Assessing a “Least-Concern” Red List Tree Species from Madagascar Used in Traditional Medicine: Morella spathulata (Myricaceae) Phyto-Compounds and Anti-Inflammatory Properties
Abstract
1. Introduction
2. Results and Discussion
2.1. Total Polyphenolic Contents (TPC)
2.2. Phytochemical Fingerprint
2.3. In Vitro Antioxidant Capacity (DPPH and FRAP)
2.4. Anti-Inflammatory and Analgesic Activity
2.4.1. Carrageenan-Induced Paw Oedema
2.4.2. Acetic Acid-Induced Writhing
2.5. Acute Toxicity
3. Materials and Methods
3.1. Plant Material
3.2. Extracts Preparation
3.3. Animals
3.4. Analgesic Writhing Test in Mice
- Group I: negative control or vehicle where mice were treated orally with normal saline solution (0.9%).
- Group II: positive control where mice were treated orally with paracetamol at a dose of 100 mg/kg body weight (b.w.).
- Groups III–V: mice were treated orally with MS_L at a dose of 100, 200, and 400 mg/kg b.w., respectively.
- Groups VI–VIII: mice were treated orally with MS_B at different doses (100, 200, and 400 mg/kg b.w., respectively).
3.5. Carrageenan-Induced Mice Paw Oedema Assay
- Group I: negative control or vehicle where mice were treated orally with normal saline solution.
- Group II: mice were treated with a dose of 10 mg/kg per indomethacin (positive control).
- Groups III–V: mice were orally treated with MS_L with a dose of 100, 200, and 400 mg/kg (b.w.), respectively.
- Groups VI–VIII: mice were treated orally with MS_B at the same doses as groups III-V.
3.6. Acute Toxicity Assay
- Group I: negative control or vehicle where mice were prepared with normal saline solution.
- Group II–V: mice were orally treated with MS_L with a dose of 250, 500, 1000, and 2000 mg/kg (b.w.), respectively.
- Group VI–IX: mice were orally treated with MS_B with a dose of 250, 500, 1000, and 2000 mg/kg (b.w.)¸ respectively.
3.7. Total Polyphenolic Content (TPC)
3.8. Anti-Radical DPPH Assay
3.9. Ferric Reducing Antioxidant Power (FRAP) Assay
3.10. HPLC Fingerprint Analysis
3.10.1. Sample Preparation
3.10.2. Chromatographic Analysis
3.11. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mahomoodally, M.F. Traditional Medicines in Africa: An Appraisal of Ten Potent African Medicinal Plants. Evid.-Based Complement. Altern. Med. 2013, 2013, 617459. [Google Scholar] [CrossRef] [PubMed]
- Wangchuk, P. Therapeutic Applications of Natural Products in Herbal Medicines, Biodiscovery Programs, and Biomedicine. J. Biol. Act. Prod. Nat. 2018, 8, 1–20. [Google Scholar] [CrossRef]
- Mesa, C.L.; Ranalison, O.; Randriantseheno, L.N.; Risuleo, G. Natural Products from Madagascar, Socio-Cultural Usage, and Potential Applications in Advanced Biomedicine: A Concise Review. Molecules 2021, 26, 4507. [Google Scholar] [CrossRef]
- Robinson, J.G. An Island of Evolutionary Exuberance. Science 2004, 304, 53. [Google Scholar] [CrossRef]
- Van Andel, T.; Carvalheiro, L.G. Why Urban Citizens in Developing Countries Use Traditional Medicines: The Case of Suriname. Evid.-Based Complement. Altern. Med. 2013, 2013, 687197. [Google Scholar] [CrossRef]
- Krause, D.W.; Sertich, J.J.; O’Connor, P.M.; Curry Rogers, K.; Rogers, R.R. The Mesozoic Biogeographic History of Gondwanan Terrestrial Vertebrates: Insights from Madagascar’s Fossil Record. Annu. Rev. Earth Planet. Sci. 2019, 47, 519–553. [Google Scholar] [CrossRef]
- Beyene, B.; Beyene, B.; Deribe, H. Review on Application and Management of Medicinal Plants for the Livelihood of the Local Community. J. Resour. Dev. Manag. 2016, 22, 33–39. [Google Scholar]
- Fokunang, C.N.; Ndikum, V.; Tabi, O.Y.; Jiofack, R.B.; Ngameni, B.; Guedje, N.M.; Tembe-Fokunang, E.A.; Tomkins, P.; Barkwan, S.; Kechia, F. Traditional Medicine: Past, Present and Future Research and Development Prospects and Integration in the National Health System of Cameroon. Afr. J. Tradit. Complement. Altern. Med. 2011, 8, 284–295. [Google Scholar] [CrossRef]
- Pedrollo, C.T.; Kinupp, V.F.; Shepard, G., Jr.; Heinrich, M. Medicinal Plants at Rio Jauaperi, Brazilian Amazon: Ethnobotanical Survey and Environmental Conservation. J. Ethnopharmacol. 2016, 186, 111–124. [Google Scholar] [CrossRef]
- Afolayan, A.J.; Adebola, P.O. In Vitro Propagation: A Biotechnological Tool Capable of Solving the Problem of Medicinal Plants Decimation in South Africa. Afr. J. Biotechnol. 2004, 3, 683–687. [Google Scholar]
- Tropicos. Available online: https://www.tropicos.org/name/50135463 (accessed on 13 June 2024).
