Exploring the Antidiabetic Potential of Salvia officinalis Using Network Pharmacology, Molecular Docking and ADME/Drug-Likeness Predictions
Abstract
1. Introduction
2. Results
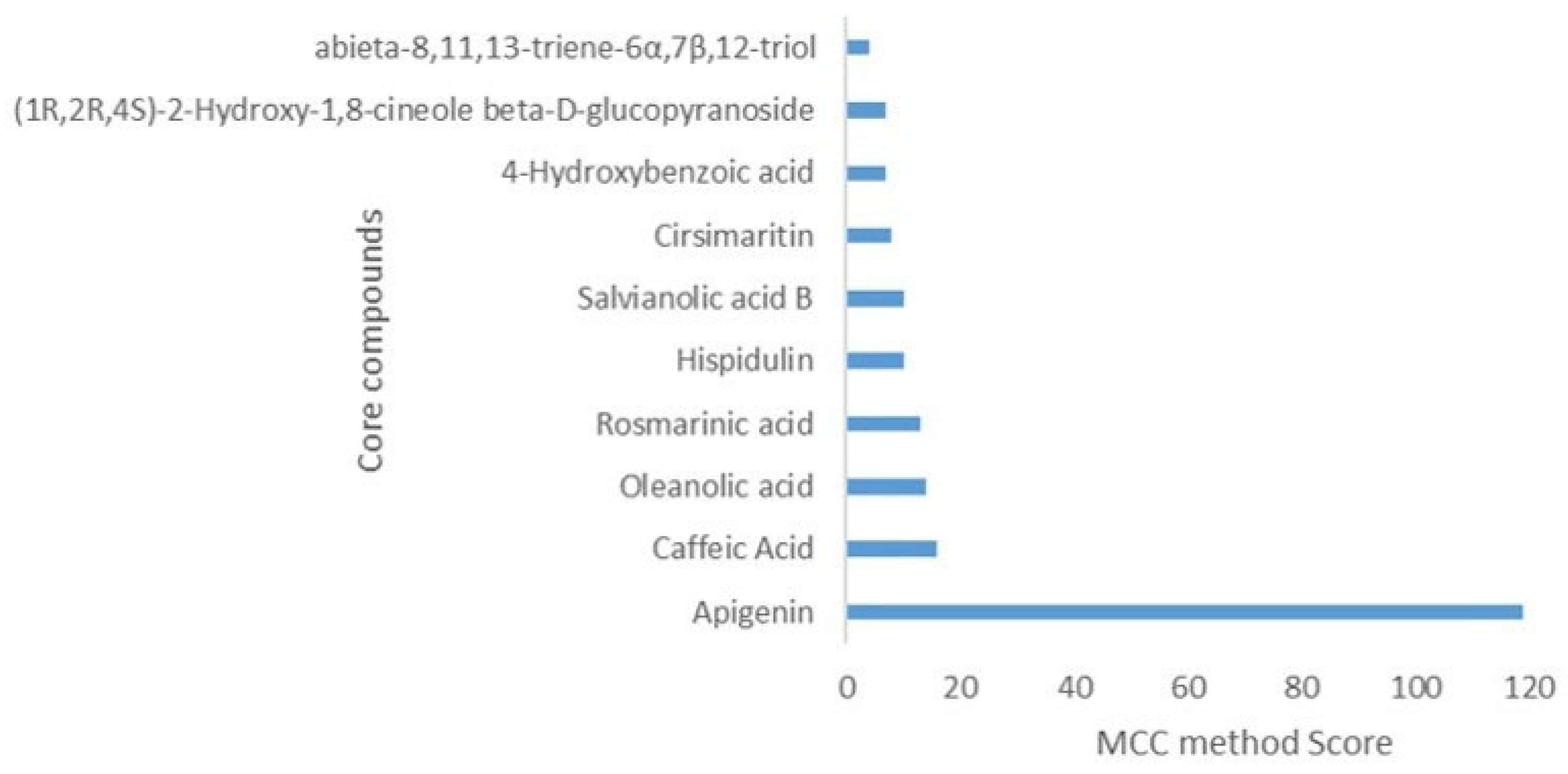
2.1. Compounds Mining and Ranking
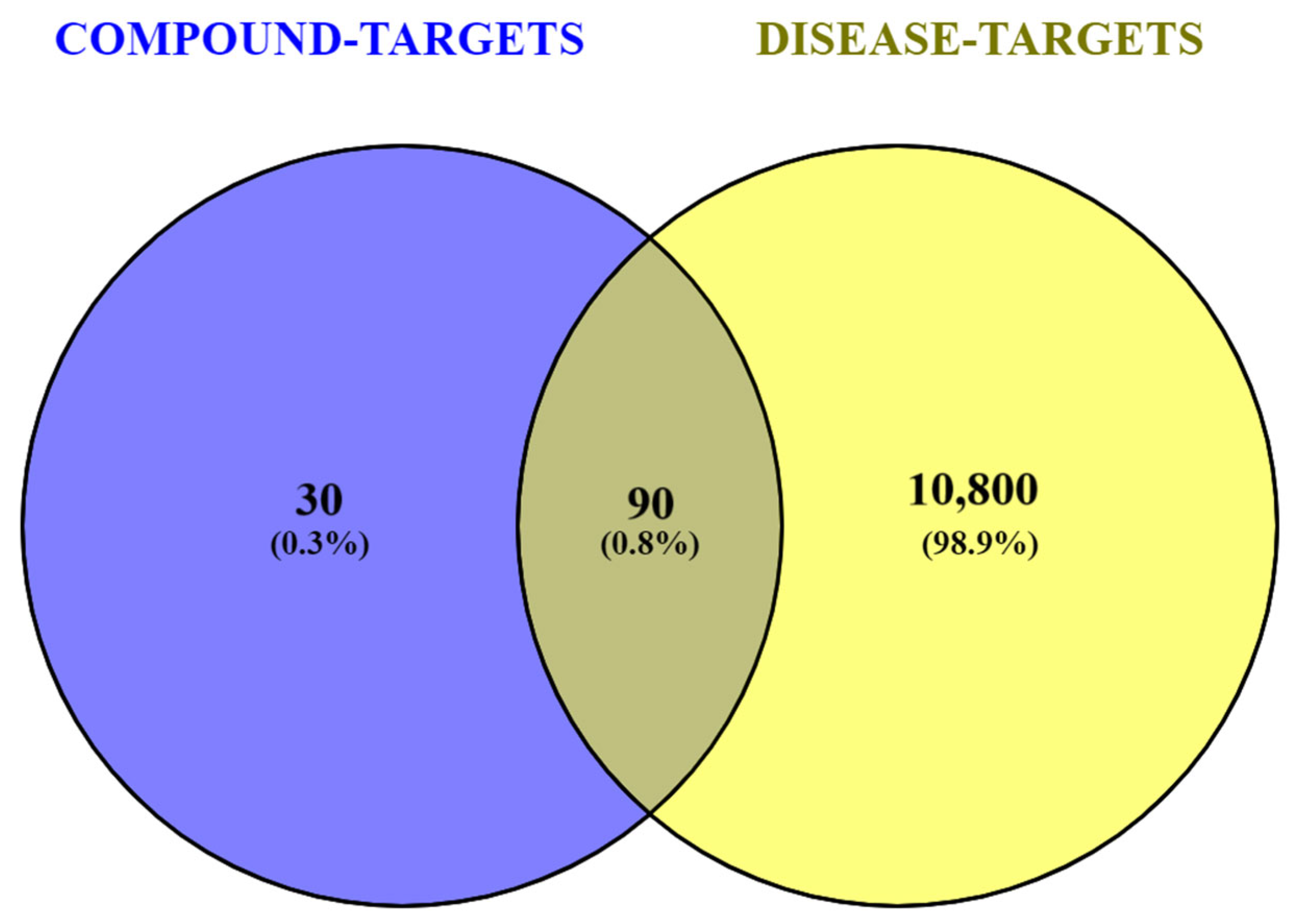
2.2. Compounds- and Disease-Associated Targets
2.3. Constructed Networks
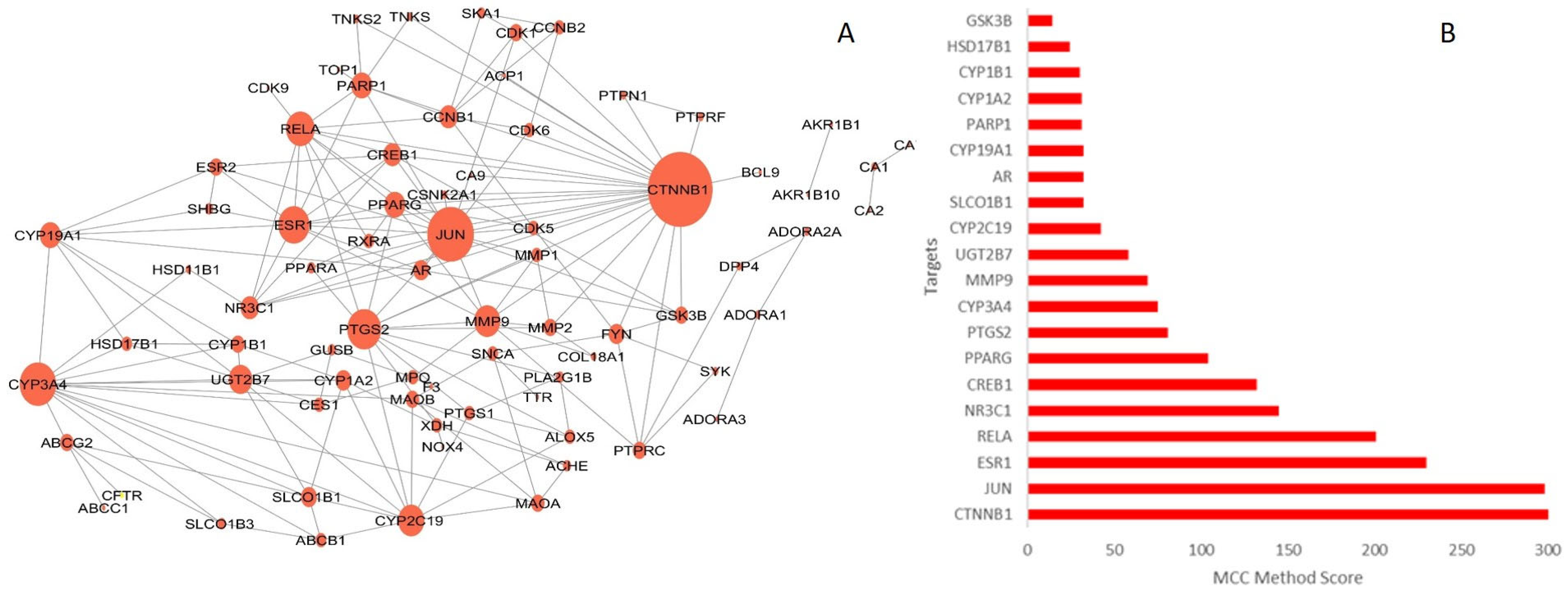
2.3.1. Protein–Protein Interaction (PPI) Network
2.3.2. Compound-Target (CT) Network
2.3.3. Target-Pathways (TP) Network
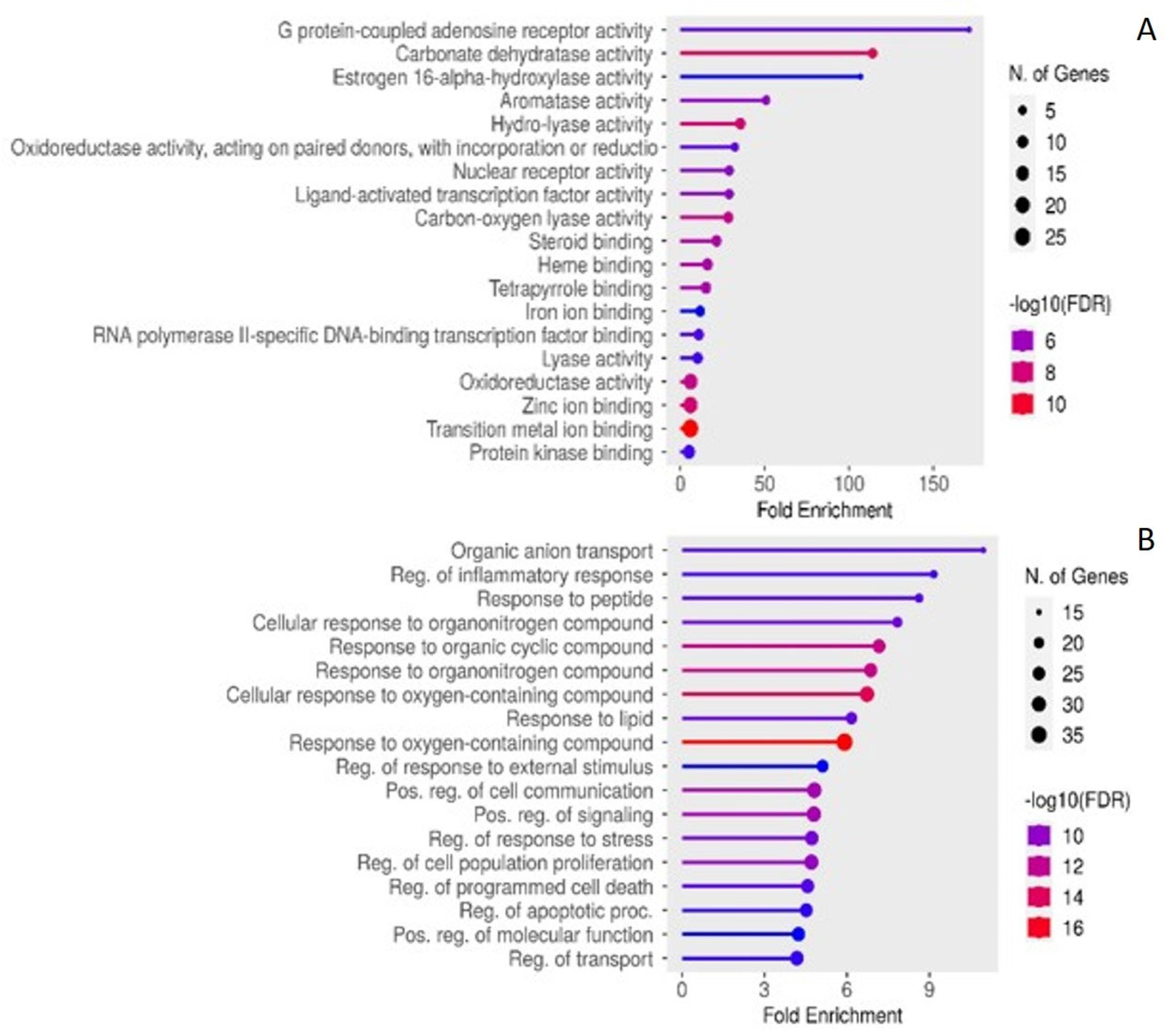
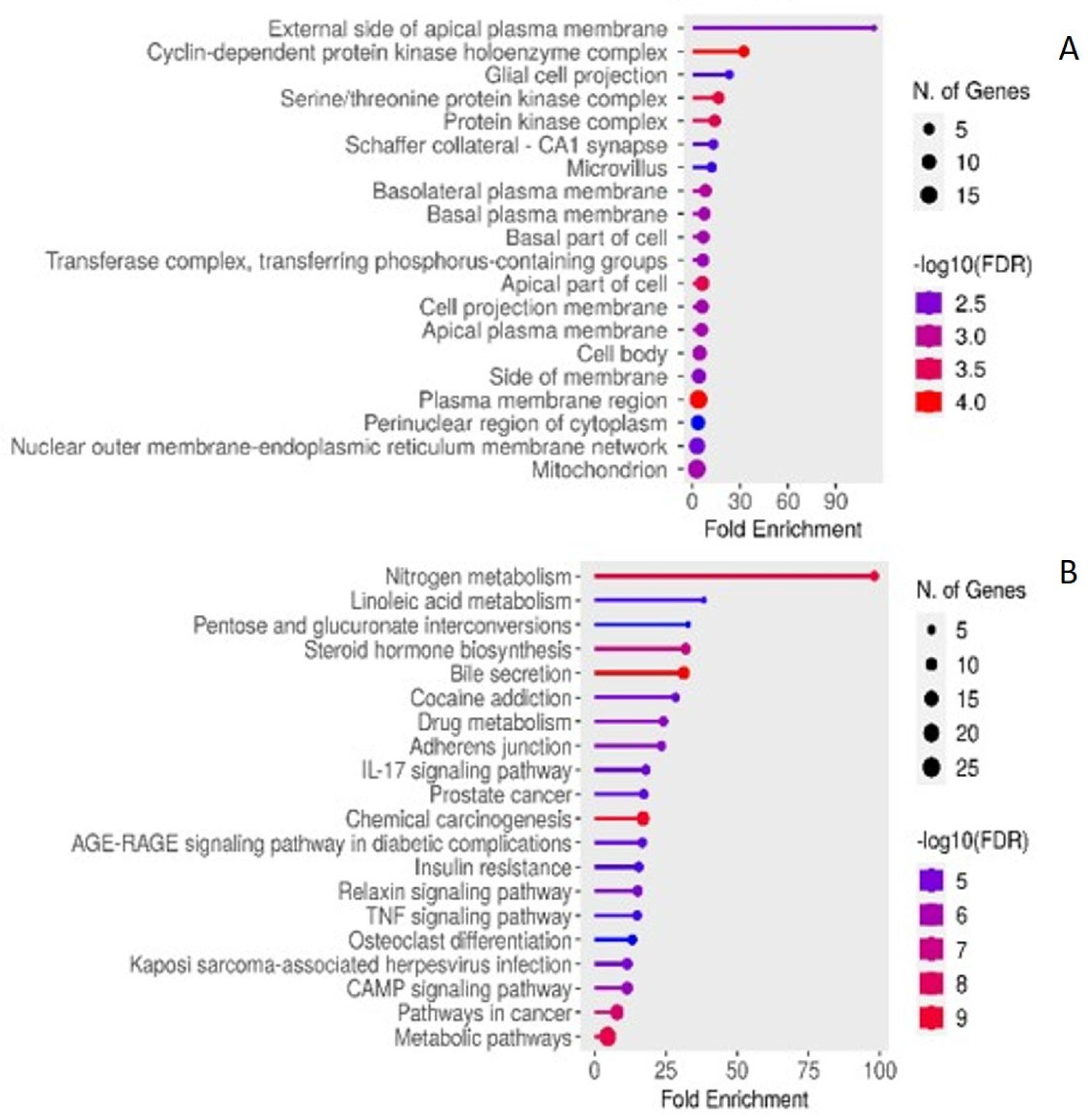
2.4. Enriched KEGG Pathwas and GO
2.5. Molecular Docking
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Compounds Mining
5.2. Ranking of Mined Compounds
5.3. Identification of Compounds- and Disease-Associated Targets
5.4. Network Construction and Analysis
5.4.1. Construction of Protein–Protein Interaction (PPI) Network
5.4.2. Construction of Compound-Target (CT) Network
5.4.3. Construction of Target-Pathways (TP) Network
5.5. GO and KEGG Pathway Enrichment Analysis
5.6. Molecular Docking: Preparation and Simulation
5.6.1. Protein Target Preparation
5.6.2. Binding/Docking Site Prediction
5.6.3. Validation of Docking
5.6.4. Docking Simulations
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Khan, M.A.B.; Hashim, M.J.; King, J.K.; Govender, R.D.; Mustafa, H.; Al Kaabi, J. Epidemiology of Type 2 Diabetes—Global Burden of Disease and Forecasted Trends. J. Epidemiol. Glob. Health 2020, 10, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Perreault, L.; Skyler, J.S.; Rosenstock, J. Novel therapies with precision mechanisms for type 2 diabetes mellitus. Nat. Rev. Endocrinol. 2021, 17, 364–377. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Kriti, M.; Anamika, K.S.; Sarma, D.K.; Verma, V.; Nagpal, R.; Mohania, D.; Tiwari, R.; Kumar, M. Deciphering the complex interplay of risk factors in type 2 diabetes mellitus: A comprehensive review. Metabol. Open 2024, 22, 100287. [Google Scholar] [CrossRef] [PubMed]
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef]
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.N.; Mbanya, J.C.; et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 2022, 183, 109119. [Google Scholar] [CrossRef]
- DeFronzo, R.A.; Ferrannini, E.; Groop, L.; Henry, R.R.; Herman, W.H.; Holst, J.J.; Hu, F.B.; Kahn, C.R.; Raz, I.; Shulman, G.I.; et al. Type 2 diabetes mellitus. Nat. Rev. Dis. Primers 2015, 1, 15019. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.; Liu, S.; Gao, M.; Wang, W.; Chen, K.; Huang, L.; Liu, Y. Diabetic vascular diseases: Molecular mechanisms and therapeutic strategies. Signal Transduct. Target. Ther. 2023, 8, 152. [Google Scholar] [CrossRef]
- Ansari, P.; Samia, J.F.; Khan, J.T.; Rafi, M.R.; Rahman, M.S.; Rahman, A.B.; Abdel-Wahab, Y.H.A.; Seidel, V. Protective Effects of Medicinal Plant-Based Foods Against Diabetes: A Review on Pharmacology, Phytochemistry, and Molecular Mechanisms. Nutrients 2023, 15, 3266. [Google Scholar] [CrossRef]
- Furman, B.L.; Candasamy, M.; Bhattamisra, S.K.; Veettil, S.K. Reduction of blood glucose by plant extracts and their use in the treatment of diabetes mellitus; discrepancies in effectiveness between animal and human studies. J. Ethnopharmacol. 2020, 247, 112264. [Google Scholar] [CrossRef]
- Alam, S.; Dhar, A.; Hasan, M.; Richi, F.T.; Emon, N.U.; Aziz, M.A.; Mamun, A.A.; Chowdhury, M.N.R.; Hossain, M.J.; Kim, J.K.; et al. Antidiabetic Potential of Commonly Available Fruit Plants in Bangladesh: Updates on Prospective Phytochemicals and Their Reported MoAs. Molecules 2022, 27, 8709. [Google Scholar] [CrossRef]
- Ansari, P.; Akther, S.; Hannan, J.M.A.; Seidel, V.; Nujat, N.J.; Abdel-Wahab, Y.H.A. Pharmacologically Active Phytomolecules Isolated from Traditional Antidiabetic Plants and Their Therapeutic Role for the Management of Diabetes Mellitus. Molecules 2022, 27, 4278. [Google Scholar] [CrossRef] [PubMed]
- Ansari, P.; Choudhury, S.T.; Seidel, V.; Rahman, A.B.; Aziz, M.A.; Richi, A.E.; Rahman, A.; Jafrin, U.H.; Hannan, J.M.A.; Abdel-Wahab, Y.H.A. Therapeutic Potential of Quercetin in the Management of Type-2 Diabetes Mellitus. Life 2022, 12, 1146. [Google Scholar] [CrossRef] [PubMed]
- Asante, D.B.; Wiafe, G.A. Therapeutic Benefit of Vernonia amygdalina in the Treatment of Diabetes and Its Associated Complications in Preclinical Studies. J. Diabetes Res. 2023, 2023, 3159352. [Google Scholar] [CrossRef]
- Chhabria, S.; Mathur, S.; Vadakan, S.; Sahoo, D.K.; Mishra, P.; Paital, B. A Review on Phytochemical and Pharmacological Facets of Tropical Ethnomedicinal Plants as Reformed DPP-IV Inhibitors to Regulate Incretin Activity. Front. Endocrinol. 2022, 13, 1027237. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Ghosh, A.; Majie, A.; Karmakar, V.; Das, S.; Dinda, S.C.; Bose, A.; Gorain, B. Terpenoids as potential phytoconstituent in the treatment of diabetes: From preclinical to clinical advancement. Phytomedicine 2024, 129, 155638. [Google Scholar] [CrossRef] [PubMed]
- Raal, A.; Orav, A.; Arak, E. Composition of the essential oil of Salvia officinalis L. from various European countries. Nat. Prod. Res. 2007, 21, 406–411. [Google Scholar] [CrossRef] [PubMed]
- Delamare, A.P.L.; Moschen-Pistorello, I.T.; Artico, L.; Atti-Serafini, L.; Echeverrigaray, S. Antibacterial activity of the essential oils of Salvia officinalis L. and Salvia triloba L. cultivated in South Brazil. Food Chem. 2007, 100, 603–608. [Google Scholar] [CrossRef]
- Ghorbani, A.; Esmaeilizadeh, M. Pharmacological properties of Salvia officinalis and its components. J. Tradit. Complement. Med. 2017, 7, 433–440. [Google Scholar] [CrossRef]
- Horváthová, E.; Srančíková, A.; Regendová-Sedláčková, E.; Melušová, M.; Meluš, V.; Netriová, J.; Krajčovičová, Z.; Slameňová, D.; Pastorek, M.; Kozics, K. Enriching the drinking water of rats with extracts of Salvia officinalis and Thymus vulgaris increases their resistance to oxidative stress. Mutagenesis 2016, 31, 51–59. [Google Scholar] [CrossRef]
- Mansourabadi, A.H.; Sadeghi, H.M.; Razavi, N.; Rezvani, E. Anti-inflammatory and analgesic properties of salvigenin, Salvia officinalis flavonoid extracted. Adv. Herb. Med. 2016, 2, 31–41. [Google Scholar]
- Bozin, B.; Mimica-Dukic, N.; Samojlik, I.; Jovin, E. Antimicrobial and Antioxidant Properties of Rosemary and Sage (Rosmarinus officinalis L. and Salvia officinalis L., Lamiaceae) Essential Oils. J. Agric. Food Chem. 2007, 55, 7879–7885. [Google Scholar] [CrossRef] [PubMed]
- Khedher, M.R.; Hammami, M.; Arch, J.R.S.; Hislop, D.C.; Eze, D.; Wargent, E.T.; Kępczyńska, M.A.; Zaibi, M.S. Preventive Effects of Salvia officinalis Leaf Extract on Insulin Resistance and Inflammation in a Model of High Fat Diet-Induced Obesity in Mice that Responds to Rosiglitazone. PeerJ 2018, 6, e4166. [Google Scholar] [CrossRef] [PubMed]
