Nitrogen Level Impacts the Dynamic Changes in Nitrogen Metabolism, and Carbohydrate and Anthocyanin Biosynthesis Improves the Kernel Nutritional Quality of Purple Waxy Maize
Abstract
1. Introduction
2. Materials and Methods
2.1. Material Planting and Preparing
2.2. Anthocyanin Quantification
2.3. N Metabolism Product Content Quantification
2.3.1. The Total N Content
2.3.2. Nitrite Content
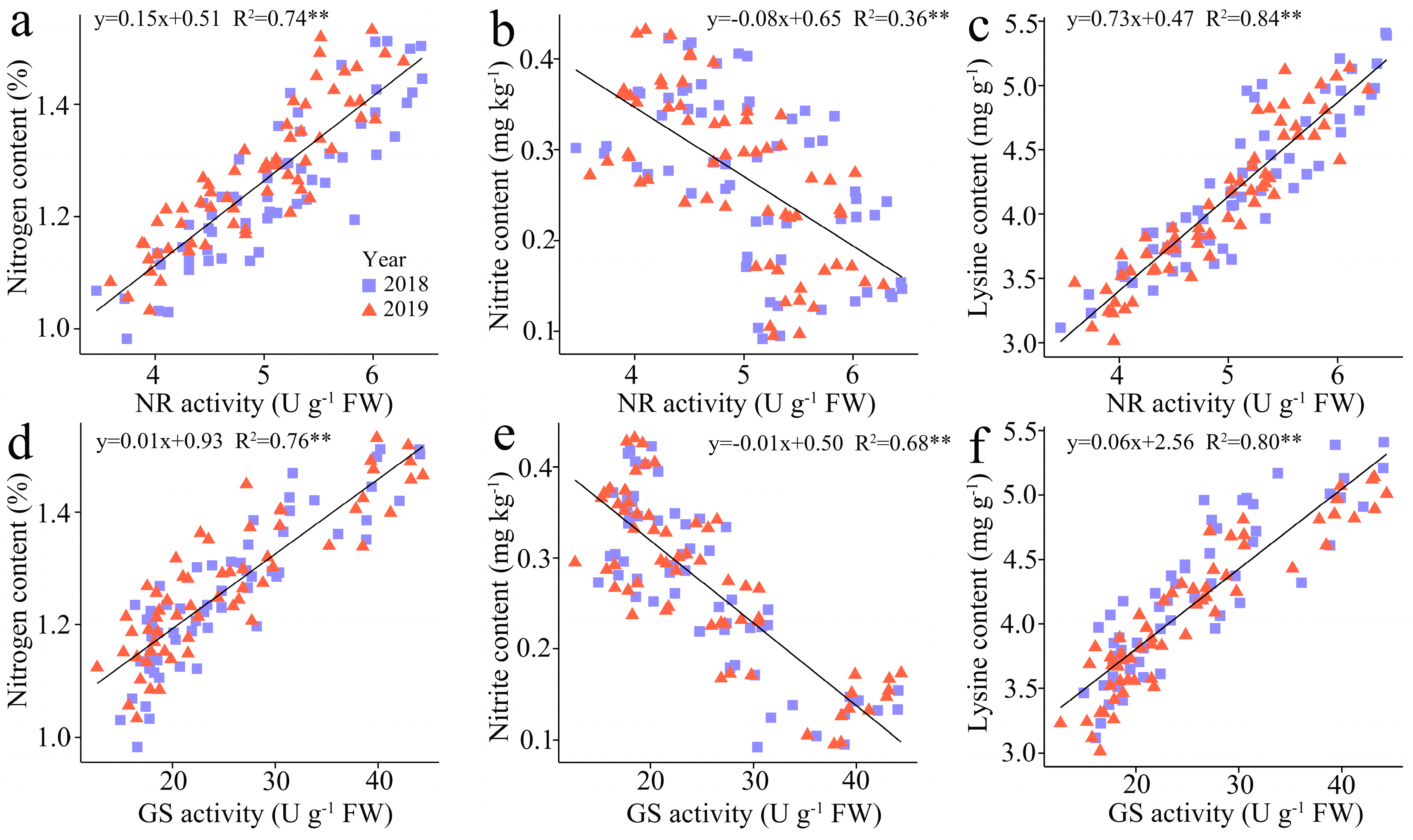
2.3.3. Lysine Content
2.4. Carbohydrate Content Determination
2.4.1. Soluble Sugars
2.4.2. Amylopectin Content
2.5. Determination of Enzyme Activities
2.5.1. Anthocyanin Metabolism Enzyme
2.5.2. Nitrate Reductase (NR) and Glutamine Synthetase (GS)
2.5.3. Carbon-Metabolism-Related Enzymes
2.6. Data Analysis
3. Results
3.1. Analysis of Variances (ANOVA)
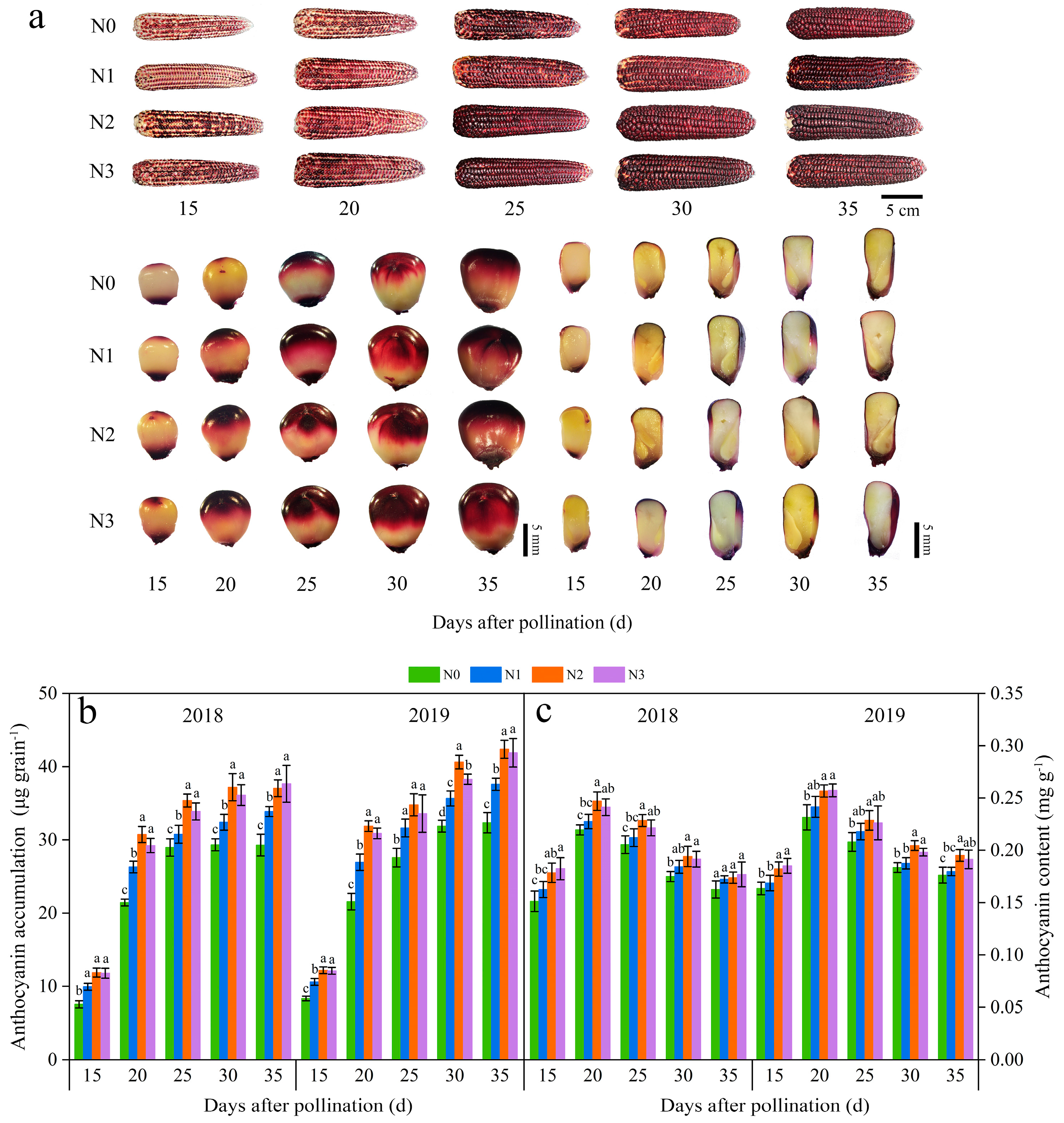
3.2. Dynamic Changes of Anthocyanin Biosynthesis in Grains of Purple Waxy Maize under Different N Application Rates
3.2.1. The Seed Color and Anthocyanin Accumulation
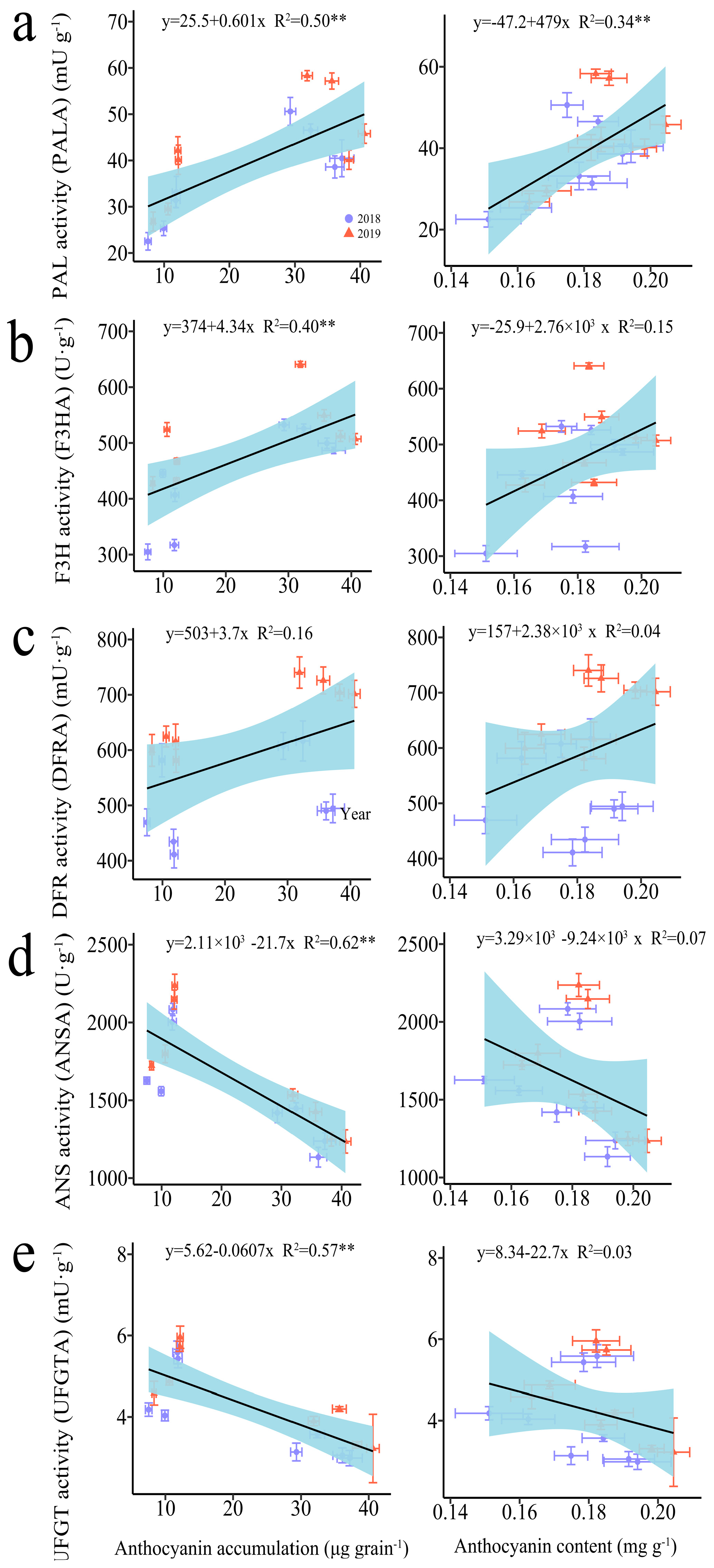
3.2.2. The Anthocyanin-Biosynthesis-Related Enzymes
3.2.3. The Regression Analysis
3.3. Dynamic Changes in N Metabolism in Grains of Purple Waxy Maize under Different N Application Rates
3.3.1. The Total N Content (TNC)
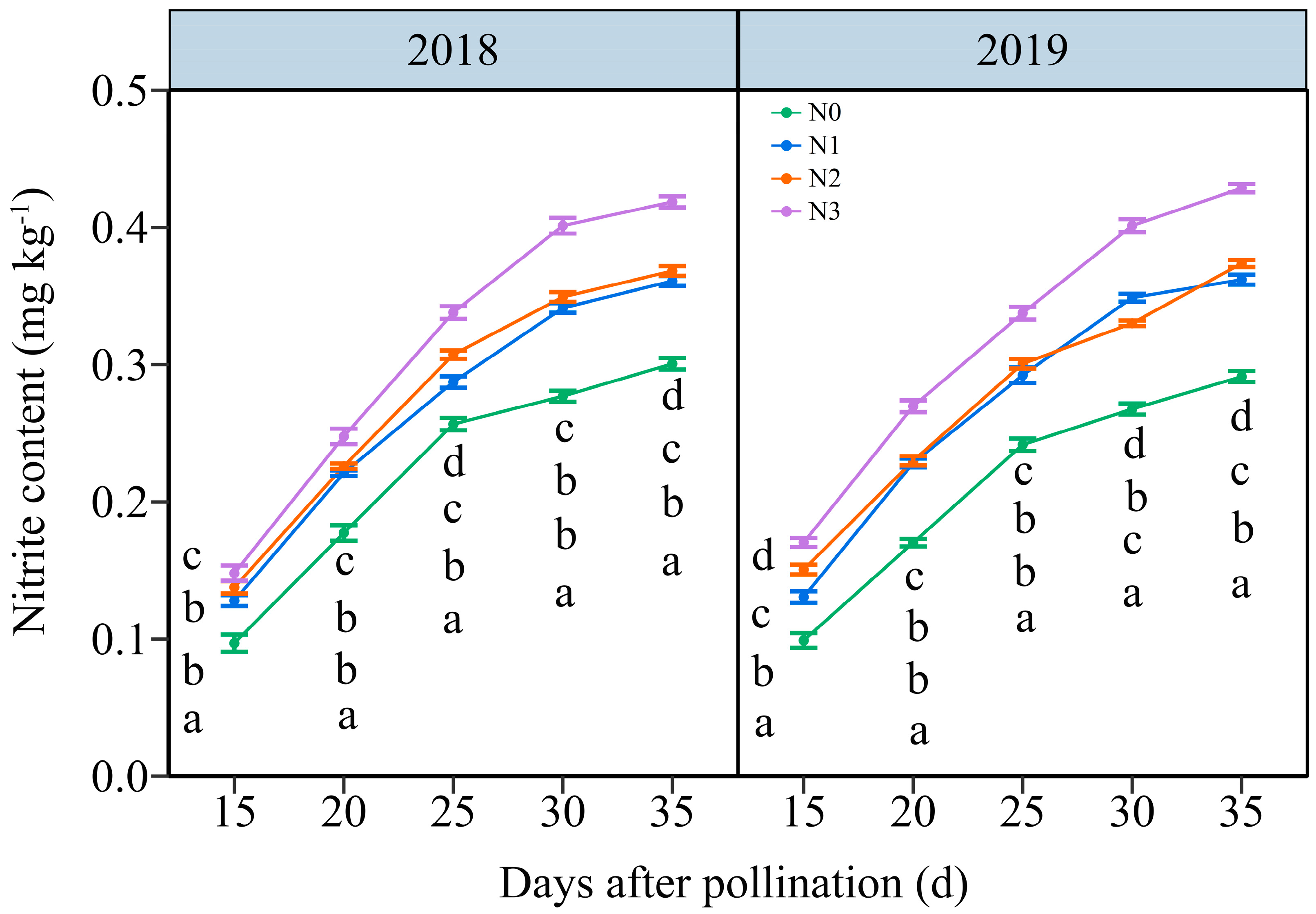
3.3.2. The Nitrite Content (NC)
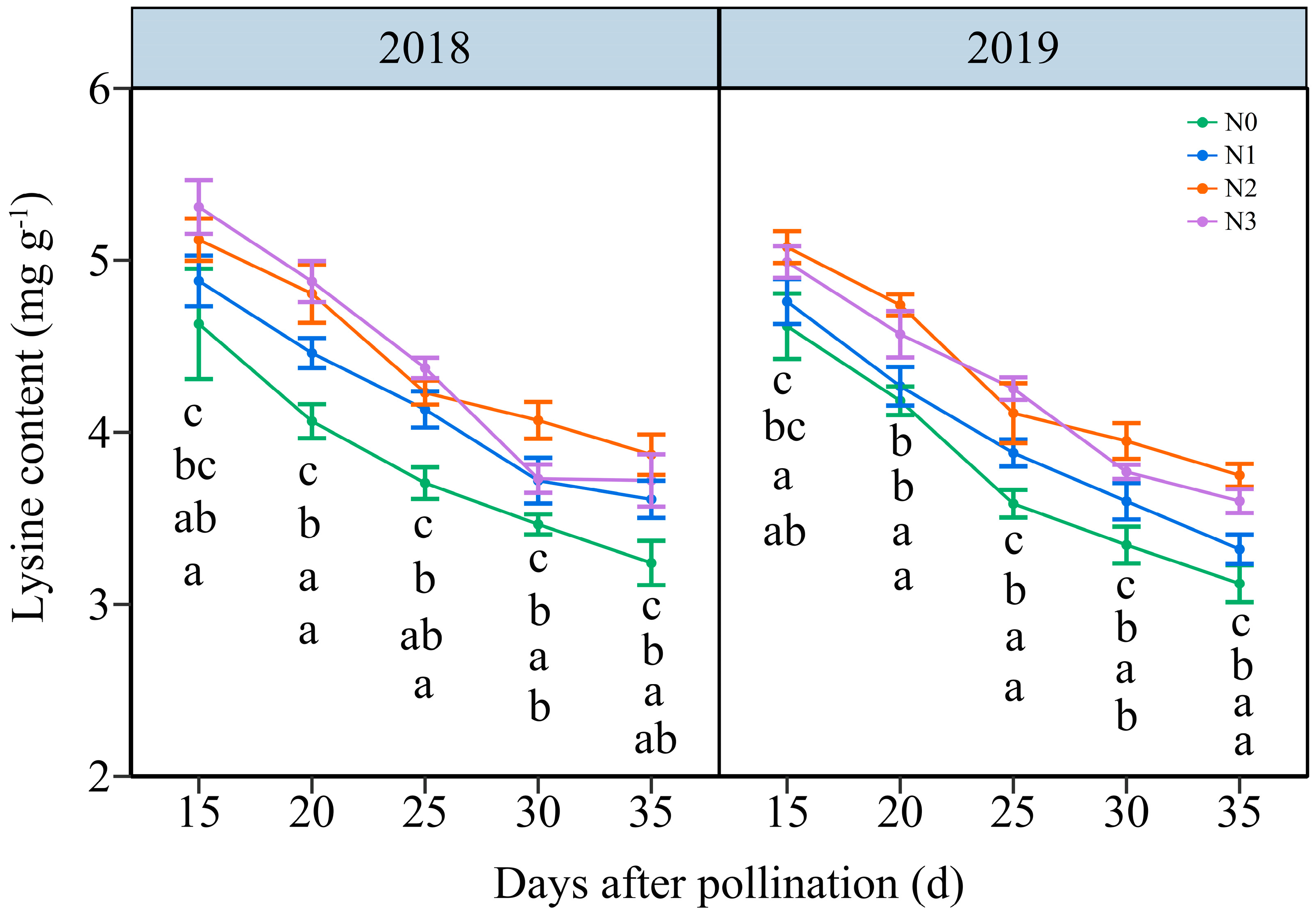
3.3.3. The Lysine Content (LC)
3.3.4. Nitrate Reductase (NR) and Glutamine Synthetase (GS) Activity
3.3.5. The Regression Analysis
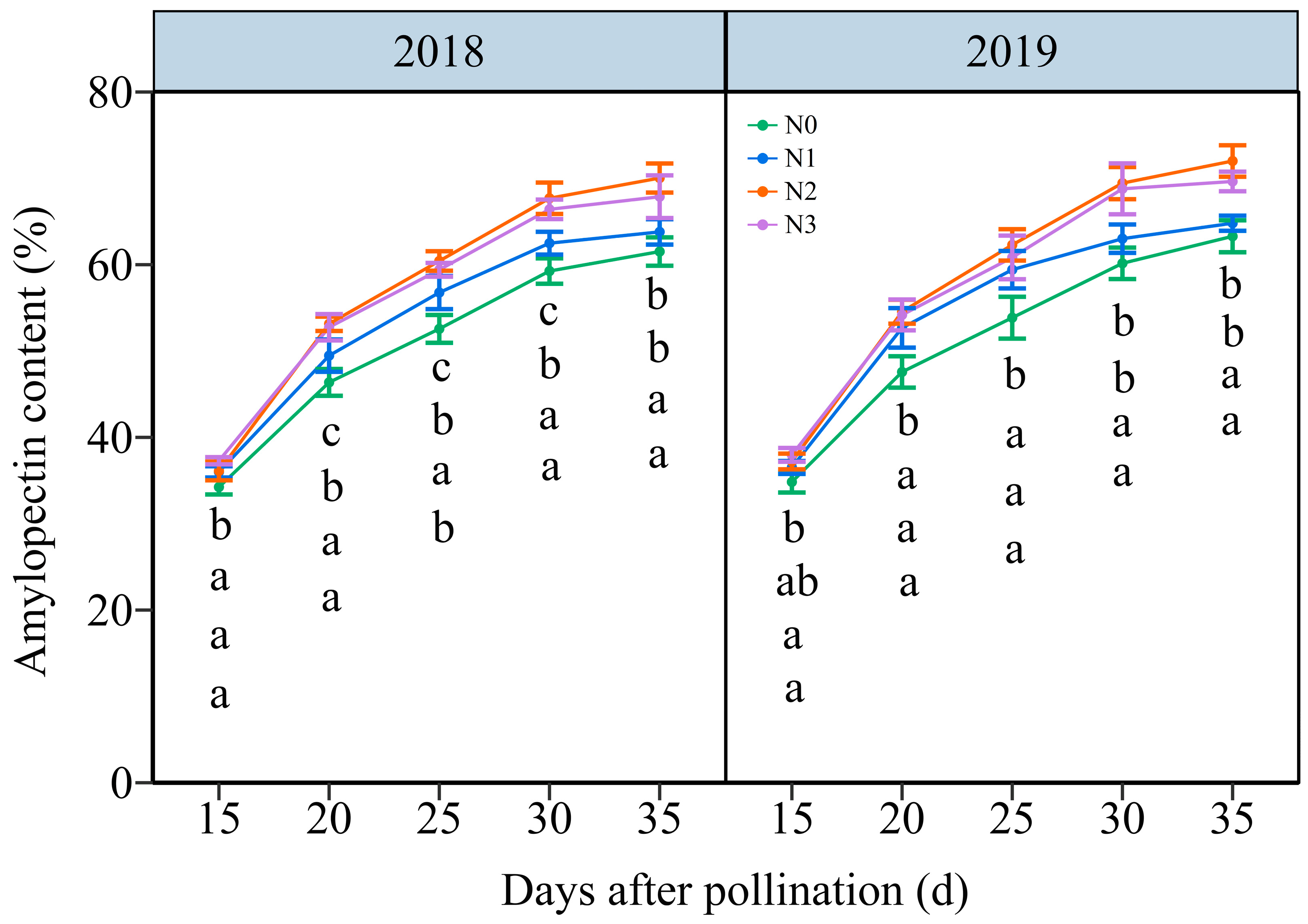
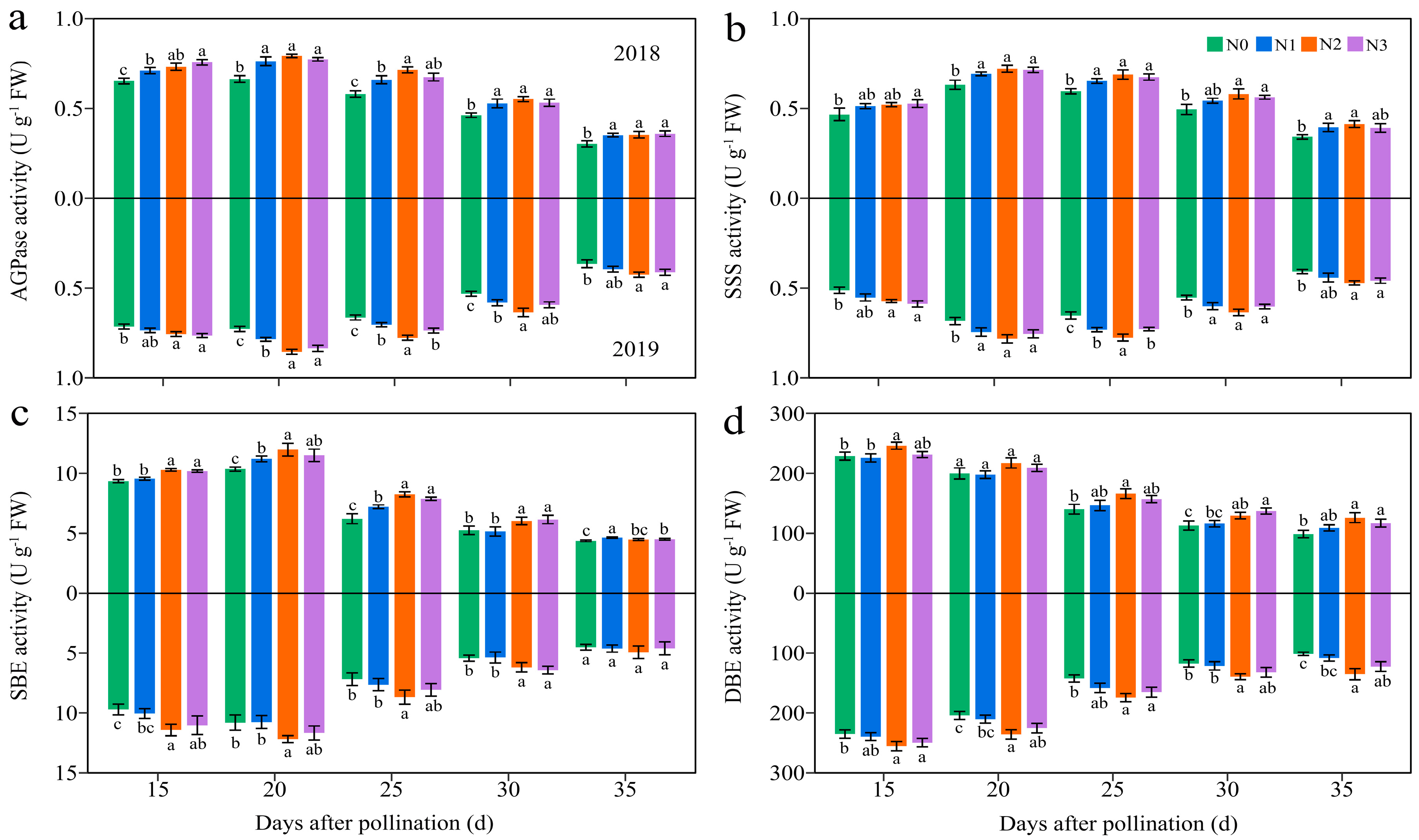
3.4. Dynamic Changes in Carbohydrate Biosynthesis in Grains of Purple Waxy Maize under Different N Application Rates
3.4.1. The Soluble Sugar Content (SSC)
3.4.2. The Amylopectin Content (AC)
3.4.3. Carbohydrate-Metabolism-Related Enzymes
3.4.4. The Regression Analysis
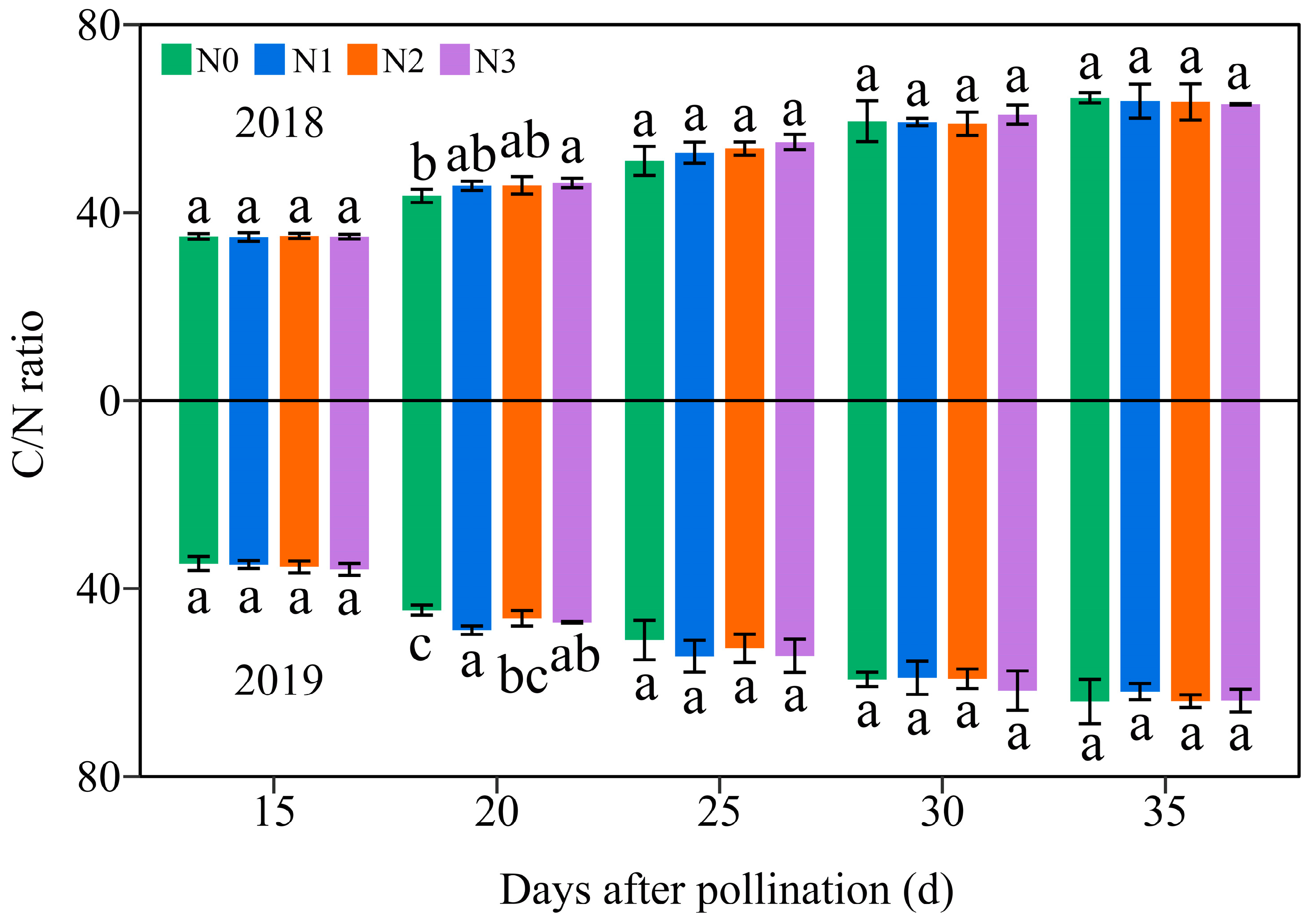
3.5. Effects of the N Levels on the Dynamic C/N Ratio in Grains of Purple Waxy Maize
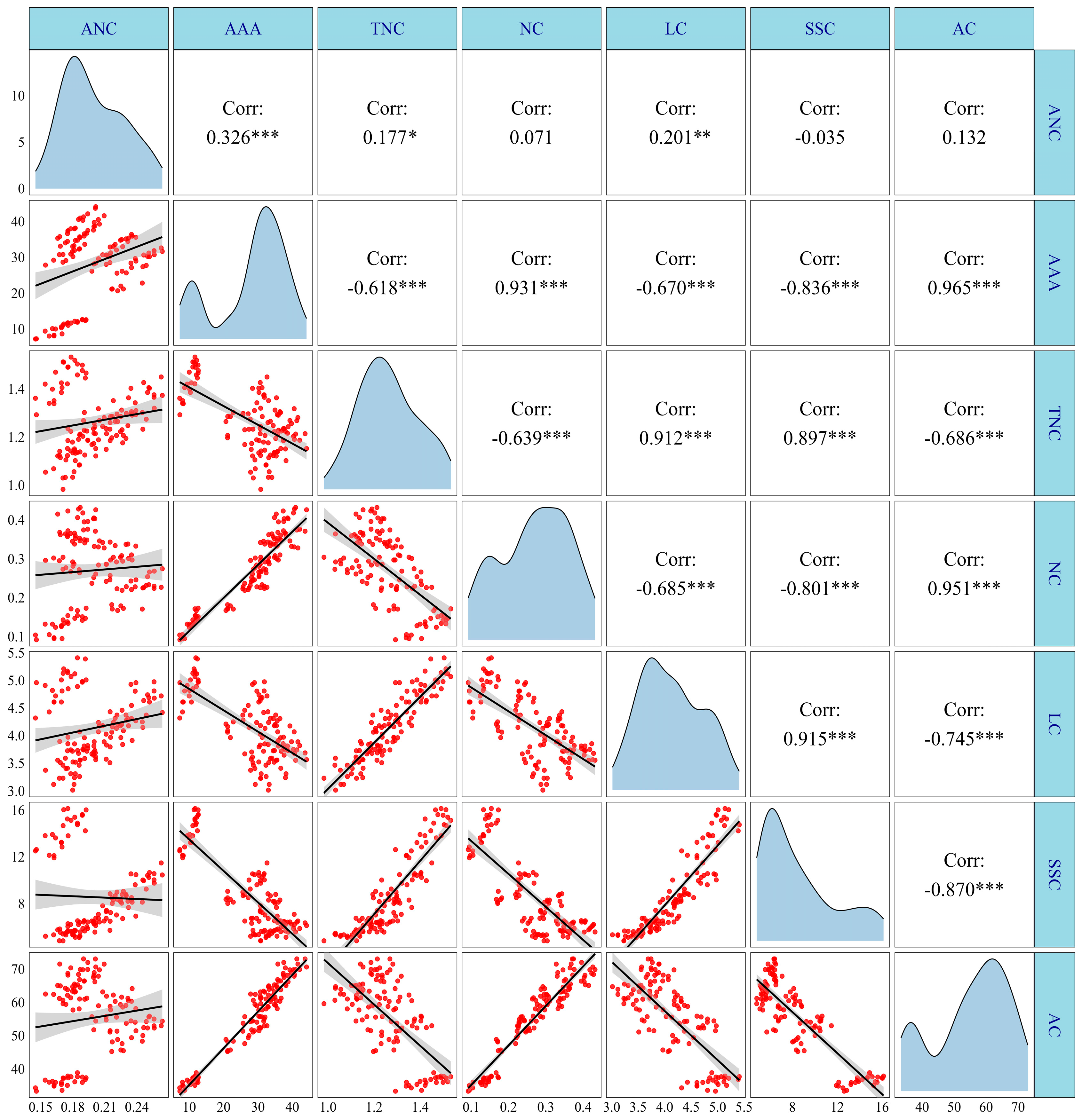
3.6. Correlation Analysis
4. Discussion
4.1. The Appropriate N Application Rate Promotes Nitrogen Metabolism in Waxy Maize Kernels
4.2. The Reasonable Application of N Induces Carbohydrate Biosynthesis
4.3. The Reasonable Application of N Promotes Anthocyanin Biosynthesis in the Kernels of Purple Waxy Maize
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kang, Y.; Chen, M.; Wan, S. Effects of drip irrigation with saline water on waxy maize (Zea mays L. var. ceratina Kulesh) in North China Plain. Agric. Water Manag. 2010, 97, 1303–1309. [Google Scholar] [CrossRef]
- Schwartz, D.; Whistler, R.L. History and Future of Starch, In Starch, 3rd ed.; Academic Press: Cambridge, MA, USA, 2009; pp. 1–10. [Google Scholar]
- Choe, E.; Rocheford, T.R. Genetic and QTL analysis of pericarp thickness and ear architecture traits of Korean waxy corn germplasm. Euphytica 2012, 183, 243–260. [Google Scholar] [CrossRef]
- Lim, S.; Yi, G. Investigating seed mineral composition in Korean landrace maize(Zea mays L.)and its kernel texture specificity. J. Integr. Agric. 2019, 18, 1996–2005. [Google Scholar] [CrossRef]