- Ribeiro-Barros, A.I.; Catarino, S.; Moura, I.; Ramalho, J.C.; Romeiras, M.M.; Ghodhbane-Gtari, F. Actinorhizal Trees and Shrubs from Africa: Distribution, Conservation and Uses. Antonie Leeuwenhoek 2019, 112, 31–46. [Google Scholar] [CrossRef]
- Polhill, R.M.; Verdcourt, B. Myricaceae Flora Zambesiaca 9 (3). Incl. Pict. 2006, 256–258. [Google Scholar]
- Burrows, J.E.; Burrows, S.; Schmidt, E.; Lotter, M.; Wilson, E.O. Trees and Shrubs Mozambique; Print Matters Heritage: Cape Town, South Africa, 2018; ISBN 0-9922403-6-0. [Google Scholar]
- JSTOR. Available online: https://plants.jstor.org/stable/10.5555/al.ap.flora.ftea004367 (accessed on 13 June 2024).
- IUCN Morella spathulata: Daniels, A.: The IUCN Red List of Threatened Species. Available online: https://dx.doi.org/10.2305/IUCN.UK.2021-1.RLTS.T189308817A189308836.en (accessed on 24 June 2024).
- Rainha, N.; Koci, K.; Coelho, A.V.; Lima, E.; Baptista, J.; Fernandes-Ferreira, M. HPLC–UV–ESI-MS Analysis of Phenolic Compounds and Antioxidant Properties of Hypericum Undulatum Shoot Cultures and Wild-Growing Plants. Phytochemistry 2013, 86, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Donno, D.; Mellano, M.G.; Riondato, I.; De Biaggi, M.; Andriamaniraka, H.; Gamba, G.; Beccaro, G.L. Traditional and Unconventional Dried Fruit Snacks as a Source of Health-Promoting Compounds. Antioxidants 2019, 8, 396. [Google Scholar] [CrossRef]
- Bristy, A.T.; Islam, T.; Ahmed, R.; Hossain, J.; Reza, H.M.; Jain, P. Evaluation of Total Phenolic Content, HPLC Analysis, and Antioxidant Potential of Three Local Varieties of Mushroom: A Comparative Study. Int. J. Food Sci. 2022, 2022, 3834936. [Google Scholar] [CrossRef] [PubMed]
- Puertas-Mejía, M.A.; Gutierrez-Villegas, M.I.; Mejía-Giraldo, J.C.; Winkler, R.; Rojano, B. In Vitro UV Absorption Properties and Radical Scavenging Capacity of Morella parvifolia (Benth.) Parra-Os. Extracts. Braz. J. Pharm. Sci. 2018, 54, e17498. [Google Scholar] [CrossRef]
- Tanase, C.; Coșarcă, S.; Muntean, D.-L. A Critical Review of Phenolic Compounds Extracted from the Bark of Woody Vascular Plants and Their Potential Biological Activity. Molecules 2019, 24, 1182. [Google Scholar] [CrossRef]
- Piątczak, E.; Dybowska, M.; Płuciennik, E.; Kośla, K.; Kolniak-Ostek, J.; Kalinowska-Lis, U. Identification and Accumulation of Phenolic Compounds in the Leaves and Bark of Salix alba (L.) and Their Biological Potential. Biomolecules 2020, 10, 1391. [Google Scholar] [CrossRef]
- Kumari, A.; Parida, A.K.; Rangani, J.; Panda, A. Antioxidant Activities, Metabolic Profiling, Proximate Analysis, Mineral Nutrient Composition of Salvadora persica Fruit Unravel a Potential Functional Food and a Natural Source of Pharmaceuticals. Front. Pharmacol. 2017, 8, 232579. [Google Scholar] [CrossRef]
- Berton, S.B.; Cabral, M.R.; de Jesus, G.A.; Sarragiotto, M.H.; Pilau, E.J.; Martins, A.F.; Bonafe, E.G.; Matsushita, M. Ultra-High-Performance Liquid Chromatography Supports a New Reaction Mechanism between Free Radicals and Ferulic Acid with Antimicrobial and Antioxidant Activities. Ind. Crops Prod. 2020, 154, 112701. [Google Scholar] [CrossRef]