- Eidi, M.; Eidi, A.; Zamanizadeh, H. Effect of Salvia officinalis L. leaves on serum glucose and insulin in healthy and streptozotocin-induced diabetic rats. J. Ethnopharmacol. 2005, 100, 310–313. [Google Scholar] [CrossRef] [PubMed]
- Behradmanesh, S.; Derees, F.; Rafieian-Kopaei, M. Effect of Salvia officinalis on Diabetic Patients. J. Renal Inj. Prev. 2013, 2, 51–54. [Google Scholar] [CrossRef]
- Kianbakht, S.; Dabaghian, F.H. Improved glycemic control and lipid profile in hyperlipidemic type 2 diabetic patients consuming Salvia officinalis L. leaf extract: A randomized placebo. Controlled clinical trial. Complement. Ther. Med. 2013, 21, 441–446. [Google Scholar] [CrossRef]
- Hopkins, A.L. Network pharmacology. Nat. Biotechnol. 2007, 25, 1110–1111. [Google Scholar] [CrossRef]
- Zhou, Z.; Chen, B.; Chen, S.; Lin, M.; Chen, Y.; Jin, S.; Chen, W.; Zhang, Y. Applications of Network Pharmacology in Traditional Chinese Medicine Research. Evid.-Based Complement. Alternat. Med. 2020, 2020, 1646905. [Google Scholar] [CrossRef]
- Li, S.; Fan, T.P.; Jia, W.; Lu, A.; Zhang, W. Network pharmacology in traditional Chinese medicine. Evid.-Based Complement. Altern. Med. 2014, 2014, 138460. [Google Scholar] [CrossRef]
- Li, W.; Yuan, G.; Pan, Y.; Wang, C.; Chen, H. Network pharmacology studies on the bioactive compounds and action mechanisms of natural products for the treatment of diabetes mellitus: A review. Front. Pharmacol. 2017, 8, 74. [Google Scholar] [CrossRef]
- An, W.; Huang, Y.; Chen, S.; Teng, T.; Shi, Y.; Sun, Z.; Xu, Y. Mechanisms of Rhizoma Coptidis Against Type 2 Diabetes Mellitus Explored by Network Pharmacology Combined with Molecular Docking and Experimental Validation. Sci. Rep. 2021, 11, 20849. [Google Scholar] [CrossRef]
- Ge, Q.; Chen, L.; Tang, M.; Zhang, S.; Liu, L.; Gao, L.; Ma, S.; Kong, M.; Yao, Q.; Feng, F.; et al. Analysis of mulberry leaf components in the treatment of diabetes using network pharmacology. Eur. J. Pharmacol. 2018, 833, 50–62. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Li, Z.; Wang, Y.; Zhang, B.; Liu, G.; Liu, J. Network pharmacology and metabolomics study on the intervention of traditional Chinese medicine Huanglian Decoction in rats with type 2 diabetes mellitus. J. Ethnopharmacol. 2020, 258, 112842. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Zhu, F.; Shen, M.; Qiu, L.; Tang, M.; Xia, H.; Chen, L.; Yuan, Y.; Ma, S.; Chen, K. Network pharmacology-based analysis on bioactive anti-diabetic compounds in Potentilla discolor Bunge. J. Ethnopharmacol. 2019, 241, 111905. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Niu, L.; Liu, Y.; Pang, M.; Lu, W.; Xia, C.; Wang, Q. Study on the Mechanism of Gegen Qinlian Decoction for Treating Type II Diabetes Mellitus by Integrating Network Pharmacology and Pharmacological Evaluation. J. Ethnopharmacol. 2020, 262, 113129. [Google Scholar] [CrossRef]
- Zhu, C.; Cai, T.; Jin, Y.; Chen, J.; Liu, G.; Xu, N.; Shen, R.; Chen, Y.; Han, L.; Wang, S.; et al. Artificial intelligence and network pharmacology based investigation of pharmacological mechanism and substance basis of Xiaokewan in treating diabetes. Pharmacol. Res. 2020, 159, 104935. [Google Scholar] [CrossRef]
- Fang, J.; Wang, L.; Wu, T.; Yang, C.; Gao, L.; Cai, H.; Liu, J.; Fang, S.; Chen, Y.; Tan, W.; et al. Network pharmacology-based study on the mechanism of action for herbal medicines in Alzheimer treatment. J. Ethnopharmacol. 2017, 196, 281–292. [Google Scholar] [CrossRef]
- Nazir, S.S.; Goel, D.; Vohora, D. A network pharmacology based approach to decipher the pharmacological mechanisms of Salvia officinalis in neurodegenerative disorders. Res. Sq. 2023. [Google Scholar] [CrossRef]
- Malik, A.; Morya, R.K.; Bhadada, S.K.; Rana, S. Type 1 diabetes mellitus: Complex interplay of oxidative stress, cytokines, gastrointestinal motility and small intestinal bacterial overgrowth. Eur. J. Clin. Investig. 2018, 48, e13021. [Google Scholar] [CrossRef]
- Santiago, J.A.; Potashkin, J.A. Shared dysregulated pathways lead to Parkinson’s disease and diabetes. Trends Mol. Med. 2013, 19, 176–186. [Google Scholar] [CrossRef]
- Sanches, J.M.; Zhao, L.N.; Salehi, A.; Wollheim, C.B.; Kaldis, P. Pathophysiology of type 2 diabetes and the impact of altered metabolic interorgan crosstalk. FEBS J. 2023, 290, 620–648. [Google Scholar] [CrossRef]
- Ononamadu, C.J.; Alhassan, A.J.; Ibrahim, A.; Imam, A.A.; Ihegboro, G.O.; Owolarafe, T.A.; Sule, M.S. Methanol-Extract/Fractions of Dacryodes edulis Leaves Ameliorate Hyperglycemia and Associated Oxidative Stress in Streptozotocin-Induced Diabetic Wistar Rats. J. Evid. Based Integr. Med. 2019, 24, 2515690X19843832. [Google Scholar] [CrossRef] [PubMed]
- Zięba, A.; Stępnicki, P.; Matosiuk, D.; Kaczor, A.A. What are the challenges with multi-targeted drug design for complex diseases? Expert Opin. Drug Discov. 2022, 17, 673–683. [Google Scholar] [CrossRef] [PubMed]
- Cabri, W.; Cantelmi, P.; Corbisiero, D.; Fantoni, T.; Ferrazzano, L.; Martelli, G.; Mattellone, A.; Tolomelli, A. Therapeutic Peptides Targeting PPI in Clinical Development: Overview, Mechanism of Action and Perspectives. Front. Mol. Biosci. 2021, 8, 697586. [Google Scholar] [CrossRef] [PubMed]
- Rout, T.; Mohapatra, A.; Kar, M.; Patra, S.; Muduly, D. Centrality Measures and Their Applications in Network Analysis: Unveiling Important Elements and Their Impact. Procedia Comput. Sci. 2024, 235, 2756–2765. [Google Scholar] [CrossRef]
- Chaudhury, A.; Duvoor, C.; Reddy Dendi, V.S.; Kraleti, S.; Chada, A.; Ravilla, R.; Marco, A.; Shekhawat, N.S.; Montales, M.T.; Kuriakose, K.; et al. Clinical Review of Antidiabetic Drugs: Implications for Type 2 Diabetes Mellitus Management. Front. Endocrinol. 2017, 8, 6. [Google Scholar] [CrossRef] [PubMed]
- Nakao, S.; Ogtata, Y.; Shimizu, E.; Yamazaki, M.; Furuyama, S.; Sugiya, H. Tumor necrosis factor alpha (TNF-alpha)-induced prostaglandin E2 release is mediated by the activation of cyclooxygenase-2 (COX-2) transcription via NFkappaB in human gingival fibroblasts. Mol. Cell. Biochem. 2002, 238, 11–18. [Google Scholar] [CrossRef]
- Ke, B.; Zhao, Z.; Ye, X.; Gao, Z.; Manganiello, V.; Wu, B.; Ye, J. Inactivation of NF-κB p65 (RelA) in Liver Improves Insulin Sensitivity and Inhibits cAMP/PKA Pathway. Diabetes 2015, 64, 3355–3362. [Google Scholar] [CrossRef]
- Wang, X.; Tao, Y.; Huang, Y.; Zhan, K.; Xue, M.; Wang, Y.; Ruan, D.; Liang, Y.; Huang, X.; Lin, J.; et al. Catalase Ameliorates Diabetes-Induced Cardiac Injury through Reduced p65/RelA-Mediated Transcription of BECN1. J. Cell. Mol. Med. 2017, 21, 3420–3434. [Google Scholar] [CrossRef]
- Zammit, N.W.; Wong, Y.Y.; Walters, S.N.; Warren, J.; Barry, S.C.; Grey, S.T. RELA Governs a Network of Islet-Specific Metabolic Genes Necessary for Beta Cell Function. Diabetologia 2023, 66, 1516–1531. [Google Scholar] [CrossRef]
