Application of ZnO NPs, SiO2 NPs and Date Pollen Extract as Partial Substitutes to Nitrogen, Phosphorus, and Potassium Fertilizers for Sweet Basil Production
Abstract
1. Introduction
2. Results
2.1. Vegetative Growth Parameters
2.2. Essential Oil Productivity
2.3. Essential Oil Composition
2.4. Total Phenolic Compounds (TPCs) and Antioxidant Activity (AOA)
3. Discussion
4. Materials and Methods
4.1. Soil Analysis of the Experimental Site
4.2. Plant Preparation
4.3. Fertilizer Types
4.3.1. NPK Fertilizers
4.3.2. Nanoparticles
Synthesis of NPs of ZnO and SiO2
4.3.3. Date Palm Pollen Extract (DPPE)
4.4. The Fertilization Treatments
4.5. Experimental Layout
4.5.1. Vegetative Growth Traits
4.5.2. Essential Oil Percentage and Yield
4.5.3. Gas Chromatography/Mass Spectrometry Analysis of Oil
4.5.4. Total Phenols and Antioxidant Activity
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Esetlili, B.Ç.; Öztürk, B.; Çobanoğlu, Ö.; Anaç, D. Sweet basil (Ocimum basilicum L.) and potassium fertilization. J. Plant Nutrit. 2016, 39, 35–44. [Google Scholar] [CrossRef]
- Ekren, S.; Sönmez, Ç.; Özçakal, E.; Kurttaş, Y.S.K.; Bayram, E.; Gürgülü, H. The effect of different irrigation water levels on yield and quality characteristics of purple basil (Ocimum basilicum L.). Agric. Water Manag. 2012, 109, 155–161. [Google Scholar] [CrossRef]
- Khalid, K.A.; Hendawy, S.; El-Gezawy, E. Ocimum basilicum L. production under organic farming. Res. J. Agric. Biolog. Sci. 2006, 2, 25–32. [Google Scholar]
- Hanif, M.A.; Al-Maskari, M.Y.; Al-Maskari, A.; Al-Shukaili, A.; Al-Maskari, A.Y.; Al-Sabahi, J.N. Essential oil composition, antimicrobial and antioxidant activities of unexplored Omani basil. J. Med. Plants Res. 2011, 5, 751–757. [Google Scholar]
- Simon, J.E.; Morales, M.R.; Phippen, W.B.; Vieira, R.F.; Hao, Z. Basil: A source of aroma compounds and a popular culinary and ornamental herb. Perspect. New Crops New Uses 1999, 16, 499–505. [Google Scholar]
- Bufalo, J.; Cantrell, C.L.; Astatkie, T.; Zheljazkov, V.D.; Gawde, A.; Boaro, C.S.F. Organic versus conventional fertilization effects on sweet basil (Ocimum basilicum L.) growth in a greenhouse system. Ind. Crops Prod. 2015, 74, 249–254. [Google Scholar] [CrossRef][Green Version]
- Muráriková, A.; Ťažký, A.; Neugebauerová, J.; Planková, A.; Jampílek, J.; Mučaji, P.; Mikuš, P. Characterization of essential oil composition in different basil species and pot cultures by a GC-MS method. Molecules 2017, 22, 1221. [Google Scholar] [CrossRef]
- Hawkesford, M.; Horst, W.; Kichey, T.; Lambers, H.; Schjoerring, J.; Møller, I.S.; White, P. Functions of macronutrients. In Marschner’s Mineral Nutrition of Higher Plants; Elsevier: Amsterdam, The Netherlands, 2012; pp. 135–189. [Google Scholar]
- Jones, D.L.; Cross, P.; Withers, P.J.; DeLuca, T.H.; Robinson, D.A.; Quilliam, R.S.; Harris, I.M.; Chadwick, D.R.; Edwards-Jones, G. Nutrient stripping: The global disparity between food security and soil nutrient stocks. J. Appl. Ecol. 2013, 50, 851–862. [Google Scholar] [CrossRef]
- Zhang, X.; Davidson, E.A.; Mauzerall, D.L.; Searchinger, T.D.; Dumas, P.; Shen, Y. Managing nitrogen for sustainable development. Nature 2015, 528, 51–59. [Google Scholar] [CrossRef]
- Congreves, K.; Van Eerd, L. Nitrogen cycling and management in intensive horticultural systems. Nutr. Cycl. Agroecosyst. 2015, 102, 299–318. [Google Scholar] [CrossRef]
- Savci, S. Investigation of effect of chemical fertilizers on environment. APCBEE Procedia 2012, 1, 287–292. [Google Scholar] [CrossRef]
- Faizan, M.; Bhat, J.A.; Chen, C.; Alyemeni, M.N.; Wijaya, L.; Ahmad, P.; Yu, F. Zinc oxide nanoparticles (ZnO-NPs) induce salt tolerance by improving the antioxidant system and photosynthetic machinery in tomato. Plant Physiol. Biochem. 2021, 161, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Adrees, M.; Khan, Z.S.; Hafeez, M.; Rizwan, M.; Hussain, K.; Asrar, M.; Alyemeni, M.N.; Wijaya, L.; Ali, S. Foliar exposure of zinc oxide nanoparticles improved the growth of wheat (Triticum aestivum L.) and decreased cadmium concentration in grains under simultaneous Cd and water deficient stress. Ecotox. Environ. Saf. 2021, 208, 111627. [Google Scholar] [CrossRef] [PubMed]
