Non-Structural Carbohydrates Accumulation in Seedlings Improved Flowering Quality of Tree Peony under Forcing Culture Conditions, with Roots Playing a Crucial Role
Abstract
1. Introduction
2. Results
2.1. Changes in the Photosynthetic Capacity of Leaves
2.2. Differences in Morphology and NSCs accumulation of Potted Seedlings
2.3. Differences in Flowering Quality under Forcing Culture Conditions
2.4. Correlations Analysis of Growth Parameters of Potted Seedlings and Off-Season Flower
2.5. Correlation Network Analysis between Growth Parameter of Potted Seedling and Off-Season Flowering
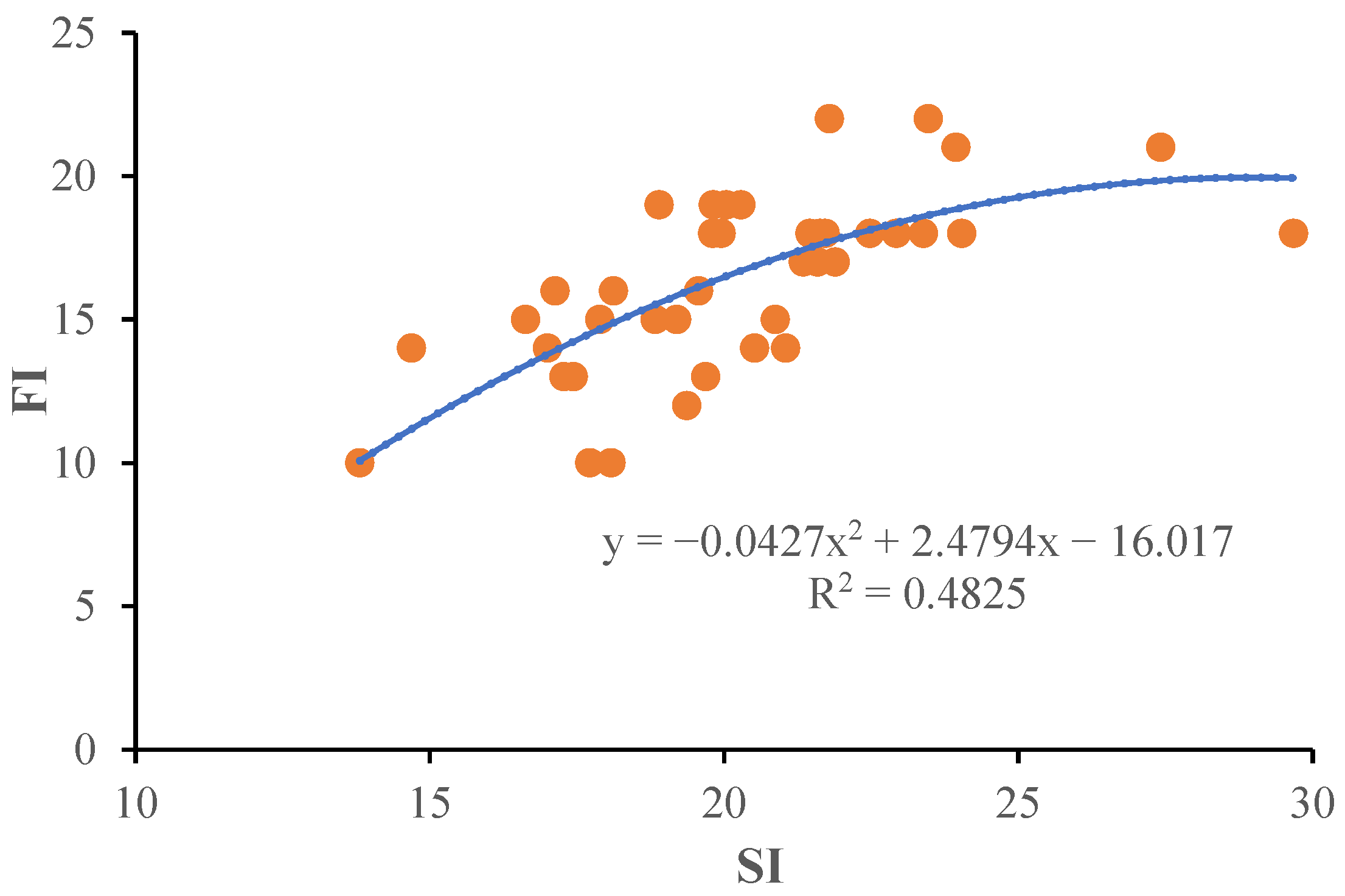
2.6. Dependency between SI and FI on Roots’ NSCs accumulation
3. Discussion
3.1. Seedling Quality Depends upon NSCs accumulation of Potted Tree Peony
3.2. NSCs Are Matter and Energy Sources and Signaling Triggers of Off-Season Tree Peony
3.3. Roots NSCs Remobilization Is a Milestone in Forcing Culture Conditions
4. Materials and Methods
4.1. Experimental Site and Design
4.2. Potted Seedling Growth and Flowering Regulation
4.3. Measurement of Photosynthesis Performance
4.4. Determination of Antioxidant Performance
4.5. Seedlings’ Morphological Observation at Defoliation
4.6. Characteristics’ Observation of Off-Season Flowering
4.7. Determination of Non-Structural Carbohydrates
4.8. Determination of Anthocyanin and Flavonoid in Petals
4.9. Evaluation of Off-Season Flowering Quality
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhou, S.; Ma, C.; Zhou, W.B.; Gao, S.C.; Hou, D.Y.; Guo, L.L.; Shi, G.A. Selection of stable reference genes for QRT-PCR in tree peony ‘Doulv’ and functional analysis of PsCUC3. Plants 2024, 13, 1741. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Fu, L.S.; Jang, K.M. Comparative analysis on economic benefit of Xiangfeng Peony. Sci. Silvae Sin. 2019, 55, 167–174. [Google Scholar]
- Zhou, H.; Cheng, F.Y.; Wang, R.; Zhong, Y.; He, C.Y. Transcriptome comparison reveals key candidate genes responsible for the unusual reblooming trait in tree peonies. PLoS ONE 2013, 8, e79996. [Google Scholar] [CrossRef] [PubMed]
- Li, J.J. Zhongguo Mudan Shaoyao; China Forestry Publishing House: Beijing, China, 1999; pp. 15–35. [Google Scholar]
- Guan, Y.R.; Xue, J.Q.; Xue, Y.Q.; Yang, R.W.; Wang, S.L.; Zhang, X.X. Effect of exogenous GA3 on flowering quality, endogenous hormones, and hormone- and flowering-associated gene expression in forcing-cultured tree peony (Paeonia suffruticosa). J. Integr. Agric. 2019, 18, 1295–1311. [Google Scholar] [CrossRef]
- Wang, S.L.; Xue, J.Q.; Ahmadi, N.; Holloway, P.; Zhu, F.Y.; Ren, X.X.; Zhang, X.X. Molecular characterization and expression patterns of PsSVP genes reveal distinct roles in flower bud abortion and flowering in tree peony (Paeonia suffruticosa). Can. J. Plant Sci. 2014, 94, 1181–1193. [Google Scholar] [CrossRef]
- Mornya, P.M.P.; Cheng, F.Y. Effect of combined chilling and GA3 treatment on bud abortion in forced ‘Luoyanghong’ tree peony (Paeonia suffruticosa Andr.). Hortic. Plant J. 2018, 4, 250–256. [Google Scholar] [CrossRef]