- You, Y.; Park, J.; Yoon, H.-G.; Lee, Y.-H.; Hwang, K.; Lee, J.; Kim, K.; Lee, K.-W.; Shim, S.; Jun, W. Stimulatory Effects of Ferulic Acid on Endurance Exercise Capacity in Mice. Biosci. Biotechnol. Biochem. 2009, 73, 1392–1397. [Google Scholar] [CrossRef] [PubMed]
- Ascacio-Valdés, J.A.; Buenrostro-Figueroa, J.J.; Aguilera-Carbo, A.; Prado-Barragán, A.; Rodríguez-Herrera, R.; Aguilar, C.N. Ellagitannins: Biosynthesis, Biodegradation and Biological Properties. J. Med. Plants Res. 2011, 5, 4696–4703. [Google Scholar]
- Das, A.K.; Islam, M.N.; Faruk, M.O.; Ashaduzzaman, M.; Dungani, R. Review on Tannins: Extraction Processes, Applications and Possibilities. S. Afr. J. Bot. 2020, 135, 58–70. [Google Scholar] [CrossRef]
- Fioccardi, A.; Donno, D.; Razafindrakoto, Z.R.; Gamba, G.; Beccaro, G.L. First Phytochemical Study of Six Tree and Shrub Species with High Health-Promoting Potential from Madagascar: Innovative Uses for Food and Medicinal Applications. Sci. Hortic. 2022, 299, 111010. [Google Scholar] [CrossRef]
- Riondato, I.; Donno, D.; Roman, A.; Razafintsalama, V.E.; Petit, T.; Mellano, M.G.; Torti, V.; De Biaggi, M.; Rakotoniaina, E.N.; Giacoma, C.; et al. First Ethnobotanical Inventory and Phytochemical Analysis of Plant Species Used by Indigenous People Living in the Maromizaha Forest, Madagascar. J. Ethnopharmacol. 2019, 232, 73–89. [Google Scholar] [CrossRef]
- Tombozara, N.; Razafindrakoto, Z.R.; Donno, D.; Randriamampionona, D.; Ramilison-Razafimahefa, R.D.; Rakotondramanana, D.A.; Andrianjara, C.; Ramanitrahasimbola, D.; Beccaro, G.L. Phytochemical and Pharmacological Activities of Schefflera bojeri (Seem.) R. Vig.(Araliaceae). S. Afr. J. Bot. 2022, 151, 514–522. [Google Scholar] [CrossRef]
- Ríos, J.-L.; Giner, R.M.; Marín, M.; Recio, M.C. A Pharmacological Update of Ellagic Acid. Planta Med. 2018, 84, 1068–1093. [Google Scholar] [CrossRef]
- Fawzi Mahomoodally, M.; Picot-Allain, M.C.N.; Zengin, G.; Llorent-Martínez, E.J.; Abdullah, H.H.; Ak, G.; Senkardes, I.; Chiavaroli, A.; Menghini, L.; Recinella, L. Phytochemical Analysis, Network Pharmacology and in Silico Investigations on Anacamptis pyramidalis Tuber Extracts. Molecules 2020, 25, 2422. [Google Scholar] [CrossRef]
- Occhipinti, R.; Boron, W.F. Role of Carbonic Anhydrases and Inhibitors in Acid–Base Physiology: Insights from Mathematical Modeling. Int. J. Mol. Sci. 2019, 20, 3841. [Google Scholar] [CrossRef]
- Mboge, M.Y.; Mahon, B.P.; McKenna, R.; Frost, S.C. Carbonic Anhydrases: Role in pH Control and Cancer. Metabolites 2018, 8, 19. [Google Scholar] [CrossRef]
- Zhou, W.; Wang, C.; Zhang, B.; Gou, S. Hybrids of Carbonic Anhydrase and Cyclooxygenase Inhibitors Attenuate Cardiac Hypoxic Inflammatory Injuries. Eur. J. Pharmacol. 2023, 950, 175751. [Google Scholar] [CrossRef] [PubMed]
- Shore, D.M.; Reggio, P.H. The Therapeutic Potential of Orphan GPCRs, GPR35 and GPR55. Front. Pharmacol. 2015, 6, 69. [Google Scholar] [CrossRef] [PubMed]
- Boleij, A.; Fathi, P.; Dalton, W.; Park, B.; Wu, X.; Huso, D.; Allen, J.; Besharati, S.; Anders, R.A.; Housseau, F. G-Protein Coupled Receptor 35 (GPR35) Regulates the Colonic Epithelial Cell Response to Enterotoxigenic Bacteroides fragilis. Commun. Biol. 2021, 4, 585. [Google Scholar] [CrossRef]
