Determination of the Toxic Effects of Heavy Metals on the Morpho-Anatomical Responses of the Leaf of Typha latifolia as a Biomonitoring Tool
Abstract
1. Introduction
2. Results and Discussion
2.1. Physico-Chemical Characterization of Burgas Lake Waters
2.1.1. Evolution of Temperature: T °C
2.1.2. Evolution of Hydrogen Potential: pH
2.1.3. Evolution of Electrical Conductivity (EC)
2.1.4. Evolution of Turbidity
2.1.5. Evolution of the Inorganic Load
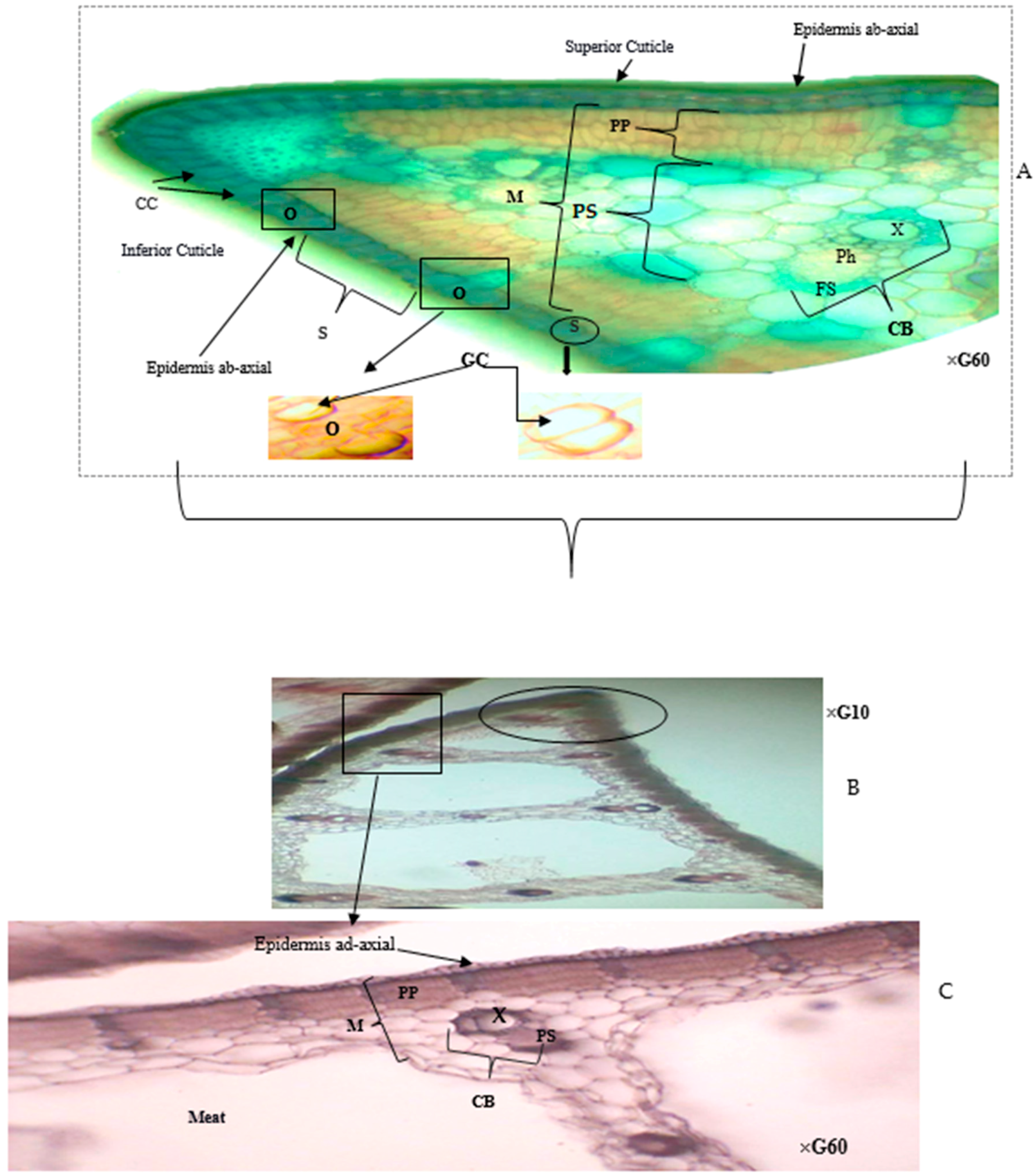
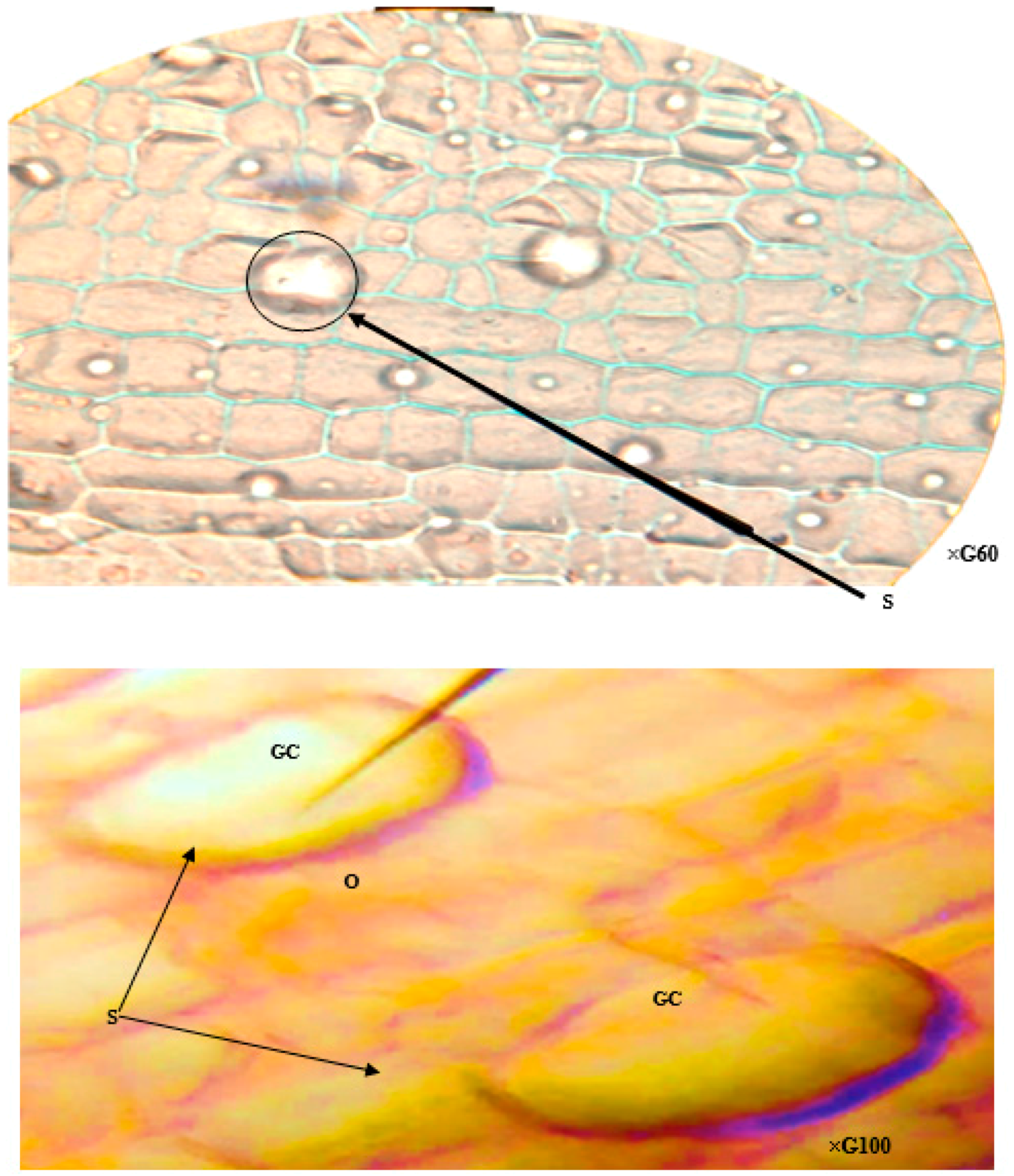
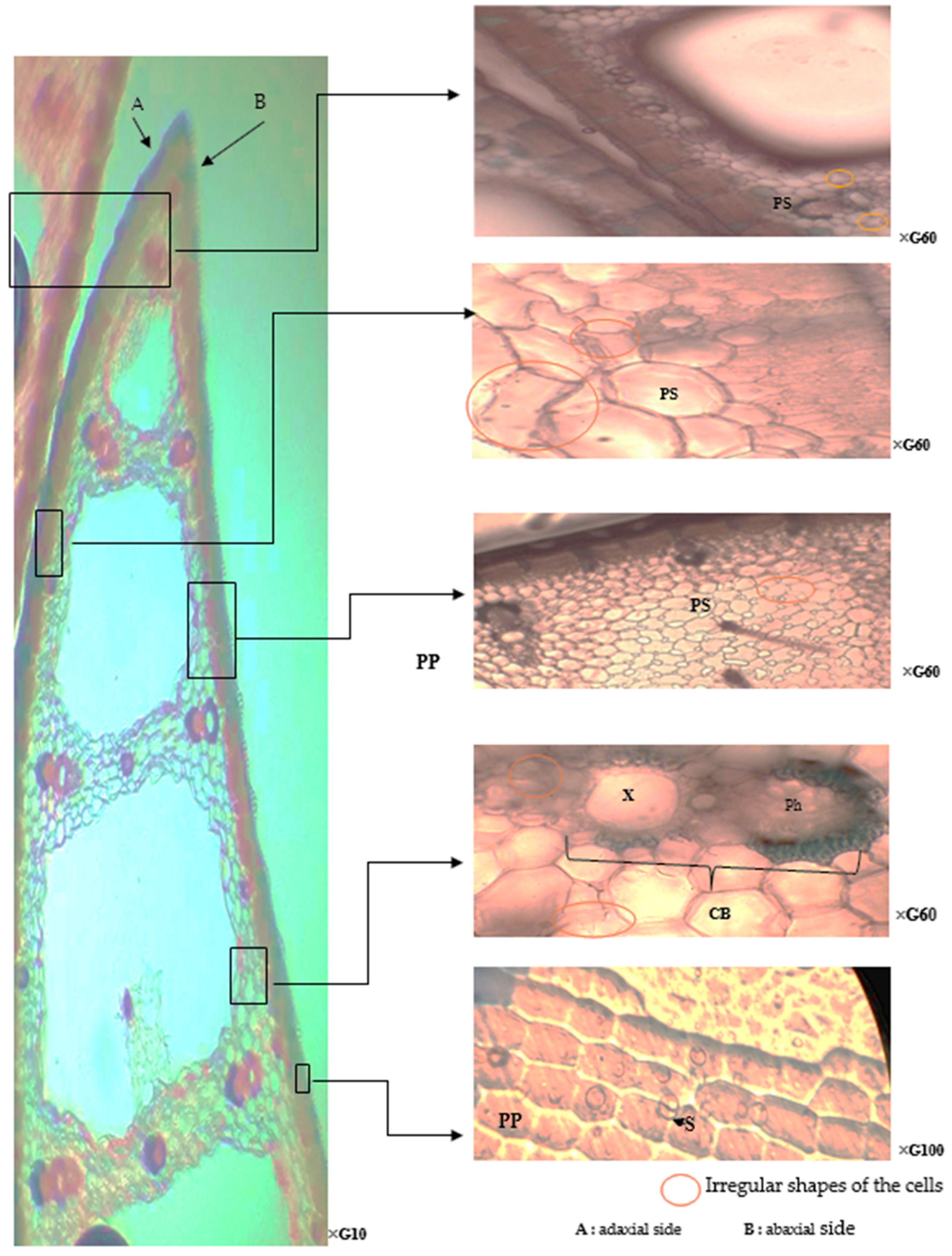
2.2. The Effect of Environmental Pollution on the Anatomical Responses of T. latifolia
