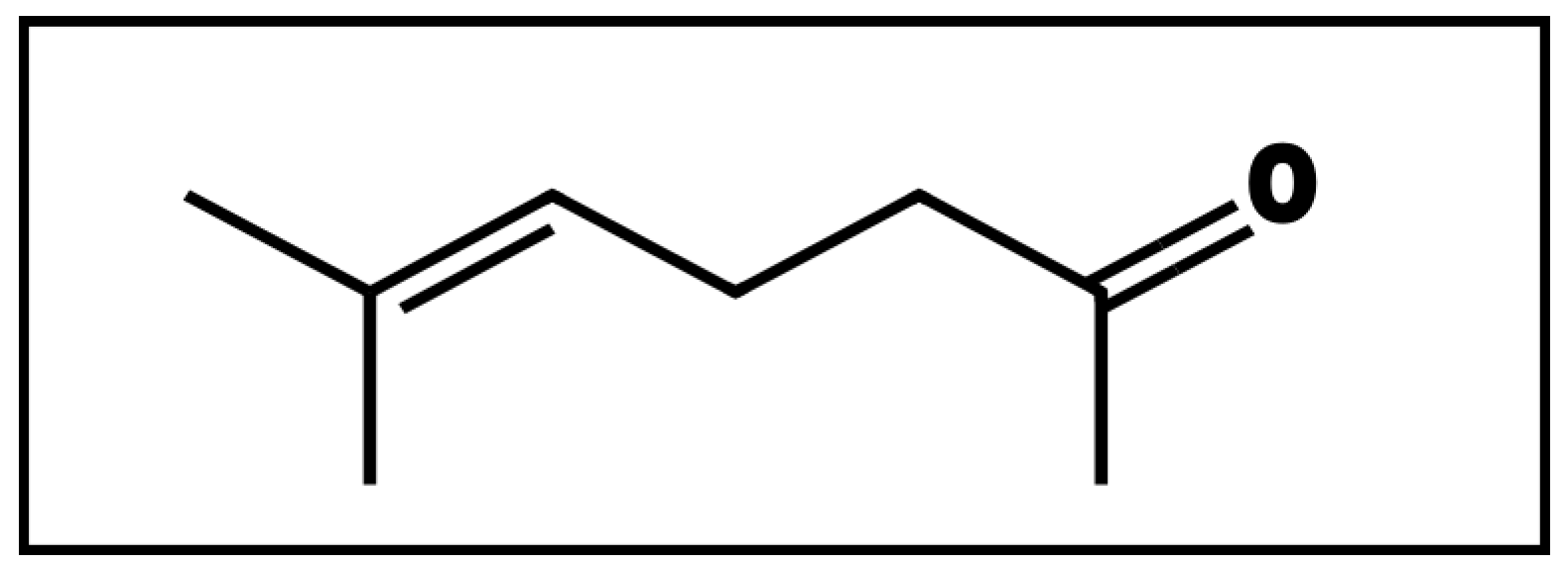
Sulcatone as a Plant-Derived Volatile Organic Compound for the Control of the Maize Weevil and Its Associated Phytopathogenic Fungi in Stored Maize
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Insect Rearing
2.3. Fungal Strains and Inoculum Preparation
2.4. Repellency Assays
2.5. Fumigant Toxicity Bioassay
2.6. Determination of Minimum Inhibitory Concentrations (MIC)
2.7. Silo-Bag Experiment
2.8. Ergosterol Extraction and Quantification
2.9. Fumonisin B1 Extraction and Quantification
2.10. Seed Germination Bioassay
2.11. Data Analyses
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brito, V.D.; Achimón, F.; Zunino, M.P.; Zygadlo, J.A.; Pizzolitto, R.P. Fungal diversity and mycotoxins detected in maize stored in silo-bags: A review. J. Sci. Food Agric. 2022, 102, 2640–2650. [Google Scholar] [CrossRef] [PubMed]
- Nwosu, L.C. Impact of age on the biological activities of Sitophilus zeamais (Coleoptera: Curculionidae) adults on stored maize: Implications for food security and pest management. J. Econ. Entomol. 2018, 111, 2454–2460. [Google Scholar] [CrossRef] [PubMed]
- Castellari, C.C.; Cendoya, M.G.; Marcos Valle, F.J.; Barrera, V.; Pacin, A.M. Factores extrínsecos e intrínsecos asociados a poblaciones fúngicas micotoxigénicas de granos de maíz (Zea mays L.) almacenados en silos bolsa en Argentina. Rev. Argent. Microbiol. 2015, 47, 350–359. [Google Scholar] [CrossRef] [PubMed]
- Barontini, J.M.; Alaniz Zanon, M.S.; Druetta, M.A.; Ruiz Posse, A.M.; Torrico, A.K.; Monge, M.d.P.; Candela, R.E.; Chulze, S.N.; Pecci, M.d.l.P.G. Differential response of maize hybrids to field infection with Aspergillus flavus and aflatoxin accumulation in the Chaco Semi-arid region of Argentina. Crop Prot. 2022, 156, 105960. [Google Scholar] [CrossRef]
- Lamboni, Y.; Hell, K. Propagation of mycotoxigenic fungi in maize stores by post-harvest insects. Int. J. Trop. Insect Sci. 2009, 29, 31–39. [Google Scholar] [CrossRef]
- Nagraj, D.; Achar, P.N.; Sreenivasa, M.Y. Current perspectives of biocontrol agents for management of Fusarium verticillioides and its fumonisin in cereals—a review. J. Fungi 2021, 7, 776. [Google Scholar] [CrossRef]
- Chen, J.; Wen, J.; Tang, Y.; Shi, J.; Mu, G.; Yan, R.; Cai, J.; Long, M. Research progress on fumonisin B1 contamination and toxicity: A review. Molecules 2021, 26, 5238. [Google Scholar] [CrossRef]
- Yilmaz, S.; Bag, H. Aflatoxin B1: Mechanism, oxidative stress, and effects on animal health. Insights Vet. Sci. 2022, 2, 017–024. [Google Scholar]
- Nesci, A.; Barra, P.; Etcheverry, M. Integrated management of insect vectors of Aspergillus flavus in stored maize, using synthetic antioxidants and natural phytochemicals. J. Stored Prod. Res. 2011, 47, 231–237. [Google Scholar] [CrossRef]
