Adaptive Seedling Strategies in Seasonally Dry Tropical Forests: A Comparative Study of Six Tree Species
Abstract
1. Introduction
2. Results
2.1. Interspecific Germination Patterns
2.2. Intraspecific Responses
3. Discussion
4. Materials and Methods
4.1. Study Area and Species
4.2. Seed Sampling and Germination
4.3. Data Analyses
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Donohue, K.; de Casas, R.R.; Burghardt, L.; Kovach, K.; Willis, C.G. Germination, Postgermination Adaptation, and Species Ecological Ranges. Annu. Rev. Ecol. Evol. Syst. 2010, 41, 293–319. [Google Scholar] [CrossRef]
- Jiménez-Alfaro, B.; Silveira, F.A.O.; Fidelis, A.; Poschlod, P.; Commander, L.E. Seed Germination Traits Can Contribute Better to Plant Community Ecology. J. Veg. Sci. 2016, 27, 637–645. [Google Scholar] [CrossRef]
- Pearson, T.R.H.; Burslem, D.F.R.; Mullins, C.E.; Dalling, J.W. Germination Ecology of Neotropical Pioneers: Interacting Effects of Environmental Conditions and Seed Size. Ecology 2002, 83, 2798–2807. [Google Scholar] [CrossRef]
- Kitajima, K.; Fenner, M. Ecology of Seedling Regeneration. In Seeds: The Ecology of Regeneration in Plant Communities; CAB International: Wallingford, UK, 2000; pp. 331–359. [Google Scholar]
- Eriksson, O. Game Theory Provides No Explanation for Seed Size Variation in Grasslands. Oecologia 2005, 144, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Khurana, E.; Sagar, R.; Singh, J.S. Seed Size: A Key Trait Determining Species Distribution and Diversity of Dry Tropical Forest in Northern India. Acta Oecologica 2006, 29, 196–204. [Google Scholar] [CrossRef]
- Leishman, M.R.; Wright, I.J.; Moles, A.T.; Westoby, M. The Evolutionary Ecology of Seed Size. In Seeds: The Ecology of Regeneration in Plant Communities; CAB International: Wallingford, UK, 2000; pp. 31–57. [Google Scholar]
- Souza, M.L.; Fagundes, M. Seed Size as Key Factor in Germination and Seedling Development of Copaifera Langsdorffi (Fabaceae). Am. J. Plant Sci. 2014, 5, 2566–2573. [Google Scholar] [CrossRef]
- Khurana, E.; Singh, J.S. Ecology of Tree Seed and Seedlings: Implications for Tropical Forest Conservation and Restoration. Curr. Sci. 2001, 80, 748–757. [Google Scholar]
- Padilla, F.M.; Miranda, J.d.D.; Pugnaire, F.I. Early Root Growth Plasticity in Seedlings of Three Mediterranean Woody Species. Plant Soil 2007, 296, 103–113. [Google Scholar] [CrossRef]
- Markesteijn, L.; Poorter, L. Seedling Root Morphology and Biomass Allocation of 62 Tropical Tree Species in Relation to Drought- and Shade-tolerance. J. Ecol. 2009, 97, 311–325. [Google Scholar] [CrossRef]
- Boonman, C.C.F.; van Langevelde, F.; Oliveras, I.; Couédon, J.; Luijken, N.; Martini, D.; Veenendaal, E.M. On the Importance of Root Traits in Seedlings of Tropical Tree Species. New Phytol. 2020, 227, 156–167. [Google Scholar] [CrossRef]
- Leishman, M.R. Does the Seed Size/Number Trade-off Model Determine Plant Community Structure? An Assessment of the Model Mechanisms and Their Generality. Oikos 2001, 93, 294–302. [Google Scholar] [CrossRef]
- Muller-Landau, H.C. The Tolerance-Fecundity Trade-off and the Maintenance of Diversity in Seed Size. Proc. Natl. Acad. Sci. USA 2010, 107, 4242–4247. [Google Scholar] [CrossRef] [PubMed]
- Dalling, J.W. Ecología de Semillas. In Ecología y Conservación de Bosques Neotropicales; Libro Universitario Regional: Cartago, Costa Rica, 2002; pp. 345–375. [Google Scholar]