2.3. Cytotoxic Effects of Heavy Metals and Bioremediation Potential
2.4. The Paradermal Sections of the Leaves
3. Materials and Methods
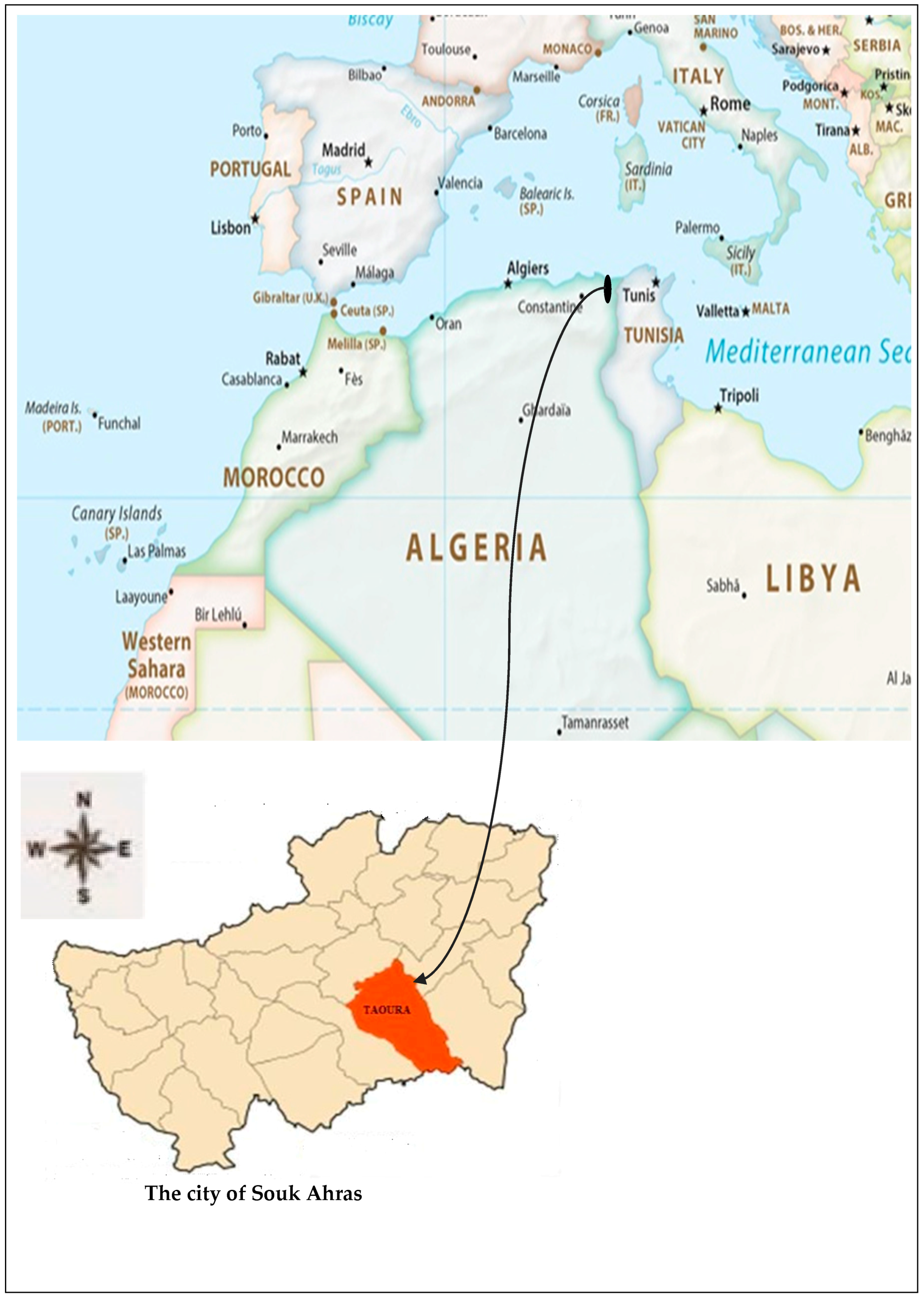
3.1. Presentation and Description of the Study Area
3.2. Water Sampling and Analysis Methods
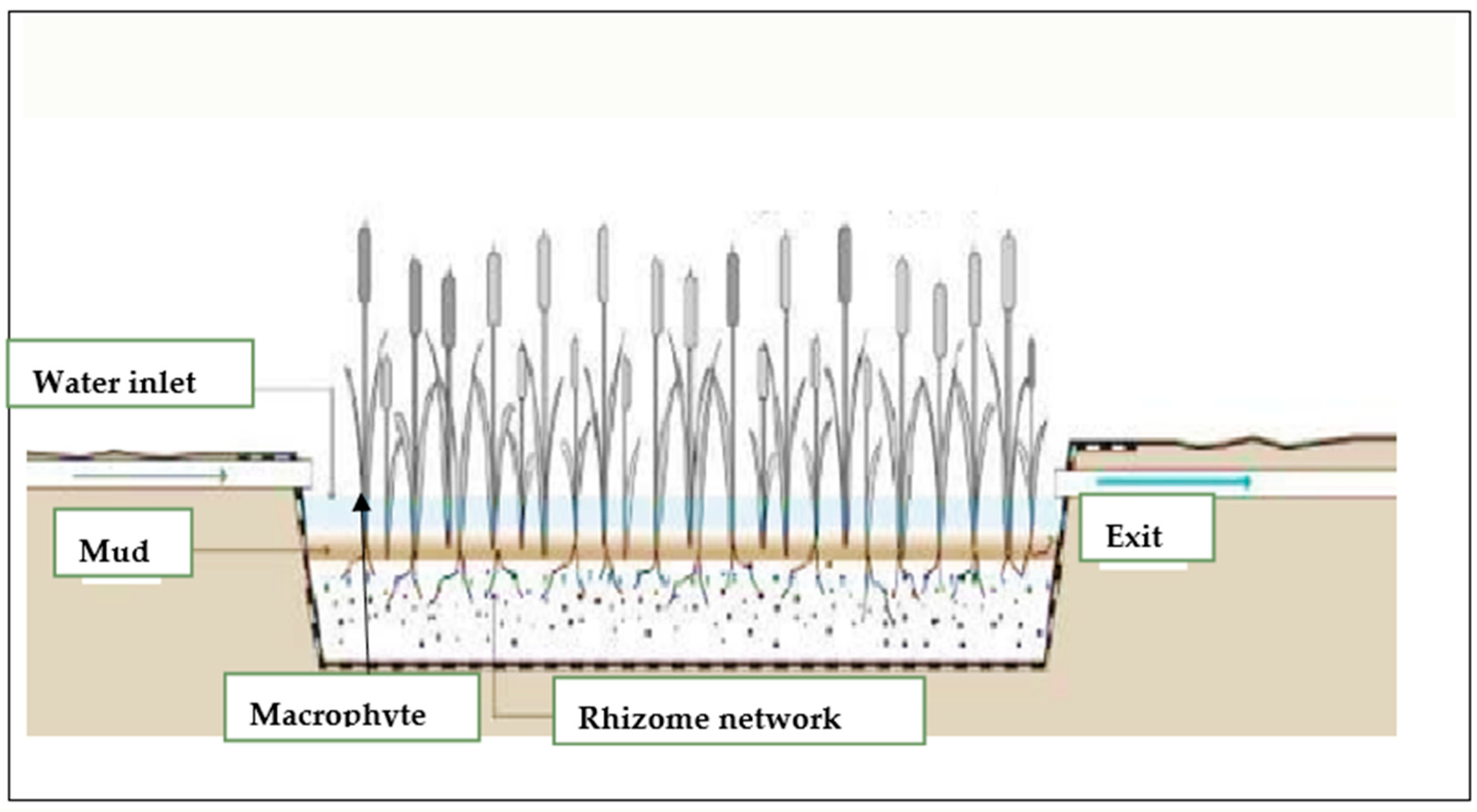
3.3. Collection and Sampling of Plants
3.4. Study Species Description
3.4.1. Selection and Preparation of Biological Material
3.4.2. Realization of Anatomical Sections and Staining
- -
- Sectioning: Using a razor blade, thin sections of the organs under study were carefully cut. The thinnest and most suitable slices were then selected for further processing.