- Kim, J.T.; Yi, G.; Chung, I.M.; Son, B.Y.; Bae, H.H.; Go, Y.S.; Ha, J.Y.; Baek, S.B.; Kim, S.L. Timing and Pattern of Anthocyanin Accumulation during Grain Filling in Purple Waxy Corn (Zea mays L.) Suggest Optimal Harvest Dates. ACS Omega 2020, 5, 15702–15708. [Google Scholar] [CrossRef] [PubMed]
- Lage, N.N.; Layosa, M.A.A.; Arbizu, S.; Chew, B.P.; Pedrosa, M.L.; Mertens-Talcott, S.; Talcott, S.; Noratto, G.D. Dark sweet cherry (Prunus avium) phenolics enriched in anthocyanins exhibit enhanced activity against the most aggressive breast cancer subtypes without toxicity to normal breast cells. J. Funct. Foods 2020, 64, 103710. [Google Scholar] [CrossRef]
- Hu, M.; Du, J.; Du, L.; Luo, Q.; Xiong, J. Anti-fatigue activity of purified anthocyanins prepared from purple passion fruit (P. edulis Sim) epicarp in mice. J. Funct. Foods 2020, 65, 103725. [Google Scholar] [CrossRef]
- Kong, J.M.; Chia, L.S.; Goh, N.K.; Chia, T.F.; Brouillard, R. Analysis and biological activities of anthocyanins. Phytochemistry 2003, 64, 923–933. [Google Scholar] [CrossRef]
- Khusnutdinov, E.; Sukhareva, A.; Panfilova, M.; Mikhaylova, E. Anthocyanin biosynthesis genes as model genes for genome editing in plants. Int. J. Mol. Sci. 2021, 22, 8752. [Google Scholar] [CrossRef]
- Gonzalez, A.; Zhao, M.; Leavitt, J.M.; Lloyd, A.M. Regulation of the anthocyanin biosynthetic pathway by the TTG1/bHLH/Myb transcriptional complex in arabidopsis seedlings. Plant J. 2008, 53, 814–827. [Google Scholar] [CrossRef]
- Jaakola, L. New insights into the regulation of anthocyanin biosynthesis in fruits. Trends Plant Sci. 2013, 18, 477–483. [Google Scholar] [CrossRef]
- Shoeva, O.Y.; Glagoleva, A.Y.; Khlestkina, E.K. The factors affecting the evolution of the anthocyanin biosynthesis pathway genes in monocot and dicot plant species. BMC Plant Biol. 2017, 17, 256. [Google Scholar] [CrossRef] [PubMed]
- Cai, T.; Ge-Zhang, S.; Song, M. Anthocyanins in metabolites of purple corn. Front. Plant Sci. 2023, 14, 1154535. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Ren, Y.; Fu, H.; Li, Z.; Kong, F.; Yuan, J. Cultivar differences in carbon and nitrogen accumulation, balance, and grain yield in maize. Front. Plant Sci. 2022, 13, 992041. [Google Scholar] [CrossRef] [PubMed]
- Swank, J.C.; Below, F.E.; Lambert, R.J.; Hageman, R.H. Interaction of carbon and nitrogen metabolism in the productivity of maize 1. Plant Physiol. 1982, 70, 1185–1190. [Google Scholar] [CrossRef]
- He, P.; Zhou, W.; Jin, J. Carbon and nitrogen metabolism related to grain formation in two different senescent types of maize. J. Plant Nutr. 2004, 27, 295–311. [Google Scholar] [CrossRef]
- Liu, X.; Hu, B.; Chu, C. Nitrogen assimilation in plants: Current status and future prospects. J. Genet. Genom. = Yi Chuan Xue Bao 2022, 49, 394–404. [Google Scholar] [CrossRef]
- Smith, A.M.; Zeeman, S.C. Starch: A flexible, adaptable carbon store coupled to plant growth. Annu. Rev. Plant Biol. 2020, 71, 217–245. [Google Scholar] [CrossRef]