- García-Gómez, C.; García, S.; Obrador, A.F.; González, D.; Babín, M.; Fernández, M.D. Effects of aged ZnO NPs and soil type on Zn availability, accumulation and toxicity to pea and beet in a greenhouse experiment. Ecotoxicol. Environ. Saf. 2018, 160, 222230. [Google Scholar] [CrossRef]
- Rizwan, M.; Ali, S.; ur Rehman, M.Z.; Adrees, M.; Arshad, M.; Qayyum, M.F.; Ali, L.; Hussain, A.; Chatha, S.A.S.; Imran, M. Alleviation of cadmium accumulation in maize (Zea mays L.) by foliar spray of zinc oxide nanoparticles and biochar to contaminated soil. Environ. Poll. 2019, 248, 358–367. [Google Scholar] [CrossRef]
- Kah, M.; Kookana, R.S.; Gogos, A.; Bucheli, T.D. A critical evaluation of nanopesticides and nanofertilizers against their conventional analogues. Nat. Nanotechnol. 2018, 13, 677–684. [Google Scholar] [CrossRef]
- Cakmak, I. Enrichment of cereal grains with zinc: Agronomic or genetic biofortification? Plant Soil 2008, 302, 1–17. [Google Scholar] [CrossRef]
- Genc, Y.; McDonald, G.K.; Graham, R.D. Contribution of different mechanisms to zinc efficiency in bread wheat during early vegetative stage. Plant Soil 2006, 281, 353–367. [Google Scholar] [CrossRef]
- Marschner, P. Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Academic Press: London, UK, 2012. [Google Scholar]
- Todeschini, V.; Lingua, G.; D’agostino, G.; Carniato, F.; Roccotiello, E.; Berta, G. Effects of high zinc concentration on poplar leaves: A morphological and biochemical study. Environ. Exp. Bot. 2011, 71, 50–56. [Google Scholar] [CrossRef]
- Dimkpa, C.O.; Singh, U.; Bindraban, P.S.; Adisa, I.O.; Elmer, W.H.; Gardea-Torresdey, J.L.; White, J.C. Addition-omission of zinc, copper, and boron nano and bulk oxide particles demonstrate element and size-specific response of soybean to micronutrients exposure. Sci. Total Environ. 2019, 665, 606–616. [Google Scholar] [CrossRef]
- Smith, M.R.; Myers, S.S. Impact of anthropogenic CO2 emissions on global human nutrition. Nat. Clim. Chang. 2018, 8, 834–839. [Google Scholar] [CrossRef]
- Sommer, M.; Kaczorek, D.; Kuzyakov, Y.; Breuer, J. Silicon pools and fluxes in soils and landscapes—A review. J. Plant Nutr. Soil Sci. 2006, 169, 310–329. [Google Scholar] [CrossRef]
- Farouk, S.; Omar, M. Sweet basil growth, physiological and ultrastructural modification, and oxidative defense system under water deficit and silicon forms treatment. J. Plant Growth Regul. 2020, 39, 1307–1331. [Google Scholar] [CrossRef]
- Amooaghaie, R.; Korrani, F.M. Bacillus subtilis and vermicompost suppress damping-off disease in psyllium through nitric oxide-dependent signaling system. Russ. J. Plant Physiol. 2018, 65, 435–445. [Google Scholar] [CrossRef]
- Mohasseli, V.; Sadeghi, S. Exogenously applied sodium nitroprusside improves physiological attributes and essential oil yield of two drought susceptible and resistant specie of Thymus under reduced irrigation. Ind. Crops Prod. 2019, 130, 130–136. [Google Scholar] [CrossRef]
- Khator, K.; Shekhawat, G. Nitric oxide mitigates salt-induced oxidative stress in Brassica juncea seedlings by regulating ROS metabolism and antioxidant defense system. 3 Biotech 2020, 10, 499. [Google Scholar] [CrossRef]
- Ahanger, M.A.; Bhat, J.A.; Siddiqui, M.H.; Rinklebe, J.; Ahmad, P. Integration of silicon and secondary metabolites in plants: A significant association in stress tolerance. J. Exp. Bot. 2020, 71, 6758–6774. [Google Scholar] [CrossRef]
- Al-Helal, A.A. Electrophoretic analysis of three selected isoenzymes of date palm pollen grains. Bot. Bull. Acad. Sin. 1992, 33, 241–246. [Google Scholar]
- Basuny, A.M.; Arafat, S.M.; Soliman, H.M. Chemical analysis of olive and palm pollen: Antioxidant and antimicrobial activation properties. Wudpecker J. Food Technol. 2013, 1, 14–21. [Google Scholar]
- Hassan, H.M. Chemical composition and nutritional value of palm pollen grains. Glob. J. Biotechnol. Biochem. 2011, 6, 1–7. [Google Scholar]
- Paramo, L.A.; Feregrino-Pérez, A.A.; Guevara, R.; Mendoza, S.; Esquivel, K. Nanoparticles in agroindustry: Applications, toxicity, challenges, and trends. Nanomaterials 2020, 10, 1654. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wang, P.; Van Der Ent, A.; Cheng, M.; Jiang, H.; Lund Read, T.; Lombi, E.; Tang, C.; De Jonge, M.D.; Menzies, N.W. Absorption of foliar-applied Zn in sunflower (Helianthus annuus): Importance of the cuticle, stomata and trichomes. Ann. Bot. 2019, 123, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wang, P.; Lombi, E.; Cheng, M.; Tang, C.; Howard, D.L.; Menzies, N.W.; Kopittke, P.M. Absorption of foliar-applied Zn fertilizers by trichomes in soybean and tomato. J. Exper. Bot. 2018, 69, 2717–2729. [Google Scholar] [CrossRef]
