Effect of Bio-Fertilizer Application on Agronomic Traits, Yield, and Nutrient Uptake of Barley (Hordeum vulgare) in Saline Soil
Abstract
1. Introduction
2. Results
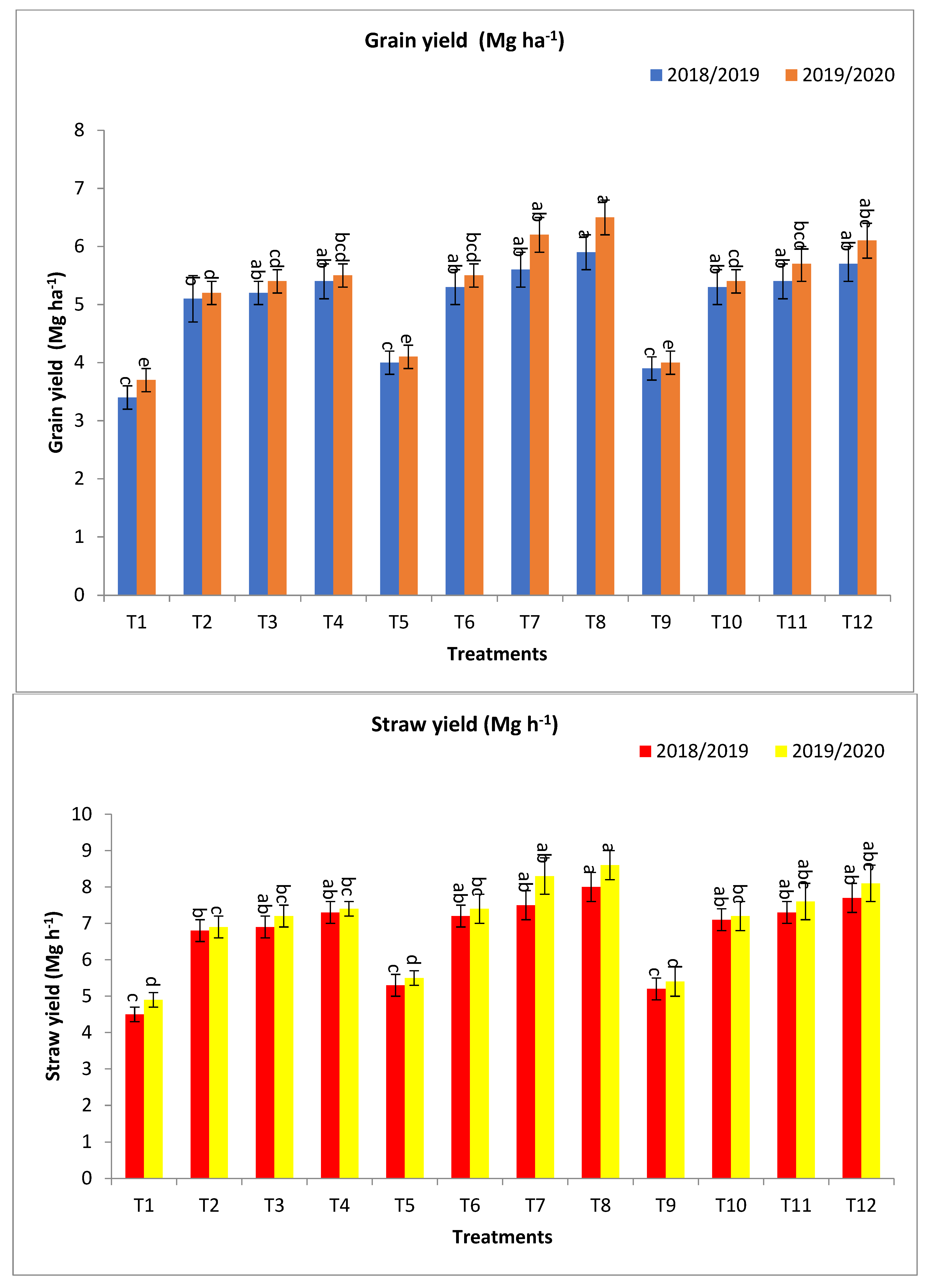
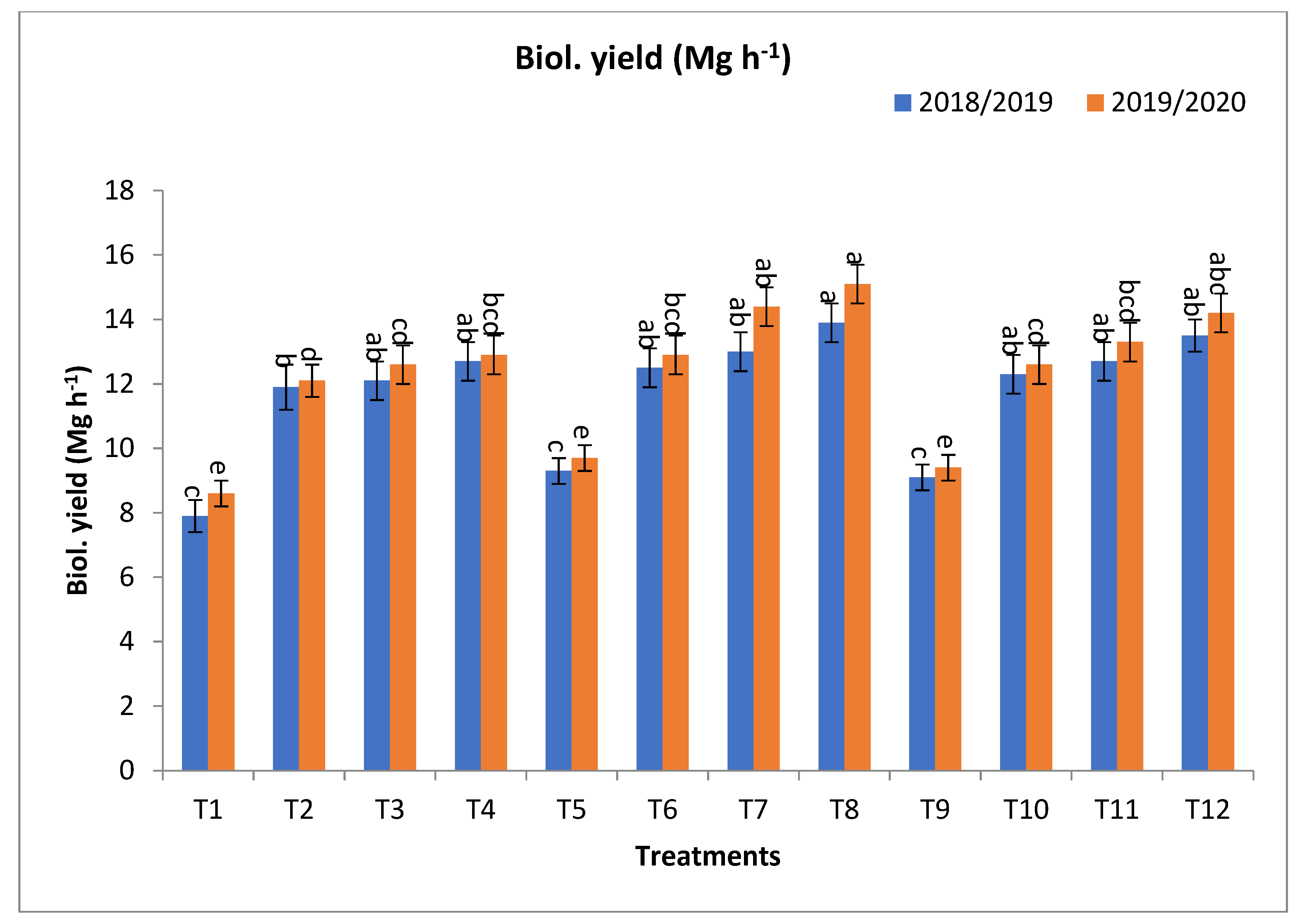
2.1. The Effect of Bio-Fertilizers and Mineral Fertilizer Rates on the Agronomic Traits of Barley Plants under Saline Conditions
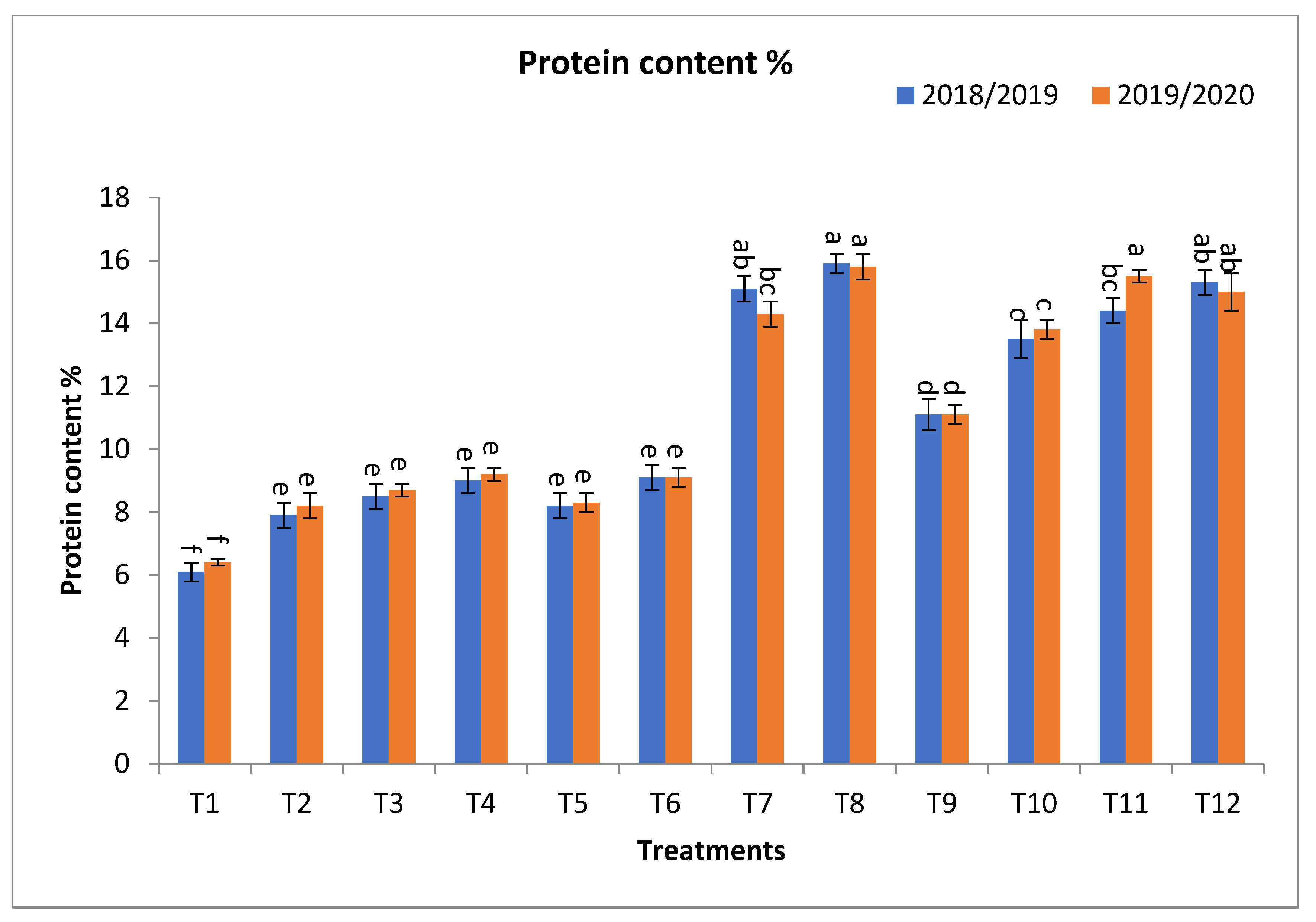
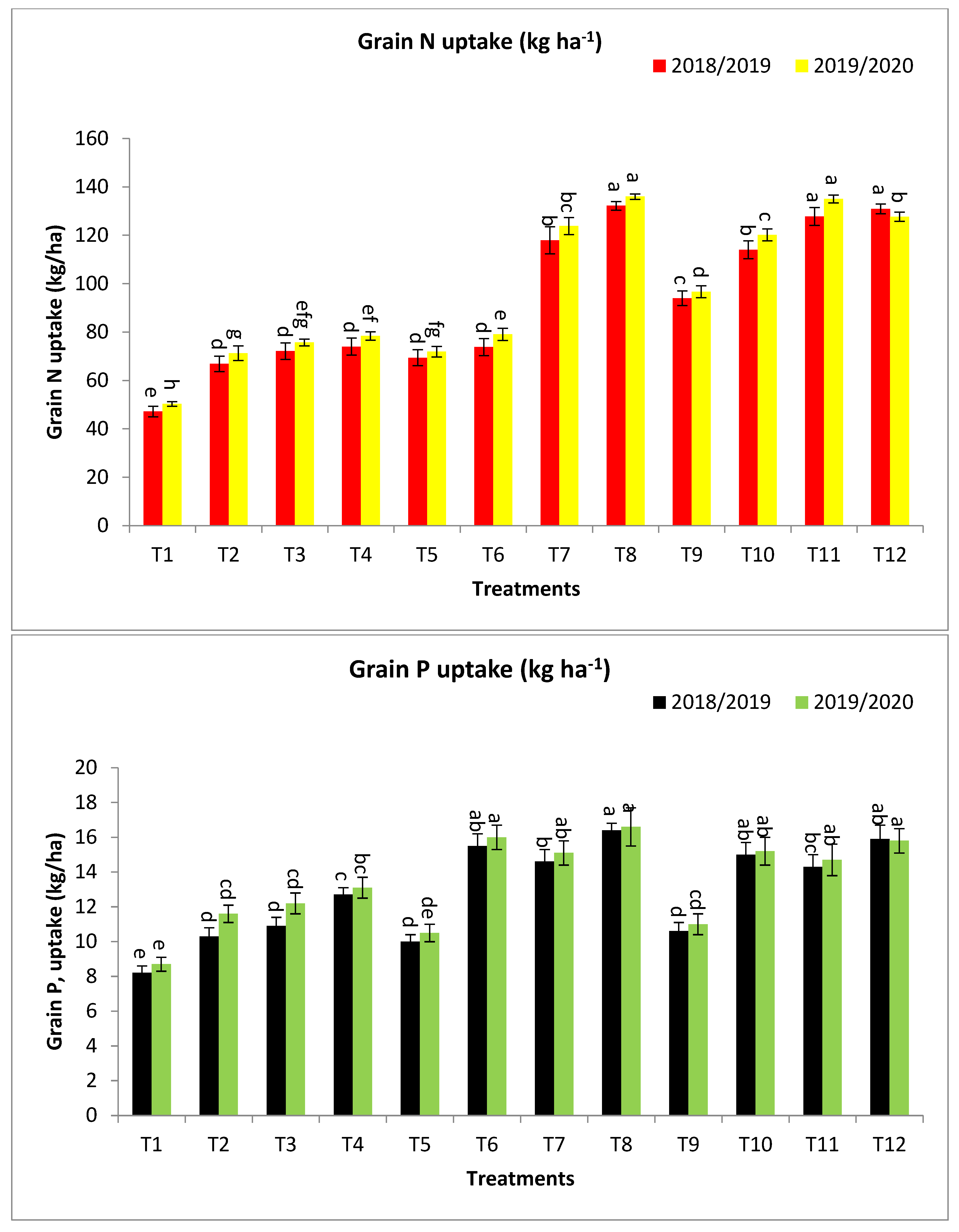
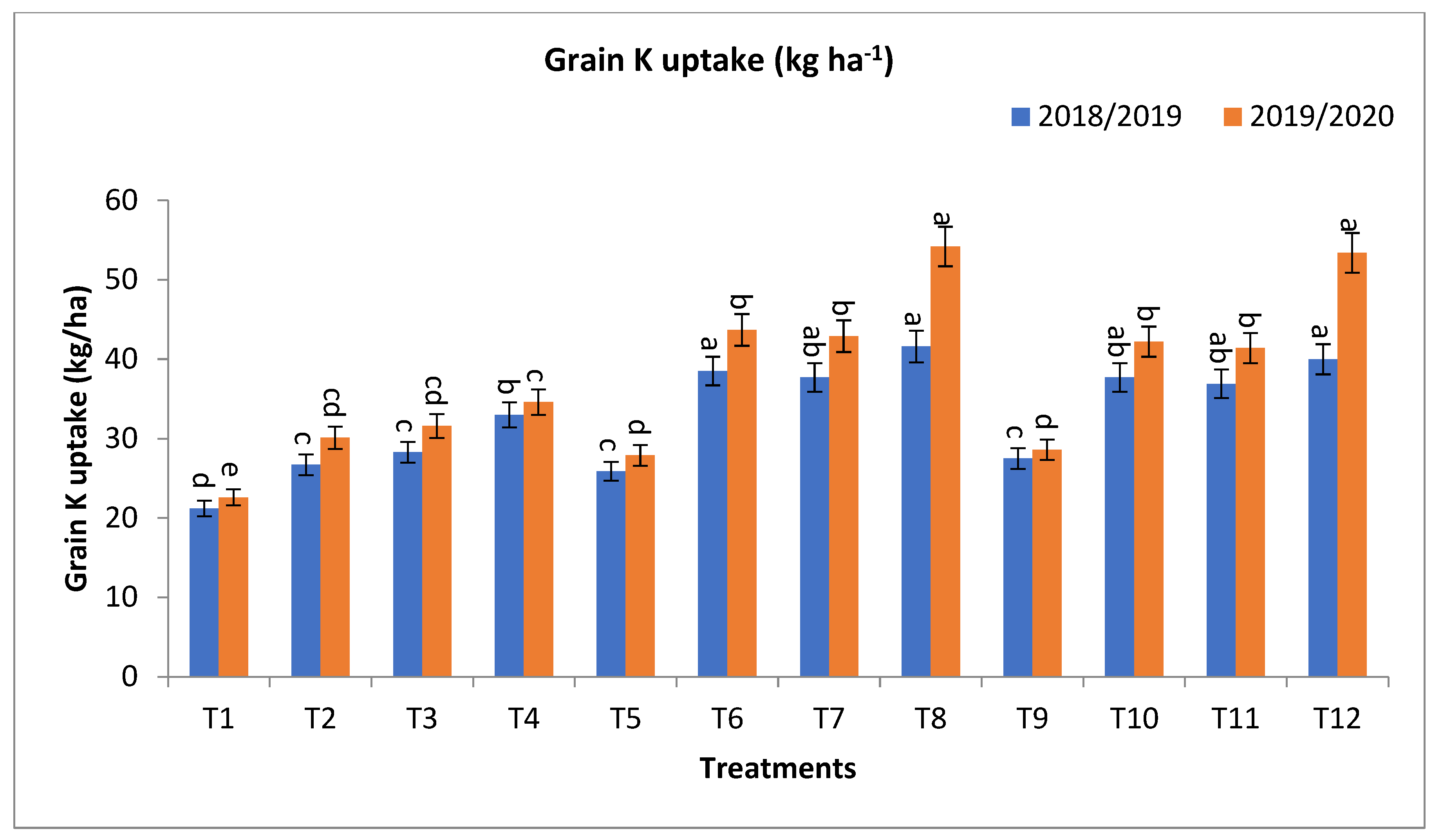
2.2. Effect of Bio-Fertilizers and Mineral Fertilizer Levels on NPK Uptake and Protein Contents
2.3. Presence of Minerals Available in the Rhizosphere of Barley Plants under Saline Conditions
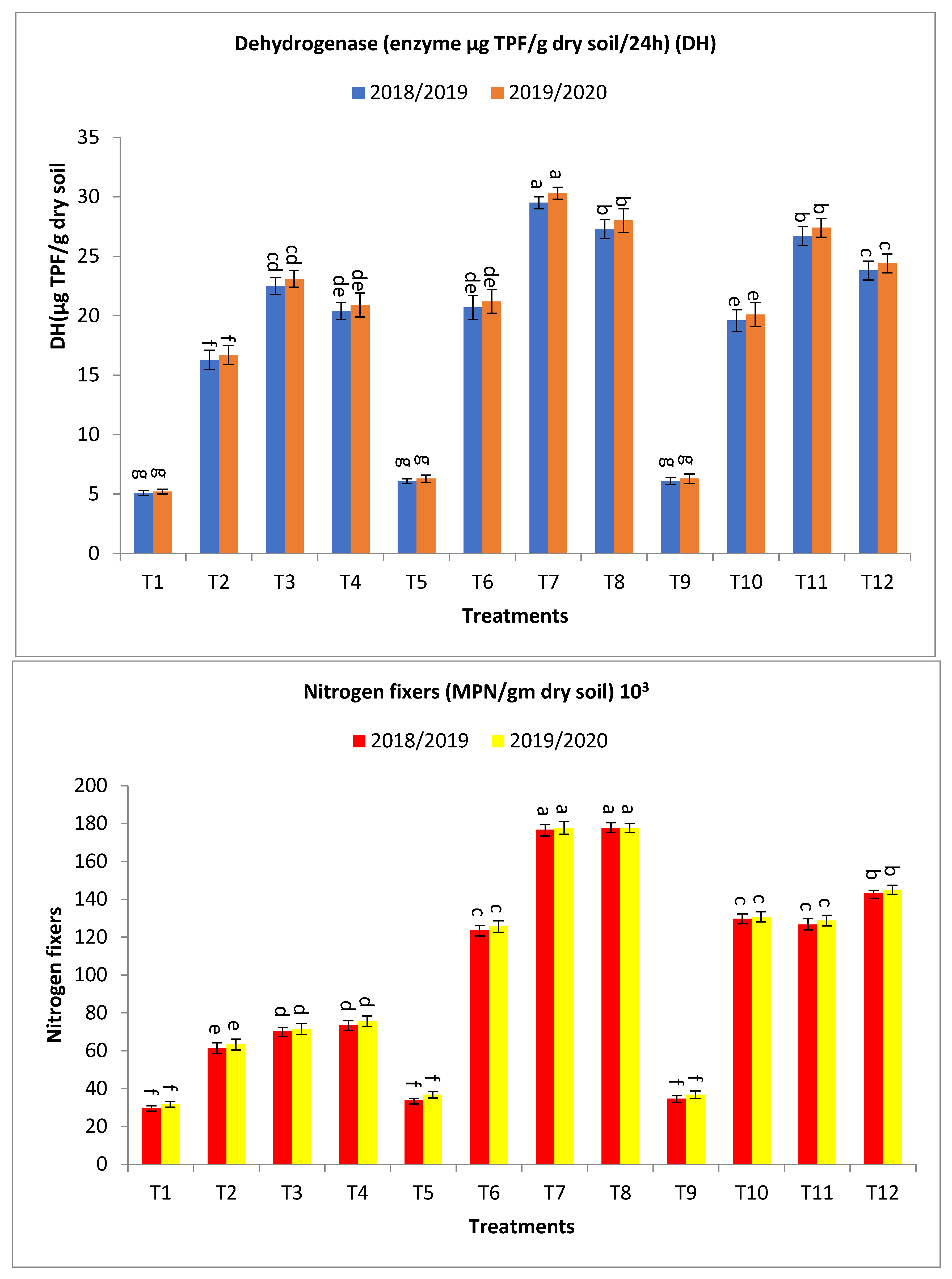
2.4. Biological Activities in the Rhizosphere of Barley Plants under Salinity-Affected Soil Conditions
3. Discussion
3.1. The Effect of Bio-Fertilizers and Mineral Fertilizer Rates on the Agronomic Traits of Barley Plants under Saline Conditions
3.2. Effect of Bio-Fertilizers and Mineral Fertilizer Levels on NPK Uptake and Protein Contents
3.3. Presence of Minerals Available in the Rhizosphere of Barley Plants under Saline Conditions
3.4. Biological Activities in the Rhizosphere of Barley Plants under Salinity-Affected Soil Conditions
4. Materials and Methods
4.1. The Experiment Site
4.2. Experimental Design and Management
4.3. Data Recorded
4.3.1. Agronomic Traits
4.3.2. Chemical Composition
4.3.3. Soil Bio-Activity
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Masrahi, A.S.; Alasmari, A.; Shahin, M.G.; Qumsani, A.T.; Oraby, H.F.; Awad-Allah, M.M.A. Role of Arbuscular Mycorrhizal Fungi and Phosphate Solubilizing Bacteria in Improving Yield, Yield Components, and Nutrients Uptake of Barley under Salinity Soil. Agriculture 2023, 13, 537. [Google Scholar] [CrossRef]
- Ebrahim, F.; Arzani, A.; Rahimmalek, M.; Sun, D.; Peng, J. Salinity Tolerance of Wild Barley Hordeum vulgare ssp. spontaneum. Plant Breed. 2019, 139, 304–316. [Google Scholar] [CrossRef]
- Hussain, M.I.; Al-Dakheel, A.J.; Chaudhry, U.K.; Khan, M.I.; ALHaithloul, H.A.S.; Alghanem, S.M.; Alaklabi, A. Morpho-Physiological Response of Barley to Assess Genotypic Differences of Salinity Tolerance under Hyper Arid Climate. Agric. Water Manag. 2022, 272, 107832. [Google Scholar] [CrossRef]
- Bencherif, K.; Dalpé, Y.; Lounès Hadj-Sahraoui, A. Arbuscular Mycorrhizal Fungi Alleviate Soil Salinity Stress in Arid and Semiarid Areas. In Soil Biology; Springer: Cham, Switzerland, 2019; pp. 375–400. [Google Scholar] [CrossRef]
- Aboelsoud, H.M.; AbdelRahman, M.A.; Kheir, A.M.; Eid, M.S.; Ammar, K.A.; Khalifa, T.H.; Scopa, A. Quantitative Estimation of Saline-Soil Amelioration Using Remote-Sensing Indices in Arid Land for Better Management. Land 2022, 11, 1041. [Google Scholar] [CrossRef]