- Larios, E.; Venable, D.L. Selection for Seed Size: The Unexpected Effects of Water Availability and Density. Funct. Ecol. 2018, 32, 2216–2224. [Google Scholar] [CrossRef]
- Lönnberg, K.; Eriksson, O. Relationships between Intra-specific Variation in Seed Size and Recruitment in Four Species in Two Contrasting Habitats. Plant Biol. 2013, 15, 601–606. [Google Scholar] [CrossRef] [PubMed]
- Jurado, E.; Westoby, M.; Westboy, M. Seedling Growth in Relation to Seed Size among Species of Arid Australia. J. Ecol. 1992, 80, 407–416. [Google Scholar] [CrossRef]
- Lloret, F.; Casanovas, C.; Peñuelas, J. Seedling Survival of Mediterranean Shrubland Species in Relation to Root:Shoot Ratio, Seed Size and Water and Nitrogen Use. Funct. Ecol. 1999, 13, 210–216. [Google Scholar] [CrossRef]
- Rees, M.; Westoby, M. Game-Theoretical Evolution of Seed Mass in Multi-Species Ecological Models. Oikos 1997, 78, 116. [Google Scholar] [CrossRef]
- Ågren, G.I.; Franklin, O. Root: Shoot Ratios, Optimization and Nitrogen Productivity. Ann. Bot. 2003, 92, 795–800. [Google Scholar] [CrossRef]
- Evans, J.R.; Poorter, H. Photosynthetic Acclimation of Plants to Growth Irradiance: The Relative Importance of Specific Leaf Area and Nitrogen Partitioning in Maximizing Carbon Gain. Plant Cell Environ. 2001, 24, 755–767. [Google Scholar] [CrossRef]
- Olff, H.; Andel, J.V.; Bakker, J.P. Biomass and Shoot/Root Allocation of Five Species from a Grassland Succession Series at Different Combinations of Light and Nutrient Supply. Funct. Ecol. 1990, 4, 193–200. [Google Scholar] [CrossRef]
- Villar, R.; Ruiz-robleto, J.; Quero, J.L.; Poorter, H.; Valladares, F.; Marañón, T. Tasas de Crecimiento En Especies Leñosas: Aspectos Funcionales e Implicaciones Ecológicas. In Ecología del Bosque Mediterráneo en un Mundo Cambiante; Ministerio de Medio Ambiente, EGRAF, S.A.: Madrid, Spain, 2004; pp. 191–227. ISBN 84-8014-552-8. [Google Scholar]
- El Atta, H.A.; Aref, I.M.; Ahmed, A.I. Seed Size Effects on the Response of Seedlings of Acacia Asak (Forssk.) Willd. to Water Stress. Pak. J. Bot. 2016, 48, 439–446. [Google Scholar]
- Martínez-González, I.; Sánchez-Velázquez, L.R.; Ruiz-Guerra, B.; Pineda-López, M.d.R.; Velázquez-Rosas, N. The Role of Seed Size in the Emergence and Survival of Seedlings in Contrasting Environments: The Case of Ceiba Aesculifolia. New For. 2021, 52, 493–507. [Google Scholar] [CrossRef]
- Khurana, E.; Singh, J.S. Influence of Seed Size on Seedling Growth of Albizia Procera under Different Soil Water Levels. Ann. Bot. 2000, 86, 1185–1192. [Google Scholar] [CrossRef]
- Garwood, N.C. Seed Germination in a Seasonal Tropical Forest in Panama: A Community Study. Ecol. Monogr. 1983, 53, 159–181. [Google Scholar] [CrossRef]
- Vieira, D.L.M.; de Lima, V.V.; Sevilha, A.C.; Scariot, A. Consequences of Dry-Season Seed Dispersal on Seedling Establishment of Dry Forest Trees: Should We Store Seeds until the Rains? For. Ecol. Manag. 2008, 256, 471–481. [Google Scholar] [CrossRef]
- Velázquez-Rosas, N.; Ruiz-Guerra, B.; Sánchez-Coronado, M.E.; Buen, A.G.-D.; Orozco-Segovia, A. Morphological Variation in Fruits and Seeds of Ceiba Aesculifolia and Its Relationship with Germination and Seedlings Biomass. Bot. Sci. 2017, 95, 81–91. [Google Scholar] [CrossRef][Green Version]