- Tan, H.; Wu, Q.; Hao, R.; Wang, C.; Zhai, J.; Li, Q.; Cui, Y.; Wu, C. Occurrence, distribution, and driving factors of current-use pesticides in commonly cultivated crops and their potential risks to non-target organisms: A case study in Hainan, China. Sci. Total Environ. 2023, 854, 158640. [Google Scholar] [CrossRef]
- Margni, M.; Rossier, D.; Crettaz, P.; Jolliet, O. Life cycle impact assessment of pesticides on human health and ecosystems. Agric. Ecosyst. Environ. 2002, 93, 379–392. [Google Scholar] [CrossRef]
- Pandey, A.K.; Kumar, P.; Singh, P.; Tripathi, N.N.; Bajpai, V.K. Essential oils: Sources of antimicrobials and food preservatives. Front. Microbiol. 2017, 7, 2161. [Google Scholar] [CrossRef] [PubMed]
- Achimón, F.; Beato, M.; Brito, V.D.; Peschiutta, M.L.; Herrera, J.M.; Merlo, C.; Pizzolitto, R.P.; Zygadlo, J.A.; Zunino, M.P. Insecticidal and repellent effects of the essential oils obtained from Argentine aromatic flora. Boletín la Soc. Argentina Botánica 2022, 57, 651–670. [Google Scholar] [CrossRef]
- Pizzolitto, R.; Herrera, J.; Zaio, Y.; Dambolena, J.; Zunino, M.; Gallucci, M.; Zygadlo, J. Bioactivities of ketones terpenes: Antifungal effect on F. verticillioides and repellents to control insect fungal vector, S. zeamais. Microorganisms 2015, 3, 851–865. [Google Scholar] [CrossRef]
- Herrera, J.M.; Zunino, M.P.; Dambolena, J.S.; Pizzolitto, R.P.; Gañan, N.A.; Lucini, E.I.; Zygadlo, J.A. Terpene ketones as natural insecticides against Sitophilus zeamais. Ind. Crops Prod. 2015, 70, 435–442. [Google Scholar] [CrossRef]
- Saadellaoui, W.; Kahlaoui, S.; Hcini, K.; Haddada, A.; Sleimi, N.; Ascrizzi, R.; Flamini, G.; Harzallah-Skhiri, F.; Stambouli-Essassi, S. Profiles of the headspace volatile organic and essential oil compounds from the Tunisian Cardaria draba (L.) Desv. and its leaf and stem epidermal micromorphology. Phyton-Int. J. Exp. Bot. 2024, 93, 725–744. [Google Scholar] [CrossRef]
- Do, D.N.; Nguyen, D.P.; Phung, V.D.; Le, X.T.; Le, T.M.; Do, V.M.; Minh, B.Q.; Luu, X.C. Fractionating of lemongrass (Cymbopogon citratus) essential oil by vacuum fractional distillation. Processes 2021, 9, 593. [Google Scholar] [CrossRef]
- Ramírez, J.; Andrade, M.D.; Vidari, G.; Gilardoni, G. Essential oil and major non-volatile secondary metabolites from the leaves of amazonian Piper subscutatum. Plants 2021, 10, 1168. [Google Scholar] [CrossRef]
- Matebie, W.A.; Zhang, W.; Xie, G. Chemical composition and antimicrobial activity of essential oil from Phytolacca dodecandra collected in Ethiopia. Molecules 2019, 24, 342. [Google Scholar] [CrossRef]
- Azizah, N.; Filaila, E.; Salahuddin, S.; Agustian, E.; Sulaswatty, A.; Artanti, N. Antibacterial and antioxidant activities of Indonesian ginger (jahe emprit) essential oil extracted by hydrodistillation. J. Kim. Terap. Indones. 2019, 20, 90–96. [Google Scholar] [CrossRef]