- Gao, Z.M.; Wang, L.Y. Study on the root growth and photosynthesis characteristics of the tree peony after rejuvenation. Forest Res. 2004, 17, 479–483. [Google Scholar]
- Wang, C.Z.; Ma, X.Y.; Li, Q.K.; Hu, Y.H.; Yang, J.; Song, Z.P. Effects of NSC in different organs and at different growth stages on the yield of oil peony Fengdan with different ages. Front. Plant Sci. 2023, 14, 1108668. [Google Scholar] [CrossRef]
- Xue, J.Q.; Li, T.T.; Wang, S.L.; Xue, Y.Q.; Liu, X.W.; Zhang, X.X. Defoliation and gibberellin synergistically induce tree peony flowering with non-structural carbohydrates as intermedia. J. Plant Physiol. 2019, 233, 31–41. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Wang, X.P.; Xue, Y.Q.; Wang, S.L.; Ren, X.X.; Xue, J.Q.; Zhang, X.X. Complete defoliation is essential for tree peony reflowering in autumn to trigger sucrose metabolism and adjust source-sink balance in buds and leaves. Sci. Hortic. 2023, 308, 111540. [Google Scholar] [CrossRef]
- Tong, N.N.; Shu, Q.Y.; Wang, B.C.; Peng, L.P.; Liu, Z.A. Histology, physiology, and transcriptomic and metabolomic profiling reveal the developmental dynamics of annual shoots in tree peonies (Paeonia suffruticosa Andr.). Hortic. Res. 2023, 10, uhad152. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.Y.; Wang, Z.C.; Shi, G.A. Effects of two forced cultivation methods on quality of potted tree peony ‘Luoyanghong’ and regulating effect of boron by root irrigation. Shandong Agric. Sci. 2022, 54, 64–70. [Google Scholar]
- Wang, Z.C.; Shi, S.Y.; Gao, S.C. Effect of water-soluble fertilizer on flowering quality of potted itoh peony ‘Bartzella’ under forcing cultivation. Acta Bot. Boreali-Occident. Sin. 2022, 42, 845–853. [Google Scholar]
- Li, M.; Hoch, G.; Körner, C. Source/sink removal affects mobile carbohydrates in Pinus cembra at the Swiss treeline. Trees 2002, 16, 331–337. [Google Scholar] [CrossRef]
- Rezaie, N.; D’Andrea, E.; Scartazza, A.; Gričar, J.; Prislan, P.; Calfapietra, C.; Battistelli, A.; Moscatello, S.; Proietti, S.; Matteucci, G. Upside down and the game of C allocation. Tree Physiol. 2023, tpad034. [Google Scholar] [CrossRef]
- Smith, M.G.; Miller, R.E.; Arndt, S.K.; Kasel, S.; Bennett, L.T. Whole-tree distribution and temporal variation of non-structural carbohydrates in broadleaf evergreen trees. Tree Physiol. 2018, 38, 570–581. [Google Scholar] [CrossRef]
- Wiley, E.; Helliker, B. A re-evaluation of carbon storage in trees lends greater support for carbon limitation to growth. New Phytol. 2012, 195, 285–289. [Google Scholar] [CrossRef]
- Reyes-Bahamonde, C.; Piper, F.I.; Cavieres, L.A. Elevational variation of the seasonal dynamic of carbohydrate reserves in an alpine plant of Mediterranean mountains. Alp. Bot. 2022, 132, 315–327. [Google Scholar] [CrossRef]
- Li, M.H.; Jiang, Y.; Wang, A.; Li, X.; Zhu, W.; Yan, C.F.; Du, Z.; Shi, Z.; Lei, J.; Schönbeck, L.; et al. Active summer carbon storage for winter persistence in trees at the cold alpine treeline. Tree Physiol. 2018, 38, 1345–1355. [Google Scholar] [CrossRef]
