Effects of Exogenous Abscisic Acid on the Physiological and Biochemical Responses of Camellia oleifera Seedlings under Drought Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials and Design
2.2. Measurement of Photosynthetic Parameters
2.3. Determination of Physiological and Biochemical Indices
2.4. Membership Function Method
2.5. RNA Extraction and Real-Time Fluorescent Quantitative Analysis
2.6. Statistical Analysis
3. Results
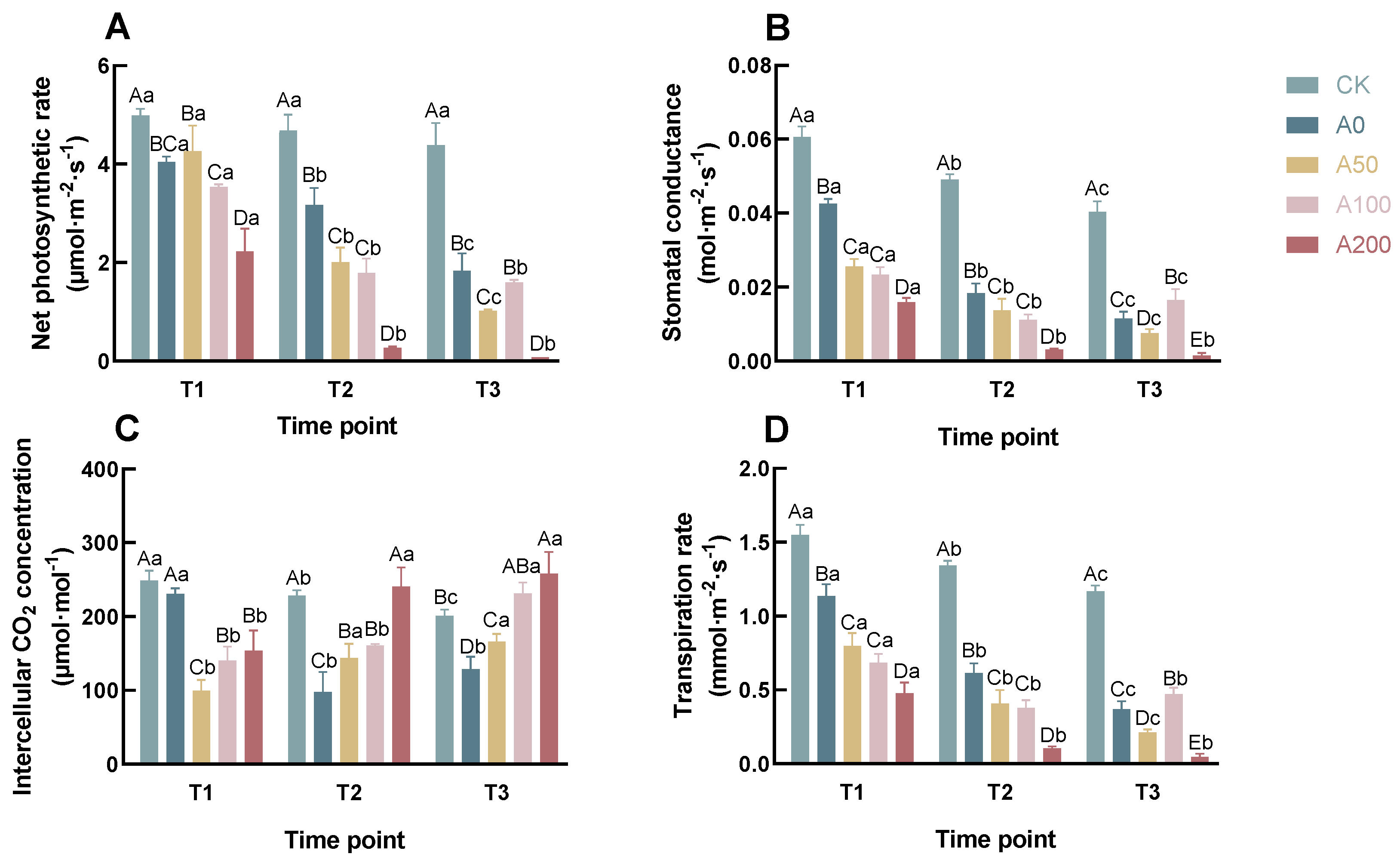
3.1. Effects of Exogenous ABA on Photosynthetic Parameters of C. oleifera Seedlings under Drought Stress
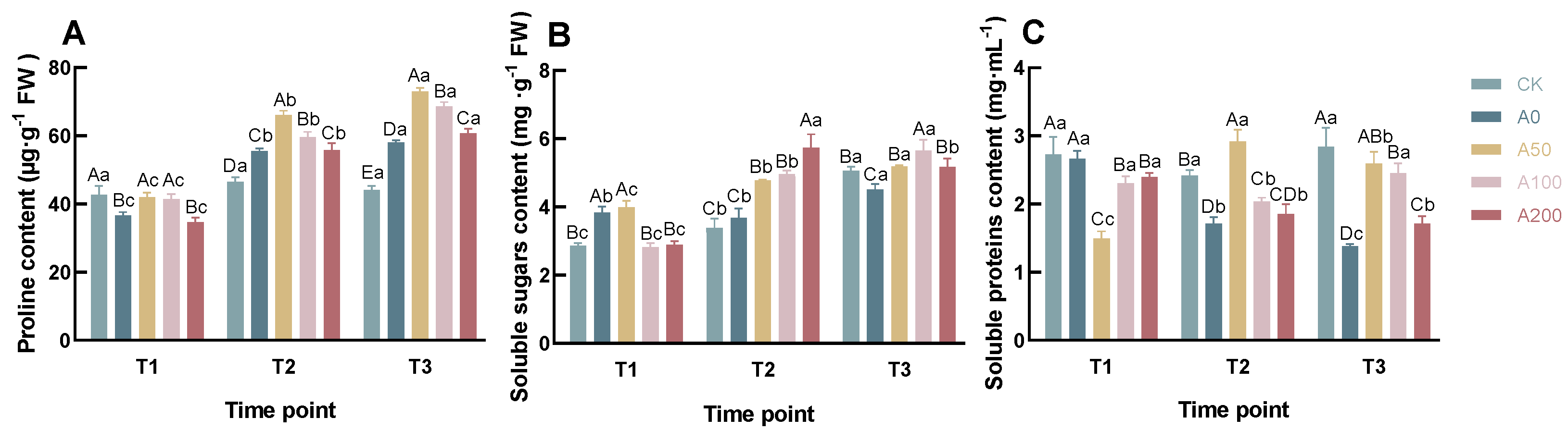
3.2. Effects of Exogenous ABA on Physiological Indicators of C. oleifera Seedlings under Drought Stress
3.3. Effects of Exogenous ABA on Gene Expression in C. oleifera Seedlings under Drought Stress
3.4. Comprehensive Evaluation through Membership Function Analysis
4. Discussion
4.1. Photosynthetic Parameters
4.2. Physiological Indicators
4.3. Gene Expression
4.4. Limitations and Future Prospects
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhong, W.; Shen, J.; Liao, X.; Liu, X.; Zhang, J.; Zhou, C.; Jin, Y. Camellia (Camellia oleifera Abel.) seed oil promotes milk fat and protein synthesis-related gene expression in bovine mammary epithelial cells. Food Sci. Nutr. 2020, 8, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Ye, H.; Rui, Y.; Chen, G.; Zhang, N. Fatty acid composition of Camellia oleifera oil. J. Verbraucherschutz Leb. 2011, 6, 9–12. [Google Scholar] [CrossRef]
- Lee, C.P.; Yen, G.C. Antioxidant activity and bioactive compounds of tea seed (Camellia oleifera Abel.) oil. J. Agric. Food Chem. 2006, 54, 779–784. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Ouyang, Z.; Chen, S.; Van Koppen, C. Role of culturally protected forests in biodiversity conservation in Southeast China. Biodivers. Conserv. 2013, 22, 531–544. [Google Scholar] [CrossRef]
- Zheng, H.; Chen, F.; Ouyang, Z.; Tu, N.; Xu, W.; Wang, X.; Miao, H.; Li, X.; Tian, Y. Impacts of reforestation approaches on runoff control in the hilly red soil region of Southern China. J. Hydrol. 2008, 356, 174–184. [Google Scholar] [CrossRef]
- Dong, B.; Lan, L.; Huang, Y.; Wei, X.; Gong, H.; Hong, W.; Huang, L.; Chen, H. Effects of drought stress on photosynthetic pigments and chlorophyll fluorescence characteristics in leaves of Camellia oleifera. Non-Wood For. Res. 2020, 38, 16–25. [Google Scholar]