- Ahmad, R.; Hussain, S.; Anjum, M.A.; Khalid, M.F.; Saqib, M.; Zakir, I.; Hassan, A.; Fahad, S.; Ahmad, S. Oxidative Stress and Antioxidant Defense Mechanisms in Plants under Salt Stress. In Plant Abiotic Stress Tolerance; Springer: Cham, Switzerland, 2019; pp. 191–205. [Google Scholar] [CrossRef]
- Mushtaq, Z.; Faizan, S.; Gulzar, B. Salt Stress, Its Impacts on Plants and the Strategies Plants Are Employing against It: A Review. J. Appl. Biol. Biotechnol. 2020, 8, 81–91. [Google Scholar] [CrossRef]
- Li, L.; Wang, J.; Qian, C.; Zhang, C.; Wang, H.; Li, W.; Zhao, H.; Ju, Y. Physiological and Molecular Responses of Apocynum venetum L. (Apocynaceae) Salt Stress. Horticulturae 2023, 9, 1010. [Google Scholar] [CrossRef]
- Delgado-Baquerizo, M.; Reich, P.B.; Trivedi, C.; Eldridge, D.J.; Abades, S.; Alfaro, F.D.; Bastida, F.; Berhe, A.A.; Cutler, N.A.; Gallardo, A.; et al. Multiple Elements of Soil Biodiversity Drive Ecosystem Functions across Biomes. Nat. Ecol. Evol. 2020, 4, 210–220. [Google Scholar] [CrossRef]
- Gebeyehu, B.; Shumiye, T.; Awoke, T. Review On: The Effect NPS Fertilizer Rate on Phenology, Growth and Yield Parameters of Food Barley (Hordeum vulgare L.). Agric. For. Fish. 2021, 10, 36. [Google Scholar] [CrossRef]
- Pahalvi, H.N.; Rafiya, L.; Rashid, S.; Nisar, B.; Kamili, A.N. Chemical Fertilizers and Their Impact on Soil Health. In Microbiota and Biofertilizers; Springer: Cham, Switzerland, 2021; Volume 2, pp. 1–20. [Google Scholar] [CrossRef]
- Cardarelli, M.; El Chami, A.; Iovieno, P.; Rouphael, Y.; Bonini, P.; Colla, G. Organic Fertilizer Sources Distinctively Modulate Productivity, Quality, Mineral Composition, and Soil Enzyme Activity of Greenhouse Lettuce Grown in Degraded Soil. Agronomy 2023, 13, 194. [Google Scholar] [CrossRef]
- Glick, B.R. Plant Growth-Promoting Bacteria: Mechanisms and Applications. Scientifica 2012, 2012, 963401. [Google Scholar] [CrossRef]
- Chandran, H.; Meena, M.; Swapnil, P. Plant Growth-Promoting Rhizobacteria as a Green Alternative for Sustainable Agriculture. Sustainability 2021, 13, 10986. [Google Scholar] [CrossRef]
- Chandler, D.; Davidson, G.; Grant, W.P.; Greaves, J.; Tatchell, G.M. Microbial Biopesticides for Integrated Crop Management: An Assessment of Environmental and Regulatory Sustainability. Trends Food Sci. Technol. 2008, 19, 275–283. [Google Scholar] [CrossRef]
- Kenneth, O.C.; Nwadibe, E.C.; Kalu, A.U.; Unah, U.V. Plant Growth Promoting Rhizobacteria (PGPR): A Novel Agent for Sustainable Food Production. Am. J. Agric. Biol. Sci. 2019, 14, 35–54. [Google Scholar] [CrossRef]
- Singh, M.; Singh, D.; Gupta, A.; Pandey, K.D.; Singh, P.K.; Kumar, A. Plant Growth Promoting Rhizobacteria. In PGPR Amelioration in Sustainable Agriculture; Elsevier: Amsterdam, The Netherlands, 2019; pp. 41–66. [Google Scholar] [CrossRef]
