Abstract
Dengue is an emerging disease of high impact on human health. Plants are an important source of new antivirals and Arachis hypogaea stands for its biological properties. The aim of this study was to evaluate the cytotoxicity and antiviral activity and elucidate the antiviral mechanism of ethanolic extracts from A. hypogaea against dengue virus 2 (DENV-2). The skin or tegument ethanolic extract (TEEs) and seed ethanolic extract (SEEs) were obtained. Cytotoxicity was evaluated by MTT and Neutral Red Uptake (NRU). Antiviral activity was evaluated at different stages of the viral replication cycle by the lysis plaque reduction method. The 50% inhibitory concentration (IC50) and selectivity index (SI) were determined. Antiviral activity was further determined by RT-qPCR. The CC50 values were 169 (NRU) and 65 (MTT) µg/mL for TEE. In addition, the CC50 values were >1400 (NRU) and 636 (MTT) µg/mL for SEE. The TEE demonstrated 99.9 ± 0.1% viral inhibition. The TEE presented an IC50 = 3.47 and SI of 48.7 (NRU) and 18.73 (MTT). Its mechanism of antiviral action is broad and it acts in the viral adsorption–penetration stage and inhibits the first steps of infection in the post-penetration stage. It is also capable of acting as virucidal and as prophylactic. Studies of RT-qPCR indicated that the TEE inhibited viral RNA synthesis. These findings suggest that the TEE from A. hypogaea could be a promising antiviral candidate for treating DENV-2 infections.
1. Introduction
Infection produced by the dengue virus (DENV) represents an emerging disease with a high impact on human health. This flavivirus is a member of the Flaviviridae family. There are four serotypes, DENV-1, DENV-2, DENV-3, and DENV-4, that are transmitted to humans by Aedes aegypti and Aedes albopictus mosquitoes. A fifth variant (DENV-5) that follows the sylvatic cycle was discovered in October 2013 [1].
DENV is an RNA virus of positive sense, linear, non-segmented, and of approximately 11 kilobases. It encodes a single polypeptide, which is processed in the endoplasmic reticulum by cellular and viral proteases to release structural proteins (C nucleocapsid protein, M membrane-associated protein, and E envelope protein) and non-structural proteins (NS1, NS2A, NS2B, NS3, NS4A, NS4B, and NS5) [2].
The DENV infection in humans ranges from an asymptomatic infection or self-limited febrile illness, called fever dengue, to severe forms, such as dengue hemorrhagic fever, which impairs hemostasis increasing vascular permeability. They also can evolve into dengue shock syndrome in some lethal cases [3]. The rapid expansion of large urban centers, absence of adequate policies for water management, poor housing, viral propagation through human travels, and inefficient vector control programs are among the factors that explain the re-emergence of this disease [4]. DENV-2 has certain disadvantages compared to others, such as its stronger association with severe disease and cross-immunity issues. In addition, its genetic variability complicates vaccine development, treatment options, and epidemiological surveillance [5].
Antiviral drugs are not available for dengue treatment. The only treatment for dengue disease consists of supportive therapies to reduce the consequences of fever, dehydration, hypotension, and bleeding [6]. Therefore, there is an urgent need for effective therapies against DENV. In this regard, plants are important and accessible potential sources of new antiviral drugs [7,8].
An antiviral agent can act by masking the viral proteins E and M necessary for adsorption and entry into the host cell or by blocking intracellular replication [9]. In addition, it can mask viral receptors on the cell surface, due to competitive binding [10]. Other attractive targets are the different cycle steps of viral replication where the agents interfere with non-structural proteins [6]. In recent years, there has been a strong tendency to use plant-based products to treat and prevent health problems [11]. Some plants with antiviral activity against dengue virus have been described [12,13].
Arachis hypogaea L., known as the peanut plant, is a legume that belongs to the Fabaceae family. It is native to South America and highly cultivated around the world [14]. Argentina is one of the main producers, which implies an elevated skin (seed coat) discharge as industrial waste that can be recovered [15,16].
This plant stands out for its several scientifically proven biological properties, such as antimicrobial, anti-inflammatory, and antioxidant properties [17,18,19]. In vivo studies indicate the absence of genotoxicity and low toxicity of the peanut skin or tegument (TEE) and seed (SEE) extracts [20]. Chemical analyses reveal potential virucidal molecules, such as proanthocyanidins and phenolic acids, which include caffeic acid in TEE and fatty acids in SEE [20].
Therefore, the current work aimed to evaluate the cytotoxicity and antiviral activity of these extracts against dengue virus serotype 2, which is one of the most aggressive genotypes worldwide. It was based on our hypothesis that peanuts have chemical compounds capable of inhibiting the multiplication of the dengue virus, exerting action in some of the stages of the viral replication cycle.
2. Results and Discussion
2.1. Cytotoxicity
The results about the cytotoxic activity of extracts are shown in Figure 1. The TEE reduced cellular viability to 50% at lower concentrations than the SEE. Therefore, the TEE demonstrayed greater toxicity than the SEE against Vero cells at 7 days of treatment. With the MTT technique, both extracts showed lower CC50 values than those obtained by the NRU technique. This suggested that the TEE and SEE exerted higher damage on mitochondria than lysosomes of these cells. Nonetheless, both extracts are cytotoxic at high concentrations.
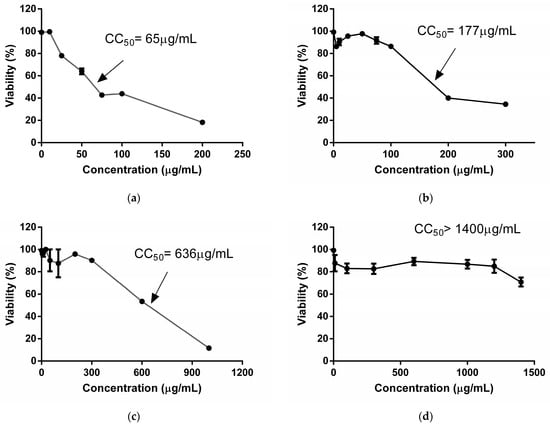
Figure 1.