- Wang, R.; Zhong, F.; Liao, W.; Li, T. Effects of Soil Moisture on Fruit Growth of Camellia oleifera. Sci. Silvae Sin. 2014, 50, 40–46. [Google Scholar]
- Laxa, M.; Liebthal, M.; Telman, W.; Chibani, K.; Dietz, K. The role of the plant antioxidant system in drought tolerance. Antioxidants 2019, 8, 94. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Ozturk, M.; Turkyilmaz Unal, B.; García-Caparrós, P.; Khursheed, A.; Gul, A.; Hasanuzzaman, M. Osmoregulation and its actions during the drought stress in plants. Physiol. Plant. 2021, 172, 1321–1335. [Google Scholar] [CrossRef]
- Devireddy, A.R.; Zandalinas, S.I.; Fichman, Y.; Mittler, R. Integration of reactive oxygen species and hormone signaling during abiotic stress. Plant J. 2021, 105, 459–476. [Google Scholar] [CrossRef] [PubMed]
- You, Y.; Wang, Y.; Zhang, S.; Sun, X.; Liu, H.; Guo, E.Y.; Du, S. Different pathways for exogenous ABA-mediated down-regulation of cadmium accumulation in plants under different iron supplies. J. Hazard. Mater. 2022, 440, 129769. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Lu, B.; Liu, L.; Duan, W.; Meng, Y.; Li, J.; Zhang, K.; Sun, H.; Zhang, Y.; Dong, H.; et al. Exogenous melatonin improves the salt tolerance of cotton by removing active oxygen and protecting photosynthetic organs. BMC Plant Biol. 2021, 21, 331. [Google Scholar] [CrossRef]
- Coelho, D.G.; de Andrade, H.M.; Marinato, C.S.; Araujo, S.C.; de Matos, L.P.; da Silva, V.M.; de Oliveira, J.A. Exogenous jasmonic acid enhances oxidative protection of Lemna valdiviana subjected to arsenic. Acta Physiol. Plant. 2020, 42, 1–9. [Google Scholar] [CrossRef]
- Ma, Q.; Wu, E.; Wang, H.; Yuan, Y.; Feng, Y.; Liu, J.; Zhao, L.; Feng, B. Exogenous 24-epibrassinolide boosts plant growth under alkaline stress from physiological and transcriptomic perspectives: The case of broomcorn millet (Panicum miliaceum L.). Ecotoxicol. Environ. Saf. 2022, 248, 114298. [Google Scholar] [CrossRef] [PubMed]
- Rady, M.M.; Boriek, S.H.; Abd El-Mageed, T.A.; Seif El-Yazal, M.A.; Ali, E.F.; Hassan, F.A.; Abdelkhalik, A. Exogenous gibberellic acid or dilute bee honey boosts drought stress tolerance in Vicia faba by rebalancing osmoprotectants, antioxidants, nutrients, and phytohormones. Plants 2021, 10, 748. [Google Scholar] [CrossRef]
- Bharath, P.; Gahir, S.; Raghavendra, A.S. Abscisic acid-induced stomatal closure: An important component of plant defense against abiotic and biotic stress. Front. Plant Sci. 2021, 12, 615114. [Google Scholar] [CrossRef]