- Verma, P.P.; Shelake, R.M.; Das, S.; Sharma, P.; Kim, J.-Y. Plant Growth-Promoting Rhizobacteria (PGPR) and Fungi (PGPF): Potential Biological Control Agents of Diseases and Pests. In Microbial Interventions in Agriculture and Environment; Springer: Singapore, 2019; pp. 281–311. [Google Scholar] [CrossRef]
- Sharma, D.; Gahtyari, N.C.; Chhabra, R.; Kumar, D. Role of Microbes in Improving Plant Growth and Soil Health for Sustainable Agriculture. In Microorganisms for Sustainability; Springer: Singapore, 2020; pp. 207–256. [Google Scholar] [CrossRef]
- Chauhan, A.; Saini, R.; Sharma, J.C. Plant Growth Promoting Rhizobacteria and Their Biological Properties for Soil Enrichment and Growth Promotion. J. Plant Nutr. 2021, 45, 273–299. [Google Scholar] [CrossRef]
- Upadhyay, S.K.; Rajput, V.D.; Kumari, A.; Espinosa-Sáiz, D.; Menéndez, E.; Minkina, T.; Dwivedi, P.; Mandzhieva, S. Plant Growth-Promoting Rhizobacteria: A Potential Bio-Asset for Restoration of Degraded Soil and Crop Productivity with Sustainable Emerging Techniques. Environ. Geochem. Health 2022, 45, 9321–9344. [Google Scholar] [CrossRef] [PubMed]
- Shahwar, D.; Mushtaq, Z.; Mushtaq, H.; Alqarawi, A.A.; Park, Y.; Alshahrani, T.S.; Faizan, S. Role of Microbial Inoculants as Bio Fertilizers for Improving Crop Productivity: A Review. Heliyon 2023, 9, e16134. [Google Scholar] [CrossRef]
- Mukherjee, B. Improving Crop Nutrition through Ecofriendly Biofertilizers: Concept, Types and Benefits in Agriculture. Int. J. Bioresour. Sci. 2023, 10, 107–116. [Google Scholar] [CrossRef]
- Patel, R.; Patel, A. Nitrogen Fixation in Nonlegume Plants and Genomics of Nitrogen-Fixing Nonrhizobium Symbioses. In The Chemical Dialogue Between Plants and Beneficial Microorganisms; Elsevier: Amsterdam, The Netherlands, 2023; pp. 145–152. [Google Scholar] [CrossRef]
- Sharma, L.; Sharma, A.K.; Ogram, A.; Singh, H. Enhancing Biological Nitrogen Fixation to Improve Soil Nutrient Status. EDIS 2023, 2023, SL501–SL506. [Google Scholar] [CrossRef]
- Shabbir, R.N.; Ali, H.; Nawaz, F.; Hussain, S.; Areeb, A.; Sarwar, N.; Ahmad, S. Use of Biofertilizers for Sustainable Crop Production. In Agronomic Crops; Springer: Cham, Switzerland, 2019; pp. 149–162. [Google Scholar] [CrossRef]
- Batista, B.D.; Singh, B.K. Realities and Hopes in the Application of Microbial Tools in Agriculture. Microb. Biotechnol. 2021, 14, 1258–1268. [Google Scholar] [CrossRef]
- Maitra, S.; Brestic, M.; Bhadra, P.; Shankar, T.; Praharaj, S.; Palai, J.B.; Shah, M.M.R.; Barek, V.; Ondrisik, P.; Skalický, M.; et al. Bioinoculants—Natural Biological Resources for Sustainable Plant Production. Microorganisms 2022, 10, 51. [Google Scholar] [CrossRef]