- Chen, W.S.; Ding, H.H.; Li, J.R.; Fu, F.W.; Li, Y.Y.; Xiao, S.Y.; Xu, D.; Lu, J.; Fang, J.P. How do montane plants manage to survive? Inferring from non-structural carbohydrates. Trees 2023, 37, 331–348. [Google Scholar] [CrossRef]
- Villar-Salvador, P.; Uscola, M.; Jacobs, D.F. The role of stored carbohydrates and nitrogen in the growth and stress tolerance of planted forest trees. New For. 2015, 46, 813–839. [Google Scholar] [CrossRef]
- Li, Z.S.; Li, X.L.; He, F. Non-structural carbohydrates contributed to cold tolerance and regeneration of Medicago sativa L. Planta 2023, 257, 116. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.Y.; Shi, T.; Zhou, S.; Gao, S.C.; Shi, G.A. Characteristics of compensatory growth and non-structural carbohydrates accumulation of potted tree peony. Acta Bot. Boreali-Occident. Sin. 2023, 43, 1732–1743. [Google Scholar]
- Zhang, Y.X.; Yu, D.; Liu, C.Y.; Gai, S.P. Dynamic of carbohydrate metabolism and the related genes highlights PPP pathway activation during chilling induced bud dormancy release in tree peony (Paeonia suffruticosa). Sci. Hortic. 2018, 242, 36–43. [Google Scholar]
- Yuan, X.; Yuan, T.; Liu, S.D. Variation in physiological and biochemical properties and DNA methylation patterns during autumn flowering of tree peony (Paeonia suffruticosa). Sci. Silvae Sin. 2021, 57, 53–67. [Google Scholar]
- Xue, Y.Q.; Liu, Z.Y.; Sun, K.R.; Zhang, X.X.; Lv, Y.M.; Xue, J.Q. The mechanism of sugar signal involved in regulating re-flowering of tree peony under forcing culture. Acta Hortic. Sin. 2023, 50, 596–606. [Google Scholar]
- Dietze, M.C.; Sala, A.; Carbone, M.S.; Czimczik, C.I.; Mantooth, J.A.; Richardson, A.D.; Vargas, R. Nonstructural carbon in woody plants. Annu. Rev. Plant Biol. 2014, 65, 667–687. [Google Scholar] [CrossRef]
- Hartmann, H.; Trumbore, S.E. Understanding the roles of nonstructural carbohydrates in forest trees-from what we can measure to what we want to know. New Phytol. 2016, 211, 386–403. [Google Scholar] [CrossRef]
- Riikonen, J.; Mäenpää, M.; Alavillamo, M.; Silfver, T.; Oksanen, E. Interactive effect of elevated temperature and O3 on antioxidant capacity and gas exchange in Betula pendula saplings. Planta 2009, 230, 419–427. [Google Scholar] [CrossRef]
- Shiraz, M.F.; Sami, F.; Siddiqui, H.; Yusuf, M.; Hayat, S. Interaction of auxin and nitric oxide improved photosynthetic efficiency and antioxidant system of brassica juncea plants under salt stress. J. Plant Growth Regul. 2020, 40, 2379–2389. [Google Scholar] [CrossRef]
- Kuptz, D.; Fleischmann, F.; Matyssek, R.; Grams, T.E. Seasonal patterns of carbon allocation to respiratory pools in 60-yr-old deciduous (Fagus sylvatica) and evergreen (Picea abies) trees assessed via whole-tree stable carbon isotope labeling. New Phytol. 2011, 191, 160–172. [Google Scholar] [CrossRef] [PubMed]
- Omari, E. Accumulation versus storage of total non-structural carbohydrates in woody plants. Trees 2022, 36, 869–881. [Google Scholar] [CrossRef]