- Melhem, H.; Kaya, B.; Ayata, C.K.; Hruz, P.; Niess, J.H. Metabolite-Sensing G Protein-Coupled Receptors Connect the Diet-Microbiota-Metabolites Axis to Inflammatory Bowel Disease. Cells 2019, 8, 450. [Google Scholar] [CrossRef]
- Scalia, S.; Marchetti, N.; Bianchi, A. Comparative Evaluation of Different Co-Antioxidants on the Photochemical-and Functional-Stability of Epigallocatechin-3-Gallate in Topical Creams Exposed to Simulated Sunlight. Molecules 2013, 18, 574–587. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.; Kim, N.; Shin, Y.; Kim, S.-Y.; Kim, Y.-J. Activity of Catechins and Their Applications. Biomed. Dermatol. 2020, 4, 8. [Google Scholar] [CrossRef]
- Paciolla, C.; Fortunato, S.; Dipierro, N.; Paradiso, A.; De Leonardis, S.; Mastropasqua, L.; De Pinto, M.C. Vitamin C in Plants: From Functions to Biofortification. Antioxidants 2019, 8, 519. [Google Scholar] [CrossRef]
- Gest, N.; Gautier, H.; Stevens, R. Ascorbate as Seen through Plant Evolution: The Rise of a Successful Molecule? J. Exp. Bot. 2013, 64, 33–53. [Google Scholar] [CrossRef]
- Carr, A.C.; Maggini, S. Vitamin C and Immune Function. Nutrients 2017, 9, 1211. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA); Turck, D.; Bresson, J.-L.; Burlingame, B.; Dean, T.; Fairweather-Tait, S.; Heinonen, M.; Hirsch-Ernst, K.-I.; Mangelsdorf, I.; McArdle, H.J. Vitamin C and Protection of DNA, Proteins and Lipids from Oxidative Damage: Evaluation of a Health Claim Pursuant to Article 14 of Regulation (EC) No 1924/2006. Volume 14, Issue 10. EFSA J. 2017, 15, e04685. [Google Scholar]
- Benali, T.; Bakrim, S.; Ghchime, R.; Benkhaira, N.; El Omari, N.; Balahbib, A.; Taha, D.; Zengin, G.; Hasan, M.M.; Bibi, S. Pharmacological Insights into the Multifaceted Biological Properties of Quinic Acid. Biotechnol. Genet. Eng. Rev. 2022, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Heikkilä, E.; Hermant, A.; Thevenet, J.; Bermont, F.; Kulkarni, S.S.; Ratajczak, J.; Santo-Domingo, J.; Dioum, E.H.; Canto, C.; Barron, D. The Plant Product Quinic Acid Activates Ca2+-dependent Mitochondrial Function and Promotes Insulin Secretion from Pancreatic Beta Cells. Br. J. Pharmacol. 2019, 176, 3250–3263. [Google Scholar] [CrossRef] [PubMed]
- Nangare, S.; Vispute, Y.; Tade, R.; Dugam, S.; Patil, P. Pharmaceutical Applications of Citric Acid. Future J. Pharm. Sci. 2021, 7, 54. [Google Scholar] [CrossRef]
- Abdel-Salam, O.M.; Shaffie, N.M.; Omara, E.A.; Yassen, N.N. Citric Acid an Antioxidant in Liver. In The Liver; Elsevier: Amsterdam, The Netherlands, 2018; pp. 183–198. [Google Scholar]
- Wang, H.; Yu, M.; Ochani, M.; Amella, C.A.; Tanovic, M.; Susarla, S.; Li, J.H.; Wang, H.; Yang, H.; Ulloa, L. Nicotinic Acetylcholine Receptor A7 Subunit Is an Essential Regulator of Inflammation. Nature 2003, 421, 384–388. [Google Scholar] [CrossRef] [PubMed]
- Schuller, H.M. Is Cancer Triggered by Altered Signalling of Nicotinic Acetylcholine Receptors? Nat. Rev. Cancer 2009, 9, 195–205. [Google Scholar] [CrossRef]
- Gu, S.; Matta, J.A.; Lord, B.; Harrington, A.W.; Sutton, S.W.; Davini, W.B.; Bredt, D.S. Brain A7 Nicotinic Acetylcholine Receptor Assembly Requires NACHO. Neuron 2016, 89, 948–955. [Google Scholar] [CrossRef]
