Interactive Effects of Blue Light and Water Turbulence on the Growth of the Green Macroalga Ulva australis (Chlorophyta)
Abstract
1. Introduction
2. Results
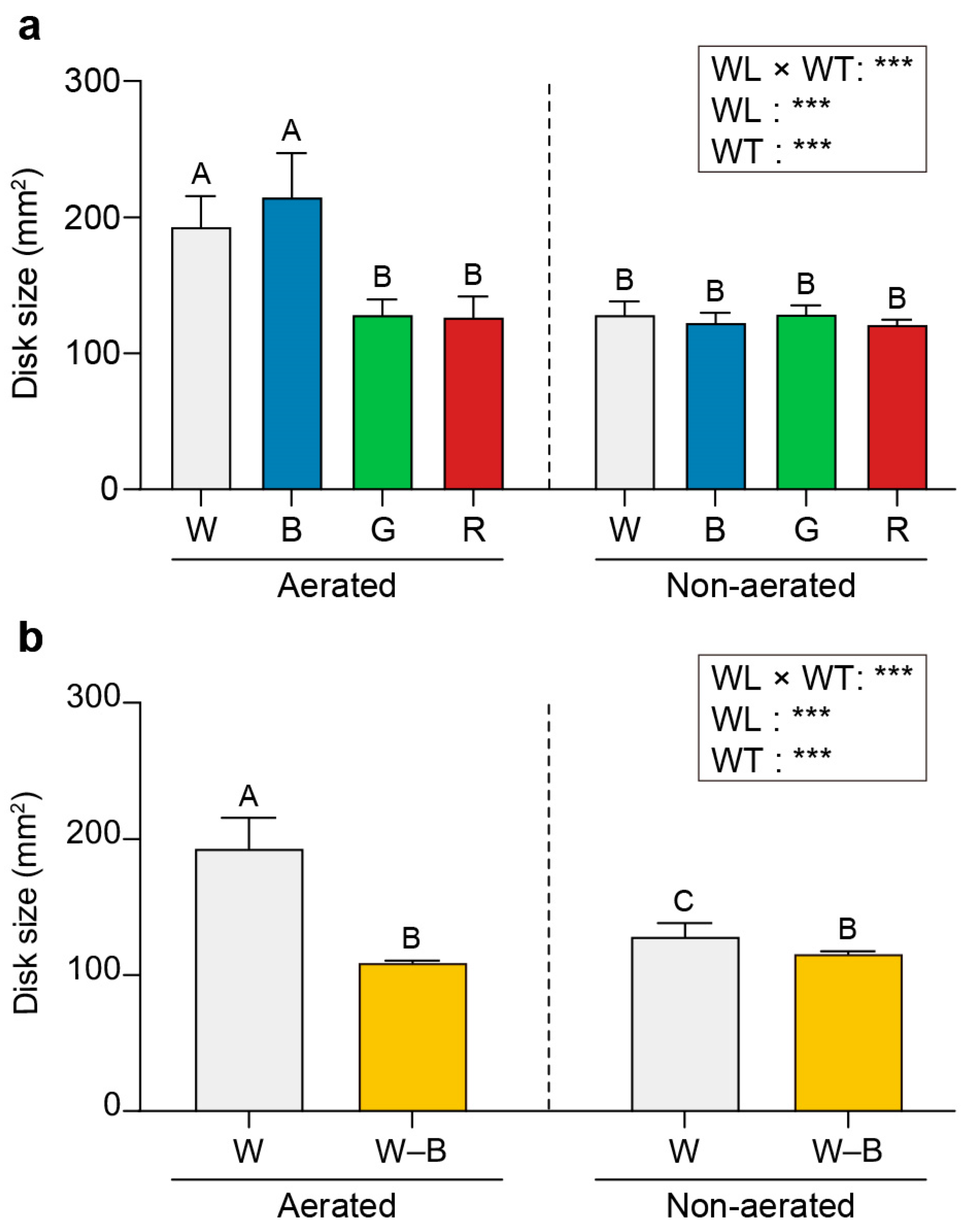
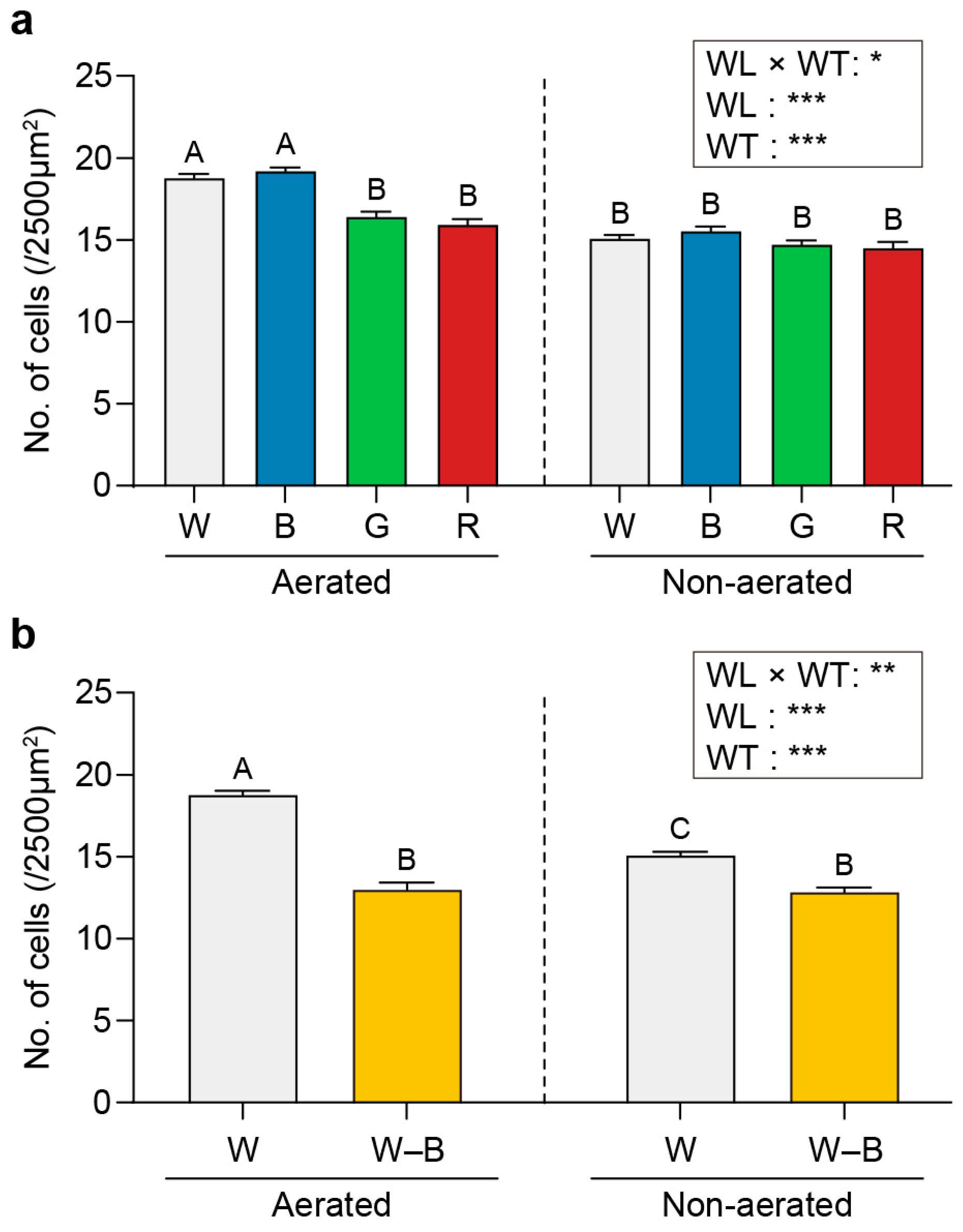
2.1. Growth and Cell Number
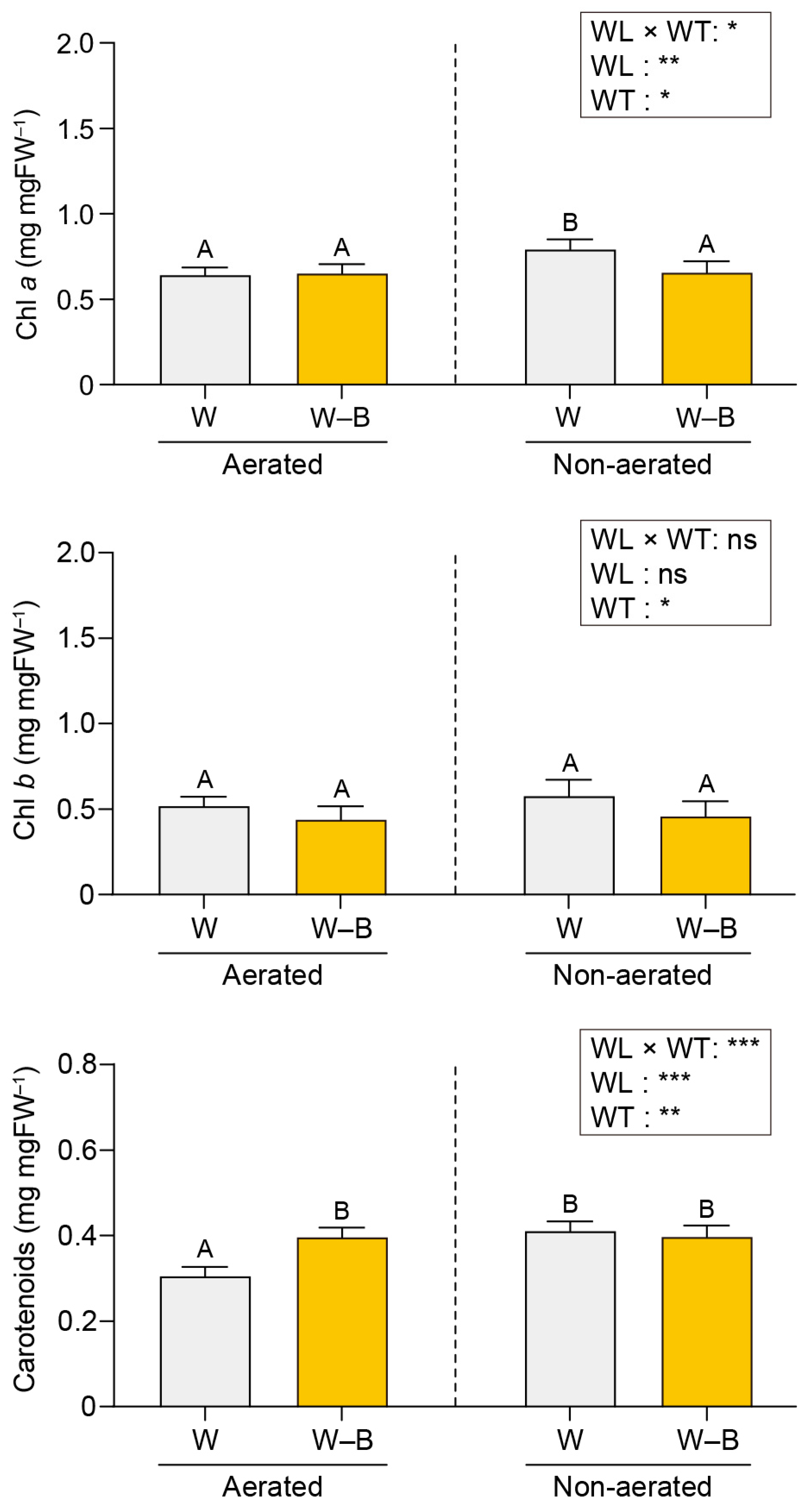
2.2. Photosynthetic Pigment Contents
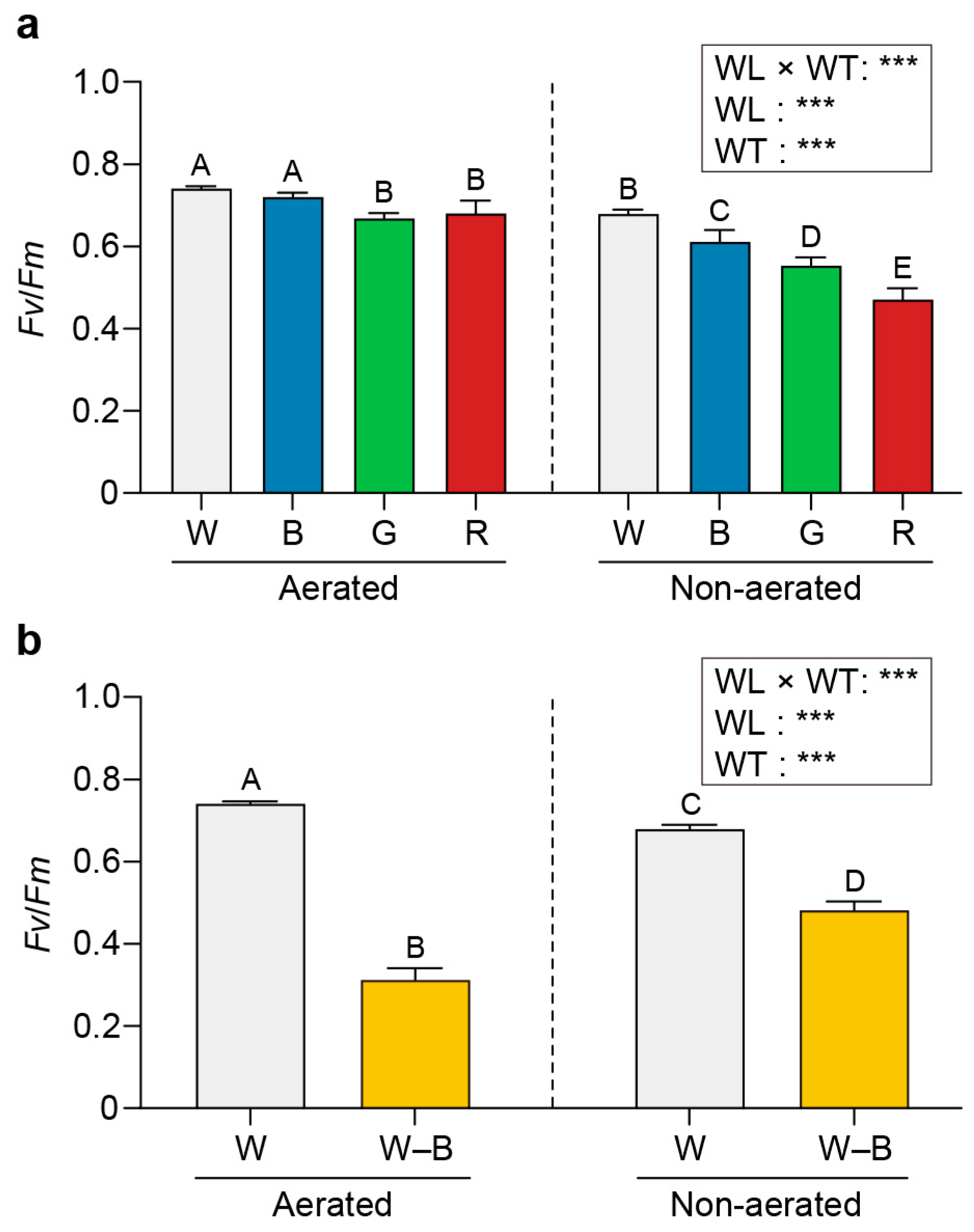
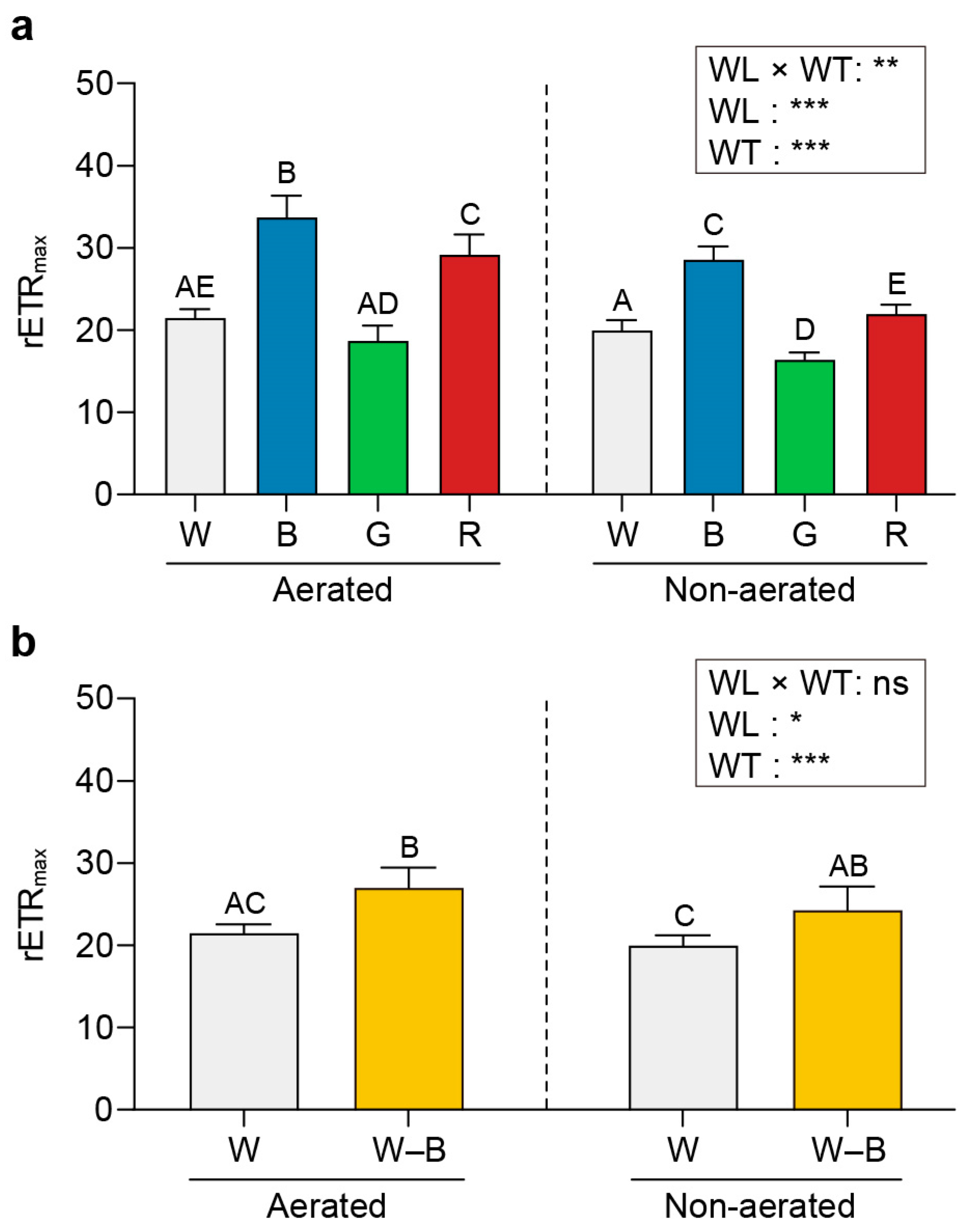
2.3. Chl a Fluorescence
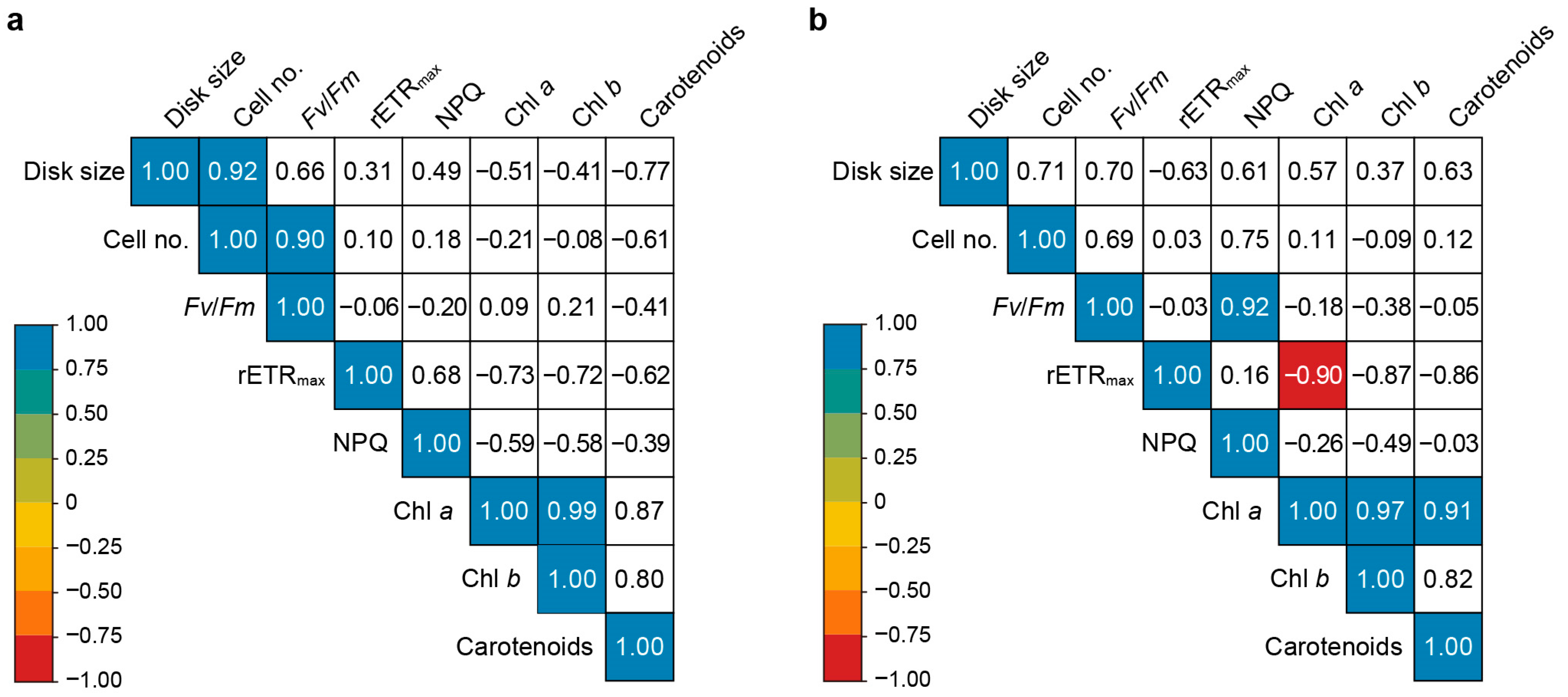
2.4. Correlation between Growth, Photosynthetic Efficiency, and Photosynthetic Pigment Content of U. australis under Different Light Wavelengths
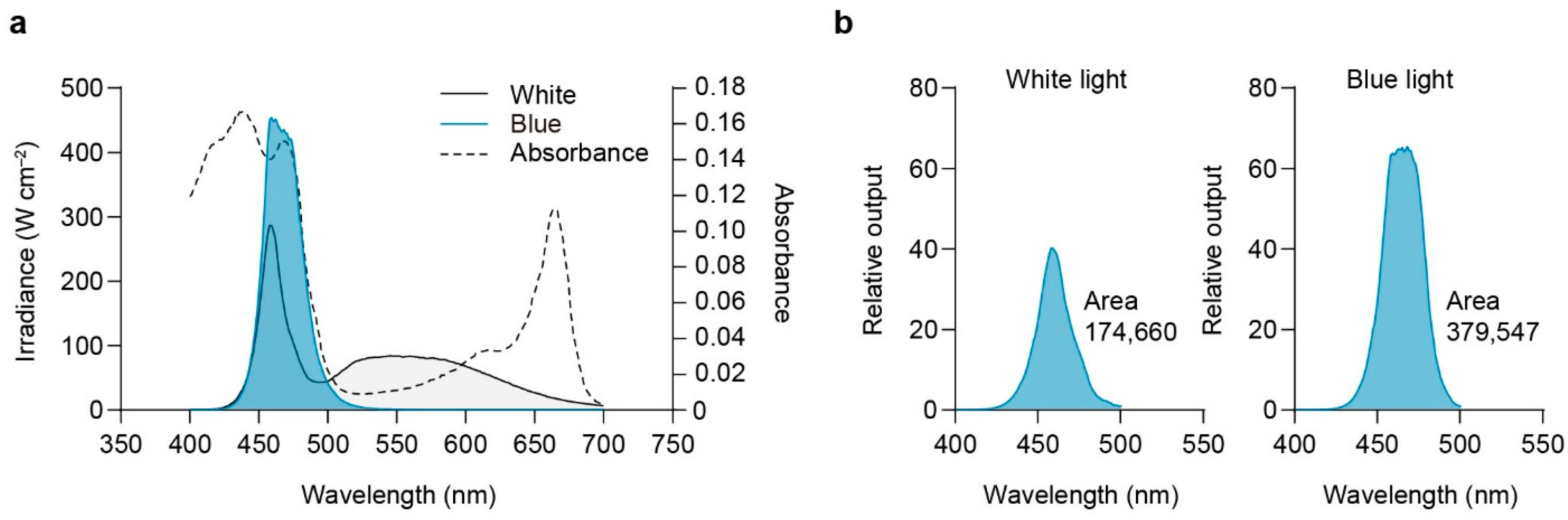
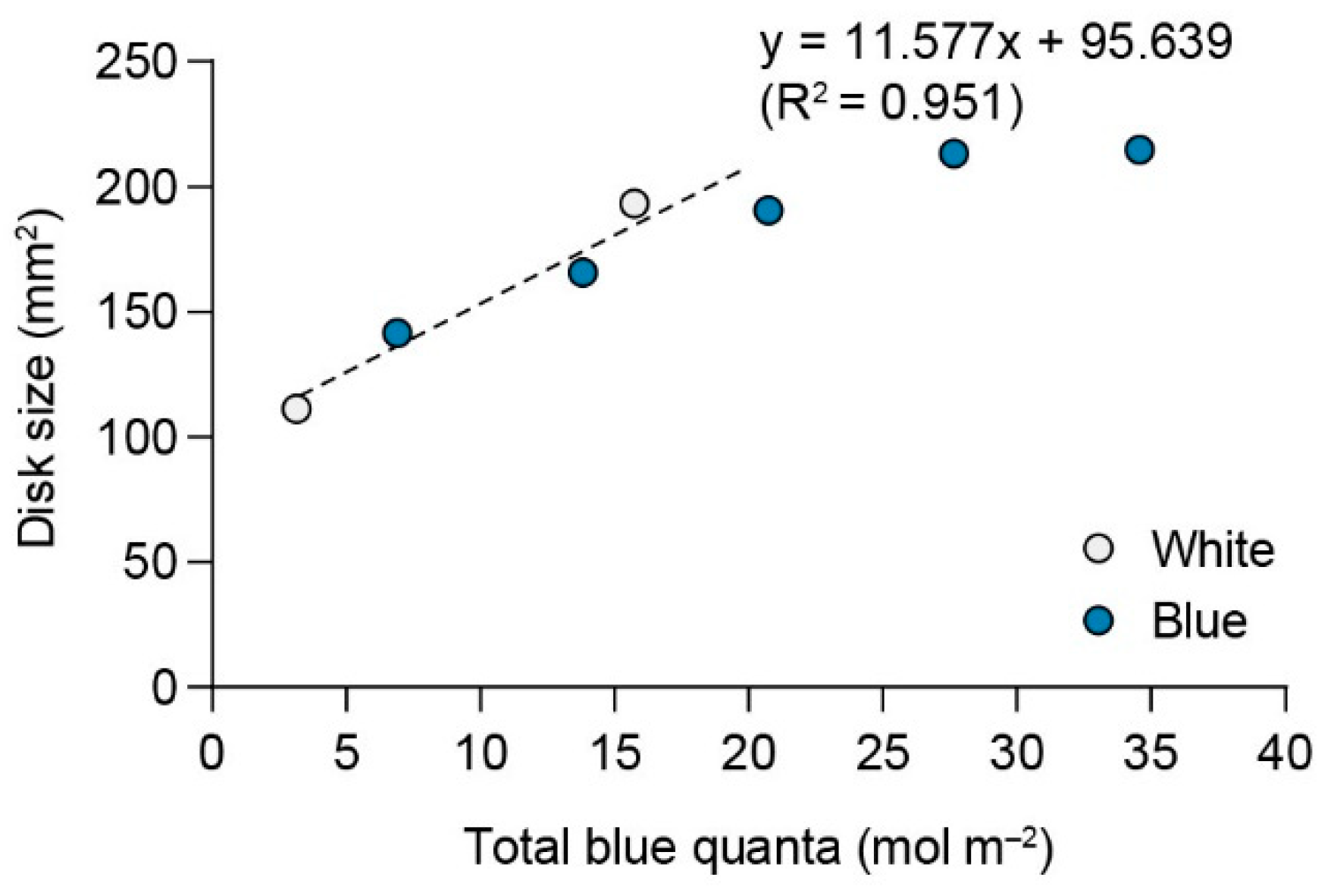
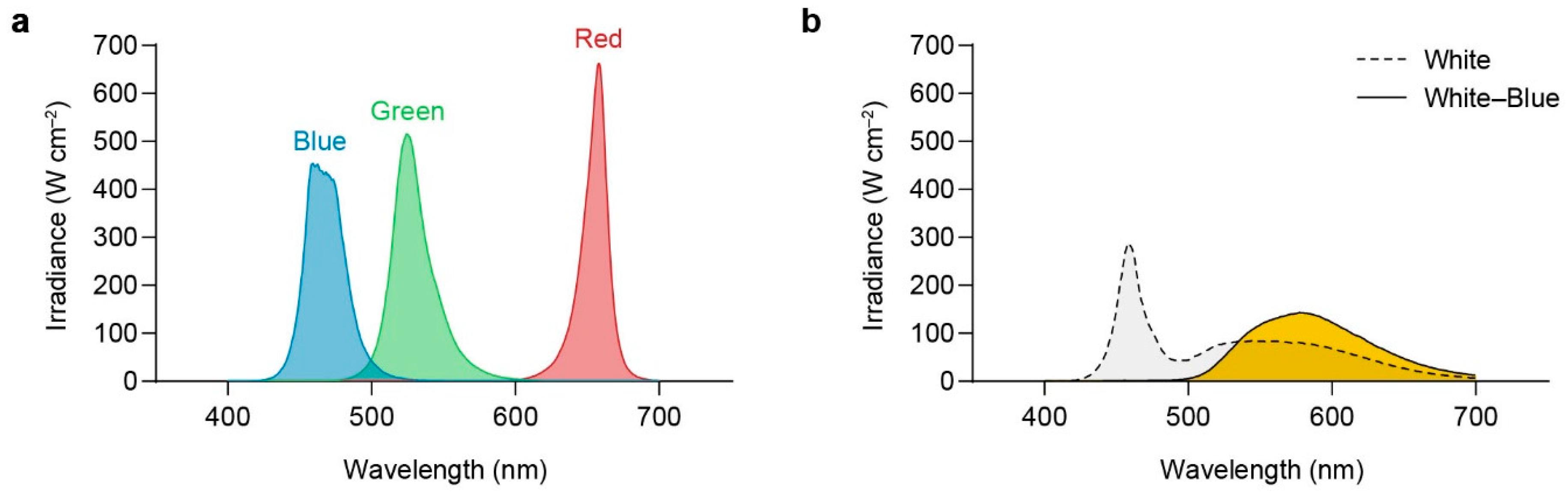
2.5. Relative Efficiency of Blue Light Quanta
2.6. Changes in the U. australis Proteome under Static or Turbulent Conditions and Blue Light Illumination
3. Discussion
3.1. Growth and Cell Number
3.2. Photosynthetic Pigment Contents
3.3. Chl a Fluorescence
3.4. Correlation between the Growth, Photosynthetic Efficiency, and Photosynthetic Pigment Contents of U. australis under Different Light Wavelengths
3.5. Relative Efficiency of Blue Light
3.6. Changes in the U. australis Proteome with or without Aeration under Blue Light
4. Materials and Methods
4.1. Laboratory Culture of U. australis
4.2. Experimental Treatments
4.3. Measuring Growth and Cell Number
4.4. Estimating Pigment Content
4.5. Measuring Chl a Fluorescence
4.6. Relative Effectiveness of the Blue Quantum
4.7. Protein Extraction from U. australis Disks
4.8. Two-Dimensional Electrophoresis and Image Acquisition
4.9. Protein Identification by MALDI-TOF/TOF
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Duarte, C.M.; Wu, J.; Xiao, X.; Bruhn, A.; Krause-Jensen, D. Can seaweed farming play a role in climate change mitigation and adaptation? Front. Mar. Sci. 2017, 4, 100. [Google Scholar] [CrossRef]
- Kraan, S. Seaweed resources, collection, and cultivation with respect to sustainability. In Sustainable Seaweed Technologies; Torres, M.D., Kraan, S., Dominguez, H., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 89–102. [Google Scholar] [CrossRef]
- Sultana, F.; Wahab, M.A.; Nahiduzzaman, M.; Mohiuddin, M.; Iqbal, M.Z.; Shakil, A.; Mamun, A.A.; Khan, M.S.R.; Wong, L.; Asaduzzaman, M. Seaweed farming for food and nutritional security, climate change mitigation and adaptation, and women empowerment: A review. Aquac. Fish. 2023, 8, 463–480. [Google Scholar] [CrossRef]
- Marques, R.; Moreira, A.; Cruz, S.; Calado, R.; Cartaxana, P. Controlling light to optimize growth and added value of the green macroalga Codium tomentosum. Front. Mar. Sci. 2022, 9, 906332. [Google Scholar] [CrossRef]
- Orfanidis, S. Light requirements for growth of six shade-acclimated Mediterranean macroalgae. Mar. Biol. 1992, 112, 511–515. [Google Scholar] [CrossRef]
