Cross-Kingdom RNA Transport Based on Extracellular Vesicles Provides Innovative Tools for Plant Protection
Abstract
1. Introduction
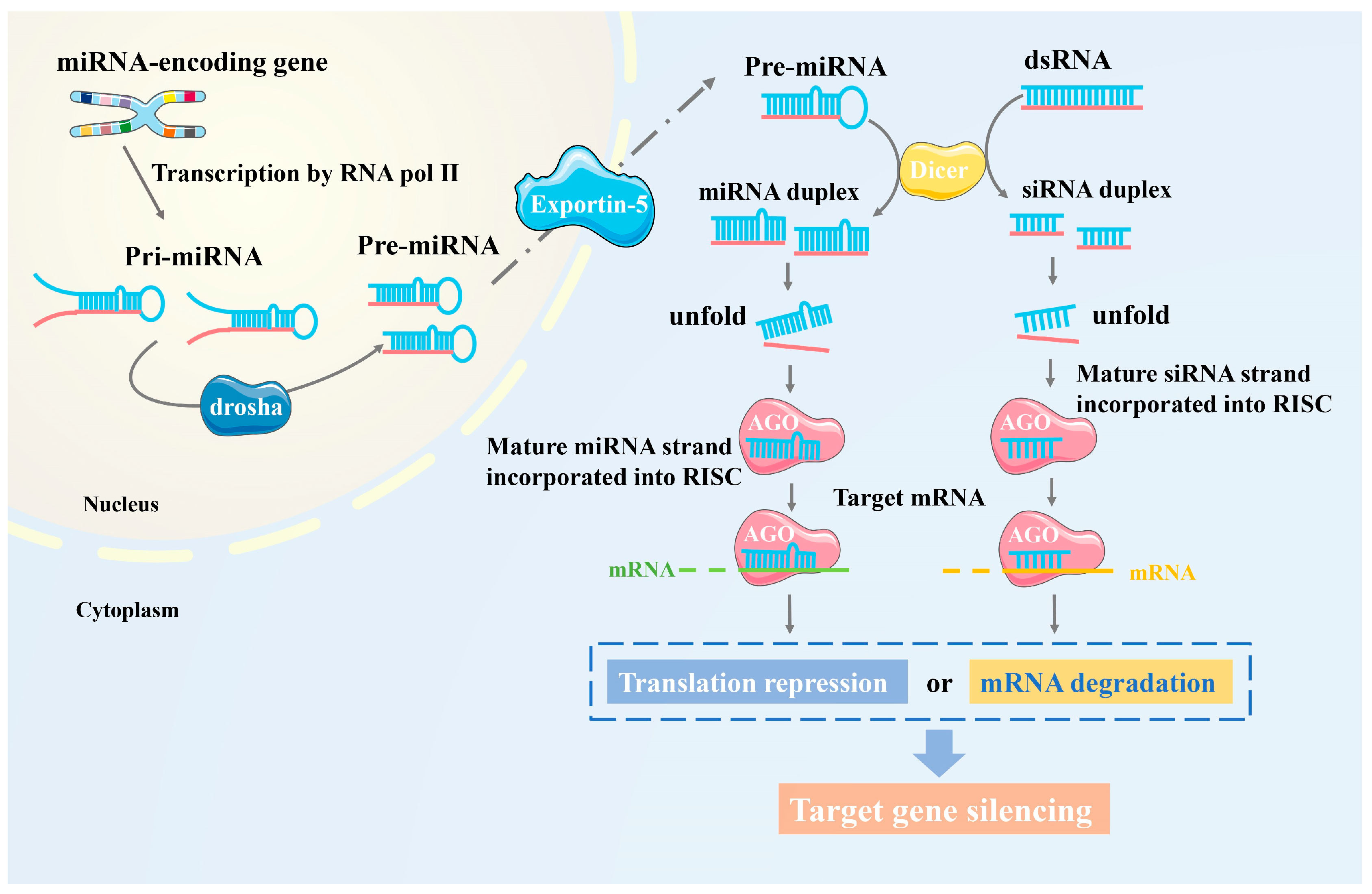
2. Regulation of Plant Disease Resistance through RNAi
3. Exogenous RNA and Environmental RNAi
4. Vesicle-Mediated RNAi-Based Cross-Kingdom Trafficking
4.1. Intercellular Communication Based on Extracellular Vesicles
4.2. RNA Components in Extracellular Vesicles
4.3. Extracellular Vesicle-Mediated RNA Transport
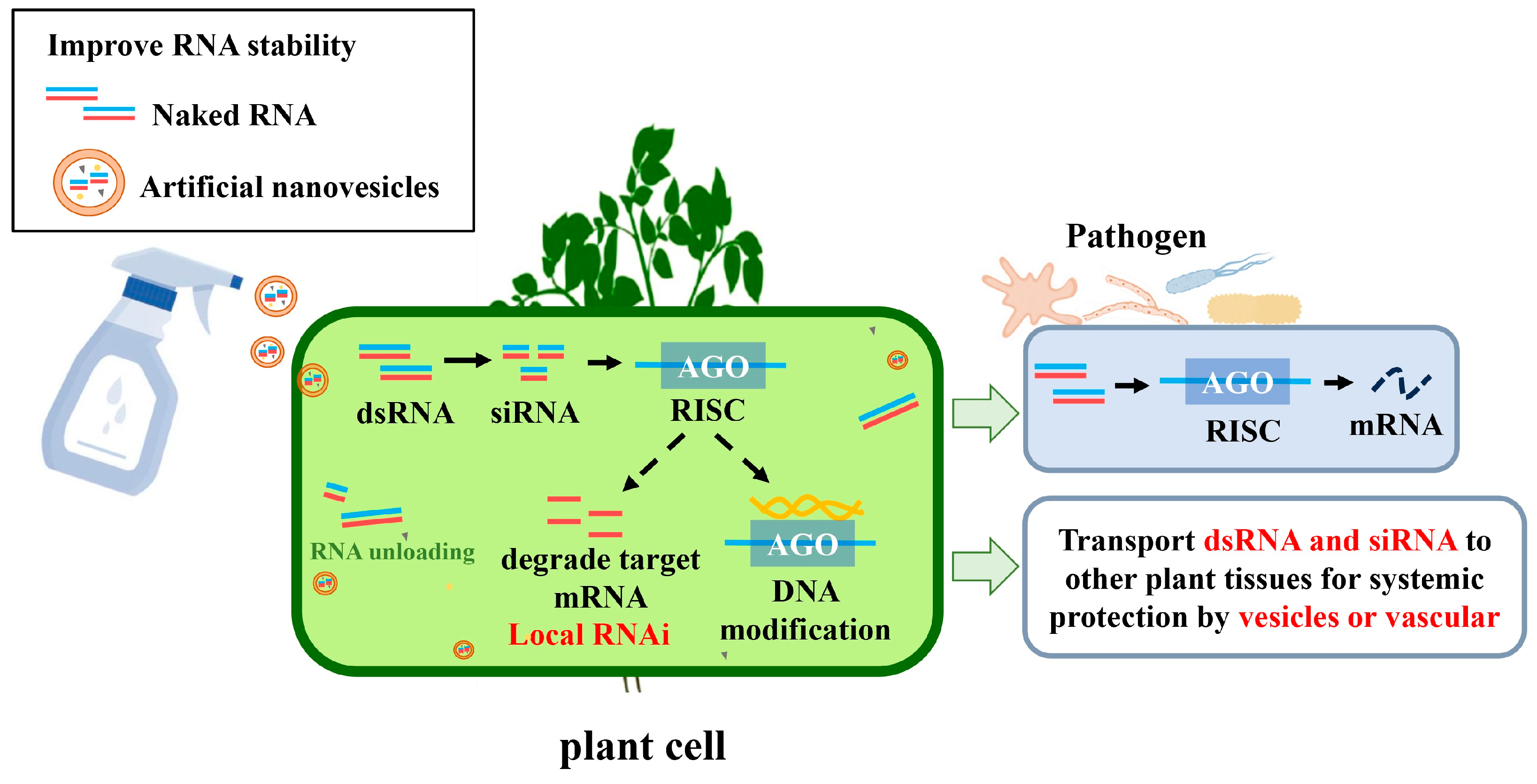
5. Strategies for Plant Protection Based on EV-RNAi
5.1. Host-Induced Gene Silencing—HIGS
5.2. Spray-Induced Gene Silencing—SIGS
6. Nanovesicles Promote SIGS as RNA Carriers
7. Discussion and Future Directions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Almeida, F.; Rodrigues, M.L.; Coelho, C. The Still Underestimated Problem of Fungal Diseases Worldwide. Front. Microbiol. 2019, 10, 214. [Google Scholar] [CrossRef] [PubMed]
- Niu, D. RNAs a New Frontier in Crop Protection. Curr. Opin. Biotechnol. 2021, 70, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.; Halilovic, L.; Shay, J.-H.; Koch, A.; Mitter, N.; Jin, H. Improving RNA-Based Crop Protection through Nanotechnology and Insights from Cross-Kingdom RNA Trafficking. Curr. Opin. Plant Biol. 2023, 76, 102441. [Google Scholar] [CrossRef]
- Rosa, C.; Kuo, Y.-W.; Wuriyanghan, H.; Falk, B.W. RNA Interference Mechanisms and Applications in Plant Pathology. Annu. Rev. Phytopathol. 2018, 56, 581–610. [Google Scholar] [CrossRef] [PubMed]
- Maizel, A.; Markmann, K.; Timmermans, M.; Wachter, A. To Move or Not to Move: Roles and Specificity of Plant RNA Mobility. Curr. Opin. Plant Biol. 2020, 57, 52–60. [Google Scholar] [CrossRef]
- Mahanty, B.; Mishra, R.; Joshi, R.K. Cross-Kingdom Small RNA Communication between Plants and Fungal Phytopathogens-Recent Updates and Prospects for Future Agriculture. RNA Biol. 2023, 20, 109–119. [Google Scholar] [CrossRef]
- Koch, A.; Wassenegger, M. Host-induced Gene Silencing—Mechanisms and Applications. New Phytol. 2021, 231, 54–59. [Google Scholar] [CrossRef]
- Ortolá, B.; Daròs, J.-A. RNA Interference in Insects: From a Natural Mechanism of Gene Expression Regulation to a Biotechnological Crop Protection Promise. Biology 2024, 13, 137. [Google Scholar] [CrossRef]
- Dubrovina, A.S.; Kiselev, K.V. Exogenous RNAs for Gene Regulation and Plant Resistance. Int. J. Mol. Sci. 2019, 20, 2282. [Google Scholar] [CrossRef]
- Betti, F.; Ladera-Carmona, M.J.; Weits, D.A.; Ferri, G.; Iacopino, S.; Novi, G.; Svezia, B.; Kunkowska, A.B.; Santaniello, A.; Piaggesi, A.; et al. Exogenous miRNAs Induce Post-Transcriptional Gene Silencing in Plants. Nat. Plants 2021, 7, 1379–1388. [Google Scholar] [CrossRef]
- Rutter, B.D. Extracellular Vesicles as Key Mediators of Plant-Microbe Interactions. Curr. Opin. Plant Biol. 2018, 44, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Li, Y.; Cao, X.; Qi, Y. MicroRNAs and Their Regulatory Roles in Plant—Environment Interactions. Annu. Rev. Plant Biol. 2019, 70, 489–525. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Yang, M.; Zhang, X. The Function of Small RNAs in Plant Biotic Stress Response. J. Integr. Plant Biol. 2016, 58, 312–327. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zamore, P.D. RNA Interference and Small RNA Analysis. Cold Spring Harb. Protoc. 2019, 4. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, S.-L.; Li, J.-L.; Hu, X.-H.; Wang, H.; Cao, X.-L.; Xu, Y.-J.; Zhao, Z.-X.; Xiao, Z.-Y.; Yang, N.; et al. Osa-miR169 Negatively Regulates Rice Immunity against the Blast Fungus Magnaporthe oryzae. Front. Plant Sci. 2017, 8, 2. [Google Scholar] [CrossRef]