- Muhammad Aslam, M.; Waseem, M.; Jakada, B.H.; Okal, E.J.; Lei, Z.; Saqib, H.S.A.; Yuan, W.; Xu, W.; Zhang, Q. Mechanisms of abscisic acid-mediated drought stress responses in plants. Int. J. Mol. Sci. 2022, 23, 1084. [Google Scholar] [CrossRef]
- Awan, S.A.; Khan, I.; Rizwan, M.; Zhang, X.; Brestic, M.; Khan, A.; El-Sheikh, M.A.; Alyemeni, M.N.; Ali, S.; Huang, L. Exogenous abscisic acid and jasmonic acid restrain polyethylene glycol-induced drought by improving the growth and antioxidative enzyme activities in pearl millet. Physiol. Plant. 2021, 172, 809–819. [Google Scholar] [CrossRef]
- Zhou, L.; Xu, H.; Mischke, S.; Meinhardt, L.W.; Zhang, D.; Zhu, X.; Li, X.; Fang, W. Exogenous abscisic acid significantly affects proteome in tea plant (Camellia sinensis) exposed to drought stress. Hortic. Res. 2014, 1, 14029. [Google Scholar] [CrossRef]
- He, Z.; Liu, C.; Zhang, Z.; Wang, R.; Chen, Y. Integration of mRNA and miRNA analysis reveals the differentially regulatory network in two different Camellia oleifera cultivars under drought stress. Front. Plant Sci. 2022, 13, 1001357. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Zhang, Z.; Xu, Y.; Wang, R.; Wang, X.; Chen, Y. Cloning of circadian clock gene LHY and its effect on photosynthesis under exogenous hormone treatment in Camellia oleifera. Non-Wood For. Res. 2021, 39, 1–9. [Google Scholar] [CrossRef]
- Zhuo, L.; Min, X.; Su, Z. Drought Resistance Identification and Screening Drought Resistance Indices for Geographical Populations within Tamarix Taklamakanensis. J. Xinjiang Norm. Univ. (Nat. Sci. Ed.) 2023, 42, 49–56. [Google Scholar] [CrossRef]
- Wang, Z.; Li, G.; Sun, H.; Ma, L.; Guo, Y.; Zhao, Z.; Gao, H.; Mei, L. Effects of drought stress on photosynthesis and photosynthetic electron transport chain in young apple tree leaves. Biol. Open 2018, 7, bio035279. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Liu, M.; Gu, W.; Chen, Z.; Gu, Y.; Pei, L.; Tian, R. Effect of drought on photosynthesis, total antioxidant capacity, bioactive component accumulation, and the transcriptome of Atractylodes lancea. BMC Plant Biol. 2021, 21, 293. [Google Scholar] [CrossRef] [PubMed]
- Ye, Q.; Wang, H.; Li, H. Arbuscular mycorrhizal fungi improve growth, photosynthetic activity, and chlorophyll fluorescence of vitis vinifera l. cv. ecolly under drought stress. Agronomy 2022, 12, 1563. [Google Scholar] [CrossRef]