- Mollá, S.; Villar-Salvador, P.; García-Fayos, P.; Rubira, J.L. Physiological and transplanting performance of Quercus ilex L. (holm oak) seedlings grown in nurseries with different winter conditions. Forest Ecol. Manag. 2006, 237, 218–226. [Google Scholar] [CrossRef]
- Heredia-Guerrero, N.; Oliet, J.A.; Villar-Salvador, P.; Benito, L.F.; Peñuelas, J. Fertilization regime interacts with fall temperature in the nursery to determine the frost and drought tolerance of the Mediterranean oak Quercus ilex subsp. ballota. For. Ecol. Manag. 2014, 331, 50–59. [Google Scholar] [CrossRef]
- Wang, Z.G.; Wang, C.K. Dynamics in nonstructural carbohydrates of Fraxinus mandshurica seedlings during a progressive shade. Trees 2023, 37, 309–317. [Google Scholar] [CrossRef]
- Mornya, P.M.P.; Cheng, F.Y.; Li, H.Y. Chronological changes in plant hormone and sugar contents in cv. Ao-Shuang autumn flowering tree peony. Hortic. Sci. 2011, 38, 104–112. [Google Scholar] [CrossRef]
- O’Brien, M.J.; Leuzinger, S.; Philipson, C.D.; Tay, J.; Hector, A. Drought survival of tropical tree seedlings enhanced by non-structural carbohydrate levels. Nat. Clim. Chang. 2014, 4, 710–714. [Google Scholar] [CrossRef]
- Ai, Z.M.; He, L.R.; Xin, Q.; Yang, T.; Liu, G.B.; Xue, S. Slope aspect affects the non-structural carbohydrates and C: N: P stoichiometry of Artemisia sacrorum on the Loess Plateau in China. Catena 2017, 152, 9–17. [Google Scholar] [CrossRef]
- Liu, W.D.; Su, J.R.; Li, S.F.; Lang, X.D.; Huang, X.B. Non-structural carbohydrates regulated by season and species in the subtropical monsoon broad-leaved evergreen forest of Yunnan Province, China. Sci. Rep. 2018, 8, 1083. [Google Scholar] [CrossRef]
- Kabeya, D. Differentiating between the adverse effects of nutrient-limitation and direct-cold-limitation on tree growth at high altitudes. Arct. Antarct. Alp. Res. 2010, 42, 430–437. [Google Scholar] [CrossRef]
- Puttonen, P. Carbohydrate reserves in Pinus sylvestris seedling needles as an attribute of seedling vigor. Scand. J. For. Res. 1986, 1, 181–193. [Google Scholar] [CrossRef]
- Grossnickle, S.C.; Ivetić, V. Root system development and field establishment: Effect of seedling quality. New For. 2022, 53, 1021–1067. [Google Scholar] [CrossRef]
- Kuo, W.; Yeh, N.; Fujimoto, N.; Lin, H.C. Physicochemical properties and fertility index of culture media containing distillery residue biochar and their applications to plug seedling. J. Fac. Agric. Kyushu Univ. 2019, 64, 127–135. [Google Scholar] [CrossRef]
- Palacio, S.; Camarero, J.J.; Maestro, M.; Alla, A.Q.; Lahoz, E.; Montserrat-Martí, G. Are storage and tree growth related? Seasonal nutrient and carbohydrate dynamics in evergreen and deciduous Mediterranean oaks. Trees 2018, 32, 777–790. [Google Scholar] [CrossRef]
- Dovis, V.L.; Machado, E.C.; Ribeiro, R.V.; Filho, J.R.M.; Sales, C.R.G. Roots are important sources of carbohydrates during flowering and fruiting in ‘Valencia’ sweet orange trees with varying fruit load. Sci. Hortic. 2014, 174, 87–95. [Google Scholar] [CrossRef]