- Wang, Z.; Xia, Y.; Lin, S.; Wang, Y.; Guo, B.; Song, X.; Ding, S.; Zheng, L.; Feng, R.; Chen, S.; et al. Osa-miR164a Targets Os60 and Negatively Regulates Rice Immunity against the Blast Fungus Magnaporthe oryzae. Plant J. 2018, 95, 584–597. [Google Scholar] [CrossRef]
- Kiselev, K.V.; Suprun, A.R.; Aleynova, O.A.; Ogneva, Z.V.; Dubrovina, A.S. Simultaneous Application of Several Exogenous dsRNAs for the Regulation of Anthocyanin biosynthesis in Arabidopsis thaliana. Plants 2024, 13, 541. [Google Scholar] [CrossRef]
- Kong, X.; Yang, M.; Le, B.H.; He, W.; Hou, Y. The Master Role of SIRNAS in Plant Immunity. Mol. Plant Pathol. 2022, 23, 1565–1574. [Google Scholar] [CrossRef]
- Meng, X.; Jin, W.; Wu, F. Novel Tomato miRNA miR1001 Initiates Cross-Species Regulation to Suppress the Conidiospore Germination and Infection Virulence of Botrytis Cinerea In Vitro. Gene 2020, 759, 145002. [Google Scholar] [CrossRef]
- Huang, C.-Y. Small RNAs—Big Players in Plant-Microbe Interactions. Cell Host Microbe 2019, 26, 173–182. [Google Scholar] [CrossRef]
- Iwakawa, H.; Tomari, Y. Life of RISC: Formation, Action, and Degradation of RNA-Induced Silencing Complex. Mol. Cell 2022, 82, 30–43. [Google Scholar] [CrossRef] [PubMed]
- Lax, C.; Tahiri, G.; Patiño-Medina, J.A.; Cánovas-Márquez, J.T.; Pérez-Ruiz, J.A.; Osorio-Concepción, M.; Navarro, E.; Calo, S. The Evolutionary Significance of RNAi in the Fungal Kingdom. Int. J. Mol. Sci. 2020, 21, 9348. [Google Scholar] [CrossRef] [PubMed]
- Duchaine, T.F.; Fabian, M.R. Mechanistic Insights into MicroRNA-Mediated Gene Silencing. Cold Spring Harb. Perspect. Biol. 2019, 11, a032771. [Google Scholar] [CrossRef] [PubMed]
- Djuranovic, S.; Nahvi, A.; Green, R. A Parsimonious Model for Gene Regulation by miRNAs. Science 2011, 331, 550–553. [Google Scholar] [CrossRef]
- Ma, X.; Kim, E.-J.; Kook, I.; Ma, F.; Voshall, A.; Moriyama, E.; Cerutti, H. Small Interfering RNA–Mediated Translation Repression Alters Ribosome Sensitivity to Inhibition by Cycloheximide in Chlamydomonas reinhardtii. Plant Cell 2013, 25, 985–998. [Google Scholar] [CrossRef]
- Zotti, M.; Dos Santos, E.A.; Cagliari, D.; Christiaens, O.; Taning, C.N.T.; Smagghe, G. RNA Interference Technology in Crop Protection against Arthropod Pests, Pathogens and Nematodes. Pest Manag. Sci 2018, 74, 1239–1250. [Google Scholar] [CrossRef]
- Kulshrestha, C.; Pathak, H.; Kumar, D.; Dave, S.; Sudan, J. Elucidating Micro RNAs Role in Different Plant-Pathogen Interactions. Mol. Biol. Rep. 2020, 47, 8219–8227. [Google Scholar] [CrossRef]
- Wang, S.; He, B.; Wu, H.; Cai, Q.; Ramírez-Sánchez, O.; Abreu-Goodger, C.; Birch, P.R.J.; Jin, H. Plant mRNAs move into a fungal pathogen via extracellular vesicles to reduce infection. Cell Host Microbe 2024, 32, 93–105.e6. [Google Scholar] [CrossRef]
- Zhang, T.; Zhao, Y.-L.; Zhao, J.-H.; Wang, S.; Jin, Y.; Chen, Z.-Q.; Fang, Y.-Y.; Hua, C.-L.; Ding, S.-W.; Guo, H.-S. Cotton Plants Export microRNAs to Inhibit Virulence Gene Expression in a Fungal Pathogen. Nat. Plants 2016, 2, 16153. [Google Scholar] [CrossRef]
- Cai, Q.; Qiao, L.; Wang, M.; He, B.; Lin, F.-M.; Palmquist, J.; Huang, S.-D.; Jin, H. Plants send small RNAs in extracellular vesicles to the fungal pathogen to silence virulence genes. Science 2018, 360, 1126–1129. [Google Scholar] [CrossRef]