- Leghari, S.J.; Wahocho, N.A.; Laghari, G.M.; HafeezLaghari, A.; MustafaBhabhan, G.; HussainTalpur, K.; Bhutto, T.A.; Wahocho, S.A.; Lashari, A.A. Role of nitrogen for plant growth and development: A review. Adv. Environ. Biol. 2016, 10, 209–219. [Google Scholar]
- Below, F.E. Nitrogen metabolism and crop productivity. In Handbook of Plant and Crop Physiology; CRC Press: Boca Raton, FL, USA, 2001; pp. 407–428. [Google Scholar]
- Paponov, I.; Sambo, P.; Erley, G.S.a.m.; Presterl, T.; Geiger, H.; Engels, C. Grain yield and kernel weight of two maize genotypes differing in nitrogen use efficiency at various levels of nitrogen and carbohydrate availability during flowering and grain filling. Plant Soil 2005, 272, 111–123. [Google Scholar] [CrossRef]
- Mueller, S.M.; Vyn, T.J. Maize plant resilience to n stress and post-silking n capacity changes over time: A review. Front. Plant Sci. 2016, 7, 53. [Google Scholar] [CrossRef]
- Chen, Y.; Xiao, C.; Wu, D.; Xia, T.; Chen, Q.; Chen, F.; Yuan, L.; Mi, G. Effects of nitrogen application rate on grain yield and grain nitrogen concentration in two maize hybrids with contrasting nitrogen remobilization efficiency. Eur. J. Agron. 2015, 62, 79–89. [Google Scholar] [CrossRef]
- Galili, G.; Amir, R. Fortifying plants with the essential amino acids lysine and methionine to improve nutritional quality. Plant Biotechnol. J. 2013, 11, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Ganie, A.H.; Ahmad, A.; Yousuf, P.Y.; Pandey, R.; Ahmad, S.; Aref, I.M.; Iqbal, M. Nitrogen-regulated changes in total amino acid profile of maize genotypes having contrasting response to nitrogen deficit. Protoplasma 2017, 254, 2143–2153. [Google Scholar] [CrossRef]
- Su, N.; Wu, Q.; Cui, J. Increased sucrose in the hypocotyls of radish sprouts contributes to nitrogen deficiency-induced anthocyanin accumulation. Front. Plant Sci. 2016, 7, 1976. [Google Scholar] [CrossRef] [PubMed]
- Yamuangmorn, S.; Dell, B.; Rerkasem, B.; Prom, U.T.C. Applying nitrogen fertilizer increased anthocyanin in vegetative shoots but not in grain of purple rice genotypes. J. Sci. Food Agric. 2018, 98, 4527–4532. [Google Scholar] [CrossRef]
- Feng, W.; Xue, W.; Zhao, Z.; Shi, Z.; Wang, W.; Wang, H.; Qiu, P.; Xue, J.-F.; Chen, B. Nitrogen fertilizer application rate affects the dynamic metabolism of nitrogen and carbohydrates in kernels of waxy maize. Front. Plant Sci. 2024, 15, 1416397. [Google Scholar] [CrossRef]
- Campbell, W.H. Nitrate reductase structure, function and regulation: Bridging the gap between biochemistry and physiology. Annu. Rev. Plant Biol. 1999, 50, 277–303. [Google Scholar] [CrossRef]
- Ronald, E.; Wrolstada, R.W.D.; Lee, J. Tracking color and pigment changes in anthocyanin products. Trends Food Sci. Technol. 2005, 16, 423–428. [Google Scholar] [CrossRef]
- Nelson, D.W.; Sommers, L.E. Determination of total nitrogen in plant material. Agron. J. 1962, 65, 109–112. [Google Scholar] [CrossRef]