- Huang, S.; Li, K.; Pan, Y.; Yu, Y.; Wernberg, T.; de Bettignies, T.; Wu, J.; Zhou, C.; Huang, Z.; Xiao, X. Artificial light source selection in seaweed production: Growth of seaweed and biosynthesis of photosynthetic pigments and soluble protein. PeerJ 2021, 9, e11351. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.; Liu, Z.; Zou, D. Growth and photosynthetic characteristics of Gracilaria lemaneiformis (Rhodophyta) and Ulva lactuca (Chlorophyta) cultured under fluorescent light and different LED light. J. Appl. Phycol. 2020, 32, 3265–3272. [Google Scholar] [CrossRef]
- Lüning, K.; Dring, M.J. Reproduction induced by blue light in female gametophytes of Laminaria saccharina. Planta 1972, 104, 252–256. [Google Scholar] [CrossRef]
- Öztaşkent, C.; Ak, İ. Effect of LED light sources on the growth and chemical composition of brown seaweed Treptacantha barbata. Aquac. Int. 2021, 29, 193–205. [Google Scholar] [CrossRef]
- Takahashi, F.; Yamagata, D.; Ishikawa, M.; Fukamatsu, Y.; Ogura, Y.; Kasahara, M.; Kiyosue, T.; Kikuyama, M.; Wada, M.; Kataoka, H. AUREOCHROME, a photoreceptor required for photomorphogenesis in stramenopiles. Proc. Natl. Acad. Sci. USA 2007, 104, 19625–19630. [Google Scholar] [CrossRef]
- Forster, R.; Dring, M.J. Interactions of blue light and inorganic carbon supply in the control of light-saturated photosynthesis in brown algae. Plant Cell Environ. 1992, 15, 241–247. [Google Scholar] [CrossRef]
- Han, T.; Kain, J.M. Blue light photoreactivation in ultraviolet-irradiated young sporophytes of Alaria esculenta and Laminaria saccharina (Phaeophyta). J. Phycol. 1993, 29, 79–81. [Google Scholar] [CrossRef]
- Miki, O.; Okumura, C.; Marzuki, M.; Tujimura, Y.; Fujii, T.; Kosugi, C.; Kato, T. Contrasting effects of blue and red LED irradiations on the growth of Sargassum horneri during the germling and immature stages. J. App. Phycol. 2017, 29, 1461–1469. [Google Scholar] [CrossRef]
- Klenell, M.; Snoeijs, P.; Pedersén, M. The involvement of a plasma membrane H+-ATPase in the blue-light enhancement of photosynthesis in Laminaria digitata (Phaeophyta). J. Phycol. 2002, 38, 1143–1149. [Google Scholar] [CrossRef]
- Dring, M.J.; Lüning, K. A photoperiodic response mediated by blue light in the brown alga Scytosiphon lomentaria. Planta 1975, 125, 25–32. [Google Scholar] [CrossRef]
- Mizuta, H.; Kai, T.; Tabuchi, K.; Yasui, H. Effects of light quality on the reproduction and morphology of sporophytes of Laminaria japonica (Phaeophyceae). Aquac. Res. 2007, 38, 1323–1329. [Google Scholar] [CrossRef]
- Wang, W.J.; Sun, X.T.; Wang, F.J. Effect of blue light on early sporophyte development of Saccharina japonica (Phaeophyta). Mar. Biol. 2010, 157, 1811–1817. [Google Scholar] [CrossRef]