- Hua, C.; Zhao, J.-H.; Guo, H.-S. Trans-Kingdom RNA Silencing in Plant-Fungal Pathogen Interactions. Mol. Plant 2018, 11, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Weiberg, A.; Wang, M.; Lin, F.-M.; Zhao, H.; Zhang, Z.; Kaloshian, I.; Huang, H.-D.; Jin, H. Fungal Small RNAs Suppress Plant Immunity by Hijacking Host RNA Interference Pathways. Science 2013, 342, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Bleackley, M.R.; Samuel, M.; Garcia-Ceron, D.; McKenna, J.A.; Lowe, R.G.T.; Pathan, M.; Zhao, K.; Ang, C.-S.; Mathivanan, S.; Anderson, M.A. Extracellular Vesicles From the Cotton Pathogen Fusarium oxysporum f. sp. Vasinfectum Induce a Phytotoxic Response in Plants. Front. Plant Sci. 2020, 10, 1610. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.; Mao, H.; Li, S.; Feng, T.; Zhang, Z.; Cheng, L.; Luo, S.; Borkovich, K.A.; Ouyang, S. Fol-milR1, a Pathogenicity Factor of Fusarium oxysporum, Confers Tomato Wilt Disease Resistance by Impairing Host Immune Responses. New Phytol. 2021, 232, 705–718. [Google Scholar] [CrossRef]
- Dunker, F.; Trutzenberg, A.; Rothenpieler, J.S.; Kuhn, S.; Pröls, R.; Schreiber, T.; Tissier, A.; Kemen, A.; Kemen, E.; Hückelhoven, R.; et al. Oomycete Small RNAs Bind to the Plant RNA-Induced Silencing Complex for Virulence. eLife 2020, 9, e56096. [Google Scholar] [CrossRef]
- Mueth, N.A.; Ramachandran, S.R.; Hulbert, S.H. Small RNAs from the Wheat Stripe Rust Fungus (Puccinia striiformis f. sp. Tritici). BMC Genom. 2015, 16, 718. [Google Scholar] [CrossRef]
- Wang, B.; Sun, Y.; Song, N.; Zhao, M.; Liu, R.; Feng, H.; Wang, X.; Kang, Z. Puccinia striiformis f. sp. Tritici microRNA-like RNA 1 (Pst-milR1), an Important Pathogenicity Factor of Pst, Impairs Wheat Resistance to Pst by Suppressing the Wheat Pathogenesis-Related 2 Gene. New Phytol. 2017, 215, 338–350. [Google Scholar] [CrossRef]
- Wu, F.; Huang, Y.; Jiang, W.; Jin, W. Genome-Wide Identification and Validation of Tomato-Encoded sRNA as the Cross-Species Antifungal Factors Targeting the Virulence Genes of Botrytis Cinerea. Front. Plant Sci. 2023, 14, 1072181. [Google Scholar] [CrossRef]
- Jiao, J.; Peng, D. Wheat microRNA1023 Suppresses Invasion of Fusarium Graminearum via Targeting and Silencing FGSG 03101. J. Plant Interact. 2018, 13, 514–521. [Google Scholar] [CrossRef]
- Wang, M.; Weiberg, A.; Lin, F.-M.; Thomma, B.P.H.J.; Huang, H.-D.; Jin, H. Bidirectional Cross-Kingdom RNAi and Fungal Uptake of External RNAs Confer Plant Protection. Nat. Plants 2016, 2, 16151. [Google Scholar] [CrossRef]
- Shahid, S.; Kim, G.; Johnson, N.R.; Wafula, E.; Wang, F.; Coruh, C.; Bernal-Galeano, V.; Phifer, T.; dePamphilis, C.W.; Westwood, J.H.; et al. MicroRNAs from the parasitic plant Cuscuta campestris target host messenger RNAs. Nature 2018, 553, 82–85. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.-S.; Li, Y.-C.; Guo, H.-S.; Zhao, J.-H. Verticillium Dahliae Secretes Small RNA to Target Host MIR157d and Retard Plant Floral Transition during Infection. Front. Plant Sci. 2022, 13, 847086. [Google Scholar] [CrossRef] [PubMed]
- Saberi, F.; Kamali, M.; Najafi, A.; Yazdanparast, A.; Moghaddam, M.M. Natural Antisense RNAs as mRNA Regulatory Elements in Bacteria: A Review on Function and Applications. Cell. Mol. Biol. Lett. 2016, 21, 6. [Google Scholar] [CrossRef] [PubMed]
- Ashwath, P.; Somanath, D.; Sannejal, A.D. CRISPR and Antisense RNA Technology: Exploiting Nature’s Tool to Restrain Virulence in Tenacious Pathogens. Mol. Biotechnol. 2023, 65, 17–27. [Google Scholar] [CrossRef]
- Jin, L.; Chen, M.; Xiang, M.; Guo, Z. RNAi-Based Antiviral Innate Immunity in Plants. Viruses 2022, 14, 432. [Google Scholar] [CrossRef]
- Koeppe, S.; Kawchuk, L.; Kalischuk, M. RNA Interference Past and Future Applications in Plants. Int. J. Mol. Sci. 2023, 24, 9755. [Google Scholar] [CrossRef]
- Wu, J.; Yang, R.; Yang, Z.; Yao, S.; Zhao, S.; Wang, Y.; Li, P.; Song, X.; Jin, L.; Zhou, T.; et al. ROS Accumulation and Antiviral Defence Control by microRNA528 in Rice. Nat. Plants 2017, 3, 16203. [Google Scholar] [CrossRef]