- Kour, D.; Rana, K.L.; Yadav, A.N.; Yadav, N.; Kumar, M.; Kumar, V.; Vyas, P.; Dhaliwal, H.S.; Saxena, A.K. Microbial Biofertilizers: Bioresources and Eco-Friendly Technologies for Agricultural and Environmental Sustainability. Biocatal. Agric. Biotechnol. 2020, 23, 101487. [Google Scholar] [CrossRef]
- Orozco-Mosqueda, M.D.C.; Flores, A.; Rojas-Sánchez, B.; Urtis-Flores, C.A.; Morales-Cedeño, L.R.; Valencia-Marin, M.F.; Chávez-Avila, S.; Rojas-Solis, D.; Santoyo, G. Plant Growth-Promoting Bacteria as Bioinoculants: Attributes and Challenges for Sustainable Crop Improvement. Agronomy 2021, 11, 1167. [Google Scholar] [CrossRef]
- Długosz, J.; Piotrowska-Długosz, A. Different Response of Carbon and P-Related Soil Properties toward Microbial Fertilizer Application. Agronomy 2023, 13, 2751. [Google Scholar] [CrossRef]
- Barman, S.; Das, S.; Bhattacharya, S.S. The Prospects of Bio-Fertilizer Technology for Productive and Sustainable Agricultural Growth. In New and Future Developments in Microbial Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2019; pp. 233–253. [Google Scholar] [CrossRef]
- Barsha, K.C.; Pandey, B.; Chand, H.; Bhusal, P.; Pandit, S.; Khanal, S. Promotion of organic and sustainable agriculture through the use of bio-fertilizers. J. Wastes Biomass Manag. 2020, 3, 1–8. [Google Scholar] [CrossRef]
- Sharma, A.K. Biofertilizer—A Key Player in Enhancing Soil Fertility and Agricultural Sustainability. Int. J. Agric. Environ. Biotechnol. 2023, 16, 121–134. [Google Scholar] [CrossRef]
- Srivastava, R. Biofertilizers Application in Agriculture: A Viable Option to Chemical Fertilizers; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2021; pp. 393–411. [Google Scholar] [CrossRef]
- Globe Newswire. Available online: https://www.globenewswire.com/en/news-release/2023/06/06/2683012/0/en/Biofertilizers-Market-Size-to-Worth-Around-USD-9-14-BN-by-2032.html (accessed on 12 June 2023).
- Nawaz, F.; Shehzad, M.A.; Majeed, S.; Ahmad, K.S.; Aqib, M.; Usmani, M.M.; Shabbir, R.N. Role of Mineral Nutrition in Improving Drought and Salinity Tolerance in Field Crops. In Agronomic Crops; Springer: Singapore, 2020; pp. 129–147. [Google Scholar] [CrossRef]
- Shrivastav, P.; Prasad, M.; Singh, T.B.; Yadav, A.; Goyal, D.; Ali, A.; Dantu, P.K. Role of Nutrients in Plant Growth and Development. In Contaminants in Agriculture; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2020; pp. 43–59. [Google Scholar] [CrossRef]
- Farhan, M.J.; Khalefah, K.M.; Altai, S.H.M.; Alsajri, F.A. The Impact of Inoculation with Azotobacter and Azospirillum Bacteria and Chemical Fertilizer on Barley Growth (Hordeum vulgare L.). In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2022; Volume 1060, p. 012001. [Google Scholar] [CrossRef]
- Tariq, A.; Zeng, F.; Graciano, C.; Ullah, A.; Sadia, S.; Ahmed, Z.; Murtaza, G.; Ismoilov, K.; Zhang, Z. Regulation of Metabolites by Nutrients in Plants. In Plant Ionomics: Sensing, Signaling, and Regulation; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2023; pp. 1–18. [Google Scholar] [CrossRef]