- -
- Bleaching: The selected sections were placed in a watch glass containing bleach. This step aimed to dissolve the cellular contents while preserving the cell walls made of pectin and cellulose.
- -
- Rinsing: To remove excess bleach, the sections were transferred to a second watch glass filled with distilled water.
- -
- Mordanting: To eliminate any remaining bleach and prepare the tissues for staining, the sections were immersed in a 1% acetic acid solution for two minutes. This step acted as a cell mordant, enhancing the staining process.
- -
- Staining: The sections were then stained in a fourth watch glass containing a mixture of equal parts Congo red and carmine alum. Congo red stains dead, lignified tissues red, while carmine alum stains living tissues and intensifies the red color with cellulose.
- -
- Dehydration: After staining, the sections were placed in 70% alcohol. This step replaced the water within the cells, helping to preserve the obtained sections.
3.4.3. Visualization and Photography
- -
- Mounting: The prepared sections were carefully placed between a slide and a coverslip, with a drop of glycerin added to enhance clarity.
- -
- Microscopic Examination: The mounted sections were then positioned under the optical microscope for visualization.
- -
- Documentation: Simultaneously with the microscopic examination, photographs of the histological sections were captured using an OPPO A53 camera (OPPO Electronics Corp., Ltd., Dongguan, China). These images allow for further analysis and record-keeping of the observed features.
3.5. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shah, V.; Daverey, A. Phytoremediation: A multidisciplinary approach to clean up heavy metal contaminated soil. Environ. Technol. Innov. 2020, 18, 100774. [Google Scholar] [CrossRef]
- Sumpter, J.P. Protecting aquatic organisms from chemicals: The harsh realities. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2009, 367, 3877–3894. [Google Scholar] [CrossRef] [PubMed]
- Gavrilescu, M.; Demnerová, K.; Aamand, J.; Agathos, S.; Fava, F. Emerging pollutants in the environment: Present and future challenges in biomonitoring, ecological risks and bioremediation. New Biotechnol. 2015, 32, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.X.; Luo, X.J.; Tan, X.X.; Tang, B.; Li, Z.R.; Mai, B.X. Legacy and emerging halogenated organic pollutants in marine organisms from the Pearl River Estuary, South China. Chemosphere 2015, 139, 565–571. [Google Scholar] [CrossRef] [PubMed]
- Arnold, K.E.; Brown, A.R.; Ankley, G.T.; Sumpter, J.P. Medicating the environment: Assessing risks of pharmaceuticals to wildlife and ecosystems. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2014, 369, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Malaj, E.; Von der Ohe, P.C.; Grote, M.; Kühne, R.; Mondy, C.P.; Usseglio Polatera, P.; Schäfer, R.B. Organic chemicals jeopardize the health of freshwater ecosystems on the continental scale. Proc. Natl. Acad. Sci. USA 2014, 111, 9549–9554. [Google Scholar] [CrossRef] [PubMed]
- Youbi, A.; Zerguine, K.; Houilia, A.; Farfar, K.; Soumati, B.; Berrebbah, H.; Djebar, M.R.; Souiki, L. Potential use of morphological deformities in Chironomus (Diptera: Chironomidae) as a bioindicator of heavy metals pollution in North-East Algeria. Environ. Sci. Pollut. Res. 2020, 27, 8611–8620. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, M.A.; Maah, J.; Yusoff, I. Heavy metals accumulation in plants growing in ex-tin mining catchment. Int. J. Environ. Sci. Technol. 2011, 8, 401–416. [Google Scholar] [CrossRef]