- Hu, W.; Zhang, J.; Yan, K.; Zhou, Z.; Zhao, W.; Zhang, X.; Pu, Y.; Yu, R. Beneficial effects of abscisic acid and melatonin in overcoming drought stress in cotton (Gossypium hirsutum L.). Physiol. Plant. 2021, 173, 2041–2054. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Wang, Z.; Yang, Y.; Li, M.; Xu, B. Abscisic acid and brassinolide combined application synergistically enhances drought tolerance and photosynthesis of tall fescue under water stress. Sci. Hortic. 2018, 228, 1–9. [Google Scholar] [CrossRef]
- Khaleghnezhad, V.; Yousefi, A.R.; Tavakoli, A.; Farajmand, B.; Mastinu, A. Concentrations-dependent effect of exogenous abscisic acid on photosynthesis, growth and phenolic content of Dracocephalum moldavica L. under drought stress. Planta 2021, 253, 127. [Google Scholar] [CrossRef]
- Li, W.; Zhang, S.; Shan, L. Responsibility of non-stomatal limitations for the reduction of photosynthesis—Response of photosynthesis and antioxidant enzyme characteristics in alfalfa (Medicago sativa L.) seedlings to water stress and rehydration. Front. Agric. China 2007, 1, 255–264. [Google Scholar] [CrossRef]
- Kanechi, M.; Uchida, N.; Yasuda, T.; Yamaguchi, T. Non-stomatal inhibition associated with inactivation of Rubisco in dehydrated coffee leaves under unshaded and shaded conditions. Plant Cell Physiol. 1996, 37, 455–460. [Google Scholar] [CrossRef]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Das, K.; Roychoudhury, A. Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front. Environ. Sci. 2014, 2, 53. [Google Scholar] [CrossRef]
- Qian, J.; Zhang, X.; Yan, Y.; Wang, N.; Ge, W.; Zhou, Q.; Yang, Y. Unravelling the molecular mechanisms of abscisic acid-mediated drought-stress alleviation in pomegranate (Punica granatum L.). Plant Physiol. Biochem. 2020, 157, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Noctor, G.; Mhamdi, A.; Foyer, C.H. The roles of reactive oxygen metabolism in drought: Not so cut and dried. Plant Physiol. 2014, 164, 1636–1648. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Zhu, H.; Zhu, H.; Tao, Y.; Liu, C.; Liu, J.; Yang, F.; Li, M. Exogenous ABA enhances the antioxidant defense system of maize by regulating the AsA-GSH cycle under drought stress. Sustainability 2022, 14, 3071. [Google Scholar] [CrossRef]
- Diaz-Vivancos, P.; de Simone, A.; Kiddle, G.; Foyer, C.H. Glutathione–linking cell proliferation to oxidative stress. Free Radic. Biol. Med. 2015, 89, 1154–1164. [Google Scholar] [CrossRef]