- Gangadhar, K.V. Response of Phosphorous and Growth Regulators on Oil Seed Crops: A Review. Int. J. Chem. Stud. 2020, 8, 2010–2016. [Google Scholar] [CrossRef]
- Singh, G.; Dahal, B. Effect of applied phosphorus on soil environment and agricultural productivity: A review. Pharma Innov. J. 2022, 11, 682–688. [Google Scholar]
- Ahmed, M.; Hasanuzzaman, M.; Raza, M.A.; Malik, A.; Ahmad, S. Plant Nutrients for Crop Growth, Development and Stress Tolerance. In Sustainable Agriculture in the Era of Climate Change; Springer: Cham, Switzerland, 2020; pp. 43–92. [Google Scholar] [CrossRef]
- Sardans, J.; Peñuelas, J. Potassium Control of Plant Functions: Ecological and Agricultural Implications. Plants 2021, 10, 419. [Google Scholar] [CrossRef]
- Mostofa, M.G.; Rahman, M.M.; Ghosh, T.K.; Kabir, A.H.; Abdelrahman, M.; Khan, M.A.; Mochida, K.; Tran, L.-S.P. Potassium in Plant Physiological Adaptation to Abiotic Stresses. Plant Physiol. Biochem. 2022, 186, 279–289. [Google Scholar] [CrossRef]
- Soni, P.G.; Basak, N.; Rai, A.K.; Sundha, P.; Chandra, P.; Yadav, R.K. Occurrence of Salinity and Drought Stresses: Status, Impact, and Management. In Salinity and Drought Tolerance in Plants; Springer: Singapore, 2023; pp. 1–28. [Google Scholar] [CrossRef]
- Etesami, H.; Maheshwari, D.K. Use of Plant Growth Promoting Rhizobacteria (PGPRs) with Multiple Plant Growth Promoting Traits in Stress Agriculture: Action Mechanisms and Future Prospects. Ecotoxicol. Environ. Saf. 2018, 156, 225–246. [Google Scholar] [CrossRef]
- Jalal, A.; da Silva Oliveira, C.E.; Galindo, F.S.; Rosa, P.A.L.; Gato, I.M.B.; de Lima, B.H.; Teixeira Filho, M.C.M. Regulatory Mechanisms of Plant Growth-Promoting Rhizobacteria and Plant Nutrition against Abiotic Stresses in Brassicaceae Family. Life 2023, 13, 211. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Sharma, S.; Nisar, S.; Choudhary, O.P. Structural Stability and Organic Matter Stabilization in Soils: Differential Impacts of Soil Salinity and Sodicity. J. Soil Sci. Plant Nutr. 2023, 23, 1751–1773. [Google Scholar] [CrossRef]
- Asoegwu, C.R.; Awuchi, C.G.; Nelson, K.C.T.; Orji, C.G.; Nwosu, O.U.; Egbufor, U.C.; Awuchi, C.G. A review on the role of biofertilizers in reducing soil pollution and increasing soil nutrients. Himal. J. Agric. 2020, 1, 34–38. [Google Scholar]
- Mahmud, A.A.; Upadhyay, S.K.; Srivastava, A.K.; Bhojiya, A.A. Biofertilizers: A Nexus between Soil Fertility and Crop Productivity under Abiotic Stress. Curr. Res. Environ. Sustain. 2021, 3, 100063. [Google Scholar] [CrossRef]
- Chakraborty, T.; Akhtar, N. Biofertilizers: Prospects and Challenges for Future. In Biofertilizers; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2021; pp. 575–590. [Google Scholar] [CrossRef]