- Narayana, B.; Sunil, K. A spectrophotometric method for the determination of nitrite and nitrate. Eurasian J. Anal. Chem. 2009, 4, 204–214. [Google Scholar]
- Bouzid, H.; Cavalcante Filho, C.; Marques, J.; Valente, A.; Gando-Ferreira, L. A New Approach on the Amino Acid Lysine Quantification by UV-Visible Spectrophotometry. Rev. Chim. 2020, 71, 159–175. [Google Scholar] [CrossRef]
- Wood, I.P.; Elliston, A.; Ryden, P.; Bancroft, I.; Roberts, I.N.; Waldron, K.W. Rapid quantification of reducing sugars in biomass hydrolysates: Improving the speed and precision of the dinitrosalicylic acid assay. Biomass Bioenergy 2012, 44, 117–121. [Google Scholar] [CrossRef]
- Jiang, D.; Cao, W.; Dai, T.; Jing, Q. Activities of key enzymes for starch synthesis in relation to growth of superior and inferior grains on winter wheat (Triticum aestivum L.) spike. Plant Growth Regulation 2003, 41, 247–257. [Google Scholar] [CrossRef]
- Nakamura, Y.; Yuki, K.; Park, S.-Y.; Ohya, T. Carbohydrate metabolism in the developing endosperm of rice grains. Plant Cell Physiol. 1989, 30, 833–839. [Google Scholar] [CrossRef]
- Yue, K.; Li, L.; Xie, J.; Liu, Y.; Xie, J.; Anwar, S.; Fudjoe, S.K. Nitrogen supply affects yield and grain filling of maize by regulating starch metabolizing enzyme activities and endogenous hormone contents. Front. Plant Sci. 2021, 12, 798119. [Google Scholar] [CrossRef]
- Olmedo Pico, L.B.; Vyn, T.J. Dry matter gains in maize kernels are dependent on their nitrogen accumulation rates and duration during grain filling. Plants 2021, 10, 1222. [Google Scholar] [CrossRef]
- Mondal, S.; Kumar, R.; Mishra, J.S.; Dass, A.; Kumar, S.; Vijay, K.V.; Kumari, M.; Khan, S.R.; Singh, V.K. Grain nitrogen content and productivity of rice and maize under variable doses of fertilizer nitrogen. Heliyon 2023, 9, e17321. [Google Scholar] [CrossRef]
- Wu, X.; Tong, L.; Kang, S.; Du, T.; Ding, R.; Li, S.; Chen, Y. Combination of suitable planting density and nitrogen rate for high yield maize and their source-sink relationship in Northwest China. J. Sci. Food Agric. 2023, 103, 5300–5311. [Google Scholar] [CrossRef]
- Rossini, M.A.; Maddonni, G.A.; Otegui, M.E. Inter-plant competition for resources in maize crops grown under contrasting nitrogen supply and density: Variability in plant and ear growth. Field Crops Res. 2011, 121, 373–380. [Google Scholar] [CrossRef]
- Zhou, T.; Zhou, Q.; Li, E.; Yuan, L.; Wang, W.; Zhang, H.; Liu, L.; Wang, Z.; Yang, J.; Gu, J. Effects of nitrogen fertilizer on structure and physicochemical properties of ‘super’ rice starch. Carbohydr. Polym. 2020, 239, 116237. [Google Scholar] [CrossRef]
- Chamizo-Ampudia, A.; Sanz-Luque, E.; Llamas, A.; Galvan, A.; Fernandez, E. Nitrate reductase regulates plant nitric oxide homeostasis. Trends Plant Sci. 2017, 22, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Miflin, B.J.; Habash, D.Z. The role of glutamine synthetase and glutamate dehydrogenase in nitrogen assimilation and possibilities for improvement in the nitrogen utilization of crops. J. Exp. Bot. 2002, 53, 979–987. [Google Scholar] [CrossRef]