- Yan, H.; He, L.; Lv, D.; Yang, J.; Yuan, Z. The Role of the Dysregulated JNK Signaling Pathway in the Pathogenesis of Human Diseases and Its Potential Therapeutic Strategies: A Comprehensive Review. Biomolecules 2024, 14, 243. [Google Scholar] [CrossRef]
- Yung, J.H.M.; Giacca, A. Role of c-Jun N-Terminal Kinase (JNK) in Obesity and Type 2 Diabetes. Cells 2020, 9, 706. [Google Scholar] [CrossRef] [PubMed]
- Ijaz, A.; Tejada, T.; Catanuto, P.; Xia, X.; Elliot, S.J.; Lenz, O.; Jauregui, A.; Saenz, M.O.; Molano, R.D.; Pileggi, A.; et al. Inhibition of C-jun N-terminal kinase improves insulin sensitivity but worsens albuminuria in experimental diabetes. Kidney Int. 2009, 75, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Martín-Vázquez, E.; Cobo-Vuilleumier, N.; López-Noriega, L.; Lorenzo, P.I.; Gauthier, B.R. The PTGS2/COX2-PGE2 signaling cascade in inflammation: Pro or anti? A case study with type 1 diabetes mellitus. Int. J. Biol. Sci. 2023, 19, 4157–4165. [Google Scholar] [CrossRef] [PubMed]
- Helmersson, J.; Vessby, B.; Larsson, A.; Basu, S. Association of type 2 diabetes with cyclooxygenase-mediated inflammation and oxidative stress in an elderly population. Circulation 2004, 109, 1729–1734. [Google Scholar] [CrossRef]
- Shanmugam, N.; Todorov, I.T.; Nair, I.; Omori, K.; Reddy, M.A.; Natarajan, R. Increased expression of cyclooxygenase-2 in human pancreatic islets treated with high glucose or ligands of the advanced glycation endproduct-specific receptor (AGER), and in islets from diabetic mice. Diabetologia 2006, 49, 100–107. [Google Scholar] [CrossRef][Green Version]
- Tan, G.S.Q.; Morton, J.I.; Wood, S.; Trevaskis, N.L.; Magliano, D.J.; Windsor, J.; Shaw, J.E.; Ilomäki, J. COX2 inhibitor use and type 2 diabetes treatment intensification: A registry-based cohort study. Diabetes Res. Clin. Pract. 2024, 207, 111082. [Google Scholar] [CrossRef]
- Bag, S.; Das, S.; Bagchi, C.; Tripathi, S.K. Aspirin Potentiates Blood Glucose Lowering Effect of Glimepiride-Pioglitazone Combination in Streptozotocin-Induced Diabetic Rats. Indian J. Pharmacol. 2014, 46, 562–564. [Google Scholar] [CrossRef]
- Herzig, S.; Long, F.; Jhala, U.S.; Hedrick, S.; Quinn, R.; Bauer, A.; Rudolph, D.; Schutz, G.; Yoon, C.; Puigserver, P.; et al. CREB regulates hepatic gluconeogenesis through the coactivator PGC-1. Nature 2001, 413, 179–183. [Google Scholar] [CrossRef]
- Xu, Y.; Song, R.; Long, W.; Guo, H.; Shi, W.; Yuan, S.; Xu, G.; Zhang, T. CREB1 Functional Polymorphisms Modulating Promoter Transcriptional Activity Are Associated with Type 2 Diabetes Mellitus Risk in Chinese Population. Gene 2018, 665, 133–140. [Google Scholar] [CrossRef]
- Erion, D.M.; Ignatova, I.D.; Yonemitsu, S.; Nagai, Y.; Chatterjee, P.; Weismann, D.; Hsiao, J.J.; Zhang, D.; Iwasaki, T.; Stark, R.; et al. Prevention of hepatic steatosis and hepatic insulin resistance by knockdown of cAMP response element-binding protein. Cell Metab. 2009, 10, 499–506. [Google Scholar] [CrossRef]
- Teli, D.M.; Gajjar, A.K. Glycogen synthase kinase-3: A potential target for diabetes. Bioorg. Med. Chem. 2023, 92, 117406. [Google Scholar] [CrossRef] [PubMed]
- Maqbool, M.; Hoda, N. GSK3 inhibitors in the therapeutic development of diabetes, cancer and neurodegeneration: Past, present and future. Curr. Pharm. Des. 2017, 23, 4332–4350. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, J.; Di, L.J. Glycogen Synthesis and Beyond: A Comprehensive Review of GSK3 as a Key Regulator of Metabolic Pathways and a Therapeutic Target for Treating Metabolic Diseases. Med. Res. Rev. 2022, 42, 946–982. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Huang, N.Q.; Yan, F.; Sun, X.; Zheng, Z.; Yang, X.; Fang, Y.; Jiang, Y. Diabetes Mellitus and Alzheimer’s Disease: GSK-3β as a Potential Link. Behav. Brain Res. 2018, 339, 57–65. [Google Scholar] [CrossRef]
- Chehimi, M.; Vidal, H.; Eljaafari, A. Pathogenic Role of IL-17-Producing Immune Cells in Obesity, and Related Inflammatory Diseases. J. Clin. Med. 2017, 6, 68. [Google Scholar] [CrossRef]
- Lebovitz, H.E.; Banerji, M.A. Treatment of insulin resistance in diabetes mellitus. Eur. J. Pharmacol. 2004, 490, 135–146. [Google Scholar] [CrossRef]
- Teijeiro, A.; Garrido, A.; Ferre, A.; Perna, C.; Djouder, N. Inhibition of the IL-17A axis in adipocytes suppresses diet-induced obesity and metabolic disorders in mice. Nat. Metab. 2021, 3, 496–512. [Google Scholar] [CrossRef]
- Qiu, A.W.; Cao, X.; Zhang, W.W.; Liu, Q.H. IL-17A is involved in diabetic inflammatory pathogenesis by its receptor IL-17RA. Exp. Biol. Med. 2021, 246, 57–65. [Google Scholar] [CrossRef]
- Abdel-Moneim, A.; Bakery, H.H.; Allam, G. The Potential Pathogenic Role of IL-17/Th17 Cells in Both Type 1 and Type 2 Diabetes Mellitus. Biomed. Pharmacother. 2018, 101, 287–292. [Google Scholar] [CrossRef]
- Shaikh, S.B.; Prabhakar Bhandary, Y. Effect of curcumin on IL-17A mediated pulmonary AMPK kinase/cyclooxygenase-2 expressions via activation of NFκB in bleomycin-induced acute lung injury in vivo. Int. Immunopharmacol. 2020, 85, 106676. [Google Scholar] [CrossRef]
- Chu, W.M. Tumor Necrosis Factor. Cancer Lett. 2013, 328, 222–225. [Google Scholar] [CrossRef] [PubMed]
- de Alvaro, C.; Teruel, T.; Hernandez, R.; Lorenzo, M. Tumor necrosis factor alpha produces insulin resistance in skeletal muscle by activation of inhibitor kappaB kinase in a p38 MAPK-dependent manner. J. Biol. Chem. 2004, 279, 17070–17078. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Yin, B.; Zhang, H.; Zhang, S.; Zeng, Q.; Wang, J.; Jiang, X.; Yuan, L.; Wang, C.Y.; Li, Z. Blockade of tumor necrosis factor (TNF) receptor type 1-mediated TNF-alpha signaling protected Wistar rats from diet-induced obesity and insulin resistance. Endocrinology 2008, 149, 2943–2951. [Google Scholar] [CrossRef] [PubMed]
- da Costa, R.M.; Neves, K.B.; Mestriner, F.L.; Louzada-Junior, P.; Bruder-Nascimento, T.; Tostes, R.C. TNF-α Induces Vascular Insulin Resistance via Positive Modulation of PTEN and Decreased Akt/eNOS/NO Signaling in High Fat Diet-Fed Mice. Cardiovasc. Diabetol. 2016, 15, 119. [Google Scholar] [CrossRef] [PubMed]
- Hartsock, A.; Nelson, W.J. Adherens and tight junctions: Structure, function and connections to the actin cytoskeleton. Biochim. Biophys. Acta 2008, 1778, 660–669. [Google Scholar] [CrossRef]
- Haidari, M.; Zhang, W.; Willerson, J.T.; Dixon, R.A. Disruption of Endothelial Adherens Junctions by High Glucose Is Mediated by Protein Kinase C-β-Dependent Vascular Endothelial Cadherin Tyrosine Phosphorylation. Cardiovasc. Diabetol. 2014, 13, 105. [Google Scholar] [CrossRef]
- Collares-Buzato, C.B.; Carvalho, C.P. Is Type 2 Diabetes Mellitus Another Intercellular Junction-Related Disorder? Exp. Biol. Med. 2022, 247, 743–755. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Q.; Kang, S.G.; Huang, K.; Tong, T. Dietary Bioactive Ingredients Modulating the cAMP Signaling in Diabetes Treatment. Nutrients 2021, 13, 3038. [Google Scholar] [CrossRef]