- Gamit, H.; Amaresan, N. Role of Methylotrophic Bacteria in Managing Abiotic Stress for Enhancing Agricultural Production: A Review. Pedosphere 2023, 33, 49–60. [Google Scholar] [CrossRef]
- Dutta, R.; Santra, H.K.; Banerjee, D. Microbial Plant Growth Promotors and Their Role in Abiotic Stress Management. In Fungal Secondary Metabolites; Elsevier: Amsterdam, The Netherlands, 2024; pp. 317–335. [Google Scholar] [CrossRef]
- Cao, M.; Narayanan, M.; Shi, X.; Chen, X.; Li, Z.; Ma, Y. Optimistic Contributions of Plant Growth-Promoting Bacteria for Sustainable Agriculture and Climate Stress Alleviation. Environ. Res. 2023, 217, 114924. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Ali, S.; Shahid, M.A.; Mustafa, A.; Sayyed, R.Z.; Curá, J.A. Insights into the Interactions among Roots, Rhizosphere, and Rhizobacteria for Improving Plant Growth and Tolerance to Abiotic Stresses: A Review. Cells 2021, 10, 1551. [Google Scholar] [CrossRef] [PubMed]
- Nivetha, N.; Lavanya, A.K.; Vikram, K.V.; Asha, A.D.; Sruthi, K.S.; Bandeppa, S.; Annapurna, K.; Paul, S. PGPR-Mediated Regulation of Antioxidants: Prospects for Abiotic Stress Management in Plants. In Antioxidants in Plant-Microbe Interaction; Springer: Singapore, 2021; pp. 471–497. [Google Scholar] [CrossRef]
- Latkovic, D.; Maksimovic, J.; Dinic, Z.; Pivic, R.; Stanojkovic, A.; Stanojkovic-Sebic, A. Case Study upon Foliar Application of Biofertilizers Affecting Microbial Biomass and Enzyme Activity in Soil and Yield Related Properties of Maize and Wheat Grains. Biology 2020, 9, 452. [Google Scholar] [CrossRef]
- Jat, M.L.; Chaplot, P.C.; Choudhary, J.; BC, D.; Meema, R.L. Effect of organic, inorganic and biofertilizer on nutrient content, uptake and nutrient status of soil in barley (Hordeum vulgare L.). Pharma Innov. J. 2021, SP-10, 301–307. [Google Scholar]
- Ramírez-Silva, J.H.; Ramírez-Jaramillo, G.; Lozano-Contreras, M.G. Bio-Fertilization Effect on the Foliar Content of Nitrogen (N), Phosphorus (P) and Potassium (K) of Two QPM Maize Varieties in Two Luvisols of Yucatan, Mexico. OALib 2022, 9, e9069. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, S.; Gaurav, A.K.; Srivastava, S.; Verma, J.P. Plant Growth-Promoting Bacteria: Biological Tools for the Mitigation of Salinity Stress in Plants. Front. Microbiol. 2020, 11, 1216. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, M.; Six, J. Soil Structure and Microbiome Functions in Agroecosystems. Nat. Reviews. Earth Environ. 2023, 4, 4–18. [Google Scholar] [CrossRef]