- Anjum, N.A.; Sofo, A.; Scopa, A.; Roychoudhury, A.; Gill, S.S.; Iqbal, M.; Lukatkin, A.S.; Pereira, E.; Duarte, A.C.; Ahmad, I. Lipids and proteins—Major targets of oxidative modifications in abiotic stressed plants. Environ. Sci. Pollut. Res. 2015, 22, 4099–4121. [Google Scholar] [CrossRef]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxidative Med. Cell. Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef]
- Hosseinifard, M.; Stefaniak, S.; Ghorbani Javid, M.; Soltani, E.; Wojtyla, Ł.; Garnczarska,, M. Contribution of exogenous proline to abiotic stresses tolerance in plants: A review. Int. J. Mol. Sci. 2022, 23, 5186. [Google Scholar] [CrossRef]
- Liang, Y.; Wei, F.; Qin, S.; Li, M.; Hu, Y.; Lin, Y.; Wei, G.; Wei, K.; Miao, J.; Zhang, Z. Sophora tonkinensis: Response and adaptation of physiological characteristics, functional traits, and secondary metabolites to drought stress. Plant Biol. 2023, 25, 1109–1120. [Google Scholar] [CrossRef]
- Alsamadany, H.; Alzahrani, Y.; Shah, Z.H. Physiomorphic and molecular-based evaluation of wheat germplasm under drought and heat stress. Front. Plant Sci. 2023, 14, 1107945. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.; Samraj, S.; Jiménez, J.; Gómez, C.; Liu, T.; Begcy, K. Genome-wide identification of heat shock factors and heat shock proteins in response to UV and high intensity light stress in lettuce. BMC Plant Biol. 2021, 21, 185. [Google Scholar] [CrossRef] [PubMed]
- Bourgine, B.; Guihur, A. Heat shock signaling in land plants: From plasma membrane sensing to the transcription of small heat shock proteins. Front. Plant Sci. 2021, 12, 710801. [Google Scholar] [CrossRef]
- Nakashima, K.; Yamaguchi-Shinozaki, K. ABA signaling in stress-response and seed development. Plant Cell Rep. 2013, 32, 959–970. [Google Scholar] [CrossRef]
- Wei, H.; Movahedi, A.; Xu, C.; Wang, P.; Sun, W.; Yin, T.; Zhuge, Q. Heterologous overexpression of the Arabidopsis SnRK2. 8 gene enhances drought and salt tolerance in Populus× euramericana cv ‘Nanlin895’. Plant Biotechnol. Rep. 2019, 13, 245–261. [Google Scholar] [CrossRef]
- Legnaioli, T.; Cuevas, J.; Mas, P. TOC1 functions as a molecular switch connecting the circadian clock with plant responses to drought. EMBO J. 2009, 28, 3745–3757. [Google Scholar] [CrossRef] [PubMed]
- Valim, H.F.; McGale, E.; Yon, F.; Halitschke, R.; Fragoso, V.; Schuman, M.C.; Baldwin, I.T. The clock gene TOC1 in shoots, not roots, determines fitness of Nicotiana attenuata under drought. Plant Physiol. 2019, 181, 305–318. [Google Scholar] [CrossRef]