- Waltona, E.F.; Boldinghb, H.L.; McLarenc, G.F.; Williamsa, M.; Jackmana, R.B. The dynamics of starch and sugar utilisation in cut peony (Paeonia lactiflora Pall.) stems during storage and vase life. Postharvest Biol. Technol. 2010, 58, 142–146. [Google Scholar] [CrossRef]
- Liu, Z.Y.; Shi, Y.T.; Xue, Y.Q.; Wang, X.P.; Huang, Z.; Xue, Q.Y.; Zhang, X.X. Non-structural carbohydrates coordinate tree peony flowering both as energy substrates and as sugar signaling triggers, with the bracts playing an essential role. Plant Physiol. Biochem. 2021, 159, 80–88. [Google Scholar] [CrossRef]
- Chapin, F.S.; Schulze, E.; Mooney, H.A. The ecology and economics of storage in plants. Annu. Rev. Ecol. Syst. 1990, 21, 423–447. [Google Scholar] [CrossRef]
- Trumbore, S.; Czimczik, C.I.; Sierra, C.A.; Muhr, J.; Xu, X. Non-structural carbon dynamics and allocation relate to growth rate and leaf habit in California oaks. Tree Physiol. 2015, 3, 1206–1222. [Google Scholar] [CrossRef]
- Gaudinski, J.B.; Torn, M.S.; Riley, W.J.; Swanston, C.W.; Trumbore, S.E.; Joslin, J.; Majdi, H.; Dawson, T.E.; Hanson, P. Use of stored carbon reserves in growth of temperate tree roots and leaf buds: Analyses using radiocarbon measurements and modeling. Glob. Chang. Biol. 2009, 15, 992–1014. [Google Scholar] [CrossRef]
- Xue, Y.Q.; Xue, J.Q.; Ren, X.X.; Li, C.Y.; Sun, K.R.; Cui, L.T.; Lyu, Y.M.; Zhang, X.X. Nutrient supply is essential for shifting tree peony reflowering ahead in autumn and sugar signaling is involved. Int. J. Mol. Sci. 2022, 23, 7703. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.L.; Liu, D.; Li, Z.Q. Effects of the coordination mechanism between roots and leaves induced by root-breaking and exogenous cytokinin spraying on the grazing tolerance of ryegrass. J. Plant Res. 2012, 125, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Rao, M.V.; Paliyath, G.; Ormrod, D.P. Ultraviolet-B- and ozone-induced biochemical changes in antioxidant enzymes of Arabidopsis thaliana. Plant Physiol. 1996, 110, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.Y.; Shi, T.; Shi, G.A.; Yang, L.F.; Fan, X.F.; Gao, S.C.; Zhang, G.N. Research on adaptability of different peony to forcing culture. Sci. Silvae Sin. 2019, 55, 54–62. [Google Scholar]
- Vig, R.G.; Huzsvai, L.; Dobos, A.; Nagy, J. Systematic measurement methods for the determination of the SPAD values of maize (Zea mays L.) canopy and potato (Solanum tuberosum L.). Commun. Soil Sci. Plant Anal. 2012, 43, 1684–1693. [Google Scholar] [CrossRef]
- Donoso, A.; Rivas, C.; Zamorano, A.; Peña, Á.; Handford, M.; Aros, D. Understanding alstroemeria pallida flower colour: Links between phenotype, anthocyanins and gene expression. Plants 2020, 10, 55. [Google Scholar] [CrossRef]
- Buysse, J.; Merckx, R. An improved colorimetric method to quantify sugar content of plant tissue. J. Exp. Bot. 1993, 44, 1627–1629. [Google Scholar] [CrossRef]