- Kotopoulou, S.; Zampelas, A.; Magriplis, E. Dietary nitrate and nitrite and human health: A narrative review by intake source. Nutr. Rev. 2022, 80, 762–773. [Google Scholar] [CrossRef] [PubMed]
- Keller, R.M.; Beaver, L.; Prater, M.C.; Hord, N.G. Dietary nitrate and nitrite concentrations in food patterns and dietary supplements. Nutr. Today 2020, 55, 218–226. [Google Scholar] [CrossRef]
- Yang, Q.; Zhao, D.; Zhang, C.; Sreenivasulu, N.; Sun, S.S.-M.; Liu, Q. Lysine biofortification of crops to promote sustained human health in the 21st century. J. Exp. Bot. 2022, 73, 1258–1267. [Google Scholar] [CrossRef]
- Prasanna, B.M.; Palacios-Rojas, N.; Hossain, F.; Muthusamy, V.; Menkir, A.; Dhliwayo, T.; Ndhlela, T.; San Vicente, F.; Nair, S.K.; Vivek, B.S. Molecular breeding for nutritionally enriched maize: Status and prospects. Front. Genet. 2020, 10, 1392. [Google Scholar] [CrossRef]
- Wani, S.H.; Vijayan, R.; Choudhary, M.; Kumar, A.; Zaid, A.; Singh, V.; Kumar, P.; Yasin, J.K. Nitrogen use efficiency (NUE): Elucidated mechanisms, mapped genes and gene networks in maize (Zea mays L.). Physiol. Mol. Biol. Plants Int. J. Funct. Plant Biol. 2021, 27, 2875–2891. [Google Scholar] [CrossRef]
- Vijayalakshmi, P.; Kiran, T.V.; Rao, Y.V.; Srikanth, B.; Rao, I.S.; Sailaja, B.; Surekha, K.; Rao, P.R.; Subrahmanyam, D.; Neeraja, C.N.; et al. Physiological approaches for increasing nitrogen use efficiency in rice. Indian J. Plant Physiol. 2013, 18, 208–222. [Google Scholar] [CrossRef]
- Ma, R.; Jiang, C.; Shou, N.; Gao, W.; Yang, X. An optimized nitrogen application rate and basal topdressing ratio improves yield, quality, and water- and N-use efficiencies for forage aaize (Zea mays L.). Agronomy 2023, 13, 181. [Google Scholar] [CrossRef]
- Li, W.; Shan, Y.; Xiao, X.; Zheng, J.; Luo, Q.; Ouyang, S.; Zhang, G. Effect of nitrogen and sulfur fertilization on accumulation characteristics and physicochemical properties of A- and B-wheat starch. J. Agric. Food Chem. 2013, 61, 2418–2425. [Google Scholar] [CrossRef]
- Lv, X.; Ding, Y.; Long, M.; Liang, W.; Gu, X.; Liu, Y.; Wen, X. Effect of foliar application of various nitrogen forms on starch accumulation and grain filling of wheat (Triticum aestivum L.) under drought stress. Front. Plant Sci. 2021, 12, 645379. [Google Scholar] [CrossRef] [PubMed]
- Bing, Y.I.; Zhou, Y.F.; Gao, M.Y.; Zhang, Z.; Yi, H.A.N.; Yang, G.D.; Wenjuan, X.U.; Huang, R.D. Effect of drought stress during flowering stage on starch accumulation and starch synthesis enzymes in sorghum grains. J. Integr. Agric. 2014, 13, 2399–2406. [Google Scholar]
- Krapp, A.; Berthomé, R.; Orsel, M.; Mercey-Boutet, S.; Yu, A.; Castaings, L.; Elftieh, S.; Major, H.; Renou, J.-P.; Daniel-Vedele, F. Arabidopsis roots and shoots show distinct temporal adaptation patterns toward nitrogen starvation. Plant Physiol. 2011, 157, 1255–1282. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Li, W.; Qi, J.; Shi, P.; Yin, Y. Starch accumulation, activities of key enzyme and gene expression in starch synthesis of wheat endosperm with different starch contents. J. Food Sci. Technol. 2014, 51, 419–429. [Google Scholar] [CrossRef]