- Ahl, H.A.H.S.-A.; Sabra, A.S.; El Gendy, A.N.G.; Aziz, E.E.; Tkachenko, K.G. Changes in content and chemical composition of Dracocephalum moldavica L. essential oil at different harvest dates. J. Med. Plants Stud. 2015, 3, 61–64. [Google Scholar]
- Hosseini, M.; Jamshidi, A.; Raeisi, M.; Azizzadeh, M. The antibacterial and antioxidant effects of clove (Syzygium aromaticum) and lemon verbena (Aloysia citriodora) essential oils. J. Human Environ. Health Promot. 2019, 5, 86–93. [Google Scholar] [CrossRef]
- Hung, Y.H.R.; Lin, H.J.; Lee, E.C.; Lu, W.J.; Lin, Y.T.; Huang, B.B.; Lin, T.C.; Lin, H.T.V. Effect of lemon essential oil on the microbial control, physicochemical properties, and aroma profiles of peeled shrimp. Lwt 2023, 173, 114340. [Google Scholar] [CrossRef]
- Herrera, J.M.; Pizzolitto, R.P.; Zunino, M.P.; Dambolena, J.S.; Zygadlo, J.A. Effect of fungal volatile organic compounds on a fungus and an insect that damage stored maize. J. Stored Prod. Res. 2015, 62, 74–78. [Google Scholar] [CrossRef]
- Achimón, F.; Pizzolitto, R.P. Volatilome of the maize phytopathogenic fungus Fusarium verticillioides: Potential applications in diagnosis and biocontrol. Pest Manag. Sci. 2024; in press. [Google Scholar] [CrossRef]
- Brito, V.D.; Achimón, F.; Zunino, M.P.; Pizzolitto, R.P. Control of Fusarium verticillioides in maize stored in silo bags with 1-octyn-3-ol. J. Stored Prod. Res. 2024, 106, 102279. [Google Scholar] [CrossRef]
- Young, J.C. Microwave-assisted extraction of the fungal metabolite ergosterol and total fatty acids. J. Agric. Food Chem. 1995, 43, 2904–2910. [Google Scholar] [CrossRef]
- Brito, V.D.; Achimón, F.; Dambolena, J.S.; Pizzolitto, R.P.; Zygadlo, J.A. Trans-2-hexen-1-ol as a tool for the control of Fusarium verticillioides in stored maize grains. J. Stored Prod. Res. 2019, 82, 123–130. [Google Scholar] [CrossRef]
- Shephard, G.S.; Sydenham, E.W.; Thiel, P.G.; Gelderblom, W.C.A. Quantitative determination of fumonisins B1 and B2 by high-performance liquid chromatography with fluorescence detection. J. Liq. Chromatogr. 1990, 13, 2077–2087. [Google Scholar] [CrossRef]
- Di Rienzo, J.A.; Guzmán, A.W.; Casanoves, F. A multiple-comparisons method based on the distribution of the root node distance of a binary tree. J. Agric. Biol. Environ. Stat. 2002, 7, 129–142. [Google Scholar] [CrossRef]
- Zunino, M.P.; Herrera, J.M.; Pizzolitto, R.P.; Rubinstein, H.R.; Zygadlo, J.A.; Dambolena, J.S. Effect of selected volatiles on two stored pests: The fungus Fusarium verticillioides and the maize weevil Sitophilus zeamais. J. Agric. Food Chem. 2015, 63, 7743–7749. [Google Scholar] [CrossRef]
- Rodríguez, A.; Magalí, B.; Usseglio, V.; Camina, J.; Zygadlo, J.A.; Dambolena, J.S.; Zunino, M.P. Phenolic compounds as controllers of Sitophilus zeamais a look at the structure-activity relationship. J. Stored Prod. Res. 2022, 99, 102038. [Google Scholar] [CrossRef]