- Farooqi ZU, R.; Qadir, A.A.; Alserae, H.; Raza, A.; Mohy-Ud-Din, W. Organic Amendment–Mediated Reclamation and Build-up of Soil Microbial Diversity in Salt-Affected Soils: Fostering Soil Biota for Shaping Rhizosphere to Enhance Soil Health and Crop Productivity. Environ. Sci. Pollut. Res. 2023, 30, 109889–109920. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Liang, B.; Zhao, H.; Zhao, Y. Impacts of Various Amendments on the Microbial Communities and Soil Organic Carbon of Coastal Saline–Alkali Soil in the Yellow River Delta. Front. Microbiol. 2023, 14, 1239855. [Google Scholar] [CrossRef] [PubMed]
- Kurokura, T.; Hiraide, S.; Shimamura, Y.; Yamane, K. PGPR Improves Yield of Strawberry Species under Less-Fertilized Conditions. Environ. Control Biol. 2017, 55, 121–128. [Google Scholar] [CrossRef]
- Salsabila, N.; Fitriatin, B.N.; Hindersah, R. The Role of Phosphate-Solubilizing Microorganisms in Soil Health and Phosphorus Cycle: A Review. Int. J. Life Sci. Agric. Res. 2023, 2, 281–287. [Google Scholar] [CrossRef]
- da Silva, L.I.; Pereira, M.C.; de Carvalho, A.M.X.; Buttrós, V.H.; Pasqual, M.; Dória, J. Phosphorus-Solubilizing Microorganisms: A Key to Sustainable Agriculture. Agriculture 2023, 13, 462. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, Y.; He, X.T.; Li, B.; Du, S. Plant Growth-Promoting Rhizobacteria: A Good Companion for Heavy Metal Phytoremediation. Chemosphere 2023, 338, 139475. [Google Scholar] [CrossRef]
- Etesami, H.; Adl, S.M. Plant Growth-Promoting Rhizobacteria (PGPR) and Their Action Mechanisms in Availability of Nutrients to Plants. In Environmental and Microbial Biotechnology; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2020; pp. 147–203. [Google Scholar] [CrossRef]
- Das, P.P.; Singh, K.R.; Nagpure, G.; Mansoori, A.; Singh, R.P.; Ghazi, I.A.; Kumar, A.; Singh, J. Plant-Soil-Microbes: A Tripartite Interaction for Nutrient Acquisition and Better Plant Growth for Sustainable Agricultural Practices. Environ. Res. 2022, 214, 113821. [Google Scholar] [CrossRef]
- Harindintwali, J.D.; Zhou, J.; Muhoza, B.; Wang, F.; Herzberger, A.; Yu, X. Integrated Eco-Strategies towards Sustainable Carbon and Nitrogen Cycling in Agriculture. J. Environ. Manag. 2021, 293, 112856. [Google Scholar] [CrossRef]
- Sahu, P.K.; Singh, D.P.; Prabha, R.; Meena, K.K.; Abhilash, P.C. Connecting Microbial Capabilities with the Soil and Plant Health: Options for Agricultural Sustainability. Ecol. Indic. 2019, 105, 601–612. [Google Scholar] [CrossRef]
- Song, L.; Niu, X.; Tian, Y.; Xiao, Y. Assessment of PAH Degradation Potential of Native Species from a Coking Plant through Identifying of the Beneficial Bacterial Community within the Rhizosphere Soil. Chemosphere 2021, 264, 128513. [Google Scholar] [CrossRef] [PubMed]
- Chowdhary, V.; Alooparampil, S.; Pandya, R.V.; Tank, J.G. Physiological Function of Phenolic Compounds in Plant Defense System. In Phenolic Compounds-Chemistry, Synthesis, Diversity, Non-Conventional Industrial, Pharmaceutical and Therapeutic Applications; IntechOpen: London, UK, 2022. [Google Scholar] [CrossRef]