- Li, H.; Liang, X.; Chen, Y.; Lian, Y.; Tian, G.; Ni, W. Effect of nitrification inhibitor DMPP on nitrogen leaching, nitrifying organisms, and enzyme activities in a rice-oilseed rape cropping system. J. Environ. Sci. 2008, 20, 149–155. [Google Scholar] [CrossRef]
- Li, Y.; Lu, W.; Lyu, D.; Su, F.; Liu, S.; Li, H.; Wang, X.; Liu, Z.; Hu, L. Effects of different nitrogen application rates on starch accumulation, starch synthase gene expression and enzyme activity in two distinctive potato cultivars. Potato Res. 2018, 61, 309–326. [Google Scholar] [CrossRef]
- Li, Z.; Ahammed, G.J. Plant stress response and adaptation via anthocyanins: A review. Plant Stress 2023, 10, 100230. [Google Scholar] [CrossRef]
- Nemie-Feyissa, D.; Olafsdottir, S.M.; Heidari, B.; Lillo, C. Nitrogen depletion and small R3-MYB transcription factors affecting anthocyanin accumulation in Arabidopsis leaves. Phytochemistry 2014, 98, 34–40. [Google Scholar] [CrossRef]
- Sun, X.; Jia, X.; Huo, L.; Che, R.; Gong, X.; Wang, P.; Ma, F. MdATG18a overexpression improves tolerance to nitrogen deficiency and regulates anthocyanin accumulation through increased autophagy in transgenic apple. Plant Cell Environ. 2018, 41, 469–480. [Google Scholar] [CrossRef]
- Soubeyrand, E.; Basteau, C.; Hilbert, G.; van Leeuwen, C.; Delrot, S.; Gomès, E. Nitrogen supply affects anthocyanin biosynthetic and regulatory genes in grapevine cv. Cabernet-Sauvignon berries. Phytochemistry 2014, 103, 38–49. [Google Scholar] [CrossRef]
- Becker, C.; Urlić, B.; Jukić Špika, M.; Kläring, H.P.; Krumbein, A.; Baldermann, S.; Goreta Ban, S.; Perica, S.; Schwarz, D. Nitrogen limited red and green leaf lettuce accumulate flavonoid glycosides, caffeic acid derivatives, and sucrose while losing chlorophylls, Β-carotene and xanthophylls. PLoS ONE 2015, 10, e0142867. [Google Scholar] [CrossRef] [PubMed]
- Thapa, M.; Liu, L.; Barkla, B.J.; Kretzschmar, T.; Rogiers, S.Y.; Rose, T.J. Nitrogen fertiliser effects on grain anthocyanin and γ-Oryzanol biosynthesis in black rice. Agriculture 2024, 14, 817. [Google Scholar] [CrossRef]
- Fritz, C.; Palacios-Rojas, N.; Feil, R.; Stitt, M. Regulation of secondary metabolism by the carbon-nitrogen status in tobacco: Nitrate inhibits large sectors of phenylpropanoid metabolism. Plant J. 2006, 46, 533–548. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.; Cho, L.H.; Tun, W.; Jeon, J.S.; An, G. Sucrose signaling in higher plants. Plant Sci. 2021, 302, 110703. [Google Scholar] [CrossRef] [PubMed]
- Solfanelli, C.; Poggi, A.; Loreti, E.; Alpi, A.; Perata, P. Sucrose-specific induction of the anthocyanin biosynthetic pathway in Arabidopsis. Plant Physiol. 2006, 140, 637–646. [Google Scholar] [CrossRef]
- Ferri, M.; Righetti, L.; Tassoni, A. Increasing sucrose concentrations promote phenylpropanoid biosynthesis in grapevine cell cultures. J. Plant Physiol. 2011, 168, 189–195. [Google Scholar] [CrossRef]