- Calvimonte, H.; Peschiutta, M.L.; Herrera, J.M.; Zunino, M.P.; Jacquat, A.G.; Usseglio, V.L.; Zygadlo, J.A. Allylic and Non-allylic alcohols against the maize weevil (Sitophilus zeamais): A promising tool for its control. Agric. Res. 2023, 12, 94–103. [Google Scholar] [CrossRef]
- Hansen, E.M.; Munson, A.S.; Wakarchuk, D.; Blackford, D.C.; Graves, A.D.; Stephens, S.S.; Moan, J.E.; Zalom, F. Advances in semiochemical repellents to mitigate host mortality from the spruce beetle (Coleoptera: Curculionidae). J. Econ. Entomol. 2019, 112, 2253–2261. [Google Scholar] [CrossRef] [PubMed]
- Stoeffler, M.; Maier, T.S.; Tolasch, T.; Steidle, J.L.M. Foreign-language skills in rove-beetles? Evidence for chemical mimicry of ant alarm pheromones in myrmecophilous Pella beetles (Coleoptera: Staphylinidae). J. Chem. Ecol. 2007, 33, 1382–1392. [Google Scholar] [CrossRef] [PubMed]
- Harvey, J.A.; Visser, B.; Lammers, M.; Marien, J.; Gershenzon, J.; Ode, P.J.; Heinen, R.; Gols, R.; Ellers, J. Ant-like traits in wingless parasitoids repel attack from wolf spiders. J. Chem. Ecol. 2018, 44, 894–904. [Google Scholar] [CrossRef]
- da Costa, J.G.; Pires, E.V.; Riffel, A.; Birkett, M.A.; Bleicher, E.; Sant’Ana, A.E.G. Differential preference of Capsicum spp. cultivars by Aphis gossypii is conferred by variation in volatile semiochemistry. Euphytica 2011, 177, 299–307. [Google Scholar] [CrossRef]
- Abdelgaleil, S.A.M.; Mohamed, M.I.E.; Badawy, M.E.I.; El-Arami, S.A.A. Fumigant and contact toxicities of monoterpenes to Sitophilus oryzae (L.) and Tribolium castaneum (Herbst) and their inhibitory effects on acetylcholinesterase activity. J. Chem. Ecol. 2009, 35, 518–525. [Google Scholar] [CrossRef]
- Lee, B.H.; Choi, W.S.; Lee, S.E.; Park, B.S. Fumigant toxicity of essential oils and their constituent compounds towards the rice weevil, Sitophilus oryzae (L.). Crop Prot. 2001, 20, 317–320. [Google Scholar] [CrossRef]
- Chaubey, M.K. Fumigant toxicity of essential oils and pure compounds against Sitophilus oryzae L. (Coleoptera: Curculionidae). Biol. Agric. Hortic. 2012, 28, 111–119. [Google Scholar] [CrossRef]
- Fang, R.; Jiang, C.H.; Wang, X.Y.; Zhang, H.M.; Liu, Z.L.; Zhou, L.; Du, S.S.; Deng, Z.W. Insecticidal activity of essential oil of Carum carvi fruits from china and its main components against two grain storage insects. Molecules 2010, 15, 9391–9402. [Google Scholar] [CrossRef]
- Yang, K.; Zhou, Y.X.; Wang, C.F.; Du, S.S.; Deng, Z.W.; Liu, Q.Z.; Liu, Z.L. Toxicity of Rhododendron anthopogonoides essential oil and its constituent compounds towards Sitophilus zeamais. Molecules 2011, 16, 7320–7330. [Google Scholar] [CrossRef] [PubMed]
- Yildirim, E.; Emsen, B.; Kordali, S. Insecticidal effects of monoterpenes on Sitophilus zeamais Motschulsky (Coleoptera: Curculionidae). J. Appl. Bot. Food Qual. 2013, 86, 198–204. [Google Scholar] [CrossRef]