- Monreal, C.; DeRosa, M.; Mallubhotla, S.; Bindraban, P.; Dimkpa, C. Nanotechnologies for increasing the crop use efficiency of fertilizer-micronutrients. Biol. Fertil. Soils 2016, 52, 423–437. [Google Scholar] [CrossRef]
- Yadegari, M. Irrigation periods and Fe, Zn foliar application on agronomic characters of Borago officinalis, Calendula officinalis, Thymus vulgaris and Alyssum desertorum. Commun. Soil Sci. Plant Anal. 2017, 48, 307–315. [Google Scholar] [CrossRef]
- Mehrabani, L.; Kamran, R.V.; Hassanpouraghdam, M.B.; Pessarakli, M. Zinc sulfate foliar application effects on some physiological characteristics and phenolic and essential oil contents of Lavandula stoechas L. under sodium chloride (NaCl) salinity conditions. Commun. Soil Sci. Plant Anal. 2017, 48, 1860–1867. [Google Scholar] [CrossRef]
- Danaee, E.; Abdossi, V. Effect of foliar application of iron, potassium, and zinc nano-chelates on nutritional value and essential oil of Basil (Ocimum basilicum L.). Food Health 2021, 4, 13–20. [Google Scholar]
- Alhasan, A.S. Effect of different NPK nano-fertilizer rates on agronomic traits, essential oil, and seed yield of basil (Ocimum basilicum L. cv Dolly) grown under field conditions. Plant Arch. 2020, 20, 2959–2962. [Google Scholar]
- El-Khateeb, M.; El-Attar, A.; Abo-Bakr, Z. Effect of nano-microelements on growth, yield and essential oil production of sweet marjoram (Origanum majorana) plants. Plant Arch. 2020, 20, 8315–8324. [Google Scholar]
- Hassanpouraghdam, M.B.; Mehrabani, L.V.; Tzortzakis, N. Foliar application of nano-zinc and iron affects physiological attributes of Rosmarinus officinalis and quietens NaCl salinity depression. J. Soil Sci. Plant Nutr. 2020, 20, 335–345. [Google Scholar] [CrossRef]
- Nemati Lafmejani, Z.; Jafari, A.A.; Moradi, P.; Ladan Moghadam, A. Application of chelate and nano-chelate zinc micronutrient onmorpho-physiological traits and essential oil compounds of peppermint (Mentha piperita L.). J. Med. Plants By-Prod. 2021, 10, 21–28. [Google Scholar]
- Mahmoodi, P.; Yarnia, M.; Rashidi, V.; Amirnia, R.; Tarinejhad, A. Effects of nano and chemical fertilizers on physiological efficiency and essential oil yield of Borago officinalis L. Appl. Ecol. Environ. Res. 2018, 16, 4773–4788. [Google Scholar] [CrossRef]
- Pirzad, A.; Barin, M. Iron and zinc interaction on leaf nutrients and the essential oil of Pimpinella anisum L. Irani. J. Plant Physiol. 2018, 8, 2507–2515. [Google Scholar]
- Azhar, B.J.; Noor, A.; Zulfiqar, A.; Zeenat, A.; Ahmad, S.; Chishti, I.; Abbas, Z.; Shakeel, S.N. Effect of ZnO, SiO2 and composite nanoparticles on Arabidopsis thaliana and involvement of ethylene and cytokinin signaling pathways. Pak. J. Bot. 2021, 53, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Amooaghaie, R.; Roohollahi, S. Effect of sodium nitroprusside on responses of Melissa officinalis to bicarbonate exposure and direct Fe deficiency stress. Photosynthetica 2017, 55, 153–163. [Google Scholar] [CrossRef]
- Siddiqui, M.H.; Al-Whaibi, M.H.; Firoz, M.; Al-Khaishany, M.Y. Role of nanoparticles in plants. In Nanotechnology and Plant Sciences; Springer: Cham, Switzerland, 2015; pp. 19–35. [Google Scholar]
- Xie, Y.; He, Y.; Irwin, P.L.; Jin, T.; Shi, X. Antibacterial activity and mechanism of action of zinc oxide nanoparticles against Campylobacter jejuni. Appl. Environ. Microbiol. 2011, 77, 2325–2331. [Google Scholar] [CrossRef] [PubMed]
- Said-Al Ahl, H.; Omer, E. Effect of spraying with zinc and/or iron on growth and chemical composition of coriander (Coriandrum sativum L.) harvested at three stages of development. J. Med. Food Plants 2009, 1, 30–46. [Google Scholar]
- Wu, Z.; Xu, S.; Shi, H.; Zhao, P.; Liu, X.; Li, F.; Deng, T.; Du, R.; Wang, X.; Wang, F. Comparison of foliar silicon and selenium on cadmium absorption, compartmentation, translocation and the antioxidant system in Chinese flowering cabbage. Ecotox. Environ. Saf. 2018, 166, 157164. [Google Scholar] [CrossRef]
- Ashfaque, F.; Inam, A.; Iqbal, S.; Sahay, S. Response of silicon on metal accumulation, photosynthetic inhibition and oxidative stress in chromium-induced mustard (Brassica juncea L.). S. Afr. J. Bot. 2017, 111, 153–160. [Google Scholar] [CrossRef]