Curves of Vero cell viability treated with the TEE (a,b) and SEE (c,d) from Arachis hypogaea, assessed by MTT reduction (a–c) and Neutral Red Uptake (b–d) after 7 days of treatment.
Among the scarce studies about the cytotoxicity of A. hypogaea, Cossetin et al. (2019) [21] used peanut leaf hydroalcoholic extracts to demonstrate the absence of cytotoxicity in human peripheral blood mononuclear cells. In the same way, Rossi et al. (2020) [16] reported viability percentages of 60% at 500 µg/mL for a peanut skin polyphenolic extract on Vero cells at 24 h of treatment.
Similarly, aqueous extracts of peanut skin, subjected to two digestion steps (P1 and P2), showed low toxicity at 48 h of treatment, with CC50 values of 9.4 mg/mL and 15.9 mg/mL in an intestinal cell model (HCT116 cell) [22].
Other authors studied the cytotoxic effect of fractions of an ethanolic extract of peanut skin on melanoma and colorectal cancer cells. These studies indicated toxicity for two fractions when treating the cells with 50 µg/mL for 72 h and determined by MTT [23]. This greater toxicity may be due to the fact that they are fractions of the crude extract.
A previous study of our group revealed that the TEE (300–1600 µg/mL) decreased Vero cellular viability to 60% after 2 days of treatment using the NRU assay. Therefore, the CC50 exceeded 1600 μg/mL, whereas the TEE CC50 was 600 μg/mL using the MTT assay. SEE CC50 values were 1600 µg/mL and >1400 µg/mL after 2 days of treatment using NRU and MTT, respectively [20]. Consequently, prolonged exposure (2 days vs. 7 days) to these extracts increased cytotoxicity, with SEEs being safer.
2.2. Antiviral Activity
The viral inhibition percentage for each extract was calculated at different stages to determine if the antiviral activity was due to (i) the induction of an antiviral state in the host cell that prevented subsequent infection (pre-treated cells), (ii) virucidal action by direct contact between extract components and DENV-2 particles that were inactivated and lost their infectivity (pre-treated viruses), (iii) an inhibitory effect at the stage of viral adsorption and penetration or (iv) an inhibitory effect at the stage of viral post-adsorption and penetration. The experimental results are summarized in Table 1, using non-cytotoxic extract concentrations.

Table 1.
Viral inhibition percentages for the TEE and SEE from A. hypogaea at different stages of the viral cycle in Vero cells.
The TEE (at 30 µg/mL) displayed antiviral properties in the four stages evaluated. It inhibited DENV-2, with percentages ranging between 84.0 ± 1.4% and 99.9 ± 0.1% (p < 0.001). Therefore, the TEE affected the intracellular stages of viral multiplication and promoted an antiviral state in Vero cells protecting them against subsequent infection. Furthermore, it interfered with viral adsorption–penetration and was virucidal. In contrast, the SEE (at 300 µg/mL) did not exert powerful antiviral activity in the stages evaluated since it presented viral inhibition percentages lower than 52%, with the SEE moderately interfering after penetration (intracellular replication or DENV-2 exocytosis).
If two extracts are compared, the TEE has a greater capacity to inhibit the dengue virus, since it can act in all stages tested and block viral production effectively. Both extracts exert the greatest antiviral action in the viral post-adsorption and penetration stage but at different concentrations, i.e., TEE at 30 µg/mL vs. SEE at 300 µg/mL.
2.3. Antiviral Activity throughout the Viral Replication Cycle
A dose–response curve was plotted and is shown in Figure 2 with the percentages of viral inhibition obtained from each extract concentration. It can be observed that increasing the TEE concentrations enhanced inhibition. The IC50 value of 3.47 μg/mL was obtained by interpolation, with a coefficient of determination greater than 0.90 (R2 = 0.96), which validated the outcome. This IC50 was related to the CC50 determined by MTT and NRU assays to obtain selectivity indices (SIs) (Table 2).
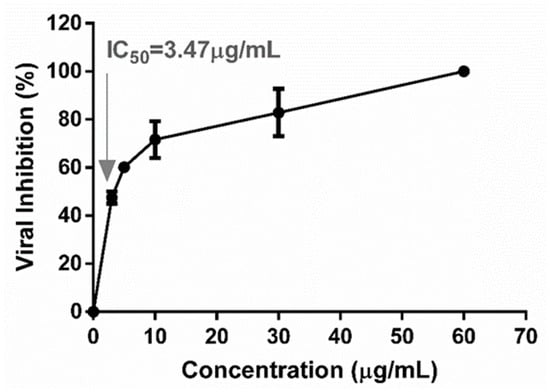
Figure 2.
Dose–response curve of DENV-2 inhibition in post-adsorption and penetration by TEE from Arachis hypogaea to determine the concentration of extract that inhibits 50% of viral infection (IC50). The arrow indicates the value IC50.

Table 2.
Selectivity indices of TEE from A. hypogaea L.
SI is a measure of the safety margin of an active principle. A greater SI reflects a greater margin (values near to 1 are dangerous). In this study, the SI values were high, which supported TEE use as a selective antiviral drug with a wide margin to be applied on eukaryotic cells.
2.4. Quantitative Real-Time PCR Assay (qRT-PCR)
The melting curves were checked and an amplification efficiency of 1.01 was determined. The cDNA concentrations in the viral control and treatments were determined by the formula y = −3.299X + 32.47 (R2 0.998) and are shown in Table 3. The percentage of viral inhibition was calculated by the formula mentioned in the method section. The concentration of 10 µg/mL of TEE inhibited viral replication in 39.83 ± 4.7% and the concentrations of 20 and 30 µg/µL managed to completely inhibit DENV-2 infection (99.9% ± 0.1) (Figure 3).