- Vollert, S.; Kaessner, N.; Heuser, A.; Hanauer, G.; Dieckmann, A.; Knaack, D.; Kley, H.P.; Beume, R.; Weiss-Haljiti, C. The glucose-lowering effects of the PDE4 inhibitors roflumilast and roflumilast-N-oxide in db/db mice. Diabetologia 2012, 55, 2779–2788. [Google Scholar] [CrossRef]
- Yang, H.; Yang, L. Targeting cAMP/PKA Pathway for Glycemic Control and Type 2 Diabetes Therapy. J. Mol. Endocrinol. 2016, 57, R93–R108. [Google Scholar] [CrossRef]
- Dalle, S.; Burcelin, R.; Gourdy, P. Specific actions of GLP-1 receptor agonists and DPP4 inhibitors for the treatment of pancreatic β-cell impairments in type 2 diabetes. Cell Signal. 2013, 25, 570–579. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.A.; Chu, Q.; Xie, J.; Foretz, M.; Viollet, B.; Birnbaum, M.J. Biguanides suppress hepatic glucagon signalling by decreasing production of cyclic AMP. Nature 2013, 494, 256–260. [Google Scholar] [CrossRef] [PubMed]
- Ookawara, M.; Nio, Y. Phosphodiesterase 4 inhibitors in diabetic nephropathy. Cell. Signal. 2022, 90, 110185. [Google Scholar] [CrossRef] [PubMed]
- Dehghan, F.; Haerian, B.S.; Muniandy, S.; Yusof, A.; Dragoo, J.L.; Salleh, N. The effect of relaxin on the musculoskeletal system. Scand. J. Med. Sci. Sports 2014, 24, e220–e229. [Google Scholar] [CrossRef] [PubMed]
- Bonner, J.S.; Lantier, L.; Hocking, K.M.; Kang, L.; Owolabi, M.; James, F.D.; Bracy, D.P.; Brophy, C.M.; Wasserman, D.H. Relaxin Treatment Reverses Insulin Resistance in Mice Fed a High-Fat Diet. Diabetes 2013, 62, 3251–3260. [Google Scholar] [CrossRef] [PubMed]
- Piperi, C.; Goumenos, A.; Adamopoulos, C.; Papavassiliou, A.G. AGE/RAGE signalling regulation by miRNAs: Associations with diabetic complications and therapeutic potential. Int. J. Biochem. Cell Biol. 2015, 60, 197–201. [Google Scholar] [CrossRef]
- Taguchi, K.; Fukami, K. RAGE signaling regulates the progression of diabetic complications. Front. Pharmacol. 2023, 14, 1128872. [Google Scholar] [CrossRef]
- Giovannucci, E.; Harlan, D.M.; Archer, M.C.; Bergenstal, R.M.; Gapstur, S.M.; Habel, L.A.; Pollak, M.; Regensteiner, J.G.; Yee, D. Diabetes and cancer: A consensus report. Diabetes Care 2010, 33, 1674–1685. [Google Scholar] [CrossRef]
- Harding, J.L.; Shaw, J.E.; Peeters, A.; Cartensen, B.; Magliano, D.J. Cancer risk among people with type 1 and type 2 diabetes: Disentangling true associations, detection bias, and reverse causation. Diabetes Care 2015, 38, 264–270. [Google Scholar] [CrossRef]
- Huo, Q.; Wang, J.; Zhang, N.; Xie, L.; Yu, H.; Li, T. Editorial: The relationship between diabetes and cancers and its underlying mechanisms. Front. Endocrinol. 2022, 13, 992569. [Google Scholar] [CrossRef]
- Zhu, B.; Qu, S. The Relationship Between Diabetes Mellitus and Cancers and Its Underlying Mechanisms. Front. Endocrinol. 2022, 13, 800995. [Google Scholar] [CrossRef] [PubMed]
- Rahman, I.; Athar, M.T.; Islam, M. Type 2 Diabetes, Obesity, and Cancer Share Some Common and Critical Pathways. Front. Oncol. 2021, 10, 600824. [Google Scholar] [CrossRef] [PubMed]
- Jung, U.J.; Cho, Y.Y.; Choi, M.S. Apigenin Ameliorates Dyslipidemia, Hepatic Steatosis and Insulin Resistance by Modulating Metabolic and Transcriptional Profiles in the Liver of High-Fat Diet-Induced Obese Mice. Nutrients 2016, 8, 305. [Google Scholar] [CrossRef] [PubMed]
- Kalivarathan, J.; Kalaivanan, K.; Chandrasekaran, S.P.; Nanda, D.; Ramachandran, V.; Venkatraman, A.C. Apigenin modulates hippocampal CREB-BDNF signaling in high fat, high fructose diet-fed rats. J. Funct. Foods 2020, 68, 103898. [Google Scholar] [CrossRef]
- Feng, X.; Weng, D.; Zhou, F.; Owen, Y.D.; Qin, H.; Zhao, J.; Huang, Y.; Chen, J.; Fu, H.; Yang, N.; et al. Activation of PPARγ by a Natural Flavonoid Modulator, Apigenin Ameliorates Obesity-Related Inflammation Via Regulation of Macrophage Polarization. EBioMedicine 2016, 9, 61–76. [Google Scholar] [CrossRef]
- Funakoshi-Tago, M.; Nakamura, K.; Tago, K.; Mashino, T.; Kasahara, T. Anti-inflammatory activity of structurally related flavonoids, Apigenin, Luteolin and Fisetin. Int. Immunopharmacol. 2011, 11, 1150–1159. [Google Scholar] [CrossRef]
- Liu, H.J.; Fan, Y.L.; Liao, H.H.; Liu, Y.; Chen, S.; Ma, Z.G.; Zhang, N.; Yang, Z.; Deng, W.; Tang, Q.Z. Apigenin alleviates STZ-induced diabetic cardiomyopathy. Mol. Cell. Biochem. 2017, 428, 9–21. [Google Scholar] [CrossRef]
- Kiraly, A.J.; Soliman, E.; Jenkins, A.; Van Dross, R.T. Apigenin inhibits COX-2, PGE2, and EP1 and also initiates terminal differentiation in the epidermis of tumor bearing mice. Prostaglandins Leukot. Essent. Fat. Acids 2016, 104, 44–53. [Google Scholar] [CrossRef]
- Fan, J.; Johnson, M.H.; Lila, M.A.; Yousef, G.; de Mejia, E.G. Berry and Citrus Phenolic Compounds Inhibit Dipeptidyl Peptidase IV: Implications in Diabetes Management. Evid.-Based Complement. Altern. Med. 2013, 2013, 479505. [Google Scholar] [CrossRef]
- Wang, X.; Yang, J.; Li, H.; Shi, S.; Peng, X. Mechanistic Study and Synergistic Effect on Inhibition of α-Amylase by Structurally Similar Flavonoids. J. Mol. Liq. 2022, 360, 119485. [Google Scholar] [CrossRef]
- Na, B.; Nguyen, P.H.; Zhao, B.T.; Vo, Q.H.; Min, B.S.; Woo, M.H. Protein tyrosine phosphatase 1B (PTP1B) inhibitory activity and glucosidase inhibitory activity of compounds isolated from Agrimonia pilosa. Pharm. Biol. 2016, 54, 474–480. [Google Scholar] [CrossRef]
- Chen, M.; Wang, K.; Zhang, Y.; Zhang, M.; Ma, Y.; Sun, H.; Jin, Z.; Zheng, H.; Jiang, H.; Yu, P.; et al. New Insights into the Biological Activities of Chrysanthemum morifolium: Natural Flavonoids Alleviate Diabetes by Targeting α-Glucosidase and the PTP-1B Signaling Pathway. Eur. J. Med. Chem. 2019, 178, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, A.; Alkhalidy, H.; Luo, J.; Moomaw, E.; Neilson, A.P.; Liu, D. Flavone Hispidulin Stimulates Glucagon-Like Peptide-1 Secretion and Ameliorates Hyperglycemia in Streptozotocin-Induced Diabetic Mice. Mol. Nutr. Food Res. 2020, 64, e1900978. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.I.; Cheng, C.I.; Kang, Y.F.; Chang, P.C.; Lin, I.P.; Kuo, Y.H.; Jhou, A.J.; Lin, M.Y.; Chen, C.Y.; Lee, C.H. Hispidulin Inhibits Neuroinflammation in Lipopolysaccharide-Activated BV2 Microglia and Attenuates the Activation of Akt, NF-κB, and STAT3 Pathway. Neurotox. Res. 2020, 38, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Qin, W.; Xi, J.; He, B.; Zhang, B.; Luan, H.; Wu, F. Ameliorative effects of hispidulin on high glucose-mediated endothelial dysfunction via inhibition of PKCβII-associated NLRP3 inflammasome activation and NF-κB signaling in endothelial cells. J. Funct. Foods 2016, 27, 392–405. [Google Scholar] [CrossRef]
- Liang, C.; Zang, J.; Ndi, C.; Semple, S.J.; Buirchell, B.; Coriani, S.; Møller, B.L.; Staerk, D. Identification of new PTP1B-inhibiting decipiene diterpenoid esters from Eremophila clarkei by high-resolution PTP1B inhibition profiling, enzyme kinetics analysis, and molecular docking. Bioorg. Chem. 2023, 139, 106744. [Google Scholar] [CrossRef]