- Islam, E.U.; Yang, X.E.; He, Z.L.; Mahmood, Q. Assessing potential dietary toxicity of heavy metals in selected vegetables and food crops. J. Zhejiang Univ. Sci. B 2007, 8, 1–13. [Google Scholar] [CrossRef]
- Li, M.; Ahammed, G.J.; Li, C.; Bao, X.; Yu, J.; Huang, C.; Yin, H.; Zhou, J. Brassinosteroid ameliorates zinc oxide nanoparticles-induced oxidative stress by improving antioxidant potential and redox homeostasis in tomato seedling. Front. Plant Sci. 2016, 7, 615. [Google Scholar] [CrossRef]
- Tamás, M.J.; Sharma, S.K.; Ibstedt, S.; Jacobson, T.; Christen, P. Heavy metals and metalloids as a cause for protein misfolding and aggregation. Biomolecules 2014, 4, 252–267. [Google Scholar] [CrossRef] [PubMed]
- Derradji, M.; Souiki, L.; Belaze, A.H.; Alayat, A.; Berrebbah, H.; Djebar, M.R. Spatial and Temporal Variation of Raw Sewage of City of Annaba Major Discharge (Northeast Algeria). Iran. J. Energy Environ. 2015, 6, 127–133. [Google Scholar]
- Mamine, N.; Grara, N.; Khaldi, F. The use of macrophyte Typha latifolia filters in the treatment of wastewaters of Medjerda river. Vasile Goldis Univ. 2019, 29, 70–81. [Google Scholar]
- Mamine, N.; Grara, N.; Khaldi, F. Monitoring and assessment of the effects of wastewater induced oxidative stress on an aquatic plant Typha latifolia. Fresenius Environ. Bull. 2021, 30, 1082–1094. [Google Scholar]
- Brix, H. Wastewater treatment in constructed wetlands system design, removal processes, and treatment performance. In Constructed Wetlands for Water Quality Improvement; Moshiri, G.A., Ed.; Lewis Publishers: Boca Raton, FL, USA; Ann Arbor, MI, USA; London, UK; Tokyo, Japan, 1993. [Google Scholar]
- Yang, Y.; Shen, Q. Phytoremediation of cadmium-contaminated wetland soil with Typha latifolia L. and the underlying mechanisms involved in the heavy-metal uptake and removal. Environ. Sci. Pollut. Res. Int. 2020, 27, 4905–4916. [Google Scholar] [CrossRef]
- Bonanno, G.; Cirelli, G.L. Comparative analysis of element concentrations and translocation in three wetland congener plants: Typha domingensis, Typha latifolia and Typha angustifolia. Ecotoxicol. Environ. Saf. 2017, 143, 92–101. [Google Scholar] [CrossRef]
- Kumari, M.; Tripathi, B.D. Efficiency of Phragmites australis and Typha latifolia for heavy metal removal from wastewater. Ecotoxicol. Environ. Saf. 2015, 112, 80–86. [Google Scholar] [CrossRef]
- Mekonnen, A.; Leta, S.; Njau, K.N. Wastewater treatment performance efficiency of constructed wetlands in African countries: A review. Water Sci. Technol. 2015, 71, 1–8. [Google Scholar] [CrossRef]
- Haddaji, D. Valorization of the Biological Activity of Phragmites australis, Typha domingensis and Bolboschoenus glaucus for the Depollution of Textile Waters. Ph.D. Thesis, University Tunisia, Tunis, Tunisia, 2020. [Google Scholar]
- Bonanno, G. Comparative performance of trace element bioaccumulation and biomonitoring in the plant species Typha domingensis, Phragmites australis and Arundo donax. Ecotoxicol. Environ. Saf. 2013, 97, 124–130. [Google Scholar] [CrossRef]
- Limit values of discharge parameters in a receiving environment. Art. n°26. Official Journal of the Algerian Republic, 23 April 2006.
- Annex specifications of treated wastewater used for irrigation purposes. Official Journal of the Algerian Republic 2012, 41, 18–21.