Table 3.
Ct values and concentrations of viral cDNA obtained in the viral control and treatments with TEE from A. hypogaea.
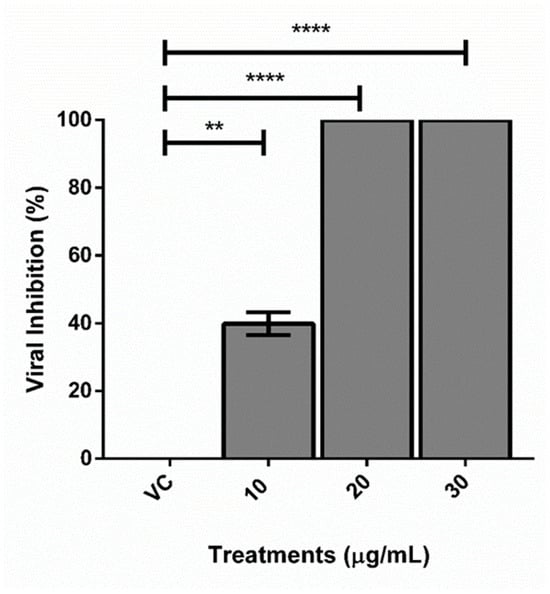
Figure 3.
Inhibition of DENV-2 virus (%) treated with TEE from Arachis hypogaea at different concentrations (10, 20, and 30 µg/mL). VC: viral control. ** p < 0.001; **** p < 0.00001.
These results indicate that the action targets of the TEE from A. hypogaea in the post-adsorption and penetration stage of DENV-2 are the initial steps of infection, including endosomal pathways or viral stripping, and not the subsequent steps of the multiplication cycle, which include maturation, assembly or viral exocytosis.
The results determined by the molecular technique are consistent with those determined by the lysis plate reduction technique, since 100% viral inhibition was achieved in the treatment with 30 µg/mL of TEE in the same evaluation stage.
In 2018, Makau et al. carried out a study of antiviral activity of the ethanol extract of tegument from A. hypogaea [18]. They achieved effective inhibition of influenza virus type A and B, with an IC50 corresponding to 1.3 μg/mL in the replication early stages. This shows that the peanut tegument has a great antiviral capacity, since with low concentrations, it achieved the inhibition of DENV-2 and influenza viruses.
On the other hand, the antiviral activity of the TEE from A. hypogaea is related to the ethanolic extract’s activity from Cassia alata (Fabaceae), since it showed an IC50 less than 10 μg/mL and an SI of 32.3 in the post-adsorption stage of the DENV-2 virus [24]
Ramalingam et al. (2018) [13] evaluated the antiviral action mechanism of an ethanolic extract from Andrographis paniculata (Burm.f.) Nees (Acanthaceae) against the dengue virus and demonstrated that it acted through the inhibition of viral RNA synthesis. Therefore, this action mode is similar to that shown by the TEE, since both exert antiviral action in the initial steps of infection after adsorption and penetration of the virus. However, the TEE from A. hypogaea was able to completely inhibit viral multiplication at 30 μg/mL and the ethanolic extract from A. paniculata reduced virus replication by 52% at a 100 μg/mL concentration.
In the section below, we elucidate the probable active compound(s) responsible for the antiviral activity against dengue virus serotype 2. In previous studies on the chemical composition of the TEE of A. hypogaea, we identified 74.33 mg GAE/g of total phenols by the Folin–Ciocalteu test (7.43% of its composition) [20]. Makau et al. (2018) [18] also determined the presence of polyphenols in the ethanolic extract of tegument from the peanut plant and they proposed that these components could be responsible for the great inhibitory power that the extract showed against the replication of influenza viruses.
On the other hand, Clain et al. (2019) [25] reported that a polyphenol-rich extract from Psiloxylon mauritianum inhibits the early stages of dengue virus and Zika virus infection.
Also, we demonstrated the presence of proanthocyanidins and the absence of phytosterols in the TEE [20]. In the same way, other authors have stated that in peanut skin extracts, the presence of proantocyanidins is due to polymerizations of catechin or epicatechin. The most common proanthocyanidins found in the TEE are A-type procyanidins [26].
Various investigations have indicated that proanthocyanidins possess antiviral capacities. For example, Terlizzi et al. (2016) [27] found that proanthocyanidins are capable of reducing Herpes simplex virus. Other studies showed that proanthocyanidins were also active against Rotavirus [28]. In the same way, activity-guided isolation of a methanol leaf extract of Kratom (Mitragyna speciose) led to the identification of B-type procyanidin condensed tannins of (-)-epicatechin as virucidal compounds against SARS-CoV-2. The fraction containing condensed tannins exhibited virucidal activity with an EC50 value of 8.38 μg/mL and a selectivity index (SI) value > 23.86 [29].
In addition, recent studies of dynamic molecular simulation (in silico, molecular docking) have suggested the proanthocyanidins’ potential to inhibit the coronavirus disease (COVID-19 global pandemic) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The mechanism proposed consists of the ability of the proanthocyanidins/procyanidins to bind with enzymes and proteins involved in the virus replication cycle, the SARSCoV-2 spike protein (S), ACE2 receptor, and the transmembrane serine protein (TMPRSS2), destabilizing the binding between the virus and human cell, and preventing virus replication. In addition, these authors suggest that the activity of proanthocyanidins/procyanidins may alleviate the severity of COVID-19 symptoms and modulate the immune response [30].
Furthermore, we identified the presence of an organic acid, caffeic acid, with a percentage corresponding to 2.46% by HPLC-ESI-MS/MS analysis [20]. Also, other authors have indicated the occurrence of phenolic acids such as vanillic, caffeic, p-coumaric and tartaric acids in peanut skin extracts [26].