- Luo, J.R.; Duan, J.J.; Huo, D.; Shi, Q.Q.; Niu, L.X.; Zhang, Y.L. Transcriptomic analysis reveals transcription factors related to leaf anthocyanin biosynthesis in Paeonia qiui. Molecules 2017, 22, 2186. [Google Scholar] [CrossRef]
- He, J.M.; Yin, T.P.; Chen, Y.; Cai, L.; Tai, Z.G.; Li, Z.J.; Liu, C.S.; Wang, Y.R.; Ding, Z.T. Phenolic compounds and antioxidant activities of edible flowers of Pyrus pashia. J. Funct. Foods 2015, 17, 371–379. [Google Scholar] [CrossRef]
- GB/T 27646-2011; Potted Tree Peony. National Forestry Administration: Beijing, China, 2011.



| Groups | Pn /(µmolCO2 ·m−2·s−1) | Gs /(mmolH2O ·m−2·s−1) | Ci /(µmol ·mol−1) | Tr /(mmolH2O ·m−2·s−1) | WUE /(µmolCO2 ·mmol−1 H2O) | LA /(cm2 ·plant−1) | POD Activity/(U·g−1·h−1) | MDA Content /(µmol·g−1) |
|---|---|---|---|---|---|---|---|---|
| T0 | 10.21 ± 1.51 | 0.09 ± 0.01 b | 200.8 ± 18.0 bc | 2.89 ± 0.34 de | 3.54 ± 0.35 a | 1725.6 ± 263.5 a | 41.4 ± 13.6 c | 9.79 ± 0.29 ab |
| T1 | 9.75 ± 2.23 | 0.09 ± 0.04 b | 194.4 ± 29.1 c | 2.81 ± 0.97 e | 3.58 ± 0.45 a | 1554.1 ± 77.6 ab | 62.5 ± 11.0 b | 10.07 ± 0.57 a |
| T2 | 10.81 ± 1.79 | 0.10 ± 0.02 ab | 195.7 ± 28.5 c | 3.08 ± 0.59 cde | 3.57 ± 0.58 a | 1345.4 ± 81.4 bc | 63.7 ± 15.1 b | 8.54 ± 0.37 de |
| T3 | 11.01 ± 1.65 | 0.10 ± 0.02 ab | 198.1 ± 17.7 c | 3.27 ± 0.62 bcde | 3.40 ± 0.25 a | 1792.3 ± 209.7 a | 82.7 ± 14.2 a | 8.14 ± 0.37 ef |
| T4 | 11.41 ± 1.08 | 0.10 ± 0.02 ab | 196.2 ± 17.9 c | 3.36 ± 0.46 abcd | 3.42 ± 0.26 a | 1269.2 ± 244.1 cd | 19.8 ± 2.6 d | 9.10 ± 0.33 cd |
| T5 | 10.60 ± 2.44 | 0.12 ± 0.03 a | 225.0 ± 13.9 a | 3.78 ± 0.85 ab | 2.82 ± 0.23 bc | 1237.0 ± 40.5 cd | 27.9 ± 8.8 cd | 9.28 ± 0.38 bc |
| T6 | 10.52 ± 1.05 | 0.11 ± 0.02 ab | 222.9 ± 14.9 a | 4.02 ± 0.47 a | 2.63 ± 0.28 c | 1434.7 ± 203.5 bc | 33.5 ± 11.0 cd | 8.02 ± 0.39 ef |
| T7 | 10.63 ± 1.06 | 0.11 ± 0.02 ab | 210.7 ± 11.3 abc | 3.84 ± 0.47 ab | 2.78 ± 0.15 bc | 1028.6 ± 112.3 d | 26.4 ± 6.2 cd | 10.36 ± 0.4 a |
| T8 | 10.69 ± 1.56 | 0.10 ± 0.02 ab | 189.4 ± 18.8 c | 3.53 ± 0.67 abcd | 3.06 ± 0.26 b | 1097.5 ± 157.0 d | 15.7 ± 10.2 d | 7.94 ± 0.35 ef |
| T9 | 10.64 ± 1.49 | 0.11 ± 0.02 ab | 220.0 ± 24.3 ab | 3.65 ± 0.49 abc | 2.94 ± 0.43 bc | 1219.2 ± 209.9 cd | 19.8 ± 4.6 d | 7.55 ± 0.13 f |
| Groups | SL /mm | SD /mm | WPB /(g·plant−1) | NSCAS /(g·plant−1) | NSCAR /(g·plant−1) | NSCAT /(g·plant−1) | SI |
|---|---|---|---|---|---|---|---|
| T0 | 111.6 ± 11.9 a | 6.2 ± 0.1 ab | 344.8 ± 39.2 a | 1.42 ± 0.06 h | 81.3 ± 4.3 b | 82.7 ± 4.2 b | 19.2 ± 1.7 bcd |
| T1 | 102.0 ± 9.0 ab | 6.3 ± 0.3 ab | 332.9 ± 17.3 ab | 2.46 ± 0.03 b | 79.1 ± 2.8 b | 81.6 ± 2.8 b | 20.8 ± 2.4 bc |
| T2 | 88.2 ± 4.0 bc | 5.4 ± 0.1 cd | 345.4 ± 39.2 a | 2.92 ± 0.12 a | 80.3 ± 6.2 b | 83.2 ± 6.3 b | 21.2 ± 2.5 bc |
| T3 | 91.4 ± 7.6 bc | 6.4 ± 0.3 a | 370.7 ± 15.1 a | 2.01 ± 0.06 d | 89.0 ± 0.5 a | 91.0 ± 0.5 a | 26.2 ± 2.9 a |
| T4 | 93.4 ± 14.7 bc | 5.8 ± 0.7 bc | 321.6 ± 32.9 ab | 1.93 ± 0.08 de | 71.4 ± 3.0 c | 73.4 ± 3.0 c | 20.2 ± 2.3 bc |
| T5 | 87.2 ± 12.0 bc | 5.3 ± 0.2 d | 333.3 ± 24.9 ab | 1.84 ± 0.07 ef | 69.9 ± 1.1 c | 71.7 ± 1.2 c | 20.3 ± 1.5 bc |