- Rodier, J.; Legube, B.; Merlet, N.; Brunet, R. Water Testing: Natural Waters, Wastewater, Seawater, 9th ed.; Dunod: Paris, France, 2009; pp. 100–110. [Google Scholar]
- Khoufi, S.; Aloui, F.; Sayadi, S. Anaerobic digestion of olive mill wastewater after Ca(OH)2 pretreatment and reuse adapted. In Proceedings of the International Conference on Wastewater Treatment and Reuse Adapted to Mediterranean Area (WATRAMA), CNUDST, Tunis, Tunisia, 25–28 October 2000. [Google Scholar]
- Ndzomo, G.T.; Ndoumou, D.O.; Awah, A.T. Effect of Fe2+, Mn2+, Zn2+, and Pb2+ on H+/K+ fluxes in excised Pistia stratiotes roots. Biol. Plant. Prague 1994, 36, 591–597. [Google Scholar]
- Jedicke, A.; Furch, B.; Saint, P.U.; Schlueter, U.B. Increase in the oxygen concentration in Amazon waters resulting from the root exudation of two notorious water plants, Eichhornia crassipes (Pontederiaceae) and Pistia stratiotes (Araceae). Amazoniana 1989, 11, 53–70. [Google Scholar]
- Bowes, G.; Beer, S. Physiological plant processes: Photosynthesis. In Aquatic Plants for Water Treatment and Resource Recovery; Reddy, K.R., Smith, W.H., Eds.; Magnolia Publishing: Orlando, FL, USA, 1987; pp. 311–337. [Google Scholar]
- FAO (Food and Agriculture Organization). Water Quality for Agriculture; Irrigation and Drainage Paper 29 Rev. 1; Ayers, R.S., Westcot, D.W., Eds.; FAO: Rome, Italy, 1985. [Google Scholar]
- Chafai, D. Micromycetes of Wadi Sediments and Industrial Effluents from Eastern Algeria. Ph.D. Thesis, Joseph Fourier University, Grenoble, France, 1996. [Google Scholar]
- Xu, Q.; Renault, S.; Goltz, D.; Yuan, Q. Phytoremediation of waste dumping site soil and landfill leachate by using cattail (Typha latifolia). Environ. Technol. 2020, 41, 1101–1106. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Hänninen, O. Pentachlorophenol: Uptake/elimination kinetics and metabolism in an aquatic plant, Eichhornia crassipes. Environ. Toxicol. Chem. Int. J. 1994, 13, 763–773. [Google Scholar] [CrossRef]
- WHO (World Health Organization). Wastewater Use in Agriculture and Aquaculture: Recommendations for Sanitation; Technical Report Series, n8778; WHO: Geneva, Switzerland, 1989. [Google Scholar]
- Amir, W.; Farid, M.; Ishaq, H.K.; Farid, S.; Zubair, M.; Alharby, H.F.; Bamagoos, A.A.; Rizwan, M.; Raza, N.; Hakeem, K.R.; et al. Accumulation potential and tolerance response of Typha latifolia L. under citric acid assisted phytoextraction of lead and mercury. Chemosphere 2020, 257, 127247. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Z.T.; Wang, H. Copper toxicity and bioaccumulation in Chinese cabbage (Brassica pekinensis Rupr). Environ. Toxicol. 2005, 20, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, T.; Shah, S.K.; Ullah, F.; Mehmood, S.; Zeb, M.A. Analysis of deformable distortion in the architecture of leaf xylary vessel elements of Carthamus oxycantha caused by heavy metals stress using image registration. Microsc. Res. Tech. 2020, 83, 843–849. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Zhao, Y.; Tian, K.; He, X.; Jia, Y.; He, Z.; Wang, W.; Xiang, C.; Tian, X. Insight into nitrogen and phosphorus enrichment on cadmium phytoextraction of hydroponically grown Salix matsudana Koidz cuttings. Environ. Sci. Pollut. Res. Int. 2020, 27, 8406–8417. [Google Scholar] [CrossRef]
- Nguyen, T.Q.; Sesin, V.; Kisiala, A.; Emery, R.J.N. Phytohormonal Roles in Plant Responses to Heavy Metal Stress: Implications for Using Macrophytes in Phytoremediation of Aquatic Ecosystems. Environ. Toxicol. Chem. 2021, 40, 7–22. [Google Scholar] [CrossRef]
- Ben Salem, Z.; Laffray, X.; Al-Ashoor, A.; Ayadi, H.; Aleya, L. Metals and metalloid bioconcentrations in the tissues of Typha latifolia grown in the four interconnected ponds of a domestic landfill site. J. Environ. Sci. 2017, 54, 56–68. [Google Scholar] [CrossRef] [PubMed]
- Brunet, J.; Reppelin, A.; Varralut, G.; Terryn, N.; Zuily-Fodil, Y. Lead accumulation in the roots of grass pea (Lathyrus sativus L.). Comptes Rendus Biol. 2008, 331, 859–864. [Google Scholar] [CrossRef] [PubMed]
- Castro, E.M.; Pereira, F.J.; Paiva, R. Histologia Vegetal: Estrutura e Função de Órgãos Vegetativos; Universidade Federal de Lavras, UFLA: Lavras, Brazil, 2009; p. 234. [Google Scholar]
- Doheny-Adams, T.; Hunt, L.; Franks, P.J.; Beerling, D.J.; Gray, J.E. Genetic manipulation of stomatal density influences stomatal size, plant growth and tolerance to restricted water supply across a growth carbon dioxide gradient. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2012, 367, 547–555. [Google Scholar] [CrossRef] [PubMed]
- Brodribb, T.J.; McAdam, S.A.; Carins Murphy, M.R. Xylem and stomata, coordinated through time and space. Plant Cell Environ. 2017, 40, 872–880. [Google Scholar] [CrossRef] [PubMed]
- Available online: http://timein.org/algeria/souk-ahras/map (accessed on 6 June 2023).
