; Condrain-Morel, D.; Bourret, G.; Martinez-Gomez, N.C.; Lee, J.A.; Foster, J.A.; Stolyar, S.; Shapiro, B.J.; et al. Comprehensive phylogenomics of methylobacterium reveals four evolutionary distinct groups and underappreciated phyllosphere diversity. Genome Biol. Evol. 2022, 14, evac123. [Google Scholar] [CrossRef]
- Palberg, D.; Kisiała, A.; Jorge, G.L.; Emery, R.J.N. A survey of Methylobacterium species and strains reveals widespread production and varying profiles of cytokinin phytohormones. BMC Microbiol. 2022, 22, 49. [Google Scholar] [CrossRef]
- Vera, R.T.; García, A.J.B.; Álvarez, F.J.C.; Ruiz, J.M.; Martín, F.F. Application and effectiveness of Methylobacterium symbioticum as a biological inoculant in maize and strawberry crops. Folia Microbiol. 2024, 69, 121–131. [Google Scholar] [CrossRef]
- Barrera, S.E.; Sarango-Flóres, S.-W.; Montenegro-Gómez, S.-P. The phyllosphere microbiome and its potential application in horticultural crops. A review. Rev. Colomb. Cienc. Hortíc. 2019, 13, 384–396. [Google Scholar] [CrossRef]
- Jinal, H.N.; Amaresan, N.; Sankaranarayanan, A. Methylobacterium. In Beneficial Microbes in Agro-Ecology: Bacteria and Fungi; Amaresan, N., Kumar, M.S., Annapurna, K., Kumar, K., Sankaranarayanan, A., Eds.; Academic Press: London, UK; Elsevier: Amsterdam, The Netherland, 2020; pp. 509–519. [Google Scholar]
- Srivastava, A.; Dixit, R.; Chand, N.; Kumar, P. Overview of methylotrophic microorganisms in agriculture. Bio Sci. Res. Bull. 2022, 38, 65–71. [Google Scholar] [CrossRef]
- Abanda-Nkpwatt, D.; Müsch, M.; Tschiersch, J.; Boettner, M.; Schwab, W. Molecular interaction between Methylobacterium extorquens and seedlings: Growth promotion, methanol consumption, and localization of the methanol emission site. J. Exp. Bot. 2006, 57, 4025–4032. [Google Scholar] [CrossRef]
- IPMA (Instituto Português do Mar e da Atmosfera). Normais Climatológicas [Climate Normals]. 2024. Available online: https://www.ipma.pt/pt/oclima/normais.clima/ (accessed on 15 August 2024).
- WRB. World Reference Base for Soil Resources 2014, Update 2015. In International Soil Classification System for Naming Soils and Creating Legends for Soil Maps; World Soil Resources Reports No. 106; FAO: Rome, Italy, 2015. [Google Scholar]
- Meier, U. Growth Stages of Mono and Dicotyledonous Plants; Federal Biological Research Centre for Agriculture and Forestry: Berlin, Germany, 2018. [Google Scholar]
- Van Reeuwijk, L.P. Procedures for Soil Analysis, Technical Paper 9, 6th ed.; ISRIC: Wageningen, The Netherlands; FAO of the United Nations: Rome, Italy, 2002. [Google Scholar]
- Temminghoff, E.E.; Houba, V.J. Plant Analysis Procedures, 2nd ed.; Temminghoff, E.E., Houba, V.J., Eds.; Kluwer Academic Publishers: London, UK, 2004. [Google Scholar] [CrossRef]
- Weil, R.R.; Brady, N.C. The Nature and Properties of Soils, 15th ed.; Pearson Education Limited: Edinburg, UK, 2017. [Google Scholar]
- Hawkesford, M.J.; Cakmak, I.; Coskun, D.; De Kok, L.J.; Lambers, H.; Schjoerring, J.K.; White, P.J. Functions of macronutrients. In Marschner’s Mineral Nutrition of Plants, 4th ed.; Rengel, Z., Cakmak, I., White, P.J., Eds.; Elsevier, Ltd.: Chennai, India, 2023; pp. 201–281. [Google Scholar] [CrossRef]
- Arrobas, M.; Andrade, M.; Raimundo, S.; Mazaro, S.M.; Rodrigues, M.A. Lettuce response to the application of two commercial leonardites and their effect on soil properties in a growing medium with nitrogen as the main limiting factor. J. Plant Nutr. 2023, 46, 4280–4294. [Google Scholar] [CrossRef]