- Roriz, C.L.; Barros, L.; Carvalho, A.M.; Ferreira, I.C. HPLC-Profiles of Tocopherols, Sugars, and Organic Acids in Three Medicinal Plants Consumed as Infusions. Int. J. Food Sci. 2014, 2014, 241481. [Google Scholar] [CrossRef]
- Jeandet, P.; Formela-Luboińska, M.; Labudda, M.; Morkunas, I. The Role of Sugars in Plant Responses to Stress and Their Regulatory Function during Development. Int. J. Mol. Sci. 2022, 23, 5161. [Google Scholar] [CrossRef]
- Razafindrakoto, Z.R.; Donno, D.; Tombozara, N.; Andriamaniraka, H.; Andrianjara, C.; Ramanitrahasimbola, D.; Beccaro, G.L. Antioxidant, Anti-Inflammatory, and Antidiabetic Activities of Leaves and Stems of Uapaca bojeri Bail.(Euphorbiaceae), an Endemic Plant of Madagascar. Pharmaceuticals 2020, 13, 71. [Google Scholar] [CrossRef]
- Tombozara, N.; Donno, D.; Razafindrakoto, Z.R.; Randriamampionona, D.; Ramanitrahasimbola, D.; Andrianjara, C.; Ramilison-Razafimahefa, R.D.; Rakotondramanana, D.A.; Beccaro, G.L. The First Assessment on Antioxidant and Antidiabetic Activities of Leaves and Stems of Vaccinium secundiflorum Hook.(Ericaceae), an Endemic Plant of Madagascar. S. Afr. J. Bot. 2020, 130, 422–429. [Google Scholar] [CrossRef]
- Razafin-Drabazo, F.; Donno, D.; Tombozara, N.; Razafindrakoto, Z.R.; Rajaonarison, J.F.; Andrianjara, C.; Ramanitrahasimbola, D.; Beccaro, G.L. Phyto-Compounds and Pharmacological Activities of Lygodium lanceolatum Desv.(Schizaeaceae). S. Afr. J. Bot. 2020, 135, 225–232. [Google Scholar] [CrossRef]
- Razafindrakoto, Z.R.; Tombozara, N.; Donno, D.; Gamba, G.; Nalimanana, N.R.; Rakotondramanana, D.A.; Andrianjara, C.; Beccaro, G.L.; Ramanitrahasimbola, D. Antioxidant, Analgesic, Anti-Inflammatory and Antipyretic Properties, and Toxicity Studies of the Aerial Parts of Imperata cylindrica (L.) Beauv. S. Afr. J. Bot. 2021, 142, 222–229. [Google Scholar] [CrossRef]
- Tasleem, F.; Azhar, I.; Ali, S.N.; Perveen, S.; Mahmood, Z.A. Analgesic and Anti-Inflammatory Activities of Piper nigrum L. Asian Pac. J. Trop. Med. 2014, 7, S461–S468. [Google Scholar] [CrossRef]
- Archer, A.C.; Muthukumar, S.P.; Halami, P.M. Anti-Inflammatory Potential of Probiotic lactobacillus spp. on Carrageenan Induced Paw Edema in Wistar Rats. Int. J. Biol. Macromol. 2015, 81, 530–537. [Google Scholar] [CrossRef]
- Ray, S.D.; Ray, S.; Zia-Ul-Haq, M.; De Feo, V.; Dewanjee, S. Pharmacological Basis of the Use of the Root Bark of Zizyphus nummularia Aubrev.(Rhamnaceae) as Anti-Inflammatory Agent. BMC Complement. Altern. Med. 2015, 15, 416. [Google Scholar] [CrossRef]
- Cheng, J.; Ma, T.; Liu, W.; Wang, H.; Jiang, J.; Wei, Y.; Tian, H.; Zou, N.; Zhu, Y.; Shi, H. In in Vivo Evaluation of the Anti-Inflammatory and Analgesic Activities of Compound Muniziqi Granule in Experimental Animal Models. BMC Complement. Altern. Med. 2015, 16, 20. [Google Scholar] [CrossRef] [PubMed]
- Déciga-Campos, M.; Palacios-Espinosa, J.F.; Reyes-Ramírez, A.; Mata, R. Antinociceptive and Anti-Inflammatory Effects of Compounds Isolated from Scaphyglottis Livida and Maxillaria Densa. J. Ethnopharmacol. 2007, 114, 161–168. [Google Scholar] [CrossRef]
- Raafat, K.M. Anti-Inflammatory and Anti-Neuropathic Effects of a Novel Quinic Acid Derivative from Acanthus Syriacus. Avicenna J. Phytomed. 2019, 9, 221. [Google Scholar]