In addition, other researchers, such as Alasalvar et al. in 2015, detected caffeic acid in peanuts (2015) [31]. This organic acid is another component that stands out for its great antiviral activity. Flores-Ocelotl et al. (2018) [32] identified caffeic acid in the methanolic extract from Taraxacum officinale (Asteraceae), which exerted antiviral activity against the dengue virus. Rodríguez-Ortega et al. (2013) [33] reported the presence of caffeic acid in a chloroform extract of the leaves from Taraxacum officinale that was active against the yellow fever virus vaccine strain 17 in the viral adsorption stage.
On the other hand, caffeic acid acts against other important viruses, such as hepatitis B [34], influenza A [35], and Herpes simplex viruses [36].
All these studies suggest that the great antiviral activity of the TEE from A. hypogaea L. against DENV-2 demonstrated in the present study could be exerted by the compounds found in the extract. This evidence expands on the existing literature that addresses the antiviral activity of plant-derived products, which has enormous potential in different human health scenarios and may share methods of obtaining these products and various mechanisms [37].
3. Materials and Methods
3.1. Ethanolic Extracts of Peanut
To obtain seed ethanolic extract (SEE) and tegument ethanolic extract (TEE), a simple method of alcoholic extraction reported by García et al. (1990) [38] with modifications was followed. Arachis hypogaea L. (peanut) seeds from the cultivar Granoleico were obtained from Criadero El Carmen (General Cabrera, Córdoba, Argentina). The seeds or their tegument were macerated with 80% ethanol for 48 h at 37 °C. Products were consecutively filtered, dried at 37 °C for 3 days, and dissolved in phosphate-buffered saline (PBS). SEE and TEE stocks were filtered first with Whatman Nº 2 filters and then with 0.22 μm pore cellulose acetate filters to be sterilized. A previous phytochemical analysis of the extracts revealed the following compounds in TEE: 74.33 mg/g of phenolic compounds (including 2.46% caffeic acid and 1.39 OD at 550 nm of proanthocyanidins). On the other hand, the SEE mainly included 15.05 mg/g of phenolic compounds, linoleic acid (58.84%), oleic acid (11.31%) and palmitic acid (8.37%), together with other fatty acids at a lower content [20].
3.2. Cells and Virus
The Vero cell line derived from African green monkey kidneys (Cercopithecus aethiops ATCC CCL-81) was used. Cells were obtained commercially from the Asociación Banco Argentino de Células and were cultured in Eagle-Earle Minimum Essential Medium (MEM of Gibco, Waltham, MA, USA) supplemented with 10% (v/v) heat-inactivated fetal bovine serum (FBS) (Natocor, Córdoba, Argentina), L-glutamine (30 µg/mL) and gentamicin solution (50 µg/mL) (Sigma-Aldrich, Buenos Aires, Argentina) at 37 °C with CO2 (5%).
The dengue virus serotype-2 (DENV-2) New Guinea C strain was used. It was provided by the Universidad de Buenos Aires. The strain was maintained through propagation in Vero cell monolayers and titrated by the lysis plate formation technique [39]. The viral stock was conserved at −80 °C.
3.3. Cytotoxicity Assay In Vitro
Vero cells were plated at approximately 3 × 104 per well in the 96-well tissue culture plate and incubated for 24 h. The cell monolayers were treated with increasing concentrations of TEE and SEE (from 5 to 1600 μg/mL). The culture plate was incubated at 37 °C with CO2 (5%) for 7 days. The assay was performed in triplicate and cell monolayers with maintenance medium (MM) (containing MEM, 2% FBS) were included as cellular controls.
3.3.1. Neutral Red Uptake (NRU)-Based Assay
After incubation, cells were washed with 200 µL of PBS/well and 200 µL of the NR solution (30 μg/mL in MEM) was added. The plate was incubated at 37 °C for 3 h. Afterward, test solutions were aspirated, the monolayers were washed with PBS and the dye inside the cells was released by extraction with a mixture of acetic acid, ethanol, and water (1:50:49). Finally, the optical density values (O.D.) were measured at 540 nm in a spectrophotometer (Labsystems Multiskan MS, Vantaa, Finland). The relative viability in treatments was expressed as the percentage of captured RN reduction with respect to the control cells. A dose–response curve was constructed to determine the 50% cytotoxic concentration (CC50).
3.3.2. 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium Bromide Metabolism (MTT)-Based Assay
The assay was performed according to the methodology of Mosmann T. et al. reported in 1983 [40]. The Vybrant MTT Cell Proliferation Assay Kit (Molecular Probes Invitrogen Detection Technologies, Eugene, OR, USA) was used. After incubation, solutions were aspirated, cells were washed with 200 µL of PBS and 100 μL of MM without serum and 10 μL of the MTT solution (5 mg/mL of MTT in PBS 0.01 M, pH 7.2) was added to each well. The plate was incubated for 4 h. Then, DMSO was added to solubilize the formazan crystals and the O.D. was measured at 560 nm using a spectrophotometer. The relative viability in treatments was expressed as the percentage of MTT reduction with respect to the cell control. A dose–response curve was constructed to determine the CC50.
3.4. Activity Antiviral of Ethanolic Extracts
The antiviral activity of the TEE and SEE was evaluated at different stages to elucidate the mechanism of action, including viral adsorption and penetration; viral post-adsorption and penetration; viral pre-treatment; and cell pre-treatment [41,42].
The antiviral action was determined through the reduction in the number of foci viral in the treatment versus the viral control. The percentage of viral inhibition (VI) was calculated as follows: VI (%) = 100 − A/B × 100, where A is the mean of the viral foci number in treated wells with an extract and B is the mean of the viral foci number in control wells (without extract). Then, quantitative RT-PCR (qRT-PCR) was performed to elucidate the target of the most active extract in the dengue virus replication cycle.