- Thomazella, D.P.d.T.; Seong, K.; Mackelprang, R.; Dahlbeck, D.; Geng, Y.; Gill, U.S.; Qi, T.; Pham, J.; Giuseppe, P.; Lee, C.Y.; et al. Loss of function of a DMR6 ortholog in tomato confers broad-spectrum disease resistance. Proc. Natl. Acad. Sci. USA 2021, 118, e2026152118. [Google Scholar] [CrossRef]
- Kieu, N.P.; Lenman, M.; Wang, E.S.; Petersen, B.L.; Andreasson, E. Mutations introduced in susceptibility genes through CRISPR/Cas9 genome editing confer increased late blight resistance in potatoes. Sci. Rep. 2021, 11, 4487. [Google Scholar] [CrossRef]
- Giacomelli, L.; Zeilmaker, T.; Giovannini, O.; Salvagnin, U.; Masuero, D.; Franceschi, P.; Vrhovsek, U.; Scintilla, S.; van der Voort, J.R.; Moser, C. Simultaneous editing of two DMR6 genes in grapevine results in reduced susceptibility to downy mildew. Front. Plant Sci. 2023, 14, 1242240. [Google Scholar] [CrossRef]
- Chen, D.; Wang, S.; Qi, L.; Yin, L.; Deng, X. Galactolipid remodeling is involved in drought-induced leaf senescence in maize. Environ. Exp. Bot. 2018, 150, 57–68. [Google Scholar] [CrossRef]
- Zhang, M.; Barg, R.; Yin, M.; Gueta-Dahan, Y.; Leikin-Frenkel, A.; Salts, Y.; Shabtai, S.; Ben-Hayyim, G. Modulated fatty acid desaturation via overexpression of two distinct ω-3 desaturases differentially alters tolerance to various abiotic stresses in transgenic tobacco cells and plants. Plant J. 2005, 44, 361–371. [Google Scholar] [CrossRef]
- Liu, X.; Ma, D.; Zhang, Z.; Wang, S.; Du, S.; Deng, X.; Yin, L. Plant lipid remodeling in response to abiotic stresses. Environ. Exp. Bot. 2019, 165, 174–184. [Google Scholar] [CrossRef]
- Khosravi-Nejad, F.; Khavari-Nejad, R.A.; Moradi, F.; Najafi, F. Cytokinin and abscisic acid alleviate drought stress through changing organic acids profile, ion immolation, and fatty acid profile to improve yield of wheat (Triticum aestivum L.) cultivars. Physiol. Mol. Biol. Plants 2022, 28, 1119–1129. [Google Scholar] [CrossRef]
- Gai, Z.; Wang, Y.; Ding, Y.; Qian, W.; Qiu, C.; Xie, H.; Sun, L.; Jiang, Z.; Ma, Q.; Wang, L.; et al. Exogenous abscisic acid induces the lipid and flavonoid metabolism of tea plants under drought stress. Sci. Rep. 2020, 10, 12275. [Google Scholar] [CrossRef]
- Guschina, I.A.; Harwood, J.L.; Smith, M.; Beckett, R.P. Abscisic acid modifies the changes in lipids brought about by water stress in the moss Atrichum androgynum. New Phytol. 2002, 156, 255–264. [Google Scholar] [CrossRef]



| Gene | Forward Primer (5′-3′) | Reverse Primer (5′-3′) |