- Grodnitzky, J.A.; Coats, J.R. QSAR evaluation of monoterpenoids’ insecticidal activity. J. Agric. Food Chem. 2002, 50, 4576–4580. [Google Scholar] [CrossRef] [PubMed]
- Germinara, G.S.; De Cristofaro, A.; Rotundo, G. Bioactivity of short-chain aliphatic ketones against adults of the granary weevil, Sitophilus granarius (L.). Pest Manag. Sci. 2012, 68, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.J. The Classification of Insecticides; CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar]
- Emara, T.E. Effect of 6-methyl-5-hepten-2-one on acetylcholinesterase activity, growth and development of Spodoptera litoralis. Egypt. J. Biol. 2004, 6, 136–146. [Google Scholar]
- El-Maghrabey, M.H.; El-Shaheny, R.; El Hamd, M.A.; Al-Khateeb, L.A.; Kishikawa, N.; Kuroda, N. Aldehydes’ sources, toxicity, environmental analysis, and control in food. In Organic Pollutants, Toxicity and Solutions; Springer: Cham, Switzerland, 2022; pp. 117–151. ISBN 9783030724412. [Google Scholar]
- Eder, E.; Hoffman, C.; Bastian, H.; Deininger, C.; Scheckenbach, S. Molecular mechanisms of DNA damage initiated by α,β-unsaturated carbonyl compounds as criteria for genotoxicity and mutagenicity. Environ. Health Perspect. 1990, 88, 99–106. [Google Scholar] [CrossRef]
- Dimock, M.B.; Kennedy, G.G.; Williams, W.G. Toxicity studies of analogs of 2-tridecanone, a naturally occurring toxicant from a wild tomato. J. Chem. Ecol. 1982, 8, 837–842. [Google Scholar] [CrossRef]
- Ferreira-Castro, F.L.; Potenza, M.R.; Rocha, L.O.; Correa, B. Interaction between toxigenic fungi and weevils in corn grain samples. Food Control 2012, 26, 594–600. [Google Scholar] [CrossRef]
- Magan, N.; Hope, R.; Cairns, V.; Aldred, D. Post-harvest fungal ecology: Impact of fungal growth and mycotoxin accumulation in stored grain. Eur. J. Plant Pathol. 2003, 109, 723–730. [Google Scholar] [CrossRef]
- Khan, A.A.; Umar, U.; Ejaz, S.; Riaz, H.; Batool, M.; Chen, T.; Ahmad, F.; Saeed, Q. Insect-facilitated propagation of mycotoxic fungi in grain storages: Association of Sitotroga cerealella Olivier with Aspergillus flavus Link. Int. J. Environ. Sci. Technol. 2023, 20, 231–238. [Google Scholar] [CrossRef]
- Usseglio, V.L.; Dambolena, J.S.; Martinez, M.J.; Zunino, M.P. The role of fumonisins in the biological interaction between Fusarium verticillioides and Sitophilus zeamais. J. Chem. Ecol. 2020, 46, 1059–1068. [Google Scholar] [CrossRef] [PubMed]
- Kandasamy, D.; Gershenzon, J.; Hammerbacher, A. Volatile organic compounds emitted by fungal associates of conifer bark beetles and their potential in bark beetle control. J. Chem. Ecol. 2016, 42, 952–969. [Google Scholar] [CrossRef] [PubMed]