3.4.1. Treatment in the Viral Adsorption and Penetration Stage
Vero cells were plated at approximately 2 × 105 cells/well in the 24-well tissue culture plate and incubated for 24 h. When the monolayer was formed, 100 µL of TEE or SEE at a non-cytotoxic concentration in MM and 100 PFU of the virus were added per well and incubated at 37 °C for 1 h. Then, the non-adsorbed virus and extract were discarded, and 1 mL/well of plate medium (MP) containing MEM, FBS (2%), and gentamicin (1%) with ultrapure agarose (0.5%) was added. The system was incubated for 7 days in a humid atmosphere with CO2 (5%). Viral (only virus in MM) and cell (MM) controls were included. Treatments were performed in triplicate. After the incubation period, the cells were fixed with formaldehyde (10%) and stained with crystal violet solution (1%) to quantify the viral foci. The calculation of viral inhibition percentages was realized, as above (Section 3.4.).
3.4.2. Treatment in Viral Post-Adsorption and Penetration Stage
Vero cells were plated at approximately 2 × 105 cells/well in the 24-well tissue culture plate and incubated for 24 h. Vero cell monolayers were infected with 100 PFU/well of the virus and were incubated at 37 °C for 1 h. Then, the non-adsorbed virus was eliminated and 1 mL/well of MP with agarose, containing TEE or SEE at a non-cytotoxic concentration, was added. The system was incubated at 37 °C for 7 days in a humid atmosphere with CO2 (5%). Viral (only virus in MM) and cell (MM) controls were included. The quantification of viral foci and the calculation of viral inhibition percentages were realized, as detailed above.
3.4.3. Viral Pre-Treatment
To determine the virucidal capacity of the extracts, equal volumes of viral suspension (200 PFU) and the TEE (60 μg/mL) or SEE (600 μg/mL) were mixed in a tube. Cell and viral controls were included (tubes containing virus and MM). All treatments were incubated for 1 h at 37 °C with a humid atmosphere and CO2 (5%). Then, the cell monolayers were infected with 200 μL/well of treated and untreated virus in triplicate and incubated for 1 h at 37 °C. Subsequently, the solutions were discarded and 1 mL/well of MP with agarose was added. The system was incubated for 7 days at 37 °C in a humid atmosphere with CO2 (5%). Finally, the monolayers were fixed and stained to quantify the number of viral foci and calculate viral inhibition percentages.
3.4.4. Cell Pre-Treatment
Before infection with the virus, cell pre-treatment was performed. It consisted of adding 1 mL/well of MM containing TEE or SEE at a non-cytotoxic concentration to growing cell monolayers, followed by incubation for 1 h at 37 °C. After the extracts were removed, monolayers were washed with PBS and infected with 100 PFU/well. The system was incubated for 1 h at 37 °C. The remaining virus was removed and the cells were incubated with MP and agarose for 7 days at 37 °C in a humid atmosphere with CO2 (5%). The viral and cell controls were included. After incubation, all cultures were fixed and stained. From the obtained titles, the percentage of inhibitory action of each extract was calculated.
3.5. Quantitative Real-Time PCR Assay (qRT-PCR)
Quantitative RT-PCR was performed to determine the antiviral action mechanism of the most active extract in the viral post-adsorption and penetration stages. First, Vero cells were plated in the 12-well tissue culture plate. Then, cell monolayers were infected with 100 PFU of the virus and were incubated at 37 °C for 1 h. The non-absorbed virus was eliminated and 1 mL/well of MM containing different concentrations (10, 20, and 30 µg/mL) of the extract was added (in triplicate). The system was incubated at 37 °C for 4 days in a humid atmosphere with CO2 (5%). After the incubation period, the total intracellular RNA was extracted from viral controls and treatments, using the TRIzol reagent (Invitrogen, Buenos Aires, Argentina). The manufacturer’s instructions were followed with slight modifications. Reverse transcription was carried out with the kit “High-Capacity cDNA Reverse Transcription Kits” from ThermoFisher (RNAase OUT Recombinant Ribonuclease inhibitor, 40 U/μL (Invitrogen, Buenos Aires, Argentina)). The absolute quantification test by real-time PCR was carried out using the “Brilliant III Ultra-Fast SYBR® Green QPCR Master Mix with the Low ROX” kit, (Agilent Technologies, Buenos Aires, Argentina), compatible with Agilent Mx3000P equipment. The reaction mixture contained water, 5 µL (1×) of Brilliant III SYBR Green QPCR Master Mix with Low ROX, 0.35 µL of forward DNF (CT(A,T)TCAATATGCTGAAACGCG) and 0.35 µL of reverse D2R (CGCCACAAGGGCCATGAACAG) primers (10 μM) in a total volume of 9 µL. For each sample, 9 μL of the reaction mixture was taken and 1 μL of cDNA was added. The program consisted of a denaturation stage at 95 °C for 3 min; followed by 40 cycles of amplification (denaturation at 95 °C, 5 s; annealing-extension at 50 °C, 1 min). For absolute quantitation of the viral RNA, a standard curve was established, which was serially diluted to a tenth of cDNA from the DENV-2 viral control (known titer). The percentage of viral inhibition in treatments was determined by the formula 1 − (RNAt/RNAc) × 100, where RNAt is the RNA quantified in treatments and RNAc is the RNA quantified in the viral control [43,44].
3.6. Determination of 50% Inhibitory Concentration (IC50)
The IC50 was determined in the viral post-adsorption and penetration stages. In this way, Vero cells were plated in the 24-well tissue culture plate and after the incubation period, the monolayers were infected with 100 PFU per well of DENV-2 and incubated for 1 h at 37 °C. The residual inoculum was removed; cells were washed with PBS and MEM-0.5% agarose was added together with increasing concentrations of the active extract (1–60 μg/mL). After 7 days at 37 °C, the cultures were fixed, stained, and viral plaques were counted. The IC50 was calculated as the extract concentration that reduced the number of PFUs to 50% with respect to the viral control.