- Tuladhar, P.; Sasidharan, S.; Saudagar, P. Role of Phenols and Polyphenols in Plant Defense Response to Biotic and Abiotic Stresses; Elsevier: Amsterdam, The Netherlands, 2021; pp. 419–441. [Google Scholar] [CrossRef]
- Yan, D.; Long, X.-E.; Ye, L.; Zhang, G.; Hu, A.; Wang, D.; Ding, S. Effects of Salinity on Microbial Utilization of Straw Carbon and Microbial Residues Retention in Newly Reclaimed Coastal Soil. Eur. J. Soil Biol. 2021, 107, 103364. [Google Scholar] [CrossRef]
- Mącik, M.; Gryta, A.; Frąc, M. Biofertilizers in Agriculture: An Overview on Concepts, Strategies and Effects on Soil Microorganisms. Adv. Agron. 2020, 162, 31–87. [Google Scholar] [CrossRef]
- Bamdad, H.; Papari, S.; Lazarovits, G.; Berruti, F. Soil Amendments for Sustainable Agriculture: Microbial-Organic Fertilizers. Soil Use Manag. 2021, 38, 94–120. [Google Scholar] [CrossRef]
- Guo, X.; Du, S.; Guo, H.; Min, W. Long-Term Saline Water Drip Irrigation Alters Soil Physicochemical Properties, Bacterial Community Structure, and Nitrogen Transformations in Cotton. Appl. Soil Ecol. 2023, 182, 104719. [Google Scholar] [CrossRef]
- Cao, H.; Yang, B.; Zhang, T.; Liu, B.; Zhang, C.; Wei, X. Rural Mixed Pond Water Irrigation Affected Microbial Community and Eco-Enzymatic Stoichiometry of Soil Profiles. Appl. Soil Ecol. 2023, 188, 104863. [Google Scholar] [CrossRef]
- Kumawat, K.C.; Sharma, P.; Sirari, A.; Sharma, B.; Kumawat, G.; Nair, R.M.; Bindumadhava, H.; Kunal. Co-Existence of Halo-Tolerant Pseudomonas Fluorescens and Enterococcus Hirae with Multifunctional Growth Promoting Traits to Ameliorate Salinity Stress in Vigna Radiata. Chemosphere 2024, 349, 140953. [Google Scholar] [CrossRef]
- Orhan, F.; Efe, D.; Gormez, A. Advantages of Using Halotolerant/Halophilic Bacteria in Agriculture. In Unravelling Plant-Microbe Synergy; Elsevier: Amsterdam, The Netherlands, 2023; pp. 133–149. [Google Scholar] [CrossRef]
- Tiwari, S.; Sharma, B.; Bisht, N.; Tewari, L. Role of Beneficial Microbial Gene Pool in Mitigating Salt/Nutrient Stress of Plants in Saline Soil through Underground Phytostimulating Signalling Molecules: A Review. Pedosphere 2023, 33, 153–171. [Google Scholar] [CrossRef]
- Dahiya, U.R.; Das, J.; Bano, S. Biological Indicators of Soil Health and Biomonitoring. In Advances in Bioremediation and Phytoremediation for Sustainable Soil Management; Springer: Cham, Switzerland, 2022; pp. 327–347. [Google Scholar] [CrossRef]
- Hidri, R.; Mahmoud, O.M.-B.; Zorrig, W.; Mahmoudi, H.; Smaoui, A.; Abdelly, C.; Azcon, R.; Debez, A. Plant Growth-Promoting Rhizobacteria Alleviate High Salinity Impact on the Halophyte Suaeda Fruticosa by Modulating Antioxidant Defense and Soil Biological Activity. Front. Plant Sci. 2022, 13, 821475. [Google Scholar] [