- Mantovani, C.; de Mello Prado, R.; Pivetta, K.F.L. Silicon foliar application on nutrition and growth of Phalaenopsis and Dendrobium orchids. Sci. Hortic. 2018, 241, 83–92. [Google Scholar] [CrossRef]
- Hamzei, J.; Najjari, S.; Sadeghi, F.; Seyedi, M. Effect of foliar application of nano-iron chelate and inoculation with mesorhizobium bacteria on root nodulation, growth and yield of chickpea under rainfed conditions. Iran. J. Pulses Res. 2014, 5, 9–18. [Google Scholar]
- Al-Saidi, A.A.-R.N.; Al-Mohammad, M.H.; Al-Juthery, H.W. Effect of spraying some nano-fertilizers and their combinations on the growth and yield of fenugreek (Trigonella foenum-graecum L.). In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2022; p. 012041. [Google Scholar]
- Abou-Sreea, A.I.B.; Yassen, A.A. Pollen extracts application as a natural growth substance on Strelitzia reginae Ait. plants. Int. J. PharmTechnol. Res. 2016, 9, 16–23. [Google Scholar]
- Merwad, M.; Mostafa, E.; Saleh, M.; Mansour, A. Yield and fruit quality of Hayany date palm as affected by different pollen grain sources. Int. J. ChemTech Res. 2015, 8, 544–549. [Google Scholar]
- Singh, S.; Sawhney, V.K. Plant hormones in Brassica napus and Lycopersicon tesculentum pollen. Phytochemistry 1992, 31, 4051–4053. [Google Scholar] [CrossRef]
- Alférez, M.J.; Campos, M.S.; Haro, A.; López-Aliaga, I.; Lisbona, F.; Barrionuevo, M. Beneficial effect of pollen and/or propolis on the metabolism of iron, calcium, phosphorus, and magnesium in rats with nutritional ferropenic anemia. J. Agric. Food Chem. 2000, 48, 5715–5722. [Google Scholar]
- Russo, A.; Formisano, C.; Rigano, D.; Senatore, F.; Delfine, S.; Cardile, V.; Rosselli, S.; Bruno, M. Chemical composition and anticancer activity of essential oils of Mediterranean sage (Salvia officinalis L.) grown in different environmental conditions. Food Chem. Toxicol. 2013, 55, 42–47. [Google Scholar] [CrossRef]
- Tosun, M.; Ercisli, S.; Sengul, M.; Ozer, H.; Polat, T.; Ozturk, E. Antioxidant properties and total phenolic content of eight Salvia species from Turkey. Biolog. Res. 2009, 42, 175–181. [Google Scholar] [CrossRef]
- Ahmed, A.F.; He, N.; Xia, Z.Y.; Kang, W.Y. Total phenolic and flavoniod content and antioxidant properties of Nigella sativa L. seeds. Curr. Top. Nutraceutical Res. 2018, 16, 147–153. [Google Scholar]
- Ahmed, A.F.; Shi, M.; Liu, C.; Kang, W. Comparative analysis of antioxidant activities of essential oils and extracts of fennel (Foeniculum vulgare Mill.) seeds from Egypt and China. Food Sci. Hum. Wellness 2019, 8, 67–72. [Google Scholar] [CrossRef]
- Lin, B.; Diao, S.; Li, C.; Fang, L.; Qaio, S.; Yu, M. Effect of TMS (nanostructured silicon dioxide) on growth of Changbai larch seedlings. J. Forest. Res. 2004, 15, 138–140. [Google Scholar]
- Zafar, H.; Aziz, T.; Khan, B.; Mannan, A.; Rehman, R.U.; Zia, M. CuO and ZnO nanoparticle application in synthetic soil modulates morphology, nutritional contents, and metal analysis of Brassica nigra. ACS Omega 2020, 5, 13566–13577. [Google Scholar] [CrossRef]
- Hebbara, M.; Rajakumar, G.; Ravishankar, G.; Raghavaiah, C. Effect of salinity stress on seed yield through physiological parameters in sunflower genotypes. Helia 2003, 26, 155–160. [Google Scholar] [CrossRef]
- Pirooz, P.; Amooaghaie, R.; Ahadi, A.; Sharififar, F. Silicon-induced nitric oxide burst modulates systemic defensive responses of Salvia officinalis under copper toxicity. Plant Physiol. Biochem. 2021, 162, 752–761. [Google Scholar] [CrossRef]
- Santisree, P.; Sanivarapu, H.; Gundavarapu, S.; Sharma, K.K.; Bhatnagar-Mathur, P. Nitric oxide as a signal in inducing secondary metabolites during plant stress. In Co-Evolution of Secondary Metabolites; Springer: Cham, Switzerland, 2020; pp. 593–621. [Google Scholar]
- Farahani, H.; Sajedi, N.A.; Madani, H.; Changizi, M.; Naeini, M.R. Effect of foliar-applied silicon on flower yield and essential oil composition of damask rose (Rosa damascena Miller) under water deficit stress. Silicon 2021, 13, 4463–4472. [Google Scholar] [CrossRef]
- Mei, Y.; Sun, H.; Du, G.; Wang, X.; Lyu, D. Exogenous chlorogenic acid alleviates oxidative stress in apple leaves by enhancing antioxidant capacity. Sci. Hortic. 2020, 274, 109676. [Google Scholar] [CrossRef]