- Cui, C.; Wang, J.-J.; Zhao, J.-H.; Fang, Y.-Y.; He, X.-F.; Guo, H.-S.; Duan, C.-G. A Brassica miRNA Regulates Plant Growth and Immunity through Distinct Modes of Action. Mol. Plant 2020, 13, 231–245. [Google Scholar] [CrossRef]
- Jin, Y. Recent Advances in Understanding Plant Antiviral RNAi and Viral Suppressors of RNAi. Curr. Opin. Virol. 2021, 46, 65–72. [Google Scholar] [CrossRef]
- Atabekova, A.K.; Solovieva, A.D.; Chergintsev, D.A.; Solovyev, A.G.; Morozov, S.Y. Role of Plant Virus Movement Proteins in Suppression of Host RNAi Defense. Int. J. Mol. Sci. 2023, 24, 9049. [Google Scholar] [CrossRef]
- Chen, Y.; Schutter, K.D. Biosafety Aspects of RNAi-based Pests Control. Pest Manag. Sci. 2024, 80, 3697–3706. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.-L.; Wang, X.-J.; Lu, J.-B.; Lu, H.-B.; Ye, Z.-X.; Xu, Z.-T.; Zhang, C.; Chen, J.-P.; Li, J.-M.; Zhang, C.-X.; et al. Cross-Kingdom RNA Interference Mediated by Insect Salivary microRNAs May Suppress Plant Immunity. Proc. Natl. Acad. Sci. USA 2024, 121, e2318783121. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Khan, S.A.; Hasse, C.; Ruf, S.; Heckel, D.G.; Bock, R. Pest Control. Full Crop Protection from an Insect Pest by Expression of Long Double-Stranded RNAs in Plastids. Science 2015, 347, 991–994. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.K.B.; Hunter, W.B.; Park, A.L.; Gundersen-Rindal, D.E. Double-Stranded RNA Oral Delivery Methods to Induce RNA Interference in Phloem and Plant-Sap-Feeding Hemipteran Insects. J. Visualized. Exp. 2018, 135, e57390. [Google Scholar] [CrossRef]
- Das, P.R.; Sherif, S.M. Application of Exogenous dsRNAs-Induced RNAi in Agriculture: Challenges and Triumphs. Front. Plant Sci. 2020, 11, 946. [Google Scholar] [CrossRef]
- He, L.; Huang, Y.; Tang, X. RNAi-Based Pest Control: Production, Application and the Fate of dsRNA. Front. Bioeng. Biotechnol. 2022, 10, 1080576. [Google Scholar] [CrossRef]
- Loreti, E.; Perata, P. Mobile Plant microRNAs Allow Communication within and between Organisms. New Phytol. 2022, 235, 2176–2182. [Google Scholar] [CrossRef]
- Hoang, B.T.L.; Fletcher, S.J.; Brosnan, C.A.; Ghodke, A.B.; Manzie, N.; Mitter, N. RNAi as a Foliar Spray: Efficiency and Challenges to Field Applications. Int. J. Mol. Sci. 2022, 23, 6639. [Google Scholar] [CrossRef]
- Huang, M.; Peng, X.; Yang, L.; Yang, S.; Li, X.; Tang, S.; Li, B.; Jin, H.; Wu, B.; Liu, J.; et al. Non-Coding RNA Derived from Extracellular Vesicles in Cancer Immune Escape: Biological Functions and Potential Clinical Applications. Cancer Lett. 2021, 501, 234–246. [Google Scholar] [CrossRef]
- Stotz, H.U.; Brotherton, D.; Inal, J. Communication Is Key: Extracellular Vesicles as Mediators of Infection and Defence during Host–Microbe Interactions in Animals and Plants. FEMS Microbiol. Rev. 2022, 46, fuab044. [Google Scholar] [CrossRef]
- Garcia-Martin, R.; Wang, G.; Brandão, B.B.; Zanotto, T.M.; Shah, S.; Kumar Patel, S.; Schilling, B.; Kahn, C.R. MicroRNA Sequence Codes for Small Extracellular Vesicle Release and Cellular Retention. Nature 2022, 601, 446–451. [Google Scholar] [CrossRef] [PubMed]
- Zomer, A.; Maynard, C.; Verweij, F.J.; Kamermans, A.; Schäfer, R.; Beerling, E.; Schiffelers, R.M.; de Wit, E.; Berenguer, J.; Ellenbroek, S.I.J.; et al. In Vivo Imaging Reveals Extracellular Vesicle-Mediated Phenocopying of Metastatic Behavior. Cell 2015, 161, 1046–1057. [Google Scholar] [CrossRef] [PubMed]
- Yokoi, A.; Yoshioka, Y.; Yamamoto, Y.; Ishikawa, M.; Ikeda, S.; Kato, T.; Kiyono, T.; Takeshita, F.; Kajiyama, H.; Kikkawa, F.; et al. Malignant Extracellular Vesicles Carrying MMP1 mRNA Facilitate Peritoneal Dissemination in Ovarian Cancer. Nat. Commun. 2017, 8, 14470. [Google Scholar] [CrossRef] [PubMed]