- Huang, D.W.; Shen, S.C. Caffeic acid and cinnamic acid ameliorate glucose metabolism via modulating glycogenesis and gluconeogenesis in insulin-resistant mouse hepatocytes. J. Funct. Foods 2012, 4, 358–366. [Google Scholar] [CrossRef]
- Oboh, G.; Ademosun, A.O.; Ayeni, P.O.; Omojokun, O.S.; Bello, F. Comparative Effect of Quercetin and Rutin on α-Amylase, α-Glucosidase, and Some Pro-Oxidant-Induced Lipid Peroxidation in Rat Pancreas. Comp. Clin. Path. 2015, 24, 1103–1110. [Google Scholar] [CrossRef]
- Istyastono, E.P.; Yuniarti, N.; Prasasty, V.D.; Mungkasi, S.; Waskitha, S.S.W.; Yanuar, M.R.S.; Riswanto, F.D.O. Caffeic Acid in Spent Coffee Grounds as a Dual Inhibitor for MMP-9 and DPP-4 Enzymes. Molecules 2023, 28, 7182. [Google Scholar] [CrossRef]
- Muthusamy, V.S.; Saravanababu, C.; Ramanathan, M.; Bharathi Raja, R.; Sudhagar, S.; Anand, S.; Lakshmi, B.S. Inhibition of protein tyrosine phosphatase 1B and regulation of insulin signalling markers by caffeoyl derivatives of chicory (Cichorium intybus) salad leaves. Br. J. Nutr. 2010, 104, 813–823. [Google Scholar] [CrossRef][Green Version]
- Fu, W.; Wang, H.; Ren, X.; Yu, H.; Lei, Y.; Chen, Q. Neuroprotective effect of three caffeic acid derivatives via ameliorate oxidative stress and enhance PKA/CREB signaling pathway. Behav. Brain Res. 2017, 328, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Trang, N.T.T.; Chiu, W.C.; Feng, Y.T.; Hsieh, S.L.; Tung, D.D.; Chang, J.; Fong, T.H. Caffeic Acid Phenethyl Ester Inhibits Basal Lipolysis by Activating PPAR-Gamma and Increasing Lipid Droplet-Associated Perilipin in Mature Rat Adipocytes. Evid. Based Complement. Alternat. Med. 2022, 2022, 6007233. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, K.; Singh, S.; Burke, T.R., Jr.; Grunberger, D.; Aggarwal, B.B. Caffeic acid phenethyl ester is a potent and specific inhibitor of activation of nuclear transcription factor NF-kappa B. Proc. Natl. Acad. Sci. USA 1996, 93, 9090–9095. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Huang, X.; Xu, S.; Shen, H.; Zhang, P.; Huang, Y.; Jiang, J.; Sun, Y.; Jiang, B.; Wu, X.; et al. Discovery of Novel Hybrids of Diaryl-1,2,4-Triazoles and Caffeic Acid as Dual Inhibitors of Cyclooxygenase-2 and 5-Lipoxygenase for Cancer Therapy. Eur. J. Med. Chem. 2016, 108, 89–103. [Google Scholar] [CrossRef]
- Choi, H.G.; Tran, P.T.; Lee, J.H.; Min, B.S.; Kim, J.A. Anti-Inflammatory Activity of Caffeic Acid Derivatives Isolated from the Roots of Salvia miltiorrhiza Bunge. Arch. Pharm. Res. 2018, 41, 64–70. [Google Scholar] [CrossRef]
- Wen, Y.J.; Yin, M.C. The Anti-Inflammatory and Anti-Glycative Effects of Rosmarinic Acid in the Livers of Type 1 Diabetic Mice. BioMedicine 2017, 7, 19. [Google Scholar] [CrossRef]
- Ou, J.; Huang, J.; Wang, M.; Ou, S. Effect of Rosmarinic Acid and Carnosic Acid on AGEs Formation in Vitro. Food Chem. 2017, 221, 1057–1061. [Google Scholar] [CrossRef]
- Azhar, M.K.; Anwar, S.; Hasan, G.M.; Shamsi, A.; Islam, A.; Parvez, S.; Hassan, M.I. Comprehensive Insights into Biological Roles of Rosmarinic Acid: Implications in Diabetes, Cancer, and Neurodegenerative Diseases. Nutrients 2023, 15, 4297. [Google Scholar] [CrossRef]
- Runtuwene, J.; Cheng, K.C.; Asakawa, A.; Amitani, H.; Amitani, M.; Morinaga, A.; Takimoto, Y.; Kairupan, B.H.; Inui, A. Rosmarinic acid ameliorates hyperglycemia and insulin sensitivity in diabetic rats, potentially by modulating the expression of PEPCK and GLUT4. Drug Des. Dev. Ther. 2016, 10, 2193–2202. [Google Scholar] [CrossRef]
- Den Hartogh, D.J.; Vlavcheski, F.; Tsiani, E. Muscle Cell Insulin Resistance Is Attenuated by Rosmarinic Acid: Elucidating the Mechanisms Involved. Int. J. Mol. Sci. 2023, 24, 5094. [Google Scholar] [CrossRef]
- Han, J.; Wang, D.; Ye, L.; Li, P.; Hao, W.; Chen, X.; Ma, J.; Wang, B.; Shang, J.; Li, D.; et al. Rosmarinic Acid Protects against Inflammation and Cardiomyocyte Apoptosis during Myocardial Ischemia/Reperfusion Injury by Activating Peroxisome Proliferator-Activated Receptor Gamma. Front. Pharmacol. 2017, 8, 456. [Google Scholar] [CrossRef] [PubMed]
- Bower, A.M.; Real Hernandez, L.M.; Berhow, M.A.; de Mejia, E.G. Bioactive Compounds from Culinary Herbs Inhibit a Molecular Target for Type 2 Diabetes Management, Dipeptidyl Peptidase IV. J. Agric. Food Chem. 2014, 62, 6147–6158. [Google Scholar] [CrossRef] [PubMed]
- Salinas-Arellano, E.; Pérez-Vásquez, A.; Rivero-Cruz, I.; Torres-Colin, R.; González-Andrade, M.; Rangel-Grimaldo, M.; Mata, R. Flavonoids and Terpenoids with PTP-1B Inhibitory Properties from the Infusion of Salvia amarissima Ortega. Molecules 2020, 25, 3530. [Google Scholar] [CrossRef] [PubMed]
- Funke, I.; Melzig, M.F. Effect of different phenolic compounds on α-amylase activity: Screening by microplate-reader based kinetic assay. Die Pharmazie 2005, 60, 796–797. [Google Scholar] [PubMed]
- Tshiyoyo, K.S.; Bester, M.J.; Serem, J.C.; Apostolides, Z. In-silico reverse docking and in-vitro studies identified curcumin, 18α-glycyrrhetinic acid, rosmarinic acid, and quercetin as inhibitors of α-glucosidase and pancreatic α-amylase and lipid accumulation in HepG2 cells, important type 2 diabetes targets. J. Mol. Struct. 2022, 1266, 133492. [Google Scholar] [CrossRef]
- Teodoro, T.; Zhang, L.; Alexander, T.; Yue, J.; Vranic, M.; Volchuk, A. Regulation of glucose metabolism by IL-1β and TNFα in pancreatic β-cells. Endocrinology 2023, 164, 700–711. [Google Scholar] [CrossRef]
- Wang, W.; Chen, K.; Xia, Y.; Mo, W.; Wang, F.; Dai, W.; Niu, P. The Hepatoprotection by Oleanolic Acid Preconditioning: Focusing on PPARα Activation. PPAR Res. 2018, 2018, 3180396. [Google Scholar] [CrossRef]
- Loza-Rodríguez, H.; Estrada-Soto, S.; Alarcón-Aguilar, F.J.; Huang, F.; Aquino-Jarquín, G.; Fortis-Barrera, Á.; Giacoman-Martínez, A.; Almanza-Pérez, J.C. Oleanolic acid induces a dual agonist action on PPARγ/α and GLUT4 translocation: A pentacyclic triterpene for dyslipidemia and type 2 diabetes. Eur. J. Pharmacol. 2020, 883, 173252. [Google Scholar] [CrossRef]
- Iskender, H.; Dokumacioglu, E.; Hayirli, A.; Kapakin, K.A.T.; Bolat, I.; Kirman, E.M. Effects of oleanolic acid administration on renal NF-kB, IL-18, IL-6, YKL-40, and KIM-1 in experimental diabetic rats. Iran. J. Basic Med. Sci. 2023, 26, 1188–1193. [Google Scholar] [CrossRef]
- Zhang, S.Q.; Lin, K.L.; Law, C.Y.; Liu, B.; Fu, X.Q.; Tse, W.S.; Wong, S.S.M.; Sze, S.C.W.; Yung, K.K.L. Oleanolic Acid Enhances Neural Stem Cell Migration, Proliferation, and Differentiation in Vitro by Inhibiting GSK3β Activity. Cell Death Discov. 2018, 4, 48. [Google Scholar] [CrossRef]