- Wang, Q.; Su, H.; Yue, N.; Li, M.; Li, C.; Wang, J.; Jin, F. Dissipation and risk assessment of forchlorfenuron and its major metabolites in oriental melon under greenhouse cultivation. Ecotox. Environ. Saf. 2021, 225, 112700. [Google Scholar] [CrossRef]
- Pandey, N.; Pathak, G.C.; Sharma, C.P. Zinc is critically required for pollen function and fertilisation in lentil. J. Trace Elem. Med. Biol. 2006, 20, 89–96. [Google Scholar] [CrossRef]
- Amer, H.M.; Salah, S.H.; Salaheldin, S.; Hussein, M.S.; Abd El-Fatah, S.I. The growth, chemical composition and evaluation of antimicrobial activity of Salvia officinalis oil under partial substitution of mineral NPK fertilizer by bio-fertilizer. Mid. East. J. 2019, 8, 457–468. [Google Scholar]
- Ahmed, A.F.; Attia, F.A.; Liu, Z.; Li, C.; Wei, J.; Kang, W. Antioxidant activity and total phenolic content of essential oils and extracts of sweet basil (Ocimum basilicum L.) plants. Food Sci. Hum. Wellness 2019, 8, 299–305. [Google Scholar] [CrossRef]
- Kh, K.; Mohseni, R.; Saboora, A. Biochemical changes of Rosmarinus officinalis under salt stress. J. Stress Physiol. Biochem. 2010, 6, 114–122. [Google Scholar]
- Rady, M.; Sadak, M.S.; El-Bassiouny, H.M.S.; El-Monem, A.A.A. Alleviation the adverse effects of salinity stress in sunflower cultivars using nicotinamide and α-tocopherol. Austral. J. Basic Appl. Sci. 2011, 5, 342–355. [Google Scholar]
- Chrysargyris, A.; Michailidi, E.; Tzortzakis, N. Physiological and biochemical responses of Lavandula angustifolia to salinity under mineral foliar application. Front. Plant Sci. 2018, 9, 489. [Google Scholar] [CrossRef]
- Lingyun, Y.; Jian, W.; Chenggang, W.; Shan, L.; Shidong, Z. Effect of zinc enrichment on growth and nutritional quality in pea sprouts. J. Food Nutr. Res. 2016, 4, 100–107. [Google Scholar]
- Nourozi, E.; Hosseini, B.; Maleki, R.; Mandoulakani, B.A. Pharmaceutical important phenolic compounds overproduction and gene expression analysis in Dracocephalum kotschyi hairy roots elicited by SiO2 nanoparticles. Ind. Crops Prod. 2019, 133, 435–446. [Google Scholar] [CrossRef]
- Cools, N.; De Vos, B. Availability and evaluation of European forest soil monitoring data in the study on the effects of air pollution on forests. Iforest-Biogeosci. For. 2011, 4, 205. [Google Scholar] [CrossRef]
- Gee, G.; Bauder, J. Particle-Size Analysis 1. Methods of Soil Analysis: Part 1—Physical and Mineralogical Methods; American Society of Agronomy/Soil Science Society of America: Madison, WI, USA, 1986; Volume 5, pp. 383–411. [Google Scholar]
- Jackson, M.L. Soil Chemical Analysis; Prentice Hall of India Private Limited: New Delhi, Indian, 1973; p. 498. [Google Scholar]
- Nelson, D.W.; Sommers, L.E. Total carbon, organic carbon, and organic matter. Methods Soil. Anal. Part 3 Chem. Methods 1996, 5, 961–1010. [Google Scholar]
- Bremner, J.M.; Mulvaney, C. Nitrogen—Total Methods of Soil Analysis: Part 2. Chemical and Microbiological Properties; American Society of Agronomy/Soil Science Society of America: Madison, WI, USA, 1983; Volume 9, pp. 595–624. [Google Scholar]
- Olsen, S.; Sommers, L. Phosphorus. Methods of Soil Analysis: Part 2. Chemical and Microbiological Properties; American Society of Agronomy/Soil Science Society of America: Madison, WI, USA, 1982; pp. 421–422. [Google Scholar]
- Black, C. Methods of Soil Analysis Part 1. Chapter 18, Water Capacity; ASA Monograph Series No. 9; Klute, A., Ed.; American Society of Agronomy: Madison, WI, USA, 1965. [Google Scholar]
- Page, A.; Miller, R.; Keeney, D. Methods of Soil Analysis Part 2—Chemical and Microbiological Properties; American Society of Agronomy: Madison, WI, USA, 1982; Volume 9. [Google Scholar]
- Prasad, T.; Sudhakar, P.; Sreenivasulu, Y.; Latha, P.; Munaswamy, V.; Reddy, K.R.; Sreeprasad, T.S.; Sajanlal, P.R. Effect of nanoscale zinc oxide particles on the germination, growth and yield of peanut. J. Plant Nutr. 2012, 35, 905–927. [Google Scholar] [CrossRef]
- Adhikari, T.; Kundu, S.; Rao, A.S. Impact of SiO2 and Mo nano particles on seed germination of rice (Oryza sativa L.). Int. J. Agric. Food Sci. Technol. 2013, 4, 809–816. [Google Scholar]
- Ali, A.; Ambreen, S.; Javed, R.; Tabassum, S.; Ul Haq, I.; Zia, M. ZnO nanostructure fabrication in different solvents transforms physio-chemical, biological and photodegradable properties. Mater. Sci. Eng. C 2017, 74, 137–145. [Google Scholar] [CrossRef]