- Rodrigues, M.Â.; Raimundo, S.; Correia, C.M.; Arrobas, M. Nitrogen Fixation and Growth of Potted Olive Plants through Foliar Application of a Nitrogen-Fixing Microorganism. Horticulturae 2024, 10, 604. [Google Scholar] [CrossRef]
- Razmjooei, Z.; Etemadi, M.; Eshghi, S.; Ramezanian, A.; Mirazimi Abarghuei, F.; Alizargar, J. Potential role of foliar application of azotobacter on growth, nutritional value and quality of lettuce under different nitrogen levels. Plants 2022, 11, 406. [Google Scholar] [CrossRef]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence—A practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Afonso, S.; Arrobas, M.; Ferreira, I.Q.; Rodrigues, M.A. Assessing the potential use of two portable chlorophyll meters in diagnosing the nutritional status of plants. J. Plant Nutr. 2018, 41, 261–271. [Google Scholar] [CrossRef]
- Wicharuck, S.; Suang, S.; Chaichana, C.; Chromkaew, Y.; Mawan, N.; Soilueang, P.; Khongdee, N. The implementation of the SPAD-502 chlorophyll meter for the quantification of nitrogen content in Arabica coffee leaves. MethodsX 2024, 12, 102566. [Google Scholar] [CrossRef]
- Kirkby, E.A.; Nikolic, M.; White, P.J.; Xu, G. Mineral nutrition, yield, and source-sink relationships. In Marschner’s Mineral Nutrition of Plants, 4th ed.; Rengel, Z., Cakmak, I., White, P.J., Eds.; Elsevier, Ltd.: Chennai, India, 2023; pp. 131–200. [Google Scholar] [CrossRef]
- Bell, R. Diagnosis and prediction of deficiency and toxicity of nutrients. In Marschner’s Mineral Nutrition of Plants, 4th ed.; Rengel, Z., Cakmak, I., White, P.J., Eds.; Elsevier, Ltd.: Chennai, India, 2023; pp. 477–495. [Google Scholar] [CrossRef]
- Kawakatsu, T.F.; Takaiwa, F. Proteins. In Encyclopaedia of Applied Plant Sciences, 2nd ed.; Thomas, B., Murray, B.G., Murphy, D.J., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 100–105. [Google Scholar] [CrossRef]
- Arrobas, M.; Correia, C.M.; Rodrigues, M.Â. Methylobacterium symbioticum applied as a foliar inoculant was little effective in enhancing nitrogen fixation and lettuce dry matter yield. Sustainability 2024, 16, 4512. [Google Scholar] [CrossRef]
- Montejano-Ramírez, V.; Valencia-Cantero, E. The importance of lentils: An overview. Agriculture 2024, 14, 103. [Google Scholar] [CrossRef]
- de Vries, S.; de Vries, J. Azolla: A Model System for Symbiotic Nitrogen Fixation and Evolutionary Developmental Biology. In Current Advances in Fern Research; Fernández, H., Ed.; Springer: Cham, Switzerland, 2018. [Google Scholar] [CrossRef]
- Chanway, C.P.; Anand, R.; Yang, H. Nitrogen fixation outside and inside plant tissues. In Advances in Biology and Ecology of Nitrogen Fixation; Ohyama, T., Ed.; AvE4EvA MuViMix Records, Intechopen: London, UK, 2014; pp. 3–21. [Google Scholar] [CrossRef]
- Reed, S.C.; Cleveland, C.C.; Townsend, A.R. Functional ecology of free-living nitrogen fixation: A contemporary perspective. Annu. Rev. Ecol. Evol. Syst. 2011, 42, 489–512. [Google Scholar] [CrossRef]
- Copeland, J.K.; Yuan, L.; Layeghifard, M.; Wang, P.W.; Guttman, D.D. Seasonal community succession of the phyllosphere microbiome. Mol. Plant Microbe Interact. 2015, 28, 274–285. [Google Scholar] [CrossRef]
- Laforest-Lapointe, F.; Messier, C.; Kembel, S. Tree phyllosphere bacterial communities: Exploring the magnitude of intra- and inter-individual variation among host species. PeerJ 2016, 4, e2367. [Google Scholar] [CrossRef]
- Corteva. BlueN—Bioestimulante. Corteva Biologicals, Agriscience. 2024. Available online: https://www.corteva.pt/content/dam/dpagco/corteva/eu/pt/pt/files/folletos/DOC-BlueN-Folheto-Corteva_EU_PT.pdf (accessed on 12 March 2024).