- Han, T.; Kong, J.A.; Han, Y.S.; Kang, S.H.; Häder, D.P. UV-A/blue light-induced reactivation of spore germination in UV-B irradiated Ulva pertusa (Chlorophyta). J. Phycol. 2004, 40, 315–322. [Google Scholar] [CrossRef]
- Duggins, D.O.; Eckman, J.E.; Siddon, C.E.; Klinger, T. Population, morphometric and biomechanical studies of three understory kelps along a hydrodynamic gradient. Mar. Ecol. Prog. Ser. 2003, 265, 57–76. [Google Scholar] [CrossRef][Green Version]
- Harder, D.L.; Stevens, C.L.; Speck, T.; Hurd, C.L. The role of blade buoyancy and reconfiguration in the mechanical adaptation of the southern bull kelp. In Ecology and Biomechanics; Herrel, A., Speck, T., Rowe, N.P., Eds.; CRC Press: Boca Raton, FL, USA, 2006; pp. 61–84. [Google Scholar] [CrossRef]
- Johnson, A.S.; Koehl, M.A.R. Maintenance of dynamic strain similarity and environmental stress factor in different flow habitats: Thallus allometry and material properties of a giant kelp. J. Exp. Biol. 1994, 195, 81–410. [Google Scholar] [CrossRef]
- Kitzes, J.A.; Denny, M.W. Red algae respond to waves: Morphological and mechanical variation in Mastocarpus papillatus along a gradient of force. Biol. Bull. 2005, 208, 114–119. [Google Scholar] [CrossRef]
- Raven, J.A.; Hurd, C.L. Ecophysiology of photosynthesis in macroalgae. Photosynth. Res. 2012, 113, 105–125. [Google Scholar] [CrossRef]
- Hurd, C.L. Shaken and stirred: The fundamental role of water motion in resource acquisition and seaweed productivity. Perspect. Phycol. 2017, 4, 73–81. [Google Scholar] [CrossRef]
- Kregting, L.T.; Hepburn, C.D.; Savidge, G. Seasonal differences in the effects of oscillatory and uni-directional flow on the growth and nitrate-uptake rates of juvenile Laminaria digitata (Phaeophyceae). J. Phycol. 2015, 51, 1116–1126. [Google Scholar] [CrossRef] [PubMed]
- Leigh, E.G.; Paine, R.T.; Quinn, J.F.; Suchanek, T.H. Wave energy and intertidal productivity. Proc. Natl. Acad. Sci. USA 1987, 84, 1314–1318. [Google Scholar] [CrossRef]
- Dudgeon, S.R.; Johnson, A.S. Thick vs. thin: Thallus morphology and tissue mechanics influence differential drag and dislodgement of two co-dominant seaweeds. J. Exp. Mar. Biol. Ecol. 1992, 165, 23–43. [Google Scholar] [CrossRef]
- Gutierrez, L.M.; Fernández, C. Water motion and morphology in Chondrus crispus (Rhodophyta). J. Phycol. 1992, 28, 156–162. [Google Scholar] [CrossRef]
- Wing, S.R.; Leichter, J.J.; Perrin, C.; Rutger, S.M.; Bowman, M.H.; Cornelisen, C.D. Topographic shading and wave exposure influence morphology and ecophysiology of Ecklonia radiata (C. Agardh 1817) in Fiordland, New Zealand. Limnol. Oceanogr. 2007, 52, 1853–1864. [Google Scholar] [CrossRef]
- Kregting, L.; Blight, A.; Elsäßer, B.; Savidge, G. The influence of water motion on the growth rate of the kelp Laminaria digitata. J. Exp. Mar. Biol. Ecol. 2016, 478, 86–95. [Google Scholar] [CrossRef]