- Mittelbrunn, M.; Gutiérrez-Vázquez, C.; Villarroya-Beltri, C.; González, S.; Sánchez-Cabo, F.; González, M.Á.; Bernad, A.; Sánchez-Madrid, F. Unidirectional Transfer of microRNA-Loaded Exosomes from T Cells to Antigen-Presenting Cells. Nat. Commun. 2011, 2, 282. [Google Scholar] [CrossRef] [PubMed]
- Dosil, S.G.; Lopez-Cobo, S.; Rodriguez-Galan, A.; Fernandez-Delgado, I.; Ramirez-Huesca, M.; Milan-Rois, P.; Castellanos, M.; Somoza, A.; Gómez, M.J.; Reyburn, H.T.; et al. Natural Killer (NK) Cell-Derived Extracellular-Vesicle Shuttled microRNAs Control T Cell Responses. eLife 2022, 11, e76319. [Google Scholar] [CrossRef]
- Cai, Q.; He, B.; Wang, S.; Fletcher, S.; Niu, D.; Mitter, N.; Birch, P.R.J.; Jin, H. Message in a Bubble: Shuttling Small RNAs and Proteins Between Cells and Interacting Organisms Using Extracellular Vesicles. Annu. Rev. Plant Biol. 2021, 72, 497–524. [Google Scholar] [CrossRef]
- Buck, A.H.; Coakley, G.; Simbari, F.; McSorley, H.J.; Quintana, J.F.; Le Bihan, T.; Kumar, S.; Abreu-Goodger, C.; Lear, M.; Harcus, Y.; et al. Exosomes Secreted by Nematode Parasites Transfer Small RNAs to Mammalian Cells and Modulate Innate Immunity. Nat. Commun. 2014, 5, 5488. [Google Scholar] [CrossRef]
- Ahmadi Badi, S.; Bruno, S.P.; Moshiri, A.; Tarashi, S.; Siadat, S.D.; Masotti, A. Small RNAs in Outer Membrane Vesicles and Their Function in Host-Microbe Interactions. Front. Microbiol. 2020, 11, 1209. [Google Scholar] [CrossRef]
- Schatz, D.; Schleyer, G.; Saltvedt, M.R.; Sandaa, R.-A.; Feldmesser, E.; Vardi, A. Ecological Significance of Extracellular Vesicles in Modulating Host-Virus Interactions during Algal Blooms. ISME J. 2021, 15, 3714–3721. [Google Scholar] [CrossRef]
- Liu, G.; Kang, G.; Wang, S.; Huang, Y.; Cai, Q. Extracellular Vesicles: Emerging Players in Plant Defense against Pathogens. Front. Plant Sci. 2021, 12, 757925. [Google Scholar] [CrossRef]
- Baldrich, P.; Rutter, B.D.; Karimi, H.Z.; Podicheti, R.; Meyers, B.C.; Innes, R.W. Plant Extracellular Vesicles Contain Diverse Small RNA Species and Are Enriched in 10- to 17-Nucleotide “Tiny” RNAs. Plant Cell 2019, 31, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Munhoz Da Rocha, I.F.; Amatuzzi, R.F.; Lucena, A.C.R.; Faoro, H.; Alves, L.R. Cross-Kingdom Extracellular Vesicles EV-RNA Communication as a Mechanism for Host–Pathogen Interaction. Front. Cell. Infect. Microbiol. 2020, 10, 593160. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, K.; Breyne, K.; Ughetto, S.; Laurent, L.C.; Breakefield, X.O. RNA Delivery by Extracellular Vesicles in Mammalian Cells and Its Applications. Nat. Rev. Mol. Cell Biol. 2020, 21, 585–606. [Google Scholar] [CrossRef] [PubMed]
- You, Y.; Tian, Y.; Yang, Z.; Shi, J.; Kwak, K.J.; Tong, Y.; Estania, A.P.; Cao, J.; Hsu, W.-H.; Liu, Y.; et al. Intradermally Delivered mRNA-Encapsulating Extracellular Vesicles for Collagen-Replacement Therapy. Nat. Biomed. Eng. 2023, 7, 887–900. [Google Scholar] [CrossRef]
- Collado, A.; Gan, L.; Tengbom, J.; Kontidou, E.; Pernow, J.; Zhou, Z. Extracellular Vesicles and Their Non-Coding RNA Cargos: Emerging Players in Cardiovascular Disease. J. Physiol. 2023, 601, 4989–5009. [Google Scholar] [CrossRef]
- He, B.; Cai, Q.; Qiao, L.; Huang, C.-Y.; Wang, S.; Miao, W.; Ha, T.; Wang, Y.; Jin, H. RNA-binding proteins contribute to small RNA loading in plant extracellular vesicles. Nat. Plants 2021, 7, 342–352. [Google Scholar] [CrossRef]
- De La Canal, L.; Pinedo, M. Extracellular vesicles: A missing component in plant cell wall remodeling. J. Exp. Bot. 2018, 69, 4655–4658. [Google Scholar] [CrossRef]