- Rodier, J.; Legube, B.; Merlet, N. L’analyse de L’eau, Eaux Naturelles, Eaux Résiduaires, Eau de Mer, Chimie, Physico-Chimie, Microbiologie, Biologie, Interprétation des Résultats; Dunod: Paris, France, 2005; p. 1384. [Google Scholar]
- Bergner, I.; Jensen, U. Phytoserological contribution to the systematic placement of the Typhales. Nord. J. Bot. Mark. 1989, 8, 447–456. [Google Scholar] [CrossRef]
- Wenerick, W.R.; Stevens, S.E.; Webster, H.J.; Stark, L.R.; DeVeau, E. Tolerance of three wetland plant species to acid mine drainage: A greenhouse study. In Constructed Wetlands for Wastewater Treatment; Lewis Publishers: Chelsea, MI, USA, 1989. [Google Scholar]
- Gesberg, R.M.; Elkins, B.V.; Lyon, S.R.; Goldman, C.R. Role of aquatic plants in wastewater treatment by artificial wetlands. Water Res. 1986, 20, 363–368. [Google Scholar] [CrossRef]
- Radoux, M.; Kemp, D. Comparative treatment of domestic wastewater by three helophytic plantations and a microphyte lagoon in the same temperate climate. Acta Oecologia Appl. 1988, 9, 25–38. [Google Scholar]
- Hadad, H.R.; Maine, M.A.; Bonetto, C.A. Macrophyte growth in a pilot-scale constructed wetland for industrial wastewater treatment. Chemosphere 2006, 63, 1744–1753. [Google Scholar] [CrossRef]
- Abissy, M. Potential of Helophytic Plants in Wastewater Treatment Wastewater Treatment and the Possibility of Agricultural of Purified Water in Arid Climates. Ph.D. Thesis, Faculté des Sciences Semlalia, Université Cadi Ayyad, Marrakech, Morocco, 2000; p. 200. [Google Scholar]
- Biddlestone, A.J.; Gray, K.R.; Job, G.D. Treatment of dairy farm wastewater in engineered reed systems. Process Biochem. 1991, 26, 265–268. [Google Scholar] [CrossRef]
- Zaffran, J. Initiation to Plant Biology; Edition of Ellipses: Paris, France, 1998. [Google Scholar]
- Available online: http://www.statsoft.com (accessed on 13 June 2022).
- Dagnelie, P. Statistique Théorique et Appliquée: Tome 2, Inférence Statistique à Une et à Deux Dimensions; De Boeck Université: Bruxelles, Belgium, 2011; p. 736. [Google Scholar]

| Variable Physico-Chemical | Number of Samples | Minimum Values | Maximum Values | Mean Values ± Standard Deviation | |
|---|---|---|---|---|---|
| Parameters analyzed in the laboratory | Hg (ppm) | 12 | 0.001 | 0.060 | 0.015 ± 0.01 |
| Cd (ppm) | 12 | 0.004 | 0.07 | 0.02 ± 0.02 | |
| Cr (ppm) | 12 | 0.02 | 0.06 | 0.04 ± 0.01 | |
| Pb (ppm) | 12 | 0.001 | 0.005 | 0.003 ± 0.056 | |
| Parameters measured in situ | Turbidity (NTU) | 12 | 90.2 | 188 | 73.81 ± 12.2 |
| Oxygen dissolved (O2) (mg/L) | 12 | 1.67 | 3.37 | 2.45 ± 0.87 | |
| pH | 12 | 7.33 | 7.97 | 7.69 ± 1.18 | |
| T °C | 12 | 15.3 | 22.56 | 13.43 ± 1.54 | |
| EC (μs/cm) | 12 | 1236.56 | 1563.23 | 1385.11 ± 152.87 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mamine, N.; Grara, N.; Khaldi, F.; Maresca, V.; Aouaichia, K.; Basile, A. Determination of the Toxic Effects of Heavy Metals on the Morpho-Anatomical Responses of the Leaf of Typha latifolia as a Biomonitoring Tool. Plants 2024, 13, 176. https://doi.org/10.3390/plants13020176
Mamine N, Grara N, Khaldi F, Maresca V, Aouaichia K, Basile A. Determination of the Toxic Effects of Heavy Metals on the Morpho-Anatomical Responses of the Leaf of Typha latifolia as a Biomonitoring Tool. Plants. 2024; 13(2):176. https://doi.org/10.3390/plants13020176
Chicago/Turabian StyleMamine, Nedjma, Nedjoud Grara, Fadila Khaldi, Viviana Maresca, Khaoula Aouaichia, and Adriana Basile. 2024. "Determination of the Toxic Effects of Heavy Metals on the Morpho-Anatomical Responses of the Leaf of Typha latifolia as a Biomonitoring Tool" Plants 13, no. 2: 176. https://doi.org/10.3390/plants13020176
APA StyleMamine, N., Grara, N., Khaldi, F., Maresca, V., Aouaichia, K., & Basile, A. (2024). Determination of the Toxic Effects of Heavy Metals on the Morpho-Anatomical Responses of the Leaf of Typha latifolia as a Biomonitoring Tool. Plants, 13(2), 176. https://doi.org/10.3390/plants13020176