- Kregting, L.; Blight, A.; Elsäßer, B.; Savidge, G. The influence of water motion on the growth rate of the kelp Laminaria hyperborea. J. Exp. Mar. Biol. Ecol. 2013, 448, 337–345. [Google Scholar] [CrossRef]
- Matsumoto, F. Studies on the effect of environmental factors on the growth of ‘Nori’ (Porphyra tenera Kjellm.) with special reference to the water current. J. Fac. Fish. Anim. Hush. Hiroshima Univ. 1959, 2, 249–333. [Google Scholar]
- Santelices, B. Water movement and seasonal algal growth in Hawaii. Mar. Biol. 1977, 43, 225–235. [Google Scholar] [CrossRef]
- Santelices, B. Multiple interaction of factors in the distribution of some Hawaiian Gelidiales (Rhodophyta). Pac. Sci. 1978, 32, 119–147. [Google Scholar]
- Wheeler, W.N. Effect of boundary layer transport on the fixation of carbon by the giant kelp Macrocystis pyrifera. Mar. Biol. 1980, 56, 103–110. [Google Scholar] [CrossRef]
- Ghaderiardakani, F.; Califano, G.; Mohr, J.F.; Abreu, M.H.; Coates, J.C.; Wichard, T. Analysis of algal growth- and morphogenesis-promoting factors in an integrated multi-trophic aquaculture system for farming Ulva spp. Aquac. Environ. Interact. 2019, 11, 375–391. [Google Scholar] [CrossRef]
- Lawton, R.J.; Mata, L.; de Nys, R.; Paul, N.A. Algal bioremediation of waste waters from land-based aquaculture using Ulva: Selecting target species and strains. PLoS ONE 2013, 8, e77344. [Google Scholar] [CrossRef] [PubMed]
- Han, T.; Choi, G.W. A novel marine algal toxicity bioassay based on sporulation inhibition in the green macroalga Ulva pertusa (Chlorophyta). Aquat. Toxicol. 2005, 75, 202–212. [Google Scholar] [CrossRef] [PubMed]
- Han, T.; Han, Y.S.; Kim, K.Y.; Kim, J.H.; Shin, H.W.; Kain, J.M.; Callow, J.A.; Callow, M.E. Influences of light and UV-B on growth and sporulation of the green alga Ulva pertusa Kjellman. J. Exp. Mar. Biol. Ecol. 2003, 290, 115–131. [Google Scholar] [CrossRef]
- Han, T.; Kang, S.H.; Park, J.S.; Lee, H.K.; Brown, M.T. Physiological responses of Ulva pertusa and U. armoricana to copper exposure. Aquat. Toxicol. 2008, 86, 176–184. [Google Scholar] [CrossRef]
- Lee, H.; Brown, M.T.; Choi, S.; Pandey, L.K.; De Saeger, J.; Shin, K.; Kim, J.K.; Depuydt, S.; Han, T.; Park, J. Reappraisal of the toxicity test method using the green alga Ulva pertusa Kjellman (Chlorophyta). J. Hazard Mater. 2019, 369, 763–769. [Google Scholar] [CrossRef]
- Lee, H.; Park, J.; Shin, K.; Depuydt, S.; Choi, S.; De Saeger, J.; Han, T. Application of a programmed semi-automated Ulva pertusa bioassay for testing single toxicants and stream water quality. Aquat. Toxicol. 2020, 221, 105426. [Google Scholar] [CrossRef]