- Landry, M.P.; Mitter, N. How Nanocarriers Delivering Cargos in Plants Can Change the GMO Landscape. Nat. Nanotechnol. 2019, 14, 512–514. [Google Scholar] [CrossRef]
- Zhao, L.; Gu, C.; Gan, Y.; Shao, L.; Chen, H.; Zhu, H. Exosome-Mediated siRNA Delivery to Suppress Postoperative Breast Cancer Metastasis. J. Control. Release 2020, 318, 1–15. [Google Scholar] [CrossRef]
- Dad, H.A.; Gu, T.-W.; Zhu, A.-Q.; Huang, L.-Q.; Peng, L.-H. Plant Exosome-like Nanovesicles: Emerging Therapeutics and Drug Delivery Nanoplatforms. Mol. Ther. 2021, 29, 13–31. [Google Scholar] [CrossRef]
- Qiao, L.; Niño-Sánchez, J.; Hamby, R.; Capriotti, L.; Chen, A.; Mezzetti, B.; Jin, H. Artificial Nanovesicles for dsRNA Delivery in Spray Induced Gene Silencing for Crop Protection. Plant Biotechnol. J. 2023, 21, 854–865. [Google Scholar] [CrossRef] [PubMed]
- Degnan, R.M.; Shuey, L.S.; Radford-Smith, J.; Gardiner, D.M.; Carroll, B.J.; Mitter, N.; McTaggart, A.R.; Sawyer, A. Double-Stranded RNA Prevents and Cures Infection by Rust Fungi. Commun. Biol. 2023, 6, 1234. [Google Scholar] [CrossRef] [PubMed]
- Bilir, Ö.; Göl, D.; Hong, Y.; McDowell, J.M.; Tör, M. Small RNA-Based Plant Protection against Diseases. Front. Plant Sci. 2022, 13, 951097. [Google Scholar] [CrossRef] [PubMed]
- Taning, C.N.T.; Mezzetti, B.; Kleter, G.; Smagghe, G.; Baraldi, E. Does RNAi-Based Technology Fit within EU Sustainability Goals? Trends Biotechnol. 2021, 39, 644–647. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Du, Z.; Zhang, G.; Wang, T.; Jin, G. Advances in RNA-Silencing-Related Resistance against Viruses in Potato. Genes 2022, 13, 731. [Google Scholar] [CrossRef]
- Dalakouras, A.; Koidou, V.; Papadopoulou, K. DsRNA-Based Pesticides: Considerations for Efficiency and Risk Assessment. Chemosphere 2024, 352, 141530. [Google Scholar] [CrossRef]
- Lu, Z.; Yang, Z.; Tian, Z.; Gui, Q.; Dong, R.; Chen, C. Genome-Wide Analysis and Identification of microRNAs in Medicago Truncatula under Aluminum Stress. Front. Plant Sci. 2023, 14, 1137764. [Google Scholar] [CrossRef]
- Wang, Z.; Gao, X.; Zhong, S.; Li, Y.; Shi, M.; Zhang, B.; Zhang, S.; Shen, H.; Liu, X. Host-Induced Gene Silencing of PcCesA3 and PcOSBP1 Confers Resistance to Phytophthora capsici in Nicotiana benthamiana through NbDCL3 and NbDCL4 Processed Small Interfering RNAs. Int. J. Biol. Macromol. 2022, 222, 1665–1675. [Google Scholar] [CrossRef]
- Kaldis, A.; Berbati, M.; Melita, O.; Reppa, C.; Holeva, M.; Otten, P.; Voloudakis, A. Exogenously Applied dsRNA Molecules Deriving from the Zucchini Yellow Mosaic Virus (ZYMV) Genome Move Systemically and Protect Cucurbits against ZYMV. Mol. Plant Pathol. 2018, 19, 883–895. [Google Scholar] [CrossRef]
- Voloudakis, A.E.; Holeva, M.C.; Sarin, L.P.; Bamford, D.H.; Vargas, M.; Poranen, M.M.; Tenllado, F. Efficient Double-Stranded RNA Production Methods for Utilization in Plant Virus Control. In Plant Virology Protocols; Uyeda, I., Masuta, C., Eds.; Methods in Molecular Biology; Springer: New York, NY, USA, 2015; Volume 1236, pp. 255–274. ISBN 978-1-4939-1742-6. [Google Scholar]
- He, B.; Wang, H.; Liu, G.; Chen, A.; Calvo, A.; Cai, Q.; Jin, H. Fungal Small RNAs Ride in Extracellular Vesicles to Enter Plant Cells through Clathrin-Mediated Endocytosis. Nat. Commun. 2023, 14, 4383. [Google Scholar] [CrossRef]
- Wang, M.; Dean, R.A. Movement of Small RNAs in and between Plants and Fungi. Mol. Plant Pathol. 2020, 21, 589–601. [Google Scholar] [CrossRef] [PubMed]