- Pirie, M.; Klitgaard, B.; Pennington, R. Revision and Biogeography of Centrolobium (Leguminosae–Papilionoideae). Syst. Bot. 2009, 34, 345–359. [Google Scholar] [CrossRef]
- Qi, Y.; Wei, W.; Chen, C.; Chen, L. Plant Root-Shoot Biomass Allocation over Diverse Biomes: A Global Synthesis. Glob. Ecol. Conserv. 2019, 18, e00606. [Google Scholar] [CrossRef]
- Pélabon, C.; De Giorgi, F.; Opedal, Ø.H.; Bolstad, G.H.; Raunsgard, A.; Scott Armbruster, W. Is There More to Within-Plant Variation in Seed Size than Developmental Noise? Evol. Biol. 2021, 48, 366–377. [Google Scholar] [CrossRef]
- Engelbrecht, B.M.J.; Dalling, J.W.; Pearson, T.R.H.; Wolf, R.L.; Gálvez, D.A.; Koehler, T.; Tyree, M.T.; Kursar, T.A. Short Dry Spells in the Wet Season Increase Mortality of Tropical Pioneer Seedlings. Oecologia 2006, 148, 258–269. [Google Scholar] [CrossRef][Green Version]
- Han, X.; Zhao, Y.; Chen, Y.; Xu, J.; Jiang, C.; Wang, X.; Zhuo, R.; Lu, M.-Z.; Zhang, J.; Han, X.; et al. Lignin Biosynthesis and Accumulation in Response to Abiotic Stresses in Woody Plants. For. Res. 2022, 2, 9. [Google Scholar] [CrossRef]
- Dwivedi, U.N.; Campbell, W.H.; Yu, J.; Datla, R.S.S.; Bugos, R.C.; Chiang, V.L.; Podila, G.K. Modification of Lignin Biosynthesis in Transgenic Nicotiana through Expression of an Antisense O-Methyltransferase Gene from Populus. Plant Mol. Biol. 1994, 26, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Boudet, A.M.; Grima-Pettenati, J. Lignin Genetic Engineering. Mol. Breed. 1996, 2, 25–39. [Google Scholar] [CrossRef]
- Higuchi, T. Biosynthesis of Lignin. In Plant Carbohydrates II; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 1981; Volume 13/B, pp. 194–221. [Google Scholar]
- Negreiros, D.; Fernandes, G.W.; Silveira, F.A.O.; Chalub, C. Seedling Growth and Biomass Allocation of Endemic and Threatened Shrubs of Rupestrian Fields. Acta Oecologica 2009, 35, 301–310. [Google Scholar] [CrossRef]
- Aguirre-Gutiérrez, J.; Berenguer, E.; Oliveras Menor, I.; Bauman, D.; Corral-Rivas, J.J.; Nava-Miranda, M.G.; Both, S.; Ndong, J.E.; Ondo, F.E.; Bengone, N.N.; et al. Functional Susceptibility of Tropical Forests to Climate Change. Nat. Ecol. Evol. 2022, 6, 878–889. [Google Scholar] [CrossRef]
- Enquist, B.J.; Enquist, C.A.F. Long-Term Change within a Neotropical Forest: Assessing Differential Functional and Floristic Responses to Disturbance and Drought. Glob. Chang. Biol. 2011, 17, 1408–1424. [Google Scholar] [CrossRef]
- Miles, L.; Newton, A.C.; DeFries, R.S.; Ravilious, C.; May, I.; Blyth, S.; Kapos, V.; Gordon, J.E. A Global Overview of the Conservation Status of Tropical Dry Forests. J. Biogeogr. 2006, 33, 491–505. [Google Scholar] [CrossRef]
- Best, B. Biodiversity and Conservation in Tumbesian Ecuador and Peru; BirdLife International: Cambridge, UK, 1995. [Google Scholar]
- Sierra, R. Propuesta Preliminar de un Sistema de Clasificación de Vegetación para el Ecuador Continental; Proyecto INEFAN/GEF y EcoCiencia: Quito, Ecuador, 1999. [Google Scholar]
- Espinosa, C.; Camarero, J.; Gusmán, A. Site-Dependent Growth Responses to Climate in Two Major Tree Species from Tropical Dry Forests of Southwest Ecuador. Dendrochronologia 2018, 52, 11–19. [Google Scholar] [CrossRef]
- Rojas-Sandoval, J. Vachellia Macracantha (Porknut). Available online: https://www.cabidigitallibrary.org/doi/10.1079/cabicompendium.2318 (accessed on 22 August 2024).