- Park, J.; Brown, M.T.; Lee, H.; Choi, S.; Depuydt, S.; Häder, D.P.; Han, T. Toxicity testing using the marine macroalga Ulva pertusa: Method development and application. In Bioassays Advanced Methods and Applications; Häder, D.P., Erzinger, G.S., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 119–142. [Google Scholar] [CrossRef]
- Park, J.; Brown, M.T.; Lee, H.; Vieira, C.; Pandey, L.K.; Choi, E.; Depuydt, S.; Häder, D.P.; Han, T. UV-B radiation and the green tide-forming macroalga Ulva. In Aquatic Ecosystems in a Changing Climate; Häder, D.P., Gao, K., Eds.; CRC Press: Boca Raton, FL, USA, 2018; pp. 202–226. [Google Scholar]
- Tako, M.; Tamanaha, M.; Tamashiro, Y.; Uechi, S. Structure of ulvan isolated from the edible green seaweed, Ulva pertusa. Adv. Biosci. Biotechnol. 2015, 6, 645–655. [Google Scholar] [CrossRef]
- Li, B.; Xu, H.; Wang, X.; Wan, Y.; Jiang, N.; Qi, H.; Liu, X. Antioxidant and antihyperlipidemic activities of high sulfate content purified polysaccharide from Ulva pertusa. Int. J. Biol. Macromol. 2020, 146, 756–762. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.; Zhang, Q.; Zhao, T.; Chen, R.; Zhang, H.; Niu, X.; Li, Z. Antioxidant activity of different sulfate content derivatives of polysaccharide extracted from Ulva pertusa (Chlorophyta) in vitro. Int. J. Biol. Macromol. 2005, 37, 195–199. [Google Scholar] [CrossRef]
- Sun, Y.-Y.; Wang, H.; Guo, G.; Pu, Y.-F.; Yan, B.-L.; Wang, C.-H. Green alga Ulva pertusa—A new source of bioactive compounds with antialgal activity. Environ. Sci. Pollut. Res. Int. 2015, 22, 10351–10359. [Google Scholar] [CrossRef]
- Le, B.; Shin, J.A.; Kang, M.G.; Sun, S.; Yang, S.H.; Chung, G. Enhanced growth rate and ulvan yield of Ulva pertusa using light-emitting diodes (LEDs). Aquac. Int. 2018, 26, 937–946. [Google Scholar] [CrossRef]
- Han, T.; Han, Y.S.; Kain, J.M.; Häder, D.P. Thallus differentiation of photosynthesis, growth, reproduction, and UV-B sensitivity in the green alga Ulva pertusa (Chlorophyceae). J. Phycol. 2003, 39, 712–721. [Google Scholar] [CrossRef]
- Kuwano, K.; Abe, N.; Nishi, Y.; Seno, H.; Nishihara, G.N.; Iima, M.; Zachleder, V. Growth and cell cycle of Ulva compressa (Ulvophyceae) under LED illumination. J. Phycol. 2014, 50, 744–752. [Google Scholar] [CrossRef]
- Senger, H. The effect of blue light on plants and microorganisms. Photochem. Photobiol. 1982, 35, 911–920. [Google Scholar] [CrossRef]
- Terashima, I.; Fujita, T.; Inoue, T.; Chow, W.S.; Oguchi, R. Green light drives leaf photosynthesis more efficiently than red light in strong white light: Revisiting the enigmatic question of why leaves are green. Plant Cell Physiol. 2009, 50, 684–697. [Google Scholar] [CrossRef]
- Mariani Colombo, P.; Orsenigo, M. Sea depth effects on the algal photosynthetic apparatus II. An electron microscopic study of the photosynthetic apparatus of Halimeda tuna (Chlorophyta, Siphonales) at −0.5 m and −6.0 m sea depths. Phycologia 1977, 16, 9–17. [Google Scholar] [CrossRef]