- Korpi, A.; Järnberg, J.; Pasanen, A.L. Microbial volatile organic compounds. Crit. Rev. Toxicol. 2009, 39, 139–193. [Google Scholar] [CrossRef] [PubMed]
- Josselin, L.; Proctor, R.H.; Lippolis, V.; Cervellieri, S.; Hoylaerts, J.; De Clerck, C.; Fauconnier, M.L.; Moretti, A. Does alteration of fumonisin production in Fusarium verticillioides lead to volatolome variation? Food Chem. 2024, 438, 138004. [Google Scholar] [CrossRef]
- Achimón, F.; Brito, V.D.; Pizzolitto, R.P.; Zygadlo, J.A. Effect of carbon sources on the production of volatile organic compounds by Fusarium verticillioides. J. Fungi 2022, 8, 158. [Google Scholar] [CrossRef]
- Becker, E.M.; Herrfurth, C.; Irmisch, S.; Köllner, T.G.; Feussner, I.; Karlovsky, P.; Splivallo, R. Infection of corn ears by Fusarium spp. induces the emission of volatile sesquiterpenes. J. Agric. Food Chem. 2014, 62, 5226–5236. [Google Scholar] [CrossRef]
- Josselin, L.; De Clerck, C.; De Boevre, M.; Moretti, A.; Haïssam Jijakli, M.; Soyeurt, H.; Fauconnier, M.L. Volatile organic compounds emitted by Aspergillus flavus strains producing or not aflatoxin B1. Toxins 2021, 13, 705. [Google Scholar] [CrossRef]
- Li, H.; Kang, X.; Wang, S.; Mo, H.; Xu, D.; Zhou, W.; Hu, L. Early detection and monitoring for Aspergillus flavus contamination in maize kernels. Food Control 2021, 121, 107636. [Google Scholar] [CrossRef]
- Cartagena, E.; Marcinkevicius, K.; Luciardi, C.; Rodríguez, G.; Bardón, A.; Arena, M.E. Activity of a novel compound produced by Aspergillus parasiticus in the presence of red flour beetle Tribolium castaneum against Pseudomonas aeruginosa and coleopteran insects. J. Pest Sci. 2014, 87, 521–530. [Google Scholar] [CrossRef]
- Roze, L.V.; Chanda, A.; Laivenieks, M.; Beaudry, R.M.; Artymovich, K.A.; Koptina, A.V.; Awad, D.W.; Valeeva, D.; Jones, A.D.; Linz, J.E. Volatile profiling reveals intracellular metabolic changes in Aspergillus parasiticus: VeA regulates branched chain amino acid and ethanol metabolism. BMC Biochem. 2010, 11, 33. [Google Scholar] [CrossRef]
- Dalvi, R.R.; Salunkhe, D.K. Toxicological implications of pesticides: Their toxic effects on seeds of food plants. Toxicology 1975, 3, 269–285. [Google Scholar] [CrossRef] [PubMed]
- Brito, V.D.; Achimón, F.; Pizzolitto, R.P.; Ramírez Sánchez, A.; Gómez Torres, E.A.; Zygadlo, J.A.; Zunino, M.P. An alternative to reduce the use of the synthetic insecticide against the maize weevil Sitophilus zeamais through the synergistic action of Pimenta racemosa and Citrus sinensis essential oils with chlorpyrifos. J. Pest Sci. 2020, 94, 409–421. [Google Scholar] [CrossRef]
- Boukaew, S.; Prasertsan, P.; Sattayasamitsathit, S. Evaluation of antifungal activity of essential oils against aflatoxigenic Aspergillus flavus and their allelopathic activity from fumigation to protect maize seeds during storage. Ind. Crops Prod. 2017, 97, 558–566. [Google Scholar] [CrossRef]