- Hench, L.L.; West, J.K. The sol-gel process. Chem. Rev. 1990, 90, 33–72. [Google Scholar] [CrossRef]
- Naddaf, M.; Kafa, H.; Ghanem, I. Extraction and characterization of nano-silica from olive stones. Silicon 2020, 12, 185–192. [Google Scholar] [CrossRef]
- Pandey, A.; Dalal, S.; Dutta, S.; Dixit, A. Structural characterization of polycrystalline thin films by X-ray diffraction techniques. J. Mater. Sci. Mater. Electron. 2021, 32, 1341–1368. [Google Scholar] [CrossRef]
- Scimeca, M.; Bischetti, S.; Lamsira, H.K.; Bonfiglio, R.; Bonanno, E. Energy Dispersive X-ray (EDX) microanalysis: A powerful tool in biomedical research and diagnosis. Eur. J. Histochem. EJH 2018, 62, 2841. [Google Scholar] [CrossRef] [PubMed]
- Nagai, T.; Inoue, R.; Inoue, H.; Suzuki, N. Scavenging capacities of pollen extracts from cistus ladaniferus on autoxidation, superoxide radicals, hydroxyl radicals, and DPPH radicals. Nutr. Res. 2002, 22, 519–526. [Google Scholar] [CrossRef]
- Snedecor, G.W.; Cochran, W.G. Statistical Methods, 8th ed.; Iowa State University Digital Press: Ames, IA, USA, 1989. [Google Scholar]
- Yadava, U.L. A raped and non-destructive method to determined chlorophyll in intact leaves. HortScience 1986, 21, 1449–1450. [Google Scholar] [CrossRef]
- Elmsellem, H.; Ouadi, Y.E.; Mokhtari, M.; Bendaif, H.; Steli, H.; Aouniti, A.; Almehdi, A.M.; Abdel-Rahman, I.; Kusuma, H.S.; Hammouti, B. A natural antioxidant and an environmentally friendly inhibitor of mild steel corrosion: A commercial oil of basil (Ocimum basilicum L.). J. Chem. Technol. Metall. 2019, 54, 742–749. [Google Scholar]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. [14] Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1999; Volume 299, pp. 152–178. [Google Scholar]
- Martínez-Esplá, A.; Zapata, P.J.; Castillo, S.; Guillén, F.; Martínez-Romero, D.; Valero, D.; Serrano, M. Preharvest application of methyl jasmonate (MeJA) in two plum cultivars. 1. Improvement of fruit growth and quality attributes at harvest. Postharvest Biol. Technol. 2014, 98, 98–105. [Google Scholar] [CrossRef]
- Binsan, W.; Benjakul, S.; Visessanguan, W.; Roytrakul, S.; Tanaka, M.; Kishimura, H. Antioxidative activity of Mungoong, an extract paste, from the cephalothorax of white shrimp (Litopenaeus vannamei). Food Chem. 2008, 106, 185–193. [Google Scholar] [CrossRef]
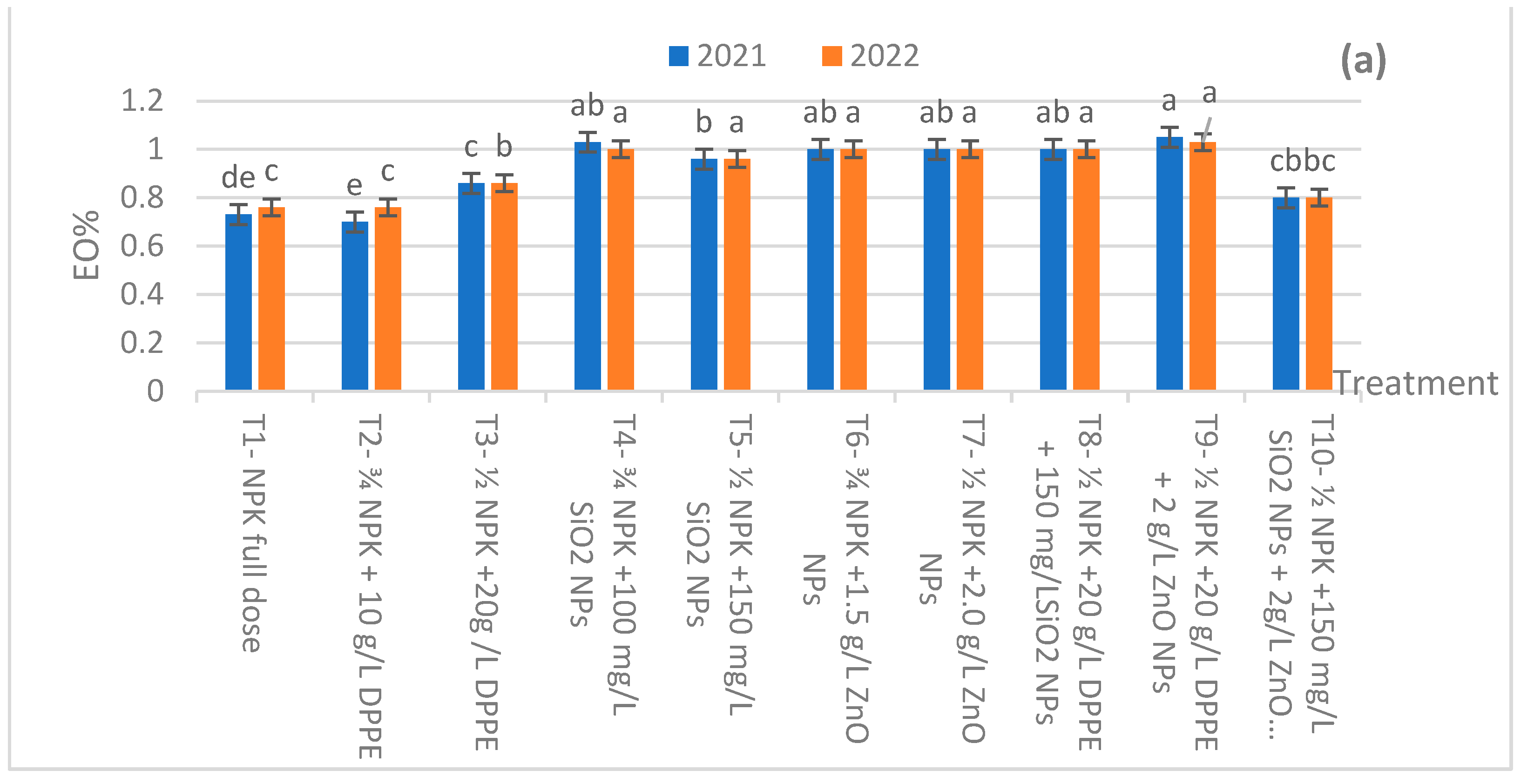

| Fertilization Treatments | Plant Height (cm) | Branch Number/Plants | ||
|---|---|---|---|---|
| 2021 | 2022 | 2021 | 2022 | |
| T1—NPK full dose | 71.66 ± 1.66 f | 72.66 ± 1.32 e | 17.33 ± 1.45 b | 17.33 ± 0.64 ab |
| T2—¾ NPK + 10 g/L DPPE | 71.33 ± 1.70 f | 74.00 ± 1.03 e | 13.33 ± 1.62 c | 12.66 ± 0.72 d |
| T3—½ NPK + 20 g/L DPPE | 74.66 ± 1.07 e | 74.66 ± 1.46 e | 20.33 ± 0.76 a | 17.00 ± 0.69 ab |
| T4—¾ NPK + 100 mg/L SiO2 NPs | 83.00 ± 1.97 b | 83.00 ± 1.66 b | 15.00 ± 0.94 c | 15.00 ± 0.14 c |
| T5—½ NPK + 150 mg/L SiO2 NPs | 70.33 ± 0.98 f | 73.66 ± 1.51 e | 13.33 ± 1.27 c | 13.00 ± 0.85 d |
| T6—¾ NPK + 1.5 g/L ZnO NPs | 83.33 ± 1.42 b | 83.66 ± 1.20 b | 17.66 ± 0.55 b | 17.66 ± 0.21 ab |
| T7—½ NPK + 2.0 g/L ZnO NPs | 89.33 ± 1.31 a | 92.33 ± 1.65 a | 18.33 ± 0.44 b | 17.66 ± 0.30 ab |
| T8—½ NPK + 20 g/L DPPE + 150 mg/L SiO2 NPs | 79.66 ± 1.34 cd | 77.00 ± 1.44 d | 21.00 ± 1.25 a | 18.00 ± 0.16 a |
| T9—½ NPK + 20 g/L DPPE + 2 g/L ZnO NPs | 78.76 ± 1.82 d | 80.33 ± 1.40 c | 21.00 ± 0.83 a | 19.00 ± 0.89 a |
| T10—½ NPK + 150 mg/L SiO2 NPs + 2 g/L ZnO NPs | 81.33 ± 1.73 bc | 82.66 ± 1.29 b | 18.33 ± 0.33 b | 17.33 ± 0.75 ab |
| Main Stem Diameter (cm) | Relative Chlorophyll Content (RCC) (SPAD Units) | |||
| T1—NPK full dose | 0.46 ± 0.04 d | 0.50 ± 0.04 d | 38.66 ± 0.49 e | 40.00 ± 0.05 f |
| T2—¾ NPK + 10 g/L DPPE | 0.60 ± 0.08 bc | 0.66 ± 0.06 ab | 42.66 ± 0.90 c | 41.00 ± 0.20 f |
| T3—½ NPK + 20 g/L DPPE | 0.63 ± 0.04 b | 0.56 ± 0.15 bcd | 43.00 ± 0.91 c | 40.66 ± 0.32 f |
| T4—¾ NPK + 100 mg/LSiO2 NPs | 0.66 ± 0.03 ab | 0.63 ± 0.003 abc | 45.00 ± 0.84 b | 43.00 ± 0.05 e |
| T5—½ NPK + 150 mg/L SiO2 NPs | 0.63 ± 0.03 b | 0.63 ± 0.03 abc | 43.33 ± 0.87 c | 43.00 ± 1.25 e |
| T6—¾ NPK + 1.5 g/L ZnO NPs | 0.50 ± 0.00 d | 0.53 ± 0.01 cd | 45.00 ± 0.36 b | 44.33 ± 0.48 cd |
| T7—½ NPK + 2.0 g/L ZnO NPs | 0.53 ± 0.05 cd | 0.56 ± 0.06 bcd | 46.00 ± 0.76 ab | 47.33 ± 0.58 a |