- Hara, M.; Oki, K.; Hoshino, K.; Kuboi, T.J.P.S. Enhancement of anthocyanin biosynthesis by sugar in radish (Raphanus sativus) hypocotyl. Plant Sci. 2003, 164, 259–265. [Google Scholar] [CrossRef]




| Trait | F Values | ||||||
|---|---|---|---|---|---|---|---|
| Year (Y) | Nitrogen Level (NL) | Filling Stage (FS) | Y × NL | Y × FS | NL × FS | Y × NL × FS | |
| Anthocyanin content (ANC) | 2.92 * | 3.85 ** | 4.16 ** | 0.01 ns | 0.08 ns | 0.16 ns | 0.07 ns |
| Anthocyanin accumulation amount (AAA) | 3.45 * | 15.84 ** | 384.87 ** | 0.06 ns | 2.22 ns | 0.41 ns | 0.04 ns |
| Total nitrogen content (TNC) | 5.53 ** | 63.28 ** | 637.92 ** | 0.28 ns | 0.51 ns | 0.62 ns | 0.09 ns |
| Nitrite content (NC) | 0.20 ns | 104.91 ** | 1723.00 ** | 1.00 ns | 1.03 ns | 6.82 ** | 0.12 ns |
| Lysine content (LC) | 21.26 ** | 93.70 ** | 1365.30 ** | 1.45 ns | 0.05 ns | 2.00 ns | 1.93 ns |
| Soluble sugar content (SSC) | 0.05 ns | 13.54 ** | 620.57 ** | 0.13 ns | 0.29 ns | 2.16 * | 0.03 ns |
| Amylopectin content (AC) | 4.34 ** | 19.95 ** | 876.85 ** | 0.02 ns | 0.08 ns | 2.19 * | 0.05 ns |
| Nitrate reductase activity (NRA) | 21.80 ** | 102.58 ** | 1150.63 ** | 6.80 ** | 0.23 ns | 2.81 ** | 1.20 ns |
| Glutamine synthetase activity (GSA) | 0.08 ns | 5.84 ** | 661.20 ** | 0.02 ns | 1.90 ns | 0.50 ns | 0.04 ns |
| AGPase activity (AGPA) | 17.47 ** | 10.24 ** | 435.23 ** | 0.27 ns | 0.63 ns | 0.19 ns | 0.07 ns |
| SSS activity (SSSA) | 8.65 ** | 3.53 ** | 31.57 ** | 0.01 ns | 0.03 ns | 0.04 ns | 0.01 ns |
| SBE activity (SBEA) | 2.75 ns | 6.74 ** | 567.21 ** | 0.14 ns | 0.47 ns | 0.82 ns | 0.03 ns |
| DBE activity (DBEA) | 11.50 ** | 22.11 ** | 1623.66 ** | 0.39 ns | 2.15 ns | 0.46 ns | 0.34 ns |
| PAL activity (PALA) | 88.09 ** | 1.45 ns | 533.19 ** | 0.23 ns | 0.03 ns | 94.29 ** | 6.25 ** |
| F3H activity (F3HA) | 600.51 ** | 677.05 ** | 2.21 ns | 44.53 ** | 13.79 ** | 222.70 ** | 28.67 ** |
| DFR activity (DFRA) | 413.34 ** | 85.22 ** | 10.40 ** | 14.36 ** | 2.45 ns | 5.23 ** | 2.43 ns |
| ANS activity (ANSA) | 48.52 ** | 646.90 ** | 7.57 ** | 4.25* | 0.91 ns | 1.43 ns | 4.57 ** |
| UFGT activity (UFGTA) | 35.55 ** | 188.76 ** | 2.27 ns | 1.84 ns | 0.01 ns | 1.17 ns | 1.15 ns |
| C/N ratio | 0.42 ns | 1.51 ns | 1386.47 ** | 0.07 ns | 0.39 ns | 0.48 ns | 0.19 ns |
| Year | Time (d) | Nitrogen Treatment | PAL Activity (mU g−1) | F3H Activity (U g−1) | DFR Activity (mU g−1) | ANS Activity (U g−1) | UFGT Activity (mU g−1) |
|---|---|---|---|---|---|---|---|
| 2018 | 15 | N0 | 22.52 ± 1.89 b | 304.76 ± 14.11 c | 469.42 ± 24.26 | 1626.49 ± 22.72 c | 4.18 ± 0.16 b |
| N1 | 25.34 ± 1.58 b | 445.55 ± 7.11 a | 581.52 ± 29.96 | 1558.18 ± 29.42 c | 4.04 ± 0.13 b | ||
| N2 | 33.17 ± 3.38 a | 406.83 ± 11.71 b | 411.13 ± 24.26 | 2083.46 ± 39.34 a | 5.43 ± 0.23 a | ||
| N3 | 31.39 ± 1.54 a | 317.08 ± 10.01 c | 434.45 ± 22.4 | 2003.37 ± 52.09 b | 5.58 ± 0.28 a | ||
| Average | 28.10 ± 0.80 | 368.55 ± 3.3 | 474.13 ± 9.92 | 1817.88 ± 22.37 | 4.81 ± 0.16 | ||
| (2.86%) | (0.90%) | (2.09%) | (1.23%) | (3.28%) | |||