- Zhang, Y.N.; Zhang, W.; Hong, D.; Shi, L.; Shen, Q.; Li, J.Y.; Li, J.; Hu, L.H. Oleanolic Acid and Its Derivatives: New Inhibitor of Protein Tyrosine Phosphatase 1B with Cellular Activities. Bioorg. Med. Chem. 2008, 16, 8697–8705. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Espinosa, J.J.; Rios, M.Y.; Paoli, P.; Flores-Morales, V.; Camici, G.; de la Rosa-Lugo, V.; Hidalgo-Figueroa, S.; Navarrete-Vázquez, G.; Estrada-Soto, S. Synthesis of oleanolic acid derivatives: In vitro, in vivo and in silico studies for PTP-1B inhibition. Eur. J. Med. Chem. 2014, 87, 316–327. [Google Scholar] [CrossRef] [PubMed]
- Raoufi, S.; Baluchnejadmojarad, T.; Roghani, M.; Ghazanfari, T.; Khojasteh, F.; Mansouri, M. Antidiabetic potential of salvianolic acid B in multiple low-dose streptozotocin-induced diabetes. Pharm. Biol. 2015, 53, 1803–1809. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Pan, D.; Yan, L.; Chen, H.; Zhang, X.; Yuan, J.; Mu, B. Salvianolic acid B improved insulin resistance through suppression of hepatic ER stress in ob/ob mice. Biochem. Biophys. Res. Commun. 2020, 526, 733–737. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Zhao, W.; Zhao, D.; Wang, C.; Yu, N.; An, T.; Mo, F.; Liu, J.; Miao, J.; Lv, B.; et al. Salvianolic Acid B Improves Mitochondrial Function in 3T3-L1 Adipocytes Through a Pathway Involving PPARγ Coactivator-1α (PGC-1α). Front. Pharmacol. 2018, 9, 671. [Google Scholar] [CrossRef]
- Wang, P.; Xu, S.; Li, W.; Wang, F.; Yang, Z.; Jiang, L.; Wang, Q.; Huang, M.; Zhou, P. Salvianolic Acid B Inhibited PPARγ Expression and Attenuated Weight Gain in Mice with High-Fat Diet-Induced Obesity. Cell. Physiol. Biochem. 2014, 34, 288–298. [Google Scholar] [CrossRef]
- Chen, Y.L.; Hu, C.S.; Lin, F.Y.; Chen, Y.H.; Sheu, L.M.; Ku, H.H.; Shiao, M.S.; Chen, J.W.; Lin, S.J. Salvianolic Acid B Attenuates Cyclooxygenase-2 Expression in Vitro in LPS-Treated Human Aortic Smooth Muscle Cells and In Vivo in the Apolipoprotein-E-Deficient Mouse Aorta. J. Cell. Biochem. 2006, 98, 618–631. [Google Scholar] [CrossRef]
- Bai, X.; Fan, W.; Luo, Y.; Liu, Y.; Zhang, Y.; Liao, X. Fast Screening of Protein Tyrosine Phosphatase 1B Inhibitor from Salvia miltiorrhiza Bge by Cell Display-Based Ligand Fishing. Molecules 2022, 27, 7896. [Google Scholar] [CrossRef]
- Paudel, P.; Seong, S.H.; Zhou, Y.; Park, C.H.; Yokozawa, T.; Jung, H.A.; Choi, J.S. Rosmarinic Acid Derivatives’ Inhibition of Glycogen Synthase Kinase-3β Is the Pharmacological Basis of Kangen-Karyu in Alzheimer’s Disease. Molecules 2018, 23, 2919. [Google Scholar] [CrossRef]
- Wang, B.; Sun, J.; Shi, Y.; Le, G. Salvianolic Acid B Inhibits High-Fat Diet-Induced Inflammation by Activating the Nrf2 Pathway. J. Food Sci. 2017, 82, 1953–1960. [Google Scholar] [CrossRef]
- Agu, P.C.; Afiukwa, C.A.; Orji, O.U.; Ezeh, E.M.; Ofoke, I.H.; Ogbu, C.O.; Ugwuja, E.I.; Aja, P.M. Molecular Docking as a Tool for the Discovery of Molecular Targets of Nutraceuticals in Disease Management. Sci. Rep. 2023, 13, 13398. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.Y.; Zhang, H.X.; Mezei, M.; Cui, M. Molecular docking: A powerful approach for structure-based drug discovery. Curr. Comput.-Aided Drug Des. 2011, 7, 146–157. [Google Scholar] [CrossRef] [PubMed]
- Brayer, G.D.; Sidhu, G.; Maurus, R.; Rydberg, E.H.; Braun, C.; Wang, Y.; Nguyen, N.T.; Overall, C.M.; Withers, S.G. Subsite Mapping of the Human Pancreatic Alpha-Amylase Active Site Through Structural, Kinetic, and Mutagenesis Techniques. Biochemistry 2000, 39, 4778–4791. [Google Scholar] [CrossRef] [PubMed]
- Maurus, R.; Begum, A.; Williams, L.K.; Fredriksen, J.R.; Zhang, R.; Withers, S.G.; Brayer, G.D. Alternative catalytic anions differentially modulate human alpha-amylase activity and specificity. Biochemistry 2008, 47, 3332–3344. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, N.; Krishnan, K.; Connors, C.R.; Choy, M.S.; Page, R.; Peti, W.; Van Aelst, L.; Shea, S.D.; Tonks, N.K. PTP1B inhibition suggests a therapeutic strategy for Rett syndrome. J. Clin. Investig. 2015, 125, 3163–3177. [Google Scholar] [CrossRef]
- Sun, J.P.; Fedorov, A.A.; Lee, S.Y.; Guo, X.L.; Shen, K.; Lawrence, D.S.; Almo, S.C.; Zhang, Z.Y. Crystal structure of PTP1B complexed with a potent and selective bidentate inhibitor. J. Biol. Chem. 2003, 278, 12406–12414. [Google Scholar] [CrossRef]
- Kirby, M.; Yu, D.M.; O’Connor, S.; Gorrell, M.D. Inhibitor selectivity in the clinical application of dipeptidyl peptidase-4 inhibition. Clin. Sci. 2009, 118, 31–41. [Google Scholar] [CrossRef]
- Pissarnitski, D.A.; Zhao, Z.; Cole, D.; Wu, W.L.; Domalski, M.; Clader, J.W.; Scapin, G.; Voigt, J.; Soriano, A.; Kelly, T.; et al. Scaffold-hopping from xanthines to tricyclic guanines: A case study of dipeptidyl peptidase 4 (DPP4) inhibitors. Bioorg. Med. Chem. 2016, 24, 5534–5545. [Google Scholar] [CrossRef]
- Gentile, G.; Merlo, G.; Pozzan, A.; Bernasconi, G.; Bax, B.; Bamborough, P.; Bridges, A.; Carter, P.; Neu, M.; Yao, G.; et al. 5-Aryl-4-carboxamide-1,3-oxazoles: Potent and selective GSK-3 inhibitors. Bioorg. Med. Chem. Lett. 2012, 22, 1989–1994. [Google Scholar] [CrossRef]
- Pandey, M.K.; DeGrado, T.R. Glycogen Synthase Kinase-3 (GSK-3)-Targeted Therapy and Imaging. Theranostics 2016, 6, 571–593. [Google Scholar] [CrossRef]
- Pan, D.S.; Wang, W.; Liu, N.S.; Yang, Q.J.; Zhang, K.; Zhu, J.Z.; Shan, S.; Li, Z.B.; Ning, Z.Q.; Huang, L.; et al. Chiglitazar Preferentially Regulates Gene Expression via Configuration-Restricted Binding and Phosphorylation Inhibition of PPARγ. PPAR Res. 2017, 2017, 4313561. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.A.; Tan, L.; Yang, H.; Im, Y.G.; Im, Y.J. Structures of PPARγ complexed with lobeglitazone and pioglitazone reveal key determinants for the recognition of antidiabetic drugs. Sci. Rep. 2017, 7, 16837. [Google Scholar] [CrossRef] [PubMed]
- Orlando, B.J.; Malkowski, M.G. Substrate-selective Inhibition of Cyclooxygenase-2 by Fenamic Acid Derivatives Is Dependent on Peroxide Tone. J. Biol. Chem. 2016, 291, 15069–15081. [Google Scholar] [CrossRef] [PubMed]
- Rouzer, C.A.; Marnett, L.J. Structural and Chemical Biology of the Interaction of Cyclooxygenase with Substrates and Non-Steroidal Anti-Inflammatory Drugs. Chem. Rev. 2020, 120, 7592–7641. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, M.D.; Harrison, S.C. Structure of an IkappaBalpha/NF-kappaB complex. Cell 1998, 95, 749–758. [Google Scholar] [CrossRef]
- Escalante, C.R.; Shen, L.; Thanos, D.; Aggarwal, A.K. Structure of NF-kappaB p50/p65 heterodimer bound to the PRDII DNA element from the interferon-beta promoter. Structure 2002, 10, 383–391. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A Free Web Tool to Evaluate Pharmacokinetics, Drug-Likeness, and Medicinal Chemistry Friendliness of Small Molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef]
- Ugwor, E.I.; James, A.S.; Amuzat, A.I.; Ezenandu, E.O.; Ugbaja, V.C.; Ugbaja, R.N. Network pharmacology-based elucidation of bioactive compounds in propolis and putative underlying mechanisms against type-2 diabetes mellitus. Pharmacol. Res.-Mod. Chin. Med. 2022, 5, 100183. [Google Scholar] [CrossRef]