| T8—½ NPK + 20 g/LDPPE + 150 mg/L SiO2 NPs | 0.53 ± 0.05 cd | 0.53 ± 0.01 cd | 43.66 ± 0.48 c | 45.33 ± 1.13 bc |
| T9—½ NPK + 20 g/L DPPE + 2 g/L ZnO NPs | 0.73 ± 0.04 a | 0.70 ± 0.02 a | 46.66 ± 0.77 a | 46.33 ± 0.86 ab |
| T10—½ NPK + 150 mg/l SiO2 NPs + 2 g/LZnO NPs | 0.60 ± 0.01 bc | 0.60 ± 0.008 abcd | 41.33 ± 0.80 d | 43.66 ± 0.25 de |
| Fertilization Treatments | Fresh Weights of Aerial Parts/Plant (g) | Dry Weights of Aerial Parts/Plant (g) | ||
|---|---|---|---|---|
| 2021 | 2022 | 2021 | 2022 | |
| T1—NPK full dose | 821.33 ± 1.52 j | 974.00 ± 2.64 i | 165.66 ± 1.52 h | 165.00 ± 2.64 h |
| T2—¾ NPK + 10 g/L DPPE | 1085.33 ± 1.52 f | 980.66 ± 2.12 h | 181.33 ± 1.52 f | 177.66 ± 2.08 f |
| T3—½ NPK + 20 g/L DPPE | 1274.00 ± 1.73 c | 1283.00 ± 2.64 d | 210.00 ± 1.73 d | 223.33 ± 3.25 c |
| T4—¾ NPK + 100 mg/L SiO2 NPs | 965.00 ± 1 h | 1242.66 ± 2.51 e | 215.66 ± 2.08 c | 216.33 ± 3.21 d |
| T5—½ NPK + 150 mg/L SiO2 NPs | 949.66 ± 1.52 i | 1056.33 ± 2.30 g | 180.33 ± 1.52 gf | 191.33 ± 2.03 e |
| T6—¾ NPK + 1.5 g/L ZnO NPs | 1059.00 ± 1.11 g | 1311.33 ± 2.30 c | 190.33 ± 1.52 e | 194.66 ± 2.76 e |
| T7—½ NPK + 2.0 g/L ZnO NPs | 1291.66 ± 1.52 b | 1442.33 ± 2.51 b | 181.33 ± 1.52 f | 225.33 ± 2.79 c |
| T8—½ NPK + 20 g/L DPPE + 150 mg/L SiO2 NPs | 1202.00 ± 1.73 d | 1540.00 ± 2.64 a | 239.66 ± 1.52 b | 242.66 ± 3.09 b |
| T9—½ NPK + 20 g/L DPPE + 2 g/L ZnO NPs | 1386.66 ± 1.52 a | 1541.66 ± 3.05 a | 246.33 ± 1.52 a | 257.66 ± 2.12 a |
| T10—½ NPK + 150 mg/L SiO2 NPs + 2 g/L ZnO NPs | 1165.66 ± 1.15 e | 1135.66 ± 2.51 f | 178.33 ± 1.74 g | 172.66 ± 2.53 g |
| Compound Name (%) | Treatment | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Control T1 | T2 | T3 | T4 | T5 | T6 | T7 | T8 | T9 | T10 | ||
| 1 | Eucalyptol/cineole | 5.49 | 5.04 | 5.04 | 0.16 | 11.47 | 3.50 | 5.83 | 5.44 | 10.14 | 5.16 |
| 2 | Thujone | 3.69 | 1.22 | - | 0.93 | -- | - | - | - | - | - |
| 3 | Camphor/(+)-2-Bornanone | 1.25 | 0.53 | 0.48 | - | - | 0.37 | - | - | - | - |
| 4 | Estragole | 22.25 | 58.23 | 46.69 | 2.12 | 63.38 | 23.16 | 64.5 | 60.15 | 46.48 | 61.19 |
| 5 | Methyleugenol | 47.89 | 1.20 | 0.91 | 0.87 | 2.85 | 7.17 | 1.46 | 4.29 | 6.72 | 5.17 |
| 6 | Caryophyllene | 4.53 | 7.40 | 3.39 | 0.51 | 4.72 | 5.69 | 8.59 | 8.09 | 5.45 | 7.09 |
| 7 | Cis-a-Bergamotene | 1.42 | 3.60 | 3.37 | 0.23 | 2.10 | 5.85 | 2.58 | 2.70 | 5.56 | 2.44 |
| 8 | Germacrene D | 1.53 | 4.68 | 2.06 | 0.75 | 2.39 | 3.03 | 3.37 | 4.41 | 2.76 | 3.05 |
| 9 | Viridiflorol/ledol | 2.40 | 1.19 | - | - | - | - | - | - | - | - |
| 10 | (+)-BETA-PINEN | - | 0.62 | 0.51 | - | 0.43 | 0.29 | 0.62 | 0.40 | 0.97 | 0.62 |
| 11 | 1,6-OCTADIEN-3-OL,3,7DIMETHYL- | - | 2.54 | - | - | - | - | 2.05 | 2.43 | - | - |
| 12 | Trans-Sesquisabinene hydrate | - | 0.51 | - | - | - | - | - | - | - | - |
| 13 | Ç-Muurolene | - | 1.75 | 0.53 | - | 0.68 | 0.68 | - | 0.68 | 0.52 | - |
| 14 | Caryophyllene oxide | - | 0.46 | 2.57 | 0.44 | 0.87 | 3.99 | 1.15 | 1.52 | 0.65 | - |
| 15 | (-)-Caryophyllene oxide | - | - | - | 0.50 | - | - | - | - | - | 2.79 |
| 16 | p-Cymene | - | - | 0.51 | - | - | 0.44 | - | - | - | - |
| 17 | Terpineol | - | - | 0.65 | - | - | 0.72 | - | - | - | - |
| 18 | Terpinyl | - | - | 0.48 | - | - | - | - | - | - | - |
| 19 | Terpinen | - | - | - | - | - | - | - | - | 1.12 | 0.49 |
| 20 | Linalool | - | - | 19.68 | - | 3.31 | 20.44 | - | - | 10.64 | 2.94 |
| 21 | Linalool oxide | - | - | - | - | - | 0.43 | - | - | - | - |
| 22 | Cis-Geraniol | - | - | 1.07 | - | - | 1.05 | - | - | - | - |
| 23 | Cis-Verbenol | - | - | 1.52 | - | - | - | - | - | - | - |
| 24 | Cis-à-Bisabolene | - | - | 2.55 | 0.72 | - | 0.41 | - | - | - | - |
| 25 | Citral/à-Citral | - | - | 1.34 | - | - | 2.32 | - | - | - | - |
| 26 | Epiglobulol | - | - | - | 0.82 | - | 0.55 | - | - | - | - |
| 27 | Á-Pinene | - | - | - | - | 1.20 | - | - | - | - | - |
| 28 | Ocimene | - | - | - | - | - | - | - | - | 0.34 | |
| 29 | Cis-ocimene | - | - | - | - | - | 0.28 | - | - | - | |
| 30 | A-Humulene/a-Caryophyllene | - | - | 1.36 | 0.09 | - | 2.60 | 3.86 | 3.89 | 2.50 | 3.42 |
| 31 | 1H-Benzocycloheptene, 2,4a,5,6,7,8,9,9a-octahydro-3,5,5-trimethyl-9-methylene-, (4aS-cis)- | 3.03 | 6.30 | - | - | - | - | - | - | - | - |
| 32 | 1-Naphthalenepropanol, à-ethenyldecahydro-à,5,5,8a-tetrame thyl-2-methylene-, [1S-[1à(R*),4aá,8aà]]- | 2.58 | 1.03 | - | 0.85 | -- | - | - | - | - | - |