| 30 | N0 | 50.59 ± 3.02 a | 532.37 ± 10.31 a | 607.52 ± 24.41 | 1419.21 ± 62.81 a | 3.14 ± 0.22 b | |
| N1 | 46.52 ± 1.36 a | 525.91 ± 8.07 a | 616.49 ± 36.19 | 1447.48 ± 36.72 a | 3.57 ± 0.08 a | ||
| N2 | 40.47 ± 3.99 b | 486.61 ± 5.66 b | 494.53 ± 25.85 | 1237.84 ± 53.51 b | 2.99 ± 0.19 b | ||
| N3 | 38.59 ± 2.39 b | 499.52 ± 9.31 b | 490.05 ± 16.14 | 1134.19 ± 63.34 b | 3.06 ± 0.18 b | ||
| Average | 44.05 ± 1.25 | 511.10 ± 0.67 | 552.15 ± 7.44 | 1309.68 ± 37.77 | 3.19 ± 0.06 | ||
| (2.83%) | (0.13%) | (1.35%) | (2.88%) | (1.89%) | |||
| 2019 | 15 | N0 | 26.8 ± 2.05 b | 427.36 ± 12.32 c | 599.45 ± 28.76 a | 1723.07 ± 28.27 b | 4.58 ± 0.29 b |
| N1 | 29.51 ± 1.26 b | 524.15 ± 12.32 a | 624.56 ± 18.83 a | 1798.45 ± 57.12 b | 4.88 ± 0.10 b | ||
| N2 | 40.16 ± 3.16 a | 467.25 ± 5.66 b | 580.62 ± 20.9 a | 2236.57 ± 73.55 a | 5.96 ± 0.27 a | ||
| N3 | 42.14 ± 2.99 a | 432.06 ± 5.66 c | 615.59 ± 31.41 a | 2147.06 ± 61.20 a | 5.73 ± 0.12 a | ||
| Average | 34.65 ± 1.85 | 462.71 ± 2.83 | 605.06 ± 22.23 | 1976.29 ± 35.35 | 5.29 ± 0.05 | ||
| (5.35%) | (0.61%) | (3.67%) | (1.79%) | (0.91%) | |||
| 30 | N0 | 58.32 ± 1.1 a | 640.89 ± 5.38 a | 740.24 ± 28.34 a | 1534.63 ± 38.92 a | 3.90 ± 0.11 ab | |
| N1 | 57.17 ± 1.75 a | 549.38 ± 10.31 b | 725.89 ± 24.41 a | 1426.28 ± 60.38 b | 4.20 ± 0.06 a | ||
| N2 | 45.79 ± 2.09 b | 507.14 ± 9.69 c | 701.68 ± 24.41 a | 1235.48 ± 74.78 c | 3.22 ± 0.34 b | ||
| N3 | 40.16 ± 2.08 c | 512.42 ± 9.69 c | 704.37 ± 14.82 a | 1249.61 ± 44.13 c | 3.31 ± 0.08 b | ||
| Average | 50.36 ± 0.41 | 552.46 ± 0.67 | 718.05 ± 9.25 | 1361.5 ± 17.7 | 3.66 ± 0.20 | ||
| (0.82%) | (0.12%) | (1.29%) | (1.30%) | (5.57%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, W.; Xue, W.; Zhao, Z.; Wang, H.; Shi, Z.; Wang, W.; Chen, B.; Qiu, P.; Xue, J.; Sun, M. Nitrogen Level Impacts the Dynamic Changes in Nitrogen Metabolism, and Carbohydrate and Anthocyanin Biosynthesis Improves the Kernel Nutritional Quality of Purple Waxy Maize. Plants 2024, 13, 2882. https://doi.org/10.3390/plants13202882
Feng W, Xue W, Zhao Z, Wang H, Shi Z, Wang W, Chen B, Qiu P, Xue J, Sun M. Nitrogen Level Impacts the Dynamic Changes in Nitrogen Metabolism, and Carbohydrate and Anthocyanin Biosynthesis Improves the Kernel Nutritional Quality of Purple Waxy Maize. Plants. 2024; 13(20):2882. https://doi.org/10.3390/plants13202882
Chicago/Turabian StyleFeng, Wanjun, Weiwei Xue, Zequn Zhao, Haoxue Wang, Zhaokang Shi, Weijie Wang, Baoguo Chen, Peng Qiu, Jianfu Xue, and Min Sun. 2024. "Nitrogen Level Impacts the Dynamic Changes in Nitrogen Metabolism, and Carbohydrate and Anthocyanin Biosynthesis Improves the Kernel Nutritional Quality of Purple Waxy Maize" Plants 13, no. 20: 2882. https://doi.org/10.3390/plants13202882
APA StyleFeng, W., Xue, W., Zhao, Z., Wang, H., Shi, Z., Wang, W., Chen, B., Qiu, P., Xue, J., & Sun, M. (2024). Nitrogen Level Impacts the Dynamic Changes in Nitrogen Metabolism, and Carbohydrate and Anthocyanin Biosynthesis Improves the Kernel Nutritional Quality of Purple Waxy Maize. Plants, 13(20), 2882. https://doi.org/10.3390/plants13202882