- Šečić, E.; Kogel, K.-H. Requirements for Fungal Uptake of dsRNA and Gene Silencing in RNAi-Based Crop Protection Strategies. Curr. Opin. Biotechnol. 2021, 70, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Delaunois, B.; Colby, T.; Belloy, N.; Conreux, A. Large-Scale Proteomic Analysis of the Grapevine Leaf Apoplastic Fluid Reveals Mainly Stress-Related Proteins and Cell Wall Modifying Enzymes. BMC Plant Biol. 2013, 13, 24. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, Y.; Wang, Y. Apoplastic Proteases: Powerful Weapons against Pathogen Infection in Plants. Plant Commun. 2020, 1, 100085. [Google Scholar] [CrossRef] [PubMed]
- Qiao, L.; Lan, C.; Capriotti, L.; Ah-Fong, A.; Nino Sanchez, J.; Hamby, R.; Heller, J.; Zhao, H.; Glass, N.L.; Judelson, H.S.; et al. Spray-Induced Gene Silencing for Disease Control Is Dependent on the Efficiency of Pathogen RNA Uptake. Plant Biotechnol. J. 2021, 19, 1756–1768. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.T.; Davis, Z.; Chen, L.; Englaender, J.; Zomorodi, S.; Frank, J.; Bartlett, K.; Somers, E.; Carballo, S.M.; Kester, M.; et al. Minicell-Based Fungal RNAi Delivery for Sustainable Crop Protection. Microb. Biotechnol. 2021, 14, 1847–1856. [Google Scholar] [CrossRef]
- Zhou, H. Chitosan/dsRNA Polyplex Nanoparticles Advance Environmental RNA Interference Efficiency through Activating Clathrin-Dependent Endocytosis. Int. J. Biol. Macromol. 2023, 253, 127021. [Google Scholar] [CrossRef]
- Molesini, B.; Pennisi, F.; Cressoni, C.; Vitulo, N.; Dusi, V.; Speghini, A.; Pandolfini, T. Nanovector-Mediated Exogenous Delivery of dsRNA Induces Silencing of Target Genes in Very Young Tomato Flower Buds. Nanoscale Adv. 2022, 4, 4542–4553. [Google Scholar] [CrossRef]
- Thagun, C.; Horii, Y.; Mori, M.; Fujita, S.; Ohtani, M.; Tsuchiya, K.; Kodama, Y.; Odahara, M.; Numata, K. Non-Transgenic Gene Modulation via Spray Delivery of Nucleic Acid/Peptide Complexes into Plant Nuclei and Chloroplasts. ACS Nano 2022, 16, 3506–3521. [Google Scholar] [CrossRef]
- Suprun, A.R.; Kiselev, K.V.; Dubrovina, A.S. Exogenously Induced Silencing of Four MYB Transcription Repressor Genes and Activation of Anthocyanin Accumulation in Solanum lycopersicum. Int. J. Mol. Sci. 2023, 24, 9344. [Google Scholar] [CrossRef]
- Singewar, K. Double-Stranded RNA (dsRNA) Technology to Control Forest Insect Pests and Fungal Pathogens: Challenges and Opportunities. Funct. Integr. Genom. 2023, 23, 185. [Google Scholar] [CrossRef] [PubMed]
- Dalakouras, A.; Wassenegger, M.; McMillan, J.N.; Cardoza, V.; Maegele, I.; Dadami, E.; Runne, M.; Krczal, G.; Wassenegger, M. Induction of Silencing in Plants by High-Pressure Spraying of In Vitro-Synthesized Small RNAs. Front. Plant Sci. 2016, 7, 1327. [Google Scholar] [CrossRef] [PubMed]
- Vatanparast, M.; Merkel, L.; Amari, K. Exogenous Application of dsRNA in Plant Protection: Efficiency, Safety Concerns and Risk Assessment. Int. J. Mol. Sci. 2024, 25, 6530. [Google Scholar] [CrossRef] [PubMed]
- Mitter, N.; Worrall, E.A.; Robinson, K.E.; Li, P.; Jain, R.G.; Taochy, C.; Fletcher, S.J.; Carroll, B.J.; Lu, G.Q.; Xu, Z.P. Clay Nanosheets for Topical Delivery of RNAi for Sustained Protection against Plant Viruses. Nat. Plants 2017, 3, 16207. [Google Scholar] [CrossRef] [PubMed]
- Jalaluddin, N.S.M.; Asem, M.; Harikrishna, J.A. Recent Progress on Nanocarriers for Topical-Mediated RNAi Strategies for Crop Protection—A Review. Molecules 2023, 28, 2700. [Google Scholar] [CrossRef]
- Zhang, H.; Goh, N.S.; Wang, J.W.; Pinals, R.L.; González-Grandío, E.; Demirer, G.S.; Butrus, S.; Fakra, S.C.; Del Rio Flores, A.; Zhai, R.; et al. Nanoparticle Cellular Internalization Is Not Required for RNA Delivery to Mature Plant Leaves. Nat. Nanotechnol. 2022, 17, 197–205. [Google Scholar] [CrossRef]
- Abdellatef, E.; Kamal, N.M.; Tsujimoto, H. Tuning Beforehand: A Foresight on RNA Interference (RNAi) and In Vitro-Derived dsRNAs to Enhance Crop Resilience to Biotic and Abiotic Stresses. Int. J. Mol. Sci. 2021, 22, 7687. [Google Scholar] [CrossRef]