- Åkesson, C.; Lindgren, H.; Pero, R.W.; Leanderson, T.; Ivars, F. Quinic Acid Is a Biologically Active Component of the Uncaria Tomentosa Extract C-Med 100®. Int. Immunopharmacol. 2005, 5, 219–229. [Google Scholar] [CrossRef]
- Zeng, K.; Thompson, K.E.; Yates, C.R.; Miller, D.D. Synthesis and Biological Evaluation of Quinic Acid Derivatives as Anti-Inflammatory Agents. Bioorganic Med. Chem. Lett. 2009, 19, 5458–5460. [Google Scholar] [CrossRef]
- Yam, M.F.; Loh, Y.C.; Oo, C.W.; Basir, R. Overview of Neurological Mechanism of Pain Profile Used for Animal “Pain-like” Behavioral Study with Proposed Analgesic Pathways. Int. J. Mol. Sci. 2020, 21, 4355. [Google Scholar] [CrossRef] [PubMed]
- Subedi, N.K.; Rahman, S.M.; Akbar, M.A. Analgesic and Antipyretic Activities of Methanol Extract and Its Fraction from the Root of Schoenoplectus grossus. Evid.-Based Complement. Altern. Med. 2016, 2016, 3820704. [Google Scholar] [CrossRef] [PubMed]
- Konaté, K.; Bassolé, I.H.N.; Hilou, A.; Aworet-Samseny, R.R.; Souza, A.; Barro, N.; Dicko, M.H.; Datté, J.Y.; M’Batchi, B. Toxicity Assessment and Analgesic Activity Investigation of Aqueous Acetone Extracts of Sida acuta Burn f. and Sida cordifolia L.(Malvaceae), Medicinal Plants of Burkina Faso. BMC Complement. Altern. Med. 2012, 12, 120. [Google Scholar] [CrossRef] [PubMed]
- Demsie, D.G.; Yimer, E.M.; Berhe, A.H.; Altaye, B.M.; Berhe, D.F. Anti-Nociceptive and Anti-Inflammatory Activities of Crude Root Extract and Solvent Fractions of Cucumis ficifolius in Mice Model. J. Pain Res. 2019, 12, 1399–1409. [Google Scholar] [CrossRef] [PubMed]
- Tadiwos, Y.; Nedi, T.; Engidawork, E. Analgesic and Anti-Inflammatory Activities of 80% Methanol Root Extract of Jasminum abyssinicum Hochst. Ex. Dc.(Oleaceae) in Mice. J. Ethnopharmacol. 2017, 202, 281–289. [Google Scholar] [CrossRef]
- No, O.T. 423: Acute Oral Toxicity-Acute Toxic Class Method. OECD Guidel. Test. Chem. Sect. 2002, 4, 14. [Google Scholar]
- Patel, D.K.; Kumar, R.; Prasad, S.K.; Sairam, K.; Hemalatha, S. Antidiabetic and in Vitro Antioxidant Potential of Hybanthus enneaspermus (Linn) F. Muell in Streptozotocin–Induced Diabetic Rats. Asian Pac. J. Trop. Biomed. 2011, 1, 316–322. [Google Scholar] [CrossRef]
- Nagulsamy, P.; Ponnusamy, R.; Thangaraj, P. Evaluation of Antioxidant, Anti-Inflammatory, and Antiulcer Properties of Vaccinium leschenaultii Wight: A Therapeutic Supplement. J. Food Drug Anal. 2015, 23, 376–386. [Google Scholar] [CrossRef]
- Koster, R.; Anderson, W.; De Beer, E.J. Acetic Acid for Analgesic Screening. Fed. Proc. 1959, 18, 412–417. [Google Scholar]
- Morris, C.J. Carrageenan-Induced Paw Edema in the Rat and Mouse. Inflamm. Protoc. 2003, 225, 115–121. [Google Scholar]
- Slinkard, K.; Singleton, V.L. Total Phenol Analysis: Automation and Comparison with Manual Methods. Am. J. Enol. Vitic. 1977, 28, 49–55. [Google Scholar] [CrossRef]
- Donno, D.; Cerutti, A.K.; Mellano, M.G.; Prgomet, Z.; Beccaro, G.L. Serviceberry, a Berry Fruit with Growing Interest of Industry: Physicochemical and Quali-Quantitative Health-Related Compound Characterisation. J. Funct. Foods 2016, 26, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Sreejayan, N.; Rao, M.N. Free Radical Scavenging Activity of Curcuminoids. Arzneim. Forsch. 1996, 46, 169–171. [Google Scholar]