- Vesk, M.; Jeffrey, S.W. Effect of blue-green light on photosynthetic pigments and chloroplast structure in unicellular marine algae from six classes. J. Phycol. 1977, 13, 280–288. [Google Scholar] [CrossRef]
- Zhang, T.; Folta, K.M. Green light signaling and adaptive response. Plant Signal Behav. 2012, 7, 75–78. [Google Scholar] [CrossRef] [PubMed]
- Figueroa, F.L.; Niell, F.X. Red-light and blue-light photoreceptors controlling chlorophyll a synthesis in the red alga Porphyra umbilicalis and in the green alga Ulva rigida. Physiol. Plant 1989, 76, 391–397. [Google Scholar] [CrossRef]
- Ntefidou, M.; Iseki, M.; Watanabe, M.; Lebert, M.; Häder, D.P. Photoactivated adenylyl cyclase controls phototaxis in the flagellate Euglena gracilis. Plant Physiol. 2003, 133, 1517–1521. [Google Scholar] [CrossRef][Green Version]
- Tamaki, S.; Tanno, Y.; Kato, S.; Ozasa, K.; Wakazaki, M.; Sato, M.; Toyooka, K.; Maoka, T.; Ishikawa, T.; Maeda, M.; et al. Carotenoid accumulation in the eyespot apparatus required for phototaxis is independent of chloroplast development in Euglena gracilis. Plant Sci. 2020, 298, 110564. [Google Scholar] [CrossRef] [PubMed]
- Dhami, N.; Cazzonelli, C.I. Environmental impacts on carotenoid metabolism in leaves. Plant Growth Regul. 2020, 92, 455–477. [Google Scholar] [CrossRef]
- Quian-Ulloa, R.; Stange, C. Carotenoid biosynthesis and plastid development in plants: The role of light. Int. J. Mol. Sci. 2021, 22, 1184. [Google Scholar] [CrossRef]
- Park, J.; Dinh, T.B. Contrasting effects of monochromatic LED lighting on growth, pigments and photosynthesis in the commercially important cyanobacterium Arthrospira maxima. Bioresour. Technol. 2019, 291, 121846. [Google Scholar] [CrossRef]
- Chen, L.L.; Wang, H.Y.; Gong, X.C.; Zeng, Z.H.; Xue, X.Z.; Hu, Y.G. Transcriptome analysis reveals effects of red and blue light-emitting diodes (LEDs) on the growth, chlorophyll fluorescence and endogenous plant hormones of potato (Solanum tuberosum L.) plantlets cultured in vitro. J. Integr. Agri. 2021, 20, 2914–2931. [Google Scholar] [CrossRef]
- Lüning, K. Control of algal life-history by daylength and temperature. In The Shore Environment, Volume 2. Ecosystems; Price, J., Irvine, D., Farnham, W., Eds.; Academic Press: Cambridge, MA, USA, 1980; pp. 915–945. [Google Scholar]
- Han, T.; Kain, J.M. Blue light sensitivity of UV-irradiated young sporophytes of Laminaria hyperborea. J. Exp. Mar. Biol. Ecol. 1992, 158, 219–230. [Google Scholar] [CrossRef]
- Dring, M.J.; Lüning, K. Photomorphogenesis of brown algae in the laboratory and in the sea. In International Seaweed Symposium; De Gruyter: Berlin, Germany, 1981; Volume 8, pp. 159–166. [Google Scholar]