- WFO. Terminalia valverdeae A.H.Gentry. Available online: http://www.worldfloraonline.org/taxon/wfo-0001296450 (accessed on 22 August 2024).
- WFO. Erythrina velutina Willd. Available online: http://www.worldfloraonline.org/taxon/wfo-0000181214 (accessed on 22 August 2024).
- WFO. Coccoloba ruiziana Lindau. Available online: http://www.worldfloraonline.org/taxon/wfo-0000613370 (accessed on 22 August 2024).
- Jara-Guerrero, A.; De la Cruz, M.; Méndez, M. Seed Dispersal Spectrum of Woody Species in South Ecuadorian Dry Forests: Environmental Correlates and the Effect of Considering Species Abundance. Biotropica 2011, 43, 722–730. [Google Scholar] [CrossRef]
- WFO. Cynophalla flexuosa J.Presl. Available online: http://www.worldfloraonline.org/taxon/wfo-0000634340 (accessed on 22 August 2024).
- Therneau, T. A Package for Survival Analysis in R. 2020. Available online: https://cran.r-project.org/web/packages/survival/vignettes/survival.pdf (accessed on 17 July 2024).
- Harrington, D.P.; Fleming, T.R. A Class of Rank Test Procedures for Censored Survival Data. Biometrika 1982, 69, 553–566. [Google Scholar] [CrossRef]
- Lenth, R.V. Emmeans: Estimated Marginal Means, Aka Least-Squares Means. 2023. Available online: https://cran.r-project.org/web/packages/emmeans/emmeans.pdf (accessed on 20 July 2024).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.r-project.org/ (accessed on 5 December 2022).