| T6 | 99.1 ± 12.4 ab | 6.1 ± 0.4 ab | 362.6 ± 24.2 a | 2.33 ± 0.16 c | 81.3 ± 2.8 b | 83.6 ± 2.7 b | 22.4 ± 0.9 b |
| T7 | 82.8 ± 5.8 c | 4.7 ± 0.2 e | 288.0 ± 41.1 b | 1.79 ± 0.03 f | 54.0 ± 2.2 d | 55.7 ± 2.2 d | 16.4 ± 2.6 d |
| T8 | 78.7 ± 9.7 c | 4.9 ± 0.4 de | 287.7 ± 39.4 b | 1.65 ± 0.05 g | 57.4 ± 3.1 d | 59.0 ± 3.1 d | 18.0 ± 2.1 d |
| T9 | 92.7 ± 4.6 b | 5.9 ± 0.3 abc | 292.5 ± 27.9 b | 1.28 ± 0.04 i | 58.1 ± 2.1 d | 59.4 ± 2.1 d | 18.7 ± 1.7 cd |
| Groups | T0 | T1 | T2 | T3 | T4 | T5 | T6 | T7 | T8 | T9 |
|---|---|---|---|---|---|---|---|---|---|---|
| PH/(cm) | 47.8 ± 5.4 a | 44.2 ± 6.2 ab | 42.0 ± 2.7 ab | 41.5 ± 4.7 abc | 43.8 ± 3.3 ab | 45.3 ± 7.1 ab | 41.8 ± 7.3 abc | 35.0 ± 4.1 bc | 34.0 ± 3.7 c | 43.3 ± 2.1 ab |
| CW/(cm) | 57.1 ± 4.6 ab | 56.5 ± 11.2 ab | 52.3 ± 5.4 ab | 55.8 ± 4.3 ab | 57.1 ± 9.1 ab | 59.1 ± 6.0 a | 60.3 ± 8.0 a | 48.0 ± 4.0 bc | 44.8 ± 3.7 c | 56.5 ± 4.3 b |
| BN/(N) | 1.8 ± 0.5 d | 2.3 ± 0.5 cd | 3.0 ± 1.1 abc | 3.0 ± 0.0 abc | 3.5 ± 0.6 ab | 3.8 ± 1.0 a | 3.5 ± 0.6 ab | 3.3 ± 0.5 abc | 3.5 ± 0.6 ab | 2.5 ± 0.6 bcd |
| SPAD | 38.3 ± 3.5 a | 33.4 ± 2.5 bcd | 35.5 ± 1.4 ab | 35.3 ± 1.1 ab | 34.0 ± 2.6 abc | 34.5 ± 2.2 abc | 35.5 ± 3.8 ab | 28.8 ± 4.6 d | 30.1 ± 2.3 cd | 32.5 ± 3.1 bcd |
| BD/(mm) | 8.45 ± 1.54 | 7.82 ± 0.59 | 7.60 ± 0.64 | 8.00 ± 0.60 | 8.05 ± 0.56 | 8.09 ± 0.56 | 7.61 ± 0.57 | 7.21 ± 0.96 | 7.58 ± 0.85 | 8.54 ± 0.47 |
| LL/(cm) | 35.5 ± 4.2 a | 32.8 ± 2.6 ab | 31.6 ± 2.1 ab | 33.3 ± 3.1 ab | 34.1 ± 3.5 a | 36.5 ± 3.8 a | 32.1 ± 2.2 ab | 29.0 ± 3.3 b | 28.3 ± 2.3 b | 34.5 ± 2.7 a |
| LT/(mm) | 0.17 ± 0.01 | 0.17 ± 0.01 | 0.16 ± 0.01 | 0.16 ± 0.01 | 0.17 ± 0.01 | 0.15 ± 0.01 | 0.16 ± 0.01 | 0.17 ± 0.01 | 0.17 ± 0.01 | 0.17 ± 0.01 |
| CWI | 1.20 ± 0.05 c | 1.27 ± 0.10 bc | 1.24 ± 0.08 bc | 1.35 ± 0.06 ab | 1.30 ± 0.12 bc | 1.32 ± 0.09 bc | 1.45 ± 0.07 a | 1.38 ± 0.09 ab | 1.32 ± 0.04 bc | 1.31 ± 0.07 bc |
| FNP/(N) | 1.8 ± 0.5 bc | 1.3 ± 0.5 bc | 1.8 ± 0.5 bc | 2.3 ± 0.5 ab | 2.0 ± 0.8 ab | 3.0 ± 0.1 a | 2.3 ± 1.0 ab | 1.0 ± 0.1 c | 1.8 ± 1.0 bc | 1.5 ± 0.6 bc |
| FD/(mm) | 189.2 ± 4.4 ab | 198.7 ± 2.8 a | 191.4 ± 12.0 ab | 181.7 ± 16.2 ab | 175.2 ± 20.1 abc | 188.5 ± 17.8 ab | 175.7 ± 21.6 abc | 150.6 ± 10.5 c | 156.7 ± 29.5 bc | 189.7 ± 15.4 a |
| L* | 49.6 ± 5.3 c | 50.3 ± 1.8 bc | 41.1 ± 3.3 d | 45.7 ± 5.7 cd | 48.8 ± 6.9 cd | 52.8 ± 5.9 abc | 52.4 ± 8.2 abc | 58.9 ± 1.9 a | 52.8 ± 5.7 abc | 58.1 ± 3.0 ab |
| a* | 25.2 ± 3.7 bc | 27.8 ± 0.6 bc | 32.1 ± 2.5 ab | 32.1 ± 1.2 a | 26.2 ± 5.0 bc | 21.8 ± 7.0 cd | 24.2 ± 7.9 bc | 19.4 ± 5.9 cd | 19.7 ± 6.6 cd | 17.4 ± 2.8 d |
| PAC/(U·g−1) | 24.7 ± 0.7 d | 29.3 ± 0.9 c | 40.6 ± 1.5 a | 34.2 ± 1.9 b | 28.9 ± 1.2 c | 21.5 ± 0.9 e | 34.0 ± 1.6 b | 19.4 ± 0.2 f | 19.5 ± 0.7 f | 16.3 ± 0.4 g |
| PFC/(mg·g−1) | 26.9 ± 1.5 d | 28.4 ± 0.7 cd | 32.0 ± 0.8 b | 34.9 ± 2.6 a | 30.5 ± 1.3 bc | 29.3 ± 0.5 cd | 34.9 ± 2.1 a | 26.7 ± 1.0 d | 27.0 ± 0.9 d | 27.8 ± 1.9 d |
| PSSC/(mg·g−1) | 36.3 ± 2.7 ab | 36.7 ± 2.4 ab | 33.2 ± 1.2 ab | 32.8 ± 1.3 ab | 37.4 ± 3.0 a | 32.1 ± 4.8 ab | 34.6 ± 2.6 ab | 32.8 ± 1.2 ab | 32.6 ± 5.4 ab | 28.6 ± 4.4 b |