- Patil, V.S.; Harish, D.R.; Vetrivel, U.; Roy, S.; Deshpande, S.H.; Hegde, H.V. Hepatitis C Virus NS3/4A Inhibition and Host Immunomodulation by Tannins from Terminalia chebula: A Structural Perspective. Molecules 2022, 27, 1076. [Google Scholar] [CrossRef]
- Sun, J.; Liu, B.; Wang, R.; Yuan, Y.; Wang, J.; Zhang, L. Computation-Based Discovery of Potential Targets for Rheumatoid Arthritis and Related Molecular Screening and Mechanism Analysis of Traditional Chinese Medicine. Dis. Markers 2022, 2022, 1905077. [Google Scholar] [CrossRef]
- Alamri, M.A. Bioinformatics and Network Pharmacology-Based Study to Elucidate the Multi-Target Pharmacological Mechanism of the Indigenous Plants of Medina Valley in Treating HCV-Related Hepatocellular Carcinoma. Saudi Pharm. J. 2023, 31, 1125–1138. [Google Scholar] [CrossRef] [PubMed]
- El-Atawneh, S.; Goldblum, A. Activity Models of Key GPCR Families in the Central Nervous System: A Tool for Many Purposes. J. Chem. Inf. Model. 2023, 63, 3248–3262. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.Y.; Mou, X.; Liu, K.; Liu, W.H.; Xu, Y.Q.; Zhou, D. In Silico Prediction and Validation of Potential Therapeutic Genes in Pancreatic β-Cells Associated with Type 2 Diabetes. Exp. Ther. Med. 2020, 20, 60. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, A.; Yadav, P.H.; Mukunthan, K.S. Unveiling potential targeted therapeutic opportunities for co-overexpressed targeting protein for Xklp2 and Aurora-A kinase in lung adenocarcinoma. Mol. Biotechnol. 2023. [Google Scholar] [CrossRef] [PubMed]
- Ononamadu, C.J.; Abdalla, M.; Ihegboro, G.O.; Li, J.; Owolarafe, T.A.; John, T.D.; Tian, Q. In silico identification and study of potential anti-mosquito juvenile hormone binding protein (MJHBP) compounds as candidates for dengue virus—Vector insecticides. Biochem. Biophys. Rep. 2021, 28, 101178. [Google Scholar] [CrossRef]
- Ramírez, D.; Caballero, J. Is It Reliable to Take the Molecular Docking Top Scoring Position as the Best Solution without Considering Available Structural Data? Molecules 2018, 23, 1038. [Google Scholar] [CrossRef]
- Galli, C.L.; Sensi, C.; Fumagalli, A.; Parravicini, C.; Marinovich, M.; Eberini, I. A computational approach to evaluate the androgenic affinity of iprodione, procymidone, vinclozolin and their metabolites. PLoS ONE 2014, 9, e104822. [Google Scholar] [CrossRef]
- Nissink, J.W. Simple size-independent measure of ligand efficiency. J. Chem. Inf. Model. 2009, 49, 1617–1622. [Google Scholar] [CrossRef]


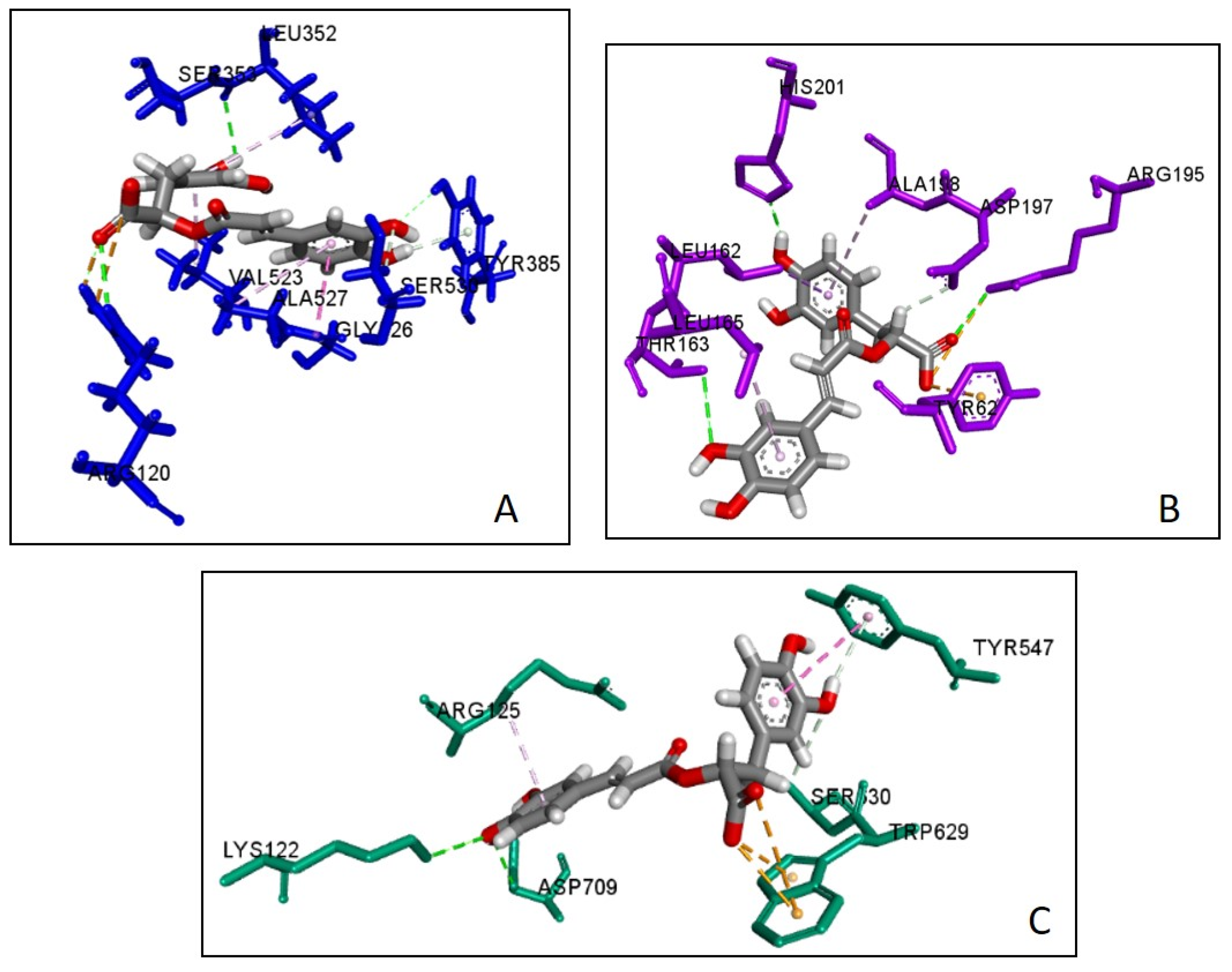

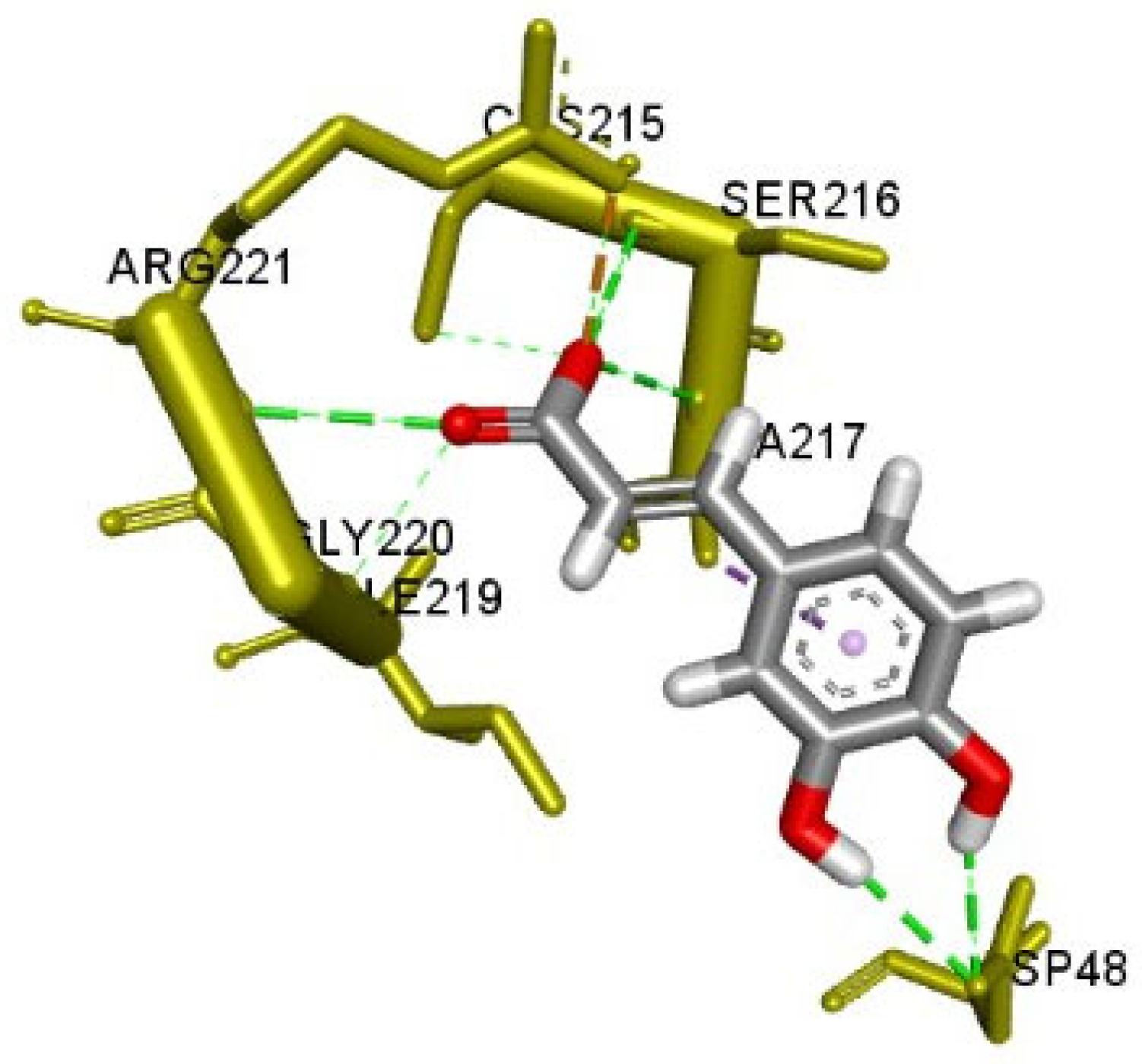
| Ligand | Docking Score | SILE Value | Interacting Amino Acid Residues |
|---|---|---|---|
| Caffeic acid | −5.1 | 2.37 | (Leu352, Tyr312) a, Arg550 d, (Leu352, Val523) c |
| Apigenin | −6.4 | 2.62 | (His90, Tyr355, Tyr385) a, (Val523, Ala527, Leu352,Val349, Ser353) c |
| Hispidulin | −6.5 | 2.58 | Ser530 a, (Leu352, Ala527, Val523, Val349, Gly526) c |
| Oleanolic acid | 8.3 | −2.91 | Tyr385 b |
| Rosmarinic acid | −7.7 | 2.90 | (Leu352, Tyr355, Arg120) a, Arg120 d, (Ser530, Tyr385) b, (Val523, Leu352, Gly526, Ala527) c |
| Salvianolic acid B | −2.3 | 0.71 | (Ser119, Tyr355) a, (Arg513, Arg120) d, (Val523, Pro86) c, Glu524 e |
| PubChem CID: 4037 (Control) | −7.0 | 2.90 | (Ser530, Tyr385) a, (Val116, Leu531, Ala527) f, (Ala527, Val523, Val349) c |
| Ligand | Docking Score | SILE Value | Interacting Amino Acid Residues |
|---|---|---|---|
| Caffeic acid | −5.0 | 2.33 | Ser289 a, (Met364, Arg288, Leu330, Cys285) c |
| Apigenin | −6.0 | 2.46 | (Ser342, Cys285) a, Arg288 b, (Arg288, Leu330, Cys285, Met364, Ile341, Gly284) c |
| Hispidulin | −6.6 | 2.60 | (Ser289, Tyr473) a, (Tyr473, His449 His323) b, (Arg288, His449, Met364,Leu330, Cys285) c |
| Oleanolic acid | −5.6 | 1.95 | Ile341 e, (Phe287, Arg280) c |
| Rosmarinic acid | −7.3 | 2.76 | (Ser342, Tyr327) a, (Cys285, Met364, Gly284 and Tyr327) c |
| Salvianolic acid B | −9.5 | 2.91 | (Ser289, Ser342, Glu343, Glu291, Cys285) a, Cys285 d, (Arg288, Leu330, Gly291, Arg 280) c |
| PubChem CID: 76965111 (Control) | −9.7 | 3.36 | (Ser289 Tyr473) a, (Cys285, Leu255) e, (Leu353, Leu330, Met348, Val339) c |
| Ligand | Docking Score | SILE Value | Interacting Amino Acid Residues |
|---|---|---|---|
| Caffeic acid | −6.0 | 2.78 | (Asp48, Ala217, Ser216, Arg221) a, Arg221 d |
| Apigenin | −5.0 | 2.03 | Asp48 e, (Tyr46, Ala217) c |
| Hispidulin | −5.4 | 2.15 | Ser216 b, Arg221 d, (Ala217, Tyr46) c |
| Oleanolic acid | −4.8 | 1.67 | Asp48 a, Lys120 d, Lys116 c, (Ala217, Tyr46, Phe182) f |
| Rosmarinic acid | −5.9 | 2.20 | (Arg221, Tyr46) a, lys120 d, (Ala217, Phe182, Arg47) c |
| Salvianolic acid B | −6.9 | 2.12 | (Asp48, Gln262, Tyr46, Ser118, Leu119) a, Asp181 b, (Lys116, Phe182, Ala217) c, Lys120 e, Lys120 d |
| PubChem CID: 91826021(Control) | −8.7 | 3.32 | (Asp48, Phe182, Ala217, Ser216, Gly220, Ile219) a, (Phe182, Ala217) c, (Arg221, Asp181) d |
| Ligand | Docking Score | SILE Value | Interacting Amino Acid Residues |
|---|---|---|---|
| Caffeic acid | −4.9 | 2.28 | (Ser630, Tyr631, Trp629) a, Tyr547 c, Lys554 d |
| Apigenin | −5.1 | 2.09 | Glu205, Arg125, Lys554) a Tyr547 c |
| Hispidulin | −5.9 | 2.32 | Asp545 a, (Trp666, Tyr547) c Arg125 d, Ser630 b, Glu205 b, Tyr662 b |
| Oleanolic acid | −6.1 | 2.15 | Arg125 d, Tyr752 b, (Trp629, Tyr547) f, Tyr547 c |
| Rosmarinic acid | −6.8 | 2.57 | (Asp709, Lys122) a, (Ser630, Tyr547) b, Trp629 e, (Arg125, Tyr547) c |
| Salvianolic acid B | −8.0 | 2.45 | (Tyr752 b, Tyr48) a, (Asn562, Trp629) b, (Arg125, Lys554) d, (Tyr547, Trp629) c |
| PubChem CID: 137348565 (Control) | −8.3 | 2.84 | (Tyr662, Tyr631) a, Ser630 b, (Val656, Val711) f, (Asp663, Glu206, Glu205) d, Trp629 c, Phe357 c |
| Ligand | Docking Score | SILE Value | Interacting Amino Acid Residues |
|---|---|---|---|
| Caffeic acid | −5.1 | 2.35 | Val135 a, Lys85 d, (Leu188, Ala83) c |
| Apigenin | −5.7 | 2.33 | (Glu97, Val135) a, Cys199 b, (Ala83, Lys85, Val110, leu188, Leu132) c |
| Hispidulin | −6.1 | 2.40 | (Glu97, Val135) a, (Cys199, Val135) b, (Ala83, Lys85, Val110, Leu188, Leu132, Met101) c, Asp200 g |
| Oleanolic acid | −6.1 | 2.15 | (Asn186, Tyr 134) a, Lys60 d, (Arg141, Cys199, Phe67) f |
| Rosmarinic acid | −6.3 | 2.37 | (Asp133, Val135) a, (Ala83, Lys199, Leu188, Val70) c, Lys85 d |
| Salvianolic acid B | −8.4 | 2.58 | (Asp181, Lys183, Thr138, Asn64, Arg141) a, (Gly63, Lys85) b (Phe67, Lys85, Val70, Cys199) c, Arg141 e |
| PubChem CID: 56643097 (Control) | −7.0 | 2.73 | Val135 a, (Ile62, Ala83, Val70, Lys85, Leu158, Phe67) c, Lys85 f |
| Ligand | Docking Score | SILE Value | Interacting Amino Acid Residues |
|---|---|---|---|
| Caffeic acid | −4.8 | 2.24 | (Asp197, Gln63) a, Trp59 e, Tyr62 c |
| Apigenin | −5.7 | 2.31 | (Asp197, Gln63) a, (Trp59, Tyr62) c |
| Hispidulin | −5.9 | 2.32 | (Asp197 a, Gln63) a, Trp59 e, (Trp59, Tyr62) c |