|---|---|---|
| CoTubulin | CCATGCCTTGGATCACATTT | TGGGGCCATTAATGTAGACG |
| CoPP2C16 | GGAGGCAAGGTGATTCAGTGG | GGCATTCGTCTTCTTTCGTTCG |
| CoPP2C51 | CAGGGTGTTTGGTGTTCTTTCC | CTCATCCTCGTCACTCCTTGTC |
| CoPP2C75 | TTCTCGGTGTTCTCGCTATGTC | GTCGCTCGCCAGTATCAGG |
| CoSNRK2.8 | GCATTCCCAACCCAAATCTACAG | GCACCAACCAACATCACATATAAGG |
| CoTOC1 | AGGAGAAGGCGAATGCTTGG | CTCTACAGGAGCAGCAGCAG |
| CoDMR6 | TCAAGGATGGCAAGTGGATGG | GGCAGAGGAAAGAGGCTATGG |
| CoaccA | CGAAACCGGTCTGGACTTCA | CTCGATCTCCGTGAAGCTCC |
| CoaccB | CCACACCACCACCTATTCCC | TCCAGAAGCCTCCAATGCTG |
| CoGK | AGTTGAGTCCACTGGCGGAGTT | ACAGCACGAGCGATGTGAGACT |
| CoFAD6 | GGCTCAGCTCAATGGCACAGTT | CGCCAATTCCAAGTCGCCTCAT |
| CoFAD7 | ATCATGGCATCCGTTGTCTGA | GGTCTTCCCAGGACTTCTACCC |
| CoFAD8 | TCAATGGCGTCAATGGGG | CGGAATCGCAGCTCGAATAT |
| Group | Membership Function Value | D | Rank | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pn | Gs | Tr | Ci | SOD | POD | MDA | Pro | GSH | SP | SS | |||
| A0 | 1 | 0.67 | 0.24 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.17 | 4 |
| A50 | 0.54 | 0.41 | 0.61 | 0.29 | 1 | 1 | 0.66 | 1 | 1 | 1 | 0.59 | 0.73 | 1 |
| A100 | 0.87 | 1 | 0 | 0.8 | 0.59 | 0.52 | 0.19 | 0.71 | 0.75 | 0.88 | 1 | 0.66 | 2 |
| A200 | 0 | 0 | 1 | 1 | 0.31 | 0.54 | 1 | 0.18 | 0.68 | 0.27 | 0.58 | 0.51 | 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, D.; Chen, Y.; Wang, R.; He, Y.; Ma, X.; Shen, J.; He, Z.; Lai, H. Effects of Exogenous Abscisic Acid on the Physiological and Biochemical Responses of Camellia oleifera Seedlings under Drought Stress. Plants 2024, 13, 225. https://doi.org/10.3390/plants13020225
Yang D, Chen Y, Wang R, He Y, Ma X, Shen J, He Z, Lai H. Effects of Exogenous Abscisic Acid on the Physiological and Biochemical Responses of Camellia oleifera Seedlings under Drought Stress. Plants. 2024; 13(2):225. https://doi.org/10.3390/plants13020225
Chicago/Turabian StyleYang, Dayu, Yongzhong Chen, Rui Wang, Yimin He, Xiaofan Ma, Jiancai Shen, Zhilong He, and Hanggui Lai. 2024. "Effects of Exogenous Abscisic Acid on the Physiological and Biochemical Responses of Camellia oleifera Seedlings under Drought Stress" Plants 13, no. 2: 225. https://doi.org/10.3390/plants13020225
APA StyleYang, D., Chen, Y., Wang, R., He, Y., Ma, X., Shen, J., He, Z., & Lai, H. (2024). Effects of Exogenous Abscisic Acid on the Physiological and Biochemical Responses of Camellia oleifera Seedlings under Drought Stress. Plants, 13(2), 225. https://doi.org/10.3390/plants13020225