- Benzie, I.F.; Strain, J.J. [2] Ferric Reducing/Antioxidant Power Assay: Direct Measure of Total Antioxidant Activity of Biological Fluids and Modified Version for Simultaneous Measurement of Total Antioxidant Power and Ascorbic Acid Concentration. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1999; Volume 299, pp. 15–27. ISBN 0076-6879. [Google Scholar]
- Donno, D.; Boggia, R.; Zunin, P.; Cerutti, A.K.; Guido, M.; Mellano, M.G.; Prgomet, Z.; Beccaro, G.L. Phytochemical Fingerprint and Chemometrics for Natural Food Preparation Pattern Recognition: An Innovative Technique in Food Supplement Quality Control. J. Food Sci. Technol. 2016, 53, 1071–1083. [Google Scholar] [CrossRef]
- Yun, B.-R.; Weon, J.B.; Lee, J.; Eom, M.R.; Ma, C.J. Simultaneous Determination of 11 Bioactive Compounds in Jaeumganghwa-Tang by High Performance Liquid Chromatography-Diode Array Detection. Pharmacogn. Mag. 2014, 10 (Suppl. S2), S256–S263. [Google Scholar]
- Gfeller, D.; Grosdidier, A.; Wirth, M.; Daina, A.; Michielin, O.; Zoete, V. SwissTargetPrediction: A Web Server for Target Prediction of Bioactive Small Molecules. Nucleic Acids Res. 2014, 42, W32–W38. [Google Scholar] [CrossRef]
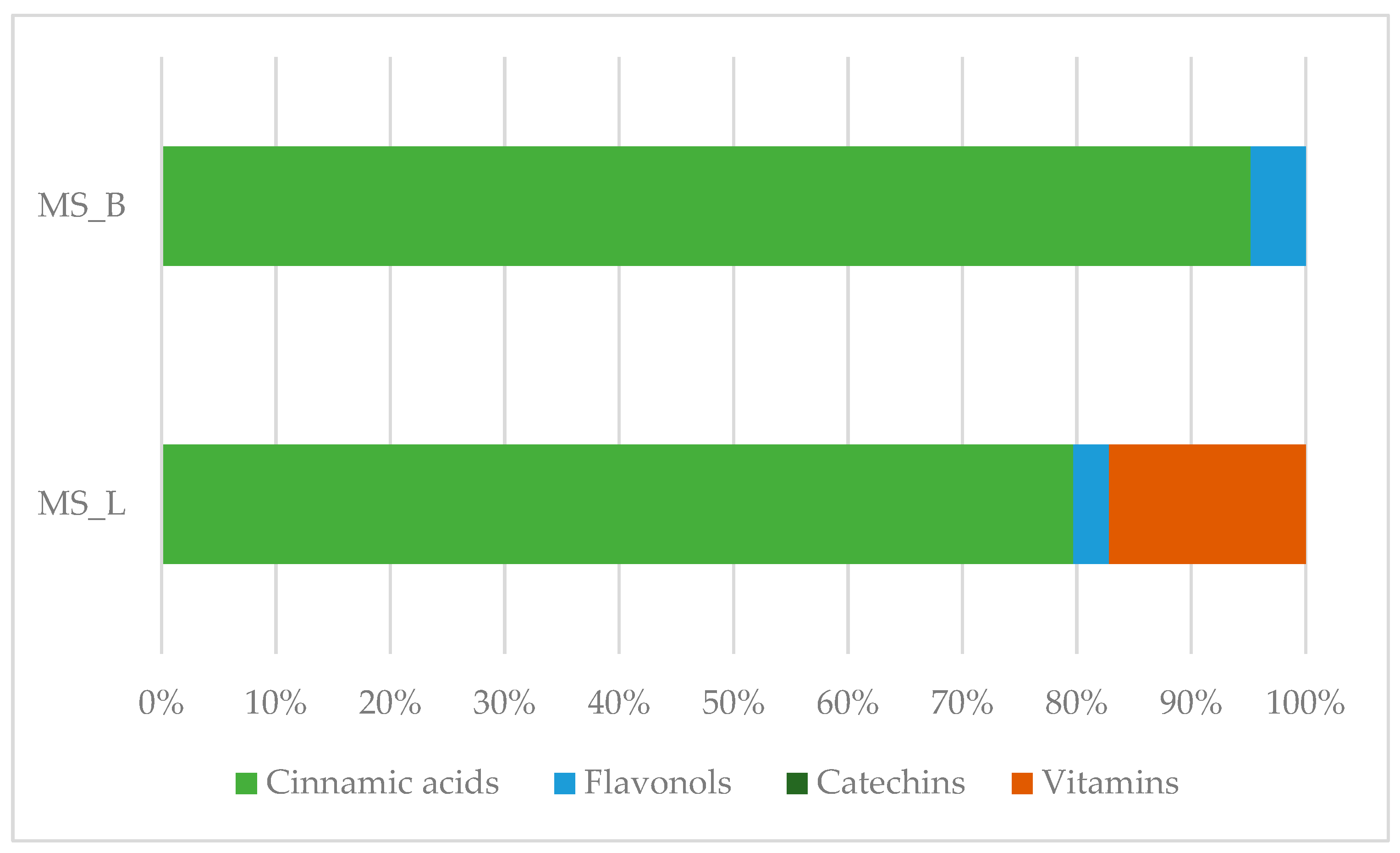
| Class | Bioactive Marker | MS_L | MS_B | Identified Compounds: Chemical Structures |
|---|---|---|---|---|
| Mean value ± SD | Mean value ± SD | |||
| (mg/100 g DW) | (mg/100 g DW) | |||
| Cinnamic acids | caffeic acid | n.d. | n.q. | |
| chlorogenic acid | n.d. | n.d. | ||
| coumaric acid | n.q. | n.d. | ||
| ferulic acid | 67.16 ± 0.73 a | 49.67 ± 2.09 b |  | |
| Flavonols | hyperoside | n.q. | n.d. | |
| isoquercitrin | n.d. | 2.5 ± 0.46 | 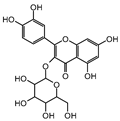 | |
| quercetin | n.d. | n.d. | ||
| quercitrin | n.d. | n.d. | ||
| rutin | 2.63 ± 1.66 | n.d. | 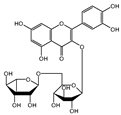 | |
| Benzoic acids | ellagic acid | 19.08 ± 1.5 a | 22.11 ± 2.26 a | 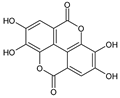 |
| gallic acid | n.d. | n.d. | ||
| Catechins | catechin | n.d. | n.d. | |
| epicatechin | n.d. | n.d. | ||
| Vitamins | ascorbic acid | 14.49 ± 0.19 | n.d. | 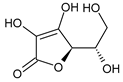 |
| dehydroascorbic acid | n.d. | n.d. | ||
| Class. | Bioactive Marker | MS_L | MS_B | Identified Compounds: Chemical Structures |
|---|---|---|---|---|
| Mean value ± SD | Mean value ± SD | |||
| (g/100 g DW) | (g/100 g DW) | |||
| Organic acids | citric acid | n.d. | 3.55 ± 0.46 | 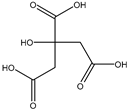 |
| malic acid | n.d. | n.d. | ||
| oxalic acid | n.d. | n.d. | ||
| quinic acid | 48.57 ± 0.68 a | 21.50 ± 0.75 b | 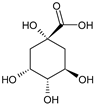 | |
| succinic acid | n.d. | n.d. | ||
| tartaric acid | n.d. | n.d. | ||
| Sugars | fructose | 1.26 ± 0.04 b | 1.75 ± 0.03 a | 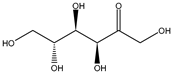 |
| glucose | 24.81 ± 0.76 b | 31.44 ± 1.22 a | 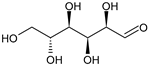 | |
| sucrose | 6.04 ± 0.54 | n.d. | 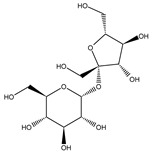 |
| Sample | FRAP (mmol Fe2+/kg DW) | Yield (%) | Equation | r2 | DPPH Assay: IC50 (µg/mL) |
|---|---|---|---|---|---|
| MS_L | 227.89 ± 12.53 a | 3.90 | y = 2.0955x − 6.1227 | 0.9994 | 26.78 ± 0.42 a |