| Oleanolic acid | −6.4 | 2.25 | Asp197 a, (Trp59, His305, Tyr62, Leu162) c, (Trp59, His305, Leu162, His 101, Ala198) f |
| Rosmarinic acid | −6.5 | 2.44 | (His201, Thr163, Arg 195 a) a, Asp197 b, (Arg195, Tyr62) d, (Ala198, Leu165, leu162) c |
| Salvianolic acid B | −7.9 | 2.42 | Gly304 a, Asp197 a, Lys200 b, Lys200 e |
| PubChem CID: 24755467 (Control) | −9.4 | 2.83 | (Gly164, Gln63, Asp300, Trp69, Glu233, Gln63, His101, Arg195, His299, Tyr62, Asn105, Ala106, Val107, Leu162) a, (Asp300, Glu233, Asp197) d |
| Ligand | Docking Score | SILE Value | Interacting Amino Acid Residues |
|---|---|---|---|
| Caffeic acid | −5.0 | 2.33 | (Arg41, Ile118) a, (Arg35, Ala43, Val91) c |
| Apigenin | −5.3 | 2.15 | (Ser42, Val91) c |
| Hispidulin | −5.3 | 2.10 | Tyr36 a, Ser42 b, (Ala43, Arg33) c, Val91 c |
| Oleanolic acid | −5.1 | 1.80 | Gly44 a, Ala43 f |
| Rosmarinic acid | −6.1 | 2.28 | (Ser44, Glu39, Gly92, Glu89, Gln119) a, Val91 c |
| Salvianolic acid B | −8.0 | 2.43 | (Ser45, Arg33) a, Lys56 e, (Arg33, Arg35) d, (Gly44, Arg 33) b, (Ala43, Lys56, Arg35, Met32) c |
| PubChem CID: 9881652 (Control) | −5.2 | 2.13 | (Ser51, Lys56, Arg35, Ser45) a, Met32 c |
| Target Protein | Ligand | Docking Score (kcal/mol) | SILE Value | Interacting Residues | Distance (Å) | Category | Type |
|---|---|---|---|---|---|---|---|
| AMY1A (2QV4) | Rosmarinic acid | −6.479 | 2.44 | Thr163 | 3.131 | H-Bond | Conventional H-Bond |
| Arg195 | 3.219 | H-Bond | Conventional H-Bond | ||||
| His201 | 2.010 | H-Bond | Conventional H-Bond | ||||
| Asp197 | 2.564 | H-Bond | Carbon H-Bond | ||||
| Leu162 | 3.910 | Hydrophobic | Pi-Sigma | ||||
| Ala198 | 5.492 | Hydrophobic | Pi-Alkyl | ||||
| Leu165 | 5.162 | Hydrophobic | Pi-Alkyl | ||||
| Arg195 | 5.297 | Electrostatic | Attractive Charge | ||||
| Tyr62 | 3.314 | Electrostatic | Pi-Anion | ||||
| DPP4 (5T4B) | Rosmarinic acid | −6.834 | 2.57 | Lys122 | 3.134 | H-Bond | Conventional H-Bond |
| Asp709 | 2.190 | H-Bond | Conventional H-Bond | ||||
| Asp709 | 2.537 | H-Bond | Conventional H-Bond | ||||
| Ser630 | 3.712 | H-Bond | Carbon H-Bond | ||||
| Tyr547 | 2.944 | H-Bond | Pi-Donor H-Bond | ||||
| Trp629 | 4.749 | Electrostatic | Pi-Anion | ||||
| Trp629 | 3.626 | Electrostatic | Pi-Anion | ||||
| Trp629 | 3.351 | Electrostatic | Pi-Anion | ||||
| Tyr547 | 5.643 | Hydrophobic | Pi-Pi T-shaped | ||||
| Arg125 | 4.342 | Hydrophobic | Pi-Alkyl | ||||
| PTGS2 (5IKQ) | Rosmarinic Acid | −7.714 | 2.90 | Tyr355 | 2.399 | H-Bond | |
| Tyr385 | 2.266 | H-Bond | |||||
| Arg120 | 2.524 | H-Bond | H-Bond | ||||
| Leu352 | 2.709 | H-Bond | H-Bond | ||||
| Ser530 | 2.700 | H-Bond | H-Bond | ||||
| Tyr385 | 2.801 | H-Bond | Pi-Donor H-Bond | ||||
| Gly526 | 3.766 | Hydrophobic | Amide-Pi Stacked | ||||
| Leu352 | 5.451 | Hydrophobic | Pi-Alkyl | ||||
| Val523 | 3.778 | Hydrophobic | Pi-Alkyl | ||||
| Ala527 | 4.950 | Hydrophobic | Pi-Alkyl | ||||
| Arg120 | 2.245 | H-Bond;Electrostatic | Salt Bridge;Attractive Charge | ||||
| Arg120 | 5.054 | Electrostatic | Attractive Charge | ||||
| PTP1B (4Y14) | Caffeic acid | −6.010 | 2.78 | Arg221 | 2.399 | H-Bond;Electrostatic | Salt Bridge |
| Ser216 | 2.266 | H-Bond | Conventional H-Bond | ||||
| Ala217 | 2.524 | H-Bond | Conventional H-Bond | ||||
| Arg221 | 2.709 | H-Bond | Conventional H-Bond | ||||
| Asp48 | 2.700 | H-Bond | Conventional H-Bond | ||||
| Asp48 | 2.801 | H-Bond | Conventional H-Bond | ||||
| Cys215 | 3.766 | H-Bond | |||||
| Gly220 | 5.451 | H-Bond | |||||
| Ala217 | 3.778 | Hydrophobic | Pi-Sigma | ||||
| GSK3B (4AFJ) | Salvianolic acid B | −8.447 | 2.58 | Asn64 | 3.083 | H-Bond | Conventional H-Bond |
| Asn64 | 3.099 | H-Bond | |||||
| Arg141 | 3.071 | H-Bond | Conventional H-Bond | ||||
| Lys183 | 2.922 | H-Bond | Conventional H-Bond | ||||
| Asp181 | 2.613 | H-Bond | Conventional H-Bond | ||||
| Thr138 | 2.808 | H-Bond | Conventional H-Bond | ||||
| Lys85 | 3.039 | H-Bond | |||||
| GLY63 | 3.702 | H-Bond | Carbon H-Bond | ||||
| ARG141 | 3.966 | Electrostatic | Pi-Cation | ||||
| VAL70 | 3.909 | Hydrophobic | Pi-Sigma | ||||
| PHE67 | 4.971 | Hydrophobic | Pi-Pi T-shaped | ||||
| LYS85 | 5.156 | Hydrophobic | Pi-Alkyl | ||||
| NF-κB (1NFI) | Salvianolic acid B | −7.963 | 2.43 | ARG33 | 2.838 | H-Bond | Conventional H-Bond |
| SER45 | 3.312 | H-Bond | Conventional H-Bond | ||||
| ARG33 | 1.790 | H-Bond | Conventional H-Bond | ||||
| ARG33 | 3.767 | H-Bond | Carbon H-Bond | ||||
| GLY44 | 3.499 | H-Bond | Carbon H-Bond | ||||
| LYS56 | 4.170 | H-Bond;Electrostatic | Pi-Cation;Pi-Donor H-Bond | ||||
| LYS56 | 3.485 | H-Bond;Electrostatic | Pi-Cation;Pi-Donor H-Bond | ||||
| ARG35 | 3.123 | H-Bond;Electrostatic | Salt Bridge | ||||
| ARG35 | 2.899 | H-Bond;Electrostatic | Salt Bridge | ||||
| ARG35 | 2.899 | H-Bond;Electrostatic | Salt Bridge | ||||
| ARG33 | 3.177 | Electrostatic | Attractive Charge | ||||
| ARG35 | 5.023 | Electrostatic | Attractive Charge | ||||
| MET32 | 3.348 | Hydrophobic | Pi-Sigma | ||||
| LYS56 | 5.233 | Hydrophobic | Pi-Alkyl | ||||
| ARG35 | 5.003 | Hydrophobic | Pi-Alkyl | ||||
| ALA43 | 4.395 | Hydrophobic | Pi-Alkyl | ||||
| PPARγ (5Y2T) | Salvianolic acid B | −9.519 | 2.91 | SER289 | 3.169 | H-Bond | |
| SER342 | 3.209 | H-Bond | |||||
| SER289 | 2.315 | H-Bond | |||||
| CYS285 | 3.054 | H-Bond | Conventional H-Bond | ||||
| CYS285 | 3.124 | H-Bond | Conventional H-Bond | ||||
| SER342 | 3.088 | H-Bond | Conventional H-Bond | ||||
| GLU343 | 2.134 | H-Bond | Conventional H-Bond | ||||
| GLU291 | 2.502 | H-Bond | Conventional H-Bond | ||||
| SER289 | 2.315 | H-Bond | Conventional H-Bond | ||||
| CYS285 | 3.709 | H-Bond | Pi-Donor H-Bond | ||||
| ARG280 | 4.644 | Hydrophobic | Amide-Pi Stacked | ||||
| ARG280 | 5.406 | Hydrophobic | Pi-Alkyl | ||||
| ARG288 | 4.900 | Hydrophobic | Pi-Alkyl | ||||
| LEU330 | 4.615 | Hydrophobic | Pi-Alkyl | ||||
| GLU291 | 3.360 | Electrostatic | Pi-Anion |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ononamadu, C.J.; Seidel, V. Exploring the Antidiabetic Potential of Salvia officinalis Using Network Pharmacology, Molecular Docking and ADME/Drug-Likeness Predictions. Plants 2024, 13, 2892. https://doi.org/10.3390/plants13202892
Ononamadu CJ, Seidel V. Exploring the Antidiabetic Potential of Salvia officinalis Using Network Pharmacology, Molecular Docking and ADME/Drug-Likeness Predictions. Plants. 2024; 13(20):2892. https://doi.org/10.3390/plants13202892
Chicago/Turabian StyleOnonamadu, Chimaobi J., and Veronique Seidel. 2024. "Exploring the Antidiabetic Potential of Salvia officinalis Using Network Pharmacology, Molecular Docking and ADME/Drug-Likeness Predictions" Plants 13, no. 20: 2892. https://doi.org/10.3390/plants13202892
APA StyleOnonamadu, C. J., & Seidel, V. (2024). Exploring the Antidiabetic Potential of Salvia officinalis Using Network Pharmacology, Molecular Docking and ADME/Drug-Likeness Predictions. Plants, 13(20), 2892. https://doi.org/10.3390/plants13202892