- Lüning, K.; Dring, M.J. Reproduction, growth and photosynthesis of gametophytes of Laminaria saccharina grown in blue and red light. Mar. Biol. 1975, 29, 195–200. [Google Scholar] [CrossRef]
- Lüning, K.; Neushul, M. Light and temperature demands for growth and reproduction of laminarian gametophytes in southern and central California. Mar. Biol. 1978, 45, 297–309. [Google Scholar] [CrossRef]
- Dring, M.J. Photocontrol of development in algae. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1988, 39, 157–174. [Google Scholar] [CrossRef]
- Yamamoto, Y. Quality control of photosystem II. Plant Cell Physiol. 2001, 42, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Andersson, I.; Backlund, A. Structure and function of Rubisco. Plant Physiol. Biochem. 2008, 46, 275–291. [Google Scholar] [CrossRef] [PubMed]
- Paul, M.J.; Jhurreea, D.; Zhang, Y.; Primavesi, L.F.; Delatte, T.; Schluepmann, H.; Wingler, A. Upregulation of biosynthetic processes associated with growth by trehalose 6-phosphate. Plant Signal Behav. 2010, 5, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Primavesi, L.F.; Jhurreea, D.; Andralojc, P.J.; Mitchell, R.A.; Powers, S.J.; Schluepmann, H.; Delatte, T.; Wingler, A.; Paul, M.J. Inhibition of SNF1-related protein kinase1 activity and regulation of metabolic pathways by trehalose-6-phosphate. Plant Physiol. 2009, 149, 1860–1871. [Google Scholar] [CrossRef]
- Wingler, A.; Fritzius, T.; Wiemken, A.; Boller, T.; Aeschbacher, R.A. Trehalose induces the ADP-glucose pyrophosphorylase gene, ApL3, and starch synthesis in Arabidopsis. Plant Physiol. 2000, 124, 105–114. [Google Scholar] [CrossRef]
- Kolbe, A.; Tiessen, A.; Schluepmann, H.; Paul, M.; Ulrich, S.; Geigenberger, P. Trehalose 6-phosphate regulates starch synthesis via posttranslational redox activation of ADP-glucose pyrophosphorylase. Proc. Natl. Acad. Sci. USA 2005, 102, 11118–11123. [Google Scholar] [CrossRef]
- Gómez, L.D.; Baud, S.; Gilday, A.; Li, Y.; Graham, I.A. Delayed embryo development in the ARABIDOPSIS TREHALOSE-6-PHOSPHATE SYNTHASE 1 mutant is associated with altered cell wall structure, decreased cell division and starch accumulation. Plant J. 2006, 46, 69–84. [Google Scholar] [CrossRef]
- Ott, F.D. Synthetic media and techniques for the xenic cultivation of marine algae and flagellata. Va. J. Sci. 1965, 16, 205–218. [Google Scholar]
- Lichtenthaler, H.K. Chlorophyll fluorescence signatures of leaves during the autumnal chlorophyll breakdown. J. Plant Physiol. 1987, 131, 101–110. [Google Scholar] [CrossRef]
- Ralph, P.J.; Gademann, R. Rapid light curves: A powerful tool to assess photosynthetic activity. Aquat. Bot. 2005, 82, 222–237. [Google Scholar] [CrossRef]
- Platt, T.; Gallegos, C.L.; Harrison, W.G. Photoinhibition of photosynthesis in natural assemblages of marine phytoplankton. J. Mar. Res. 1980, 38, 687–701. [Google Scholar]