- Qi, T.; Guo, J.; Peng, H.; Liu, P.; Kang, Z.; Guo, J. Host-Induced Gene Silencing: A Powerful Strategy to Control Diseases of Wheat and Barley. Int. J. Mol. Sci. 2019, 20, 206. [Google Scholar] [CrossRef]
- Zand Karimi, H.; Innes, R.W. Molecular Mechanisms Underlying Host-Induced Gene Silencing. Plant Cell 2022, 34, 3183–3199. [Google Scholar] [CrossRef]
- McLoughlin, A.G.; Wytinck, N.; Walker, P.L.; Girard, I.J.; Rashid, K.Y.; De Kievit, T.; Fernando, W.G.D.; Whyard, S.; Belmonte, M.F. Identification and Application of Exogenous dsRNA Confers Plant Protection against Sclerotinia sclerotiorum and Botrytis cinerea. Sci. Rep. 2018, 8, 7320. [Google Scholar] [CrossRef]
- Ray, P.; Sahu, D.; Aminedi, R.; Chandran, D. Concepts and Considerations for Enhancing RNAi Efficiency in Phytopathogenic Fungi for RNAi-Based Crop Protection Using Nanocarrier-Mediated dsRNA Delivery Systems. Front. Fungal. Biol. 2022, 3, 977502. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Zhang, R.; Zhao, J.; Qi, T.; Kang, Z.; Guo, J. Host-Induced Silencing of Fusarium graminearum Genes Enhances the Resistance of Brachypodium distachyon to Fusarium Head Blight. Front. Plant Sci. 2019, 10, 1362. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Patra, S.; Ray, S. A Combinatorial Nanobased Spray-Induced Gene Silencing Technique for Crop Protection and Improvement. ACS Omega 2023, 8, 22345–22351. [Google Scholar] [CrossRef] [PubMed]
| Host Plant | Pathogen | Goal/Target of RNAi | RNA Type | Directionality of RNA Mobility | Ref. |
|---|---|---|---|---|---|
| Lycopersicum esculentum (tomato); Arabidopsis thaliana | Botrytis cinerea | Plants | siRNA | Pathogen–host | [32] |
| Gossypoim hirsutism (cotton) | Verticillium dahliae | Fungi | miRNA | Host–pathogen | [29] |
| Lycopersicum esculentum (tomato); Arabidopsis thaliana | Botrytis cinerea | Fungi | siRNA | Host–pathogen | [40] |
| Arabidopsis thaliana | Cuscuta campestris | Plants | miRNA | Pathogen–host | [41] |
| Arabidopsis thaliana | Botrytis cinerea | Fungi | siRNA, miRNA | Host–pathogen | [30] |
| Arabidopsis thaliana | Hyaloperonospora arabidopsidis | Plants | sRNA | Pathogen–host | [35] |
| Lycopersicum esculentum (tomato) | Botrytis cinerea | Fungi | miRNA | Host–pathogen | [27] |
| Lycopersicum esculentum (tomato) | Fusarium oxysporum | Plants | mRNA | Pathogen–host | [36] |
| Arabidopsis thaliana | Verticillium dahliae | Plants | miRNA | Pathogen–host | [42] |
| Lycopersicum esculentum (tomato) | Botrytis cinerea | Fungi | siRNA | Host–pathogen | [38] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Zhou, Y.; Xu, J.; Fan, S.; Zhu, N.; Meng, Q.; Dai, S.; Yuan, X. Cross-Kingdom RNA Transport Based on Extracellular Vesicles Provides Innovative Tools for Plant Protection. Plants 2024, 13, 2712. https://doi.org/10.3390/plants13192712
Zhao Y, Zhou Y, Xu J, Fan S, Zhu N, Meng Q, Dai S, Yuan X. Cross-Kingdom RNA Transport Based on Extracellular Vesicles Provides Innovative Tools for Plant Protection. Plants. 2024; 13(19):2712. https://doi.org/10.3390/plants13192712
Chicago/Turabian StyleZhao, Yujin, Yanguang Zhou, Jingyan Xu, Sen Fan, Na Zhu, Qingling Meng, Shijie Dai, and Xiaofeng Yuan. 2024. "Cross-Kingdom RNA Transport Based on Extracellular Vesicles Provides Innovative Tools for Plant Protection" Plants 13, no. 19: 2712. https://doi.org/10.3390/plants13192712
APA StyleZhao, Y., Zhou, Y., Xu, J., Fan, S., Zhu, N., Meng, Q., Dai, S., & Yuan, X. (2024). Cross-Kingdom RNA Transport Based on Extracellular Vesicles Provides Innovative Tools for Plant Protection. Plants, 13(19), 2712. https://doi.org/10.3390/plants13192712