| MS_B | 376.18 ± 115.69 a | 9.84 | y = 2.0098x − 9.7684 | 0.9999 | 29.74 ± 0.16 a |
| Gallic acid | - | - | y = 2.7242x − 9.3331 | 0.9989 | 21.78 ± 0.11 |
| Sample | Dose (mg/kg) | Inhibition of inflammation (%) | ||||
|---|---|---|---|---|---|---|
| 30 min | 60 min | 120 min | 180 min | 240 min | ||
| Vehicle | 0 | 4.28 ± 1.87 | 9.47 ± 2.52 | 18.72 ± 2.26 | 19.99 ± 5.45 | 32.95 ± 2.77 |
| MS_L | 100 | 11.03 ± 3.21 a | 22.59 ± 2.50 b | 39.64 ± 3.15 b | 62.32 ± 4.44 b | 85.74 ± 2.49 b,c |
| 200 | 16.85 ± 5.51 b | 32.76 ± 6.23 b,c | 65.33 ± 6.95 b,c | 75.71 ± 7.74 b,c | 88.68 ± 5.77 b,c | |
| 400 | 11.89 ± 1.53 a | 29.58 ± 6.84 b | 52.44 ± 7.04 b | 80.12 ± 6.42 b,c | 94.25 ± 1.78 b,c | |
| MS_B | 100 | 6.40 ± 1.15 a | 17.43 ± 2.74 b | 29.77 ± 2.13 b,c | 43.49 ± 3.43 b | 49.88 ± 6.64 b,c |
| 200 | 12.95 ± 4.05 b | 30.21 ± 5.29 b | 59.70 ± 3.79 b | 74.57 ± 5.19 b,c | 91.41 ± 3.57 b,c | |
| 400 | 12.66 ± 2.83 b | 31.58 ± 6.95 b | 57.10 ± 8.76 b | 85.65 ± 5.83 b,c | 96.53 ± 1.90 b,c | |
| Indomethacin | 10 | 13.77 ± 3.89 b | 24.02 ± 8.60 b | 40.26 ± 7.87 b | 57.59 ± 4.14 b | 71.89 ± 1.84 b |
| Sample | Dose (mg/kg) | Writhing Number | Pain Inhibition (%) |
|---|---|---|---|
| Vehicle | - | 13.60 ± 4.46 | - |
| MS_L | 100 | 8.10 ± 2.33 a,c | 40.44 |
| 200 | 4.20 ± 2.93 b | 69.12 | |
| 400 | 3.10 ± 0.96 b | 77.21 | |
| MS_B | 100 | 10.10 ± 1.39 c | 25.74 |
| 200 | 6.00 ± 1.17 a,c | 55.88 | |
| 400 | 3.90 ± 0.65 b | 71.32 | |
| Paracetamol | 100 | 2.60 ± 1.56 b | 83.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fioccardi, A.; Donno, D.; Razafindrakoto, Z.R.; Tombozara, N.; Henintsoa, S.; Mahitasoa, E.; Torti, V.; Solofoniaina, M.; Rosso, L.; Gamba, G.; et al. Assessing a “Least-Concern” Red List Tree Species from Madagascar Used in Traditional Medicine: Morella spathulata (Myricaceae) Phyto-Compounds and Anti-Inflammatory Properties. Plants 2024, 13, 2899. https://doi.org/10.3390/plants13202899
Fioccardi A, Donno D, Razafindrakoto ZR, Tombozara N, Henintsoa S, Mahitasoa E, Torti V, Solofoniaina M, Rosso L, Gamba G, et al. Assessing a “Least-Concern” Red List Tree Species from Madagascar Used in Traditional Medicine: Morella spathulata (Myricaceae) Phyto-Compounds and Anti-Inflammatory Properties. Plants. 2024; 13(20):2899. https://doi.org/10.3390/plants13202899
Chicago/Turabian StyleFioccardi, Annachiara, Dario Donno, Zoarilala Rinah Razafindrakoto, Nantenaina Tombozara, Sylvia Henintsoa, Elyna Mahitasoa, Valeria Torti, Marcellin Solofoniaina, Lorenzo Rosso, Giovanni Gamba, and et al. 2024. "Assessing a “Least-Concern” Red List Tree Species from Madagascar Used in Traditional Medicine: Morella spathulata (Myricaceae) Phyto-Compounds and Anti-Inflammatory Properties" Plants 13, no. 20: 2899. https://doi.org/10.3390/plants13202899
APA StyleFioccardi, A., Donno, D., Razafindrakoto, Z. R., Tombozara, N., Henintsoa, S., Mahitasoa, E., Torti, V., Solofoniaina, M., Rosso, L., Gamba, G., Andrianjara, C., Ramanitrahasimbola, D., & Beccaro, G. L. (2024). Assessing a “Least-Concern” Red List Tree Species from Madagascar Used in Traditional Medicine: Morella spathulata (Myricaceae) Phyto-Compounds and Anti-Inflammatory Properties. Plants, 13(20), 2899. https://doi.org/10.3390/plants13202899