| FI | 16.0 ± 1.4 cd | 16.8 ± 1.5 cd | 18.3 ± 2.5 ab | 19.8 ± 2.1 a | 17.0 ± 2.2 abc | 18.5 ± 0.6 ab | 19.0 ± 2.0 ab | 11.5 ± 1.9 e | 13.5 ± 2.7 de | 14.0 ± 1.4 de |
| Groups | A/Root Pruning (%) | B/Rooting Agent (mg·L−1) | C/Metarhizium anisopliae (million U·mL−1) | FI |
|---|---|---|---|---|
| T1 | 25 | 250 | 10 | 16.8 ± 1.5 |
| T2 | 25 | 500 | 15 | 18.3 ± 2.5 |
| T3 | 25 | 750 | 20 | 19.8 ± 2.1 |
| T4 | 33 | 250 | 15 | 17.0 ± 2.2 |
| T5 | 33 | 500 | 20 | 18.5 ± 0.6 |
| T6 | 33 | 750 | 10 | 19.0 ± 2.0 |
| T7 | 50 | 250 | 20 | 11.5 ± 1.9 |
| T8 | 50 | 500 | 10 | 13.5 ± 2.7 |
| T9 | 50 | 750 | 15 | 14.0 ± 1.4 |
| K1 | 18.17 | 14.75 | 16.08 | |
| K2 | 17.92 | 16.75 | 16.42 | |
| K3 | 13.00 | 17.58 | 16.58 | |
| R | 5.17 | 2.83 | 0.50 | |
| Order | A > B > C | |||
| Optimal combination | A1B3C3 | |||
| Groups | A/Root Pruning (%) | B/Rooting Agent (mg·L−1) | C/Metarhizium anisopliae (million U·mL−1) |
|---|---|---|---|
| T0 (Control) | 0 (No) | 0 (No) | 0 (No) |
| T1 | 25 (Slight) | 250 (Low) | 10 (Low) |
| T2 | 25 (Slight) | 500 (Moderate) | 15 (Moderate) |
| T3 | 25 (Slight) | 750 (High) | 20 (High) |
| T4 | 33 (Moderate) | 250 (Low) | 15 (Moderate) |
| T5 | 33 (Moderate) | 500 (Moderate) | 20 (High) |
| T6 | 33 (Moderate) | 750 (High) | 10 (Low) |
| T7 | 50 (Severe) | 250 (Low) | 20 (High) |
| T8 | 50 (Severe) | 500 (Moderate) | 10 (Low) |
| T9 | 50 (Severe) | 750 (High) | 15 (Moderate) |
| Indexes | Levels | ||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| a* | <21 | 21–25 | 25–29 | 29–33 | >33 |
| FD/mm | <155 | 155–170 | 170–185 | 185–200 | >200 |
| FNP/N.plant−1 | 1 | 2 | 3 | 4 | 5 |
| SPAD | <30 | 30–32 | 32–34 | 34–36 | >36 |
| BN/N.plant−1 | 1 | 2 | 3 | 4 | >5 |
| CI | <120 | 123–130 | 130–140 | 40–150 | >150 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, S.; Shi, T.; Zhou, S.; Gao, S.; Zhao, Y.; Shi, G. Non-Structural Carbohydrates Accumulation in Seedlings Improved Flowering Quality of Tree Peony under Forcing Culture Conditions, with Roots Playing a Crucial Role. Plants 2024, 13, 2837. https://doi.org/10.3390/plants13202837
Shi S, Shi T, Zhou S, Gao S, Zhao Y, Shi G. Non-Structural Carbohydrates Accumulation in Seedlings Improved Flowering Quality of Tree Peony under Forcing Culture Conditions, with Roots Playing a Crucial Role. Plants. 2024; 13(20):2837. https://doi.org/10.3390/plants13202837
Chicago/Turabian StyleShi, Shuaiying, Tian Shi, Shuang Zhou, Shuangcheng Gao, Yuan Zhao, and Guoan Shi. 2024. "Non-Structural Carbohydrates Accumulation in Seedlings Improved Flowering Quality of Tree Peony under Forcing Culture Conditions, with Roots Playing a Crucial Role" Plants 13, no. 20: 2837. https://doi.org/10.3390/plants13202837
APA StyleShi, S., Shi, T., Zhou, S., Gao, S., Zhao, Y., & Shi, G. (2024). Non-Structural Carbohydrates Accumulation in Seedlings Improved Flowering Quality of Tree Peony under Forcing Culture Conditions, with Roots Playing a Crucial Role. Plants, 13(20), 2837. https://doi.org/10.3390/plants13202837