| Soil Properties | Field Trial | Pot Experiment |
|---|---|---|
| 1 Organic carbon (g kg−1) | 14.1 ± 0.61 | 9.1 ± 1.26 |
| 2 pH (H2O) | 5.9 ± 0.31 | 6.5 ± 0.20 |
| 3 Extract. phosphorus (mg kg−1, P2O5) | 44.0 ± 8.96 | 67.2 ± 13.78 |
| 3 Extract. potassium (mg kg−1, K2O) | 103.7 ± 11.48 | 81.2 ± 7.71 |
| 4 Exchang. calcium (cmolc kg−1) | 13.7 ± 0.92 | 9.8 ± 1.21 |
| 4 Exchang. magnesium (cmolc kg−1) | 4.9 ± 0.44 | 3.5 ± 0.16 |
| 4 Exchang. potassium (cmolc kg−1) | 1.1 ± 0.16 | 0.3 ± 0.03 |
| 4 Exchang. sodium (cmolc kg−1) | 1.5 ± 0.12 | 0.4 ± 0.04 |
| 5 Exchang. acidity (cmolc kg−1) | 0.1 ± 0.00 | 0.1 ± 0.02 |
| 6 CEC (cmolc kg−1) | 21.3 ± 1.08 | 14.1 ± 1.34 |
| 7 Sand | 562.1 ± 28.55 | 544.1 ± 24.25 |
| 7 Silt | 245.3 ± 22.89 | 206.9 ± 20.88 |
| 7 Clay | 192.7 ± 50.77 | 249.0 ± 43.31 |
| 8 Texture | Sandy loam | Sandy clay loam |
| PNC (g kg−1) | N Recovery (kg ha−1) | ANF (kg ha−1) | ||||
|---|---|---|---|---|---|---|
| 2021 | 2022 | 2021 | 2022 | 2021 | 2022 | |
| No | 9.4 a | 10.3 a | 188.8 a | 144.6 a | ||
| Yes | 10.2 a | 10.1 a | 247.5 a | 159.1 a | 58.7 | 14.5 |
| Prob. | 0.0503 | 0.7226 | 0.3008 | 0.6385 | ||
| SNC (g kg−1) | GNC (g kg−1) | N Recovery (mg pot−1) | ANF (mg pot−1) | |||||
|---|---|---|---|---|---|---|---|---|
| 2021 | 2022 | 2021 | 2022 | 2021 | 2022 | 2021 | 2022 | |
| Inoculant | ||||||||
| No | 4.2 a | 3.2 a | 9.7 a | 10.0 b | 1002.4 a | 880.4 b | ||
| Yes | 4.1 a | 3.0 b | 8.4 b | 12.0 a | 953.1 a | 1079.6 a | −49.2 | 199.2 |
| Nitrogen rate | ||||||||
| N0 | 4.4 a | 4.1 a | 13.6 a | ---- | 475.6 d | 369.9 d | −0.8 | 24.8 |
| N40 | 3.9 b | 2.8 bc | 8.3 bc | 10.7 b | 859.7 c | 685.3 c | −88.2 | −14.3 |
| N80 | 3.6 c | 2.5 c | 7.7 c | 9.7 c | 1021.2 b | 1074.1 b | −13.6 | 185.0 |
| N120 | 4.6 a | 2.9 b | 8.9 b | 12.4 a | 1565.3 a | 1790.8 a | −94.2 | 601.3 |
| Pino | 0.0811 | 0.0031 | <0.0001 | <0.0001 | 0.1968 | 0.0003 | ||
| Pnr | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | ||
| Pint | <0.0001 | 0.0221 | <0.0001 | 0.0001 | 0.6446 | 0.0004 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodrigues, M.Â.; Correia, C.M.; Arrobas, M. The Application of a Foliar Spray Containing Methylobacterium symbioticum Had a Limited Effect on Crop Yield and Nitrogen Recovery in Field and Pot-Grown Maize. Plants 2024, 13, 2909. https://doi.org/10.3390/plants13202909
Rodrigues MÂ, Correia CM, Arrobas M. The Application of a Foliar Spray Containing Methylobacterium symbioticum Had a Limited Effect on Crop Yield and Nitrogen Recovery in Field and Pot-Grown Maize. Plants. 2024; 13(20):2909. https://doi.org/10.3390/plants13202909
Chicago/Turabian StyleRodrigues, Manuel Ângelo, Carlos Manuel Correia, and Margarida Arrobas. 2024. "The Application of a Foliar Spray Containing Methylobacterium symbioticum Had a Limited Effect on Crop Yield and Nitrogen Recovery in Field and Pot-Grown Maize" Plants 13, no. 20: 2909. https://doi.org/10.3390/plants13202909
APA StyleRodrigues, M. Â., Correia, C. M., & Arrobas, M. (2024). The Application of a Foliar Spray Containing Methylobacterium symbioticum Had a Limited Effect on Crop Yield and Nitrogen Recovery in Field and Pot-Grown Maize. Plants, 13(20), 2909. https://doi.org/10.3390/plants13202909