- Bilger, W.; Björkman, O. Role of the xanthophyll cycle in photoprotection elucidated by measurements of light-induced absorbance changes, fluorescence and photosynthesis in leaves of Hedera canariensis. Photosynth. Res. 1990, 25, 173–185. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar] [CrossRef]


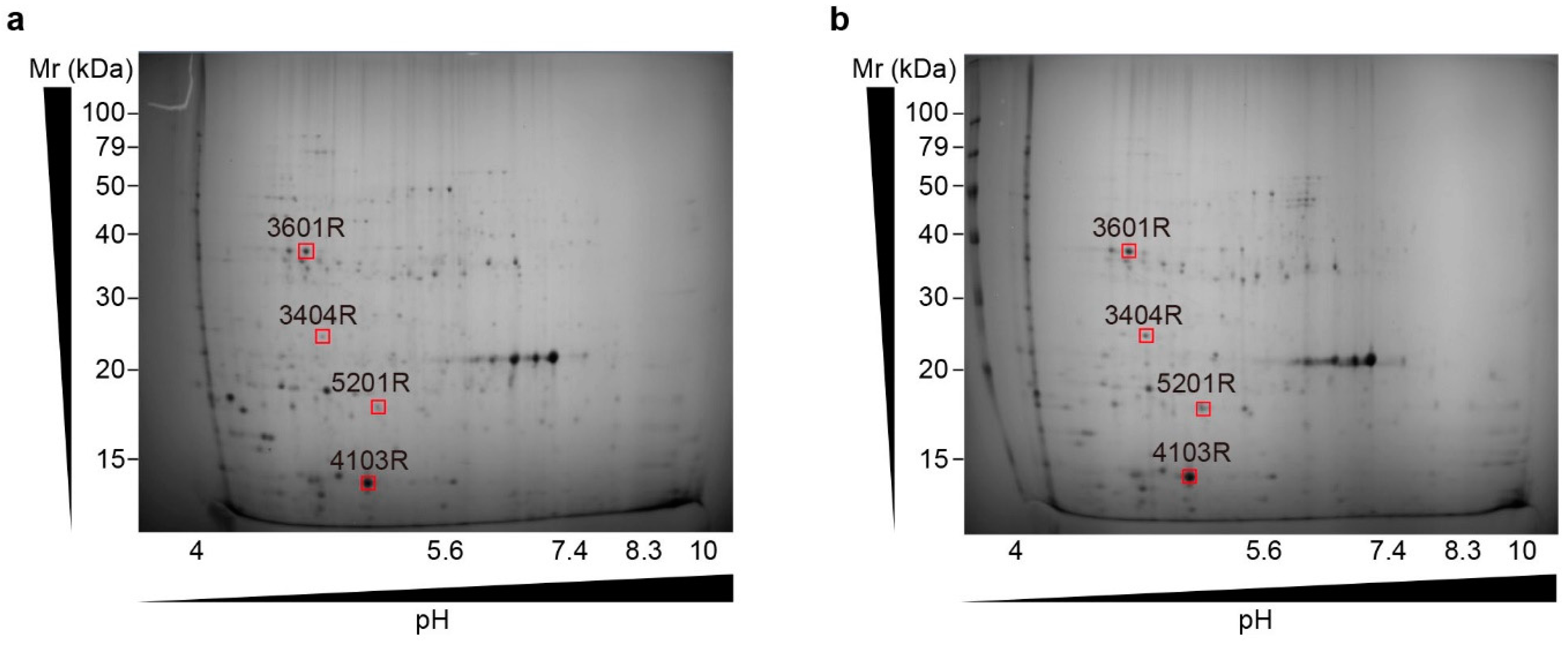
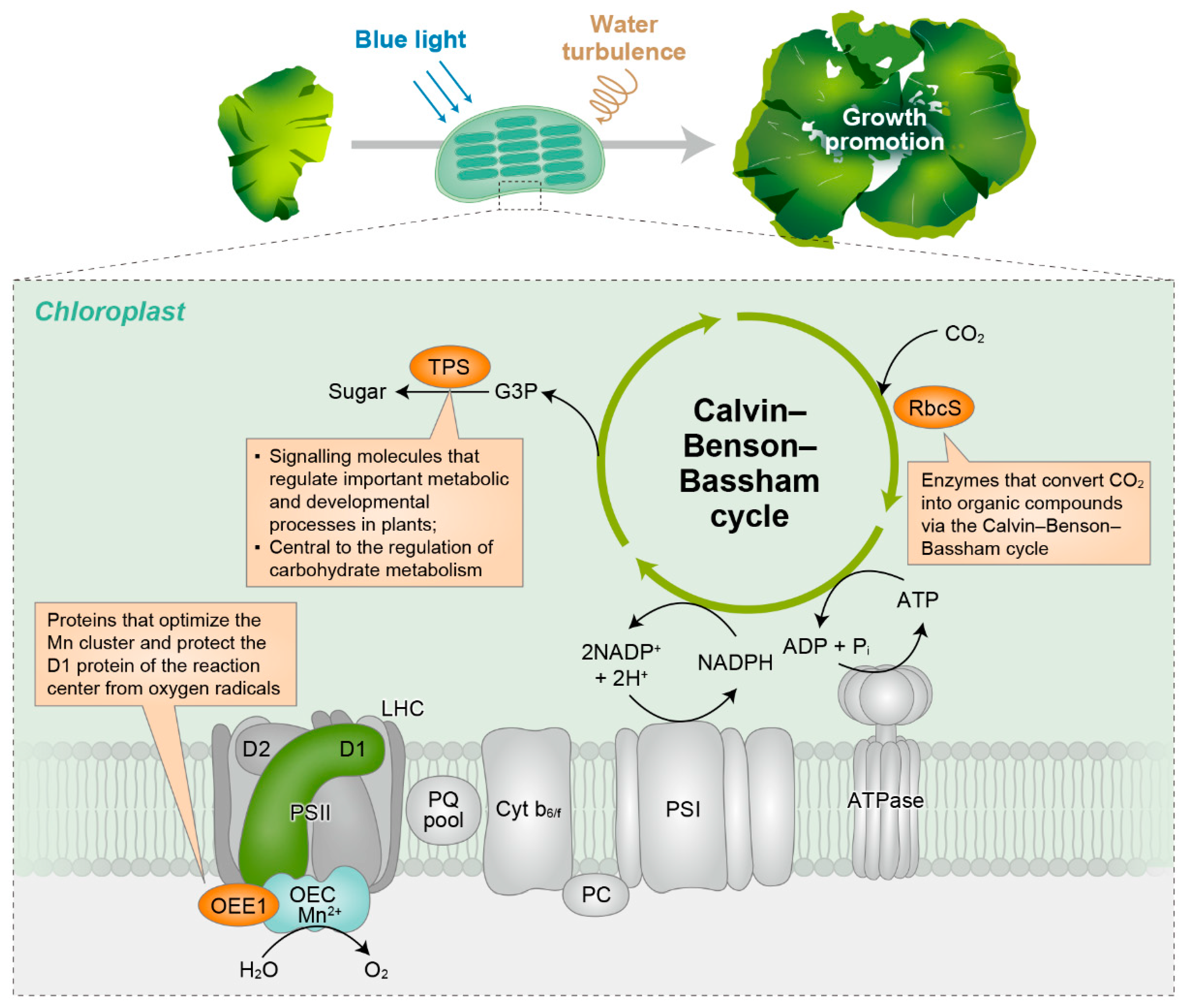
| Spot Number | Molecular Weight (kDa) | Protein Name | Gene Symbol | Description |
|---|---|---|---|---|
| 3601 | 37.2 | 30S ribosomal protein S9, chloroplast | rps9 | Housekeeping gene/reference gene |
| 4103 | 13.4 | Ribulose bisphosphate carboxylase small subunit | RbcS | An enzyme that converts CO2 into organic compounds via the Calvin–Benson–Bassham cycle |
| 5201 | 17.5 | Trehalose-6-phosphate synthase | TPS | Synthesises a signalling molecule that regulates plant metabolism and development; central to the regulation of carbohydrate metabolism |
| 3404 | 24.2 | Oxygen-evolving enhancer protein (Fragment) | OEE1 | Optimises the Mn cluster and protects the D1 protein of the reaction centre from oxygen radicals |
| Species | Quantum Requirement (mol·m−2) | Response | Reference |
|---|---|---|---|
| Alaria esculenta | 1.9 | Photoreactivation | Han and Kain [12] |
| Laminaria hyperborea | 2.5 | Photoreactivation | Han and Kain [65] |
| 2.1 | Egg formation | Dring and Lüning [66] | |
| Laminaria saccharina | 1.95 | Egg release | Lüning and Dring [15] |
| 1.2 | Photoreactivation | Han and Kain [12] | |
| Macrocystis pyrifera | 2.6 | Egg formation | Lüning and Neushul [67] |
| Scytosiphon lomentaria | 2.25 | 2D 1 growth | Dring and Lüning [68] |
| 1.97 | Hair formation | Dring and Lüning [68] | |
| Ulva australis | 1.02 | Growth | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.; Depuydt, S.; Shin, K.; De Saeger, J.; Han, T.; Park, J. Interactive Effects of Blue Light and Water Turbulence on the Growth of the Green Macroalga Ulva australis (Chlorophyta). Plants 2024, 13, 266. https://doi.org/10.3390/plants13020266
Lee H, Depuydt S, Shin K, De Saeger J, Han T, Park J. Interactive Effects of Blue Light and Water Turbulence on the Growth of the Green Macroalga Ulva australis (Chlorophyta). Plants. 2024; 13(2):266. https://doi.org/10.3390/plants13020266
Chicago/Turabian StyleLee, Hojun, Stephen Depuydt, Kisik Shin, Jonas De Saeger, Taejun Han, and Jihae Park. 2024. "Interactive Effects of Blue Light and Water Turbulence on the Growth of the Green Macroalga Ulva australis (Chlorophyta)" Plants 13, no. 2: 266. https://doi.org/10.3390/plants13020266
APA StyleLee, H., Depuydt, S., Shin, K., De Saeger, J., Han, T., & Park, J. (2024). Interactive Effects of Blue Light and Water Turbulence on the Growth of the Green Macroalga Ulva australis (Chlorophyta). Plants, 13(2), 266. https://doi.org/10.3390/plants13020266