3.7. Selectivity Index
With this value and the CC50 obtained by the cytotoxicity techniques and the IC50 obtained in the different treatments, SIs were calculated using the following formula: SI = CC50/IC50.
3.8. Data Analysis
The CC50 and IC50 were calculated from concentration-effect plots by non-linear regression analysis (Boltzmann sigmoidal) using Graph Pad Prism 6.0. The results account for the mean standard error of the mean values of three different experiments.
4. Conclusions
In conclusion, this study demonstrated that the TEE from A. hypogaea L. is a potent antiviral that completely inhibits the infection caused by the DENV-2 virus. The extract can be considered a possible antiviral drug of plant origin since it can affect the viral adsorption–penetration stage and the intracellular events of viral replication. In addition, it exerts a virucidal effect because it can act directly on the viral particle, making it defective. On the other hand, the TEE could be used as a prophylactic because it can reduce infection after cell treatment. It would be important, in the future, to investigate the antiviral activity of the pure compounds present in the TEE to understand if the anti-dengue action of the extract is due to any particular component or the synergistic activity of several of these components.
Author Contributions
Conceptualization, E.B.R. and M.C.S.; methodology, F.M.C., A.S.P., M.V.M. and M.C.S.; formal analysis, E.A.S., E.B.R. and M.C.S.; investigation, F.M.C., A.S.P., M.V.M. and M.C.S.; writing, reviewing, and editing, F.M.C., E.A.S., E.B.R., W.G. and M.C.S.; supervision, E.B.R., W.G. and M.C.S.; funding acquisition, E.A.S., W.G. and M.C.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Secretaria de Ciencia yTécnica Resolution 083/20 from Universidad Nacional de Río Cuarto; Secretaría de Ciencia y Tecnología Resolution 411/2018 from Universidad Nacional de Córdoba, and PICT 2020 SERIE A 02114 Agencia Fondo para la Investigación Científica y Tecnológica (FONCYT), Argentina.
Data Availability Statement
Datasets used can be accessed requesting to the corresponding author.
Acknowledgments
The authors thank the sources of funding. The authors would like to thank Carlos Pujol and Cybele García from the Universidad of Buenos Aires (UBA) for providing us with the dengue virus serotype 2 strain.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Mustafa, M.S.; Rasotgi, V.; Jain, S.; Gupta, V. Discovery of fifth serotype of dengue virus (DENV-5): A new public health dilemma in dengue control. Med. J. Armed Forces India 2015, 71, 67–70. [Google Scholar] [CrossRef] [PubMed]
- Velandia, M.; Castellanos, J. Virus del dengue: Estructura y ciclo viral. Infectio 2011, 15, 33–43. [Google Scholar] [CrossRef]
- Talarico, L. La entrada del virus dengue a la célula como un potencial blanco antiviral: Estudio de polisacáridos sulfatados como antivirales. Quím. Viva 2008, 7, 113–133. [Google Scholar]
- Teixeira, R.R.; Pereira, W.L.; Oliveira, A.F.; da Silva, A.M.; de Oliveira, A.S.; da Silva, M.L.; da Silva, C.C.; de Paula, S.O. Natural Products as Source of Potential Dengue Antivirals. Molecules 2014, 19, 8151–8176. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Castañeda, J.; Barreto dos Santos, F.; Martínez-Vega, R.; Galvão de Araujo, J.M.; Joint, G.; Sarti, E. Dengue in Latin America: Systematic Review of Molecular Epidemiological Trends. PLoS Negl. Trop. Dis. 2017, 11, e0005224. [Google Scholar] [CrossRef]
- Chew, M.F.; Tham, H.W.; Rajik, M.; Sharifah, S.H. Anti-dengue virus serotype 2 activity and mode of action of a novel peptide. J. Appl. Microbiol. 2015, 119, 1170–1180. [Google Scholar] [CrossRef]
- Kwon, H.J.; Kim, H.H.; Yoon, S.Y.; Ryu, Y.B.; Chang, J.S.; Cho, K.O.; Rho, M.C.; Park, S.J.; Lee, W.S. In vitro inhibitory activity of Alpinia katsumadai extracts against influenza virus infection and hemagglutination. Virol. J. 2010, 7, 307. [Google Scholar] [CrossRef]
- Zandi, K.; Taherzadeh, M.; Yaghoubi, R.; Tajbakhsh, S.; Rastian, Z.; Sartavi, K. Antiviral activity of Avicennia marina against herpes simplex virus type 1 and vaccine strain of poliovirus (An in vitro study). J. Med. Plants Res. 2009, 3, 771–775. [Google Scholar]
- Ocazionez, R.E.; Meneses, R.; Torres, F.A.; Stashenko, E. Virucidal activity of Colombian Lippia essential oils on dengue virus replication in vitro. Mem. Do Inst. Oswaldo Cruz 2010, 105, 3. [Google Scholar] [CrossRef]
- Zandi, K.; Lim, T.; Rahim, N.; Shu, M.-H.; Teoh, B.-T.; Sam, S.-S.; Danlami, M.-B.; Tan, K.-K.; Abubakar, S. Extract of Scutellaria baicalensis inhibits dengue virus replication. BMC Complement. Altern. Med. 2013, 13, 91. [Google Scholar] [CrossRef]
- Reinoso, E.; Oliva, M.M.; Pavicich, G.; Beoletto, V.; Carezzano, M.E.; Marioli, J.M.; Paletti Rovey, M.F.; Pimentel Betancourt, D.; Sabini, M.C.; Moliva, M.; et al. Una Farmacia en el Monte, 1st ed.; Ministerio de Ciencia y Tecnología de Córdoba: Córdoba, Argentina, 2019; ISBN 978-987-47203-0-6.