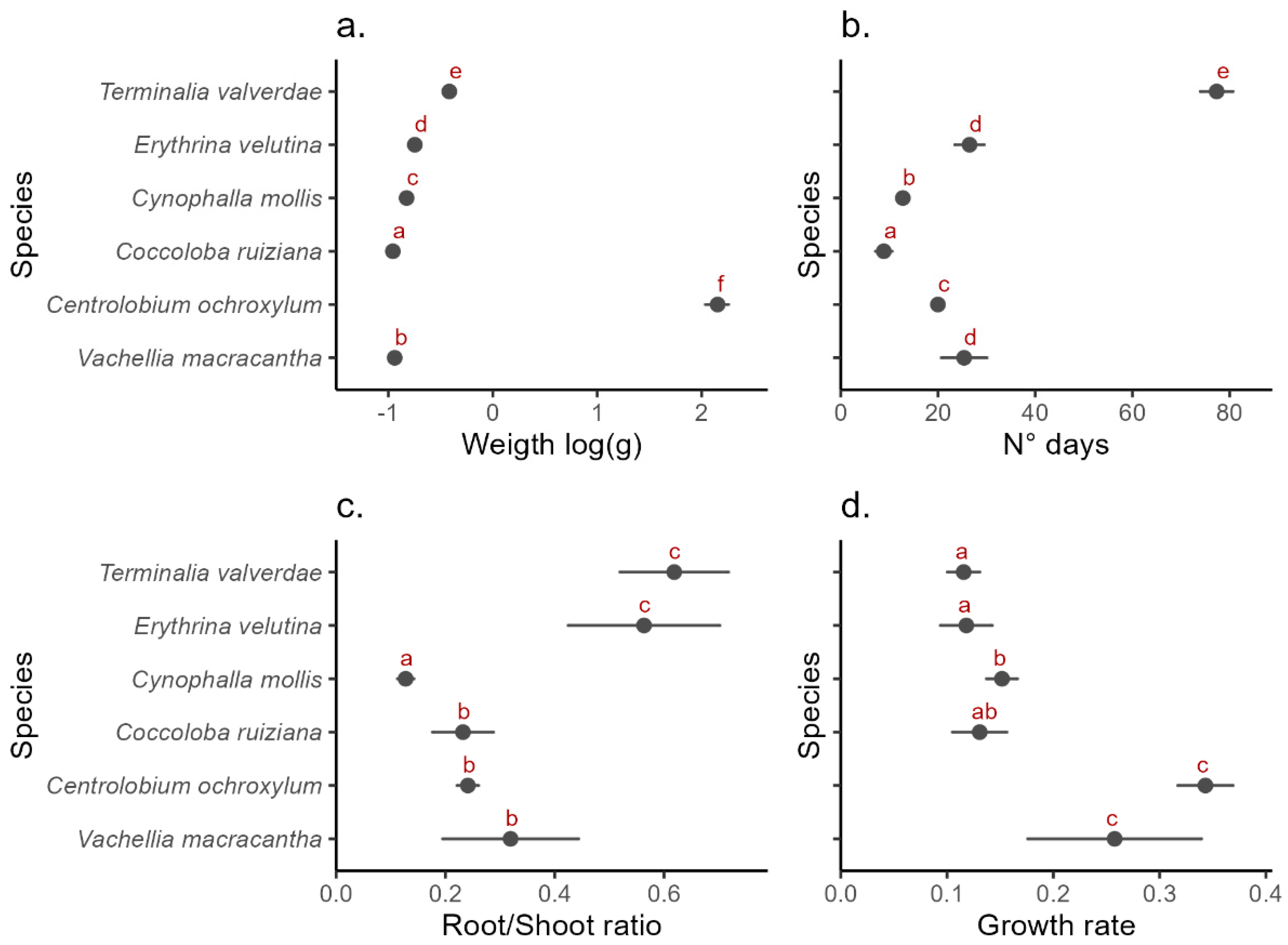
| Root/Shoot Ratio | Growth Rate | |||
|---|---|---|---|---|
| Germination Speed | 0.011 *** | (0.001) | −0.004 *** | (0.001) |
| Seed weight | 0.001 | (0.003) | 0.027 *** | (0.002) |
| N | 321 | 322 | ||
| AIC | −308.882 | −687.335 | ||
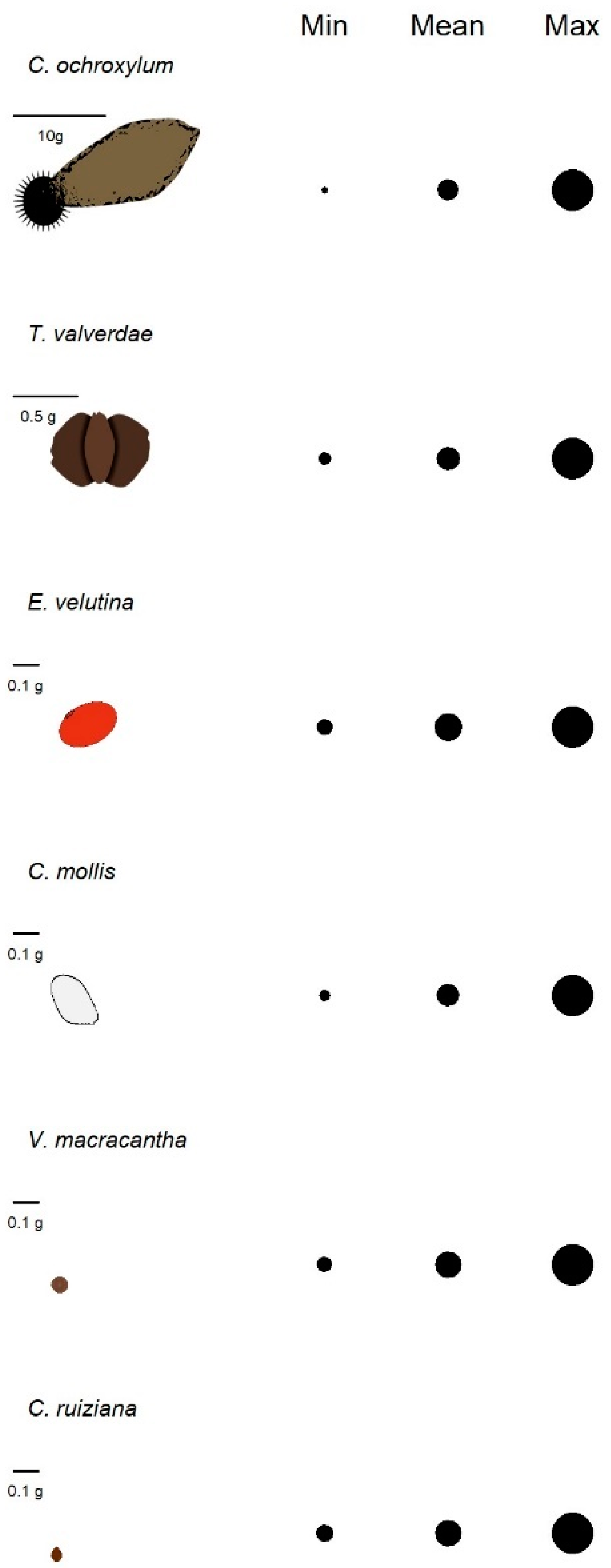
| Species | Min | Mean | Max |
|---|---|---|---|
| C. ruiziana | 5 | 9 | 16 |
| C. mollis | 10 | 13 | 31 |
| C. ochroxylum | 12 | 20 | 36 |
| A. macracantha | 7 | 25 | 50 |
| T. valverdeae | 42 | 77 | 119 |
| E. velutina | 4 | 26 | 151 |
| V. macracantha | C. ochroxylum | C. ruiziana | C. mollis | E. velutina | T. valverdae | |
|---|---|---|---|---|---|---|
| Germination speed | −0.005 ** | −0.002 | −0.004 | 0.000 | −0.004 *** | −0.006 ** |
| (0.001) | (0.002) | (0.006) | (0.001) | (0.001) | (0.002) | |
| Seed weight | −0.690 | 0.605 | −0.050 | 2.270 ** | −0.131 | |
| (1.347) | (4.796) | (0.099) | (0.643) | (0.206) | ||
| N | 8 | 139 | 21 | 87 | 19 | 47 |
| R2 | 0.855 | 0.007 | 0.033 | 0.003 | 0.721 | 0.177 |
| AIC | −25.640 | −239.347 | −40.490 | −267.666 | −16.904 | 20.605 |
| V. macracantha | C. ochroxylum | C. ruiziana | C. mollis | E. velutina | T. valverdae | |
|---|---|---|---|---|---|---|
| Germination speed | 0.001 | 0.006 ** | −0.002 | −0.002 | −0.000 * | 0.000 |