| 33 | 3-CYCLOHEXEN-1-OL, 4-METHYL-1-(1-METHYLETHYL | - | - | 3.07 | - | - | 3.00 | - | - | - | - |
| 34 | CYCLOHEXENE,4-(1,5 DIMETHYL-1,4-HEXADIENYL)-1-METHYL- | - | - | - | - | 3.69 | 4.41 | 5.54 | 5.55 | 3.85 | 5.05 |
| 35 | Cadina | - | - | - | - | - | 0.33 | - | - | - | - |
| 36 | Epicubenol | - | - | - | - | - | 0.33 | - | - | - | - |
| 37 | Patchoulene | - | - | - | - | - | 0.37 | - | - | - | - |
| 38 | BETA-ELEMEN | - | - | - | - | - | 0.51 | - | - | - | - |
| 39 | (−)-á-Bourbonene | - | - | - | - | - | 0.21 | - | - | - | - |
| 40 | Nerol acetate | - | - | - | - | - | 0.39 | - | - | - | - |
| 41 | Copaene | - | - | - | - | - | 0.32 | - | - | - | - |
| 42 | À-acorenol | - | - | - | - | - | - | 0.44 | 0.43 | 0.43 | - |
| 43 | Doconexent | - | - | - | - | - | - | - | - | 0.29 | - |
| 44 | HUMULADIENONE | - | - | - | - | - | - | - | - | - | 0.58 |
| Total compounds (%) | 96.06 | 96.3 | 97.78 | 99.99 | 97.09 | 92.84 | 99.99 | 99.98 | 98.42 | 99.99 | |
| Monoterpene hydrocarbons (%) | 0.00 | 0.62 | 0.9 | 0.44 | 1.63 | 1.01 | 0.62 | 0.4 | 1.31 | 0.62 | |
| Sesquiterpenes hydrocarbons (%) | 13.28 | 20.07 | 13.26 | 11.29 | 12.09 | 24.84 | 24.39 | 25.10 | 21.16 | 21.05 | |
| Oxygenated hydrocarbons (%) | 82.78 | 75.61 | 83.62 | 88.26 | 83.37 | 66.99 | 74.98 | 74.48 | 75.95 | 78.32 | |
| Fertilization Treatments | Total Phenols (mg GAE/g D.W) | Antioxidant Activity (µM TE/10 g D.W) |
|---|---|---|
| 2021 | 2021 | |
| T1—NPK full dose | 8.92 ± 0.01 h | 0.02542 ± 0.00 f |
| T2—¾ NPK + 10 g/L DPPE | 6.52 ± 0.01 j | 0.02804 ± 0.00 e |
| T3—½ NPK + 20 g/L DPPE | 8.32 ± 0.01 i | 0.02864 ± 0.00 ed |
| T4—¾ NPK + 100 mg/L SiO2 NPs | 9.61 ± 0.00 f | 0.02813 ± 0.00 e |
| T5—½ NPK + 150 mg/L SiO2 NPs | 10.10 ± 0.01 e | 0.03033 ± 0.00 b |
| T6—¾ NPK + 1.5 g/L ZnO NPs | 12.22 ± 0.00 a | 0.02940 ± 0.00 bcd |
| T7—½ NPK + 2.0 g/L ZnO NPs | 11.25 ± 0.01 b | 0.03321 ± 0.00 a |
| T8—½ NPK + 20 g/L DPPE + 150 mg/L SiO2 NPs | 10.83 ± 0.00 d | 0.02991 ± 0.00 cb |
| T9—½ NPK + 20 g/L DPPE + 2 g/L ZnO NPs | 9.08 ± 0.01 g | 0.02889 ± 0.00 cde |
| T10—½ NPK + 150 mg/L SiO2 NPs + 2 g/L ZnO NPs | 11.20 ± 0.01 c | 0.02948 ± 0.00 bcd |
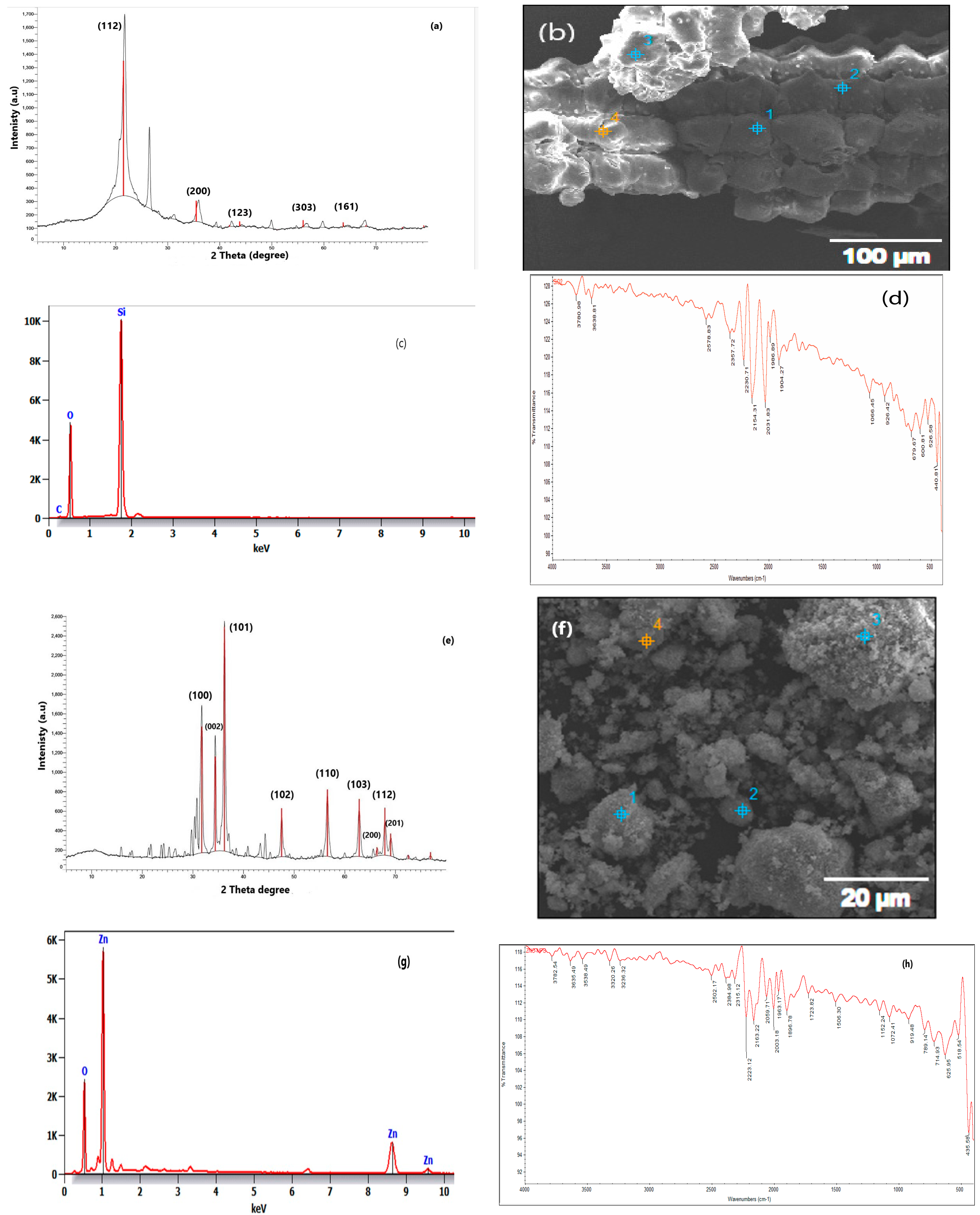
| Silica Oxide Nanoparticles | Zinc Oxide Nanoparticles | |
|---|---|---|
| Specification | ||
| Appearance | White powder | White powder |
| Average particle size | 15 ± 10 nm | 20 nm |
| Morphology | Spherical | Spherical |
| Surface area | 109.356 m2/g | 2.7534 m2/g |
| Average pore radius | 3.53198 × 101 Å | 40.5965 nm |
| Total pore volume | 1.931 × 10−2 cc/g | 0.042062 cm3/g |
| Chemical composition | silicon = 46.83%; oxygen = 53.33% | Zn = 80.34%; O = 19.6 |
| Acute toxicity | ||
| Inhalation human LD50 = 3000 mg/kg Intravenous rat LD50 = 90 mg/kg Intravenous mouse LD50 = 40 mg/kg Oral rat LD50 > 3000 mg/kg Dermal rabbit LD50 > 5000 mg/kg | The lethal dose 50 (LD50) of intravenously administration = 0.3 mg/kg in mice The LD50 of intratracheal instillation = 493.85 µg/kg in mice | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Mahrouk, E.-S.M.; Atef, E.A.M.; Gabr, M.K.; Aly, M.A.; Głowacka, A.; Ahmed, M.A.A. Application of ZnO NPs, SiO2 NPs and Date Pollen Extract as Partial Substitutes to Nitrogen, Phosphorus, and Potassium Fertilizers for Sweet Basil Production. Plants 2024, 13, 172. https://doi.org/10.3390/plants13020172
El-Mahrouk E-SM, Atef EAM, Gabr MK, Aly MA, Głowacka A, Ahmed MAA. Application of ZnO NPs, SiO2 NPs and Date Pollen Extract as Partial Substitutes to Nitrogen, Phosphorus, and Potassium Fertilizers for Sweet Basil Production. Plants. 2024; 13(2):172. https://doi.org/10.3390/plants13020172
Chicago/Turabian StyleEl-Mahrouk, El-Sayed Mohamed, Ekramy Abdel Moatamed Atef, Mohamed Kadry Gabr, Mahmoud Ahmed Aly, Aleksandra Głowacka, and Mohamed A. A. Ahmed. 2024. "Application of ZnO NPs, SiO2 NPs and Date Pollen Extract as Partial Substitutes to Nitrogen, Phosphorus, and Potassium Fertilizers for Sweet Basil Production" Plants 13, no. 2: 172. https://doi.org/10.3390/plants13020172
APA StyleEl-Mahrouk, E.-S. M., Atef, E. A. M., Gabr, M. K., Aly, M. A., Głowacka, A., & Ahmed, M. A. A. (2024). Application of ZnO NPs, SiO2 NPs and Date Pollen Extract as Partial Substitutes to Nitrogen, Phosphorus, and Potassium Fertilizers for Sweet Basil Production. Plants, 13(2), 172. https://doi.org/10.3390/plants13020172