- Hishiki, T.; Kato, F.; Tajima, S.; Toume, K.; Umezaki, M.; Takasaki, T.; Miura, T. Hirsutine, an Indole Alkaloid of Uncaria rhynchophylla, Inhibits Late Step in Dengue Virus Lifecycle. Front. Microbiol. 2017, 8, 1674. [Google Scholar] [CrossRef] [PubMed]
- Ramalingam, S.; Karupannan, S.; Padmanaban, P.; Vijayan, S.; Sheriff, K.; Palani, G.; Krishnasamy, K. Anti-dengue activity of Andrographis paniculata extracts and quantification of dengue viral inhibition by SYBR green reverse transcription polymerase chain reaction. AYU J. 2018, 39, 87–91. [Google Scholar] [CrossRef]
- Jiang, S.; Ma, Y.; Yan, D. Antioxidant and antimicrobial properties of water soluble polysaccharide from Arachis hypogaea seeds. J. Food Sci. Technol. 2014, 51, 2839–2844. [Google Scholar] [CrossRef] [PubMed]
- AÑO XXXIX—N° Edición 2017. Available online: https://www.bcr.com.ar/es/mercados/investigacion-y-desarrollo/informativo-semanal/noticias-informativo-semanal/un-nuevo (accessed on 3 September 2023).
- Rossi, Y.E.; Bohl, L.P.; Vanden Braber, N.L.; Ballatore, M.B.; Escobar, F.M.; Bodoira, R.; Maestri, D.M.; Porporatto, C.; Cavaglieri, L.R.; Montenegro, M.A. Polyphenols of peanut (Arachis hypogaea L.) skin as bioprotectors of normal cells. Studies of cytotoxicity, cytoprotection and interaction with ROS. J. Funct. Foods 2020, 67, 103862. [Google Scholar] [CrossRef]
- Larrauri, M.; Zunino, M.P.; Zygadlo, J.A.; Grosso, N.R.; Nepote, V. Chemical characterization and antioxidant properties of fractions separated from extract of peanut skin derived from different industrial processes. Ind. Crops Prod. 2016, 95, 964. [Google Scholar] [CrossRef]
- Makau, J.N.; Watanabe, K.; Mohammed, M.M.D.; Nishida, N. Antiviral Activity of Peanut (Arachis hypogaea L.) Skin Extract Against Human Influenza Viruses. J. Med. Food 2018, 21, 777–784. [Google Scholar] [CrossRef]
- Toomer, O.T. A comprehensive review of the value-added uses of peanut (Arachis hypogaea) skins and by-products. Crit. Rev. Food Sci. Nutr. 2018, 60, 341–350. [Google Scholar] [CrossRef]
- Menis Candela, F.; Giordano, W.F.; Quiroga, P.L.; Escobar, F.M.; Mañas, F.; Roma, D.A.; Larrauri, M.; Comini, L.R.; Soria, E.A.; Sabini, M.C. Evaluation of cellular safety and the chemical composition of the peanut (Arachis hypogaea L.) ethanolic extracts. Heliyon 2020, 6, e05119. [Google Scholar] [CrossRef]
- Cossetin, J.F.; da Silva Brum, E.; Casoti, R.; Camponogara, C.; Castro, R.; Maziero, D.M.; de David, C.T.; Gaube, A.C.; Ramos, A.P.; Pintos, F.G.; et al. Peanut leaf extract has antioxidant and anti-inflammatory activity but no acute toxic effects. Regul. Toxicol. Pharmacol. 2019, 107, 104407. [Google Scholar] [CrossRef]
- Cordeiro-Massironi, K.; Soares-Freitas, R.A.M.; Sampaio, G.R.; Pinaffi-Langley, A.C.d.C.; Bridi, R.; de Camargo, A.C.; Torres, E.A.F.S. In Vitro Digestion of Peanut Skin Releases Bioactive Compounds and Increases Cancer Cell Toxicity. Antioxidants 2023, 12, 1356. [Google Scholar] [CrossRef]
- Tsai, C.-H.; Huang, H.-C.; Lin, K.-J.; Liu, J.-M.; Chen, G.-L.; Yeh, Y.-H.; Lu, T.-L.; Lin, H.-W.; Lu, M.-T.; Chu, P.-C. Inhibition of Autophagy Aggravates Arachis hypogaea L. Skin Extracts-Induced Apoptosis in Cancer Cells. Int. J. Mol. Sci. 2024, 25, 1345. [Google Scholar] [CrossRef] [PubMed]
- Angelina, M.; Hanafi, M.; Suyatna, F.; Mirawati, S.; Ratnasari, S.; Dewi, B. Antiviral Effect of Sub Fraction Cassia alata Leaves Extract to Dengue Virus Serotype-2 strain New Guinea C in Human Cell Line Huh-7 it-1. IOP Conf. Ser. Earth Environ. Sci. 2017, 101, 012004. [Google Scholar] [CrossRef]
- Clain, E.; Haddad, J.G.; Koishi, A.C.; Sinigaglia, L.; Rachidi, W.; Desprès, P.; Duarte dos Santos, C.N.; Guiraud, P.; Jouvenet, N.; Kalamouni, C. The Polyphenol-Rich Extract from Psiloxylon mauritianum, an Endemic Medicinal Plant from Reunion Island, Inhibits the Early Stages of Dengue and Zika Virus Infection. Int. J. Mol. Sci. 2019, 20, 1860. [Google Scholar] [CrossRef] [PubMed]
- Bodoira, R.; Cittadini, M.C.; Velez, A.; Rossi, Y.; Montenegro, M.; Martínez, M.; Maestri, D. An overview on extraction, composition, bioactivity and food applications of peanut phenolics. Food Chem. 2022, 381, 132250. [Google Scholar] [CrossRef]
- Terlizzi, M.E.; Occhipinti, A.; Luganini, A.; Maffei, M.E.; Gribaudo, G. Inhibition of herpes simplex type 1 and type 2 infections by Oximacro, a cranberry extract with a high content of A-type proanthocyanidins (PACs-A). Antivir. Res. 2016, 132, 154–164. [Google Scholar] [CrossRef]