| (0.001) | (0.002) | (0.001) | (0.001) | (0.000) | (0.000) | |
| Seed weight | 1.260 | −0.276 | 0.339 *** | −0.493 * | 0.024 | |
| (1.779) | (1.023) | (0.089) | (0.175) | (0.031) | ||
| N | 8 | 140 | 21 | 87 | 19 | 47 |
| R2 | 0.182 | 0.069 | 0.129 | 0.185 | 0.428 | 0.041 |
| AIC | −21.190 | −198.096 | −105.383 | −286.968 | −66.277 | −158.195 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Espinosa, C.I.; Esparza, E.; Jara-Guerrero, A. Adaptive Seedling Strategies in Seasonally Dry Tropical Forests: A Comparative Study of Six Tree Species. Plants 2024, 13, 2900. https://doi.org/10.3390/plants13202900
Espinosa CI, Esparza E, Jara-Guerrero A. Adaptive Seedling Strategies in Seasonally Dry Tropical Forests: A Comparative Study of Six Tree Species. Plants. 2024; 13(20):2900. https://doi.org/10.3390/plants13202900
Chicago/Turabian StyleEspinosa, Carlos Ivan, Elvia Esparza, and Andrea Jara-Guerrero. 2024. "Adaptive Seedling Strategies in Seasonally Dry Tropical Forests: A Comparative Study of Six Tree Species" Plants 13, no. 20: 2900. https://doi.org/10.3390/plants13202900
APA StyleEspinosa, C. I., Esparza, E., & Jara-Guerrero, A. (2024). Adaptive Seedling Strategies in Seasonally Dry Tropical Forests: A Comparative Study of Six Tree Species. Plants, 13(20), 2900. https://doi.org/10.3390/plants13202900