| Fungal Species | Concentration (mM) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| MIC b | 0 (Control) | 0.03 | 0.06 | 0.13 | 0.27 | 0.54 | 1.06 | 2.12 | 4.24 | |
| F. verticillioides | 3.5 | 0.0 ± 0.0 | 23.5 ± 0.0 | 29.1 ± 0.3 | 46.3 ± 0.4 | 56.0 ± 0.6 | 63.4 ± 0.6 | 75.2 ± 0.4 | 87.2 ± 0.6 | 100.0 ± 0.0 |
| A. parasiticus | 3.8 | 0.0 ± 0.0 | 1.3 ± 0.4 | 8.4 ± 0.0 | 12.9 ± 0.6 | 20.0 ± 0.4 | 35.9 ± 1.1 | 48.5 ± 0.7 | 71.2 ± 0.8 | 100.0 ± 0.0 |
| A. flavus | 3.9 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 1.5 ± 0.2 | 13.9 ± 1.0 | 30.0 ± 0.6 | 41.3 ± 0.5 | 60.2 ± 0.7 | 100.0 ± 0.0 |
| Silo-Bag Treatment | Ergosterol (µg/g Maize) | Fumonisin B1 (µg FB1/ µg Ergosterol) | Mortality of S. zeamais (%) |
|---|---|---|---|
| F. verticillioides + A. flavus + A. parasiticus | 100.11 ± 5.60 (c) | 0.086 ± 0.015 (c) | - |
| F. verticillioides + A. flavus + A. parasiticus + S. zeamais | 142.18 ± 8.82 (d) | 0.073 ± 0.014 (c) | 32.24 ± 3.68 (b) |
| F. verticillioides + A. flavus + A. parasiticus + sulcatone | 41.82 ± 3.54 (b) | 0.032 ± 0.08 (b) | - |
| F. verticillioides + A. flavus + A. parasiticus + S. zeamais + sulcatone | 42.42 ± 5.29 (b) | 0.036 ± 0.03 (b) | 85.09 ± 1.46 (c) |
| S. zeamais | 17.88 ± 0.77 (a) | 0.00 ± 0.00 (a) | 8.76 ± 2.89 (a) |
| S. zeamais + sulcatone | 16.51 ± 1.59 (a) | 0.00 ± 0.00 (a) | 71.69 ± 1.57 (b) |
| Control (maize) | 9.87 ± 0.75 (a) | 0.00 ± 0.00 (a) | - |
| Concentration | Seed Vigour (%) |
|---|---|
| Control | 100 ± 0 (a) |
| LC95 | 94.9 ± 0.3 (a) |
| Repellent | 91.5 ± 10.6 (a) |
| 2 × MIC | 79.7 ± 1.0 (a) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Achimón, F.; Peschiutta, M.L.; Brito, V.D.; Ulla, S.B.; Pizzolitto, R.P. Sulcatone as a Plant-Derived Volatile Organic Compound for the Control of the Maize Weevil and Its Associated Phytopathogenic Fungi in Stored Maize. Plants 2024, 13, 2893. https://doi.org/10.3390/plants13202893
Achimón F, Peschiutta ML, Brito VD, Ulla SB, Pizzolitto RP. Sulcatone as a Plant-Derived Volatile Organic Compound for the Control of the Maize Weevil and Its Associated Phytopathogenic Fungi in Stored Maize. Plants. 2024; 13(20):2893. https://doi.org/10.3390/plants13202893
Chicago/Turabian StyleAchimón, Fernanda, Maria L. Peschiutta, Vanessa D. Brito, Sofia B. Ulla, and Romina P. Pizzolitto. 2024. "Sulcatone as a Plant-Derived Volatile Organic Compound for the Control of the Maize Weevil and Its Associated Phytopathogenic Fungi in Stored Maize" Plants 13, no. 20: 2893. https://doi.org/10.3390/plants13202893
APA StyleAchimón, F., Peschiutta, M. L., Brito, V. D., Ulla, S. B., & Pizzolitto, R. P. (2024). Sulcatone as a Plant-Derived Volatile Organic Compound for the Control of the Maize Weevil and Its Associated Phytopathogenic Fungi in Stored Maize. Plants, 13(20), 2893. https://doi.org/10.3390/plants13202893