- Joshi, S.S.; Howell, A.B.; D’Souza, D.H. Reduction of Enteric Viruses by Blueberry Juice and Blueberry Proanthocyanidins. Food Environ. Virol. 2016, 8, 235–243. [Google Scholar] [CrossRef]
- Sureram, S.; Chutiwitoonchai, N.; Pooprasert, T.; Sangsopha, W.; Limjiasahapong, S.; Jariyasopit, N.; Sirivatanauksorn, Y.; Khoomrung, S.; Mahidol, C.; Ruchirawat, S.; et al. Discovery of procyanidin condensed tannins of (-)-epicatechin from Kratom, Mitragyna speciosa, as virucidal agents against SARS-CoV-2. Int. J. Biol. Macromol. 2024, 273 Pt 1, 133059. [Google Scholar] [CrossRef]
- Sorita, G.D.; Leimann, F.V.; Salvador Ferreira, S.R. Phenolic Fraction from Peanut (Arachis hypogaea L.) By product: Innovative Extraction Techniques and New Encapsulation Trends for Its Valorization. Food Bioprocess Technol. 2023, 16, 726–748. [Google Scholar] [CrossRef]
- Alasalvar, C.; Bolling, B.W. Review of nut phytochemicals, fat-soluble bioactives, antioxidant components and health effects. Br. J. Nutr. 2015, 113, 68–78. [Google Scholar] [CrossRef]
- Flores-Ocelotl, M.R.; Rosas-Murrieta, N.H.; Moreno, D.A.; Vallejo-Ruiz, V.; Reyes-Leyva, J.; Domínguez, F.; Santos-López, G. Taraxacum officinale and Urtica dioica extracts inhibit dengue virus serotype 2 replication in vitro. BMC Complement. Altern. Med. 2018, 18, 95. [Google Scholar] [CrossRef]
- Rodríguez-Ortega, M.; Chumpitaz, Z.; Ríos, S.; Méndez, M.; Méndez, J.; Cabrera, G. Actividad antiviral contra el virus de la fiebre amarilla, cepa vacunal 17D, de extractos de hojas de Taraxacum officinale GH Weber ex Wiggers. Bol. Latinoam. Caribe Plantas Med. Aromát. 2013, 12, 346–355. Available online: http://saber.ucv.ve/jspui/handle/123456789/4837 (accessed on 2 November 2023).
- Wang, G.F.; Shi, L.; Ren, Y.; Liu, Q.; Liu, H.; Zhang, R.; Li, Z.; Zhu, F.; He, P.; Tang, W. Anti-hepatitis B virus activity of chlorogenic acid, quinic acid and caffeic acid in vivo and in vitro. Antivir. Res. 2009, 83, 186–190. [Google Scholar] [CrossRef] [PubMed]
- Utsunomiya, H.; Ichinose, M.; Ikeda, K.; Uozaki, M.; Morishita, J.; Kuwahara, T.; Koyama, A.H.; Yamasaki, H. Inhibition by caffeic acid of the influenza a virus multiplication in vitro. Int. J. Mol. Med. 2014, 34, 1020–1024. [Google Scholar] [CrossRef]
- Ikeda, K.; Tsujimoto, K.; Uozaki, M.; Nishide, M.; Suzuki, Y.; Koyama, A.H.; Yamasaki, H. Inhibition of multiplication of herpes simplex virus by caffeic acid. Int. J. Mol. Med. 2011, 28, 595–598. [Google Scholar] [CrossRef]
- Mohammed, F.S.; Uysal, I.; Sevindik, M. A review on antiviral plants effective against different virus types prospectsin pharmaceutical sciences. Prospect. Pharm. Sci. 2023, 21, 1–21. Available online: https://prospects.wum.edu.pl (accessed on 7 October 2024).
- García, G.H.; Campos, R.; De Torres, R.; Broussalis, A.; Ferraro, G.; Martino, V.; Coussio, J. Antiherpetic Activity of some Argentine Medicinal Plants. Fitoterapia 1990, 41, 542–546. [Google Scholar]
- Dulbecco, R. Production of plaques in monolayer tissue culture by single particles of an animal virus. Proc. Natl. Acad. Sci. USA 1962, 38, 747–752. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid for cellular colorimetric assay grow and survival application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Sabini, M.C.; Escobar, F.; Tonn, C.; Zanon, S.; Contigiani, M.; Sabini, L. Evaluation of antiviral activity of aqueous extracts from Achyrocline satureioides against West. Equine Enceph. Virus Nat. Prod. Res. 2012, 26, 405–415. [Google Scholar] [CrossRef]
- Sabini, M.C.; Cariddi, L.N.; Escobar, F.; Mañas, F.; Comini, L.; Iglesias, D.; Larrauri, M.; Núñez Montoya, S.; Sereno, J.; Contigiani, M.; et al. Potent inhibition of Western Equine Encephalitis virus by a fraction rich in flavonoids and phenolic acids obtained from Achyrocline satureioides. Rev. Bras. Farmacogn. 2016, 26, 571–578. [Google Scholar] [CrossRef]
- Gurukumar, K.; Priyadarshini, D.; Patil, J.; Bhagat, A.; Singh, A.; Shah, P.S.; Cecilia, D. Development of real time PCR for detection and quantitation of Dengue Viruses. Virol. J. 2009, 6, 10. [Google Scholar] [CrossRef]
- Prada-Arismendy, J.; Castellanos, J. Real time PCR. Application in dengue studies. Colomb. Médica 2011, 42, 243–258. Available online: https://www.redalyc.org/pdf/283/28318450016.pdf (accessed on 10 November 2022). [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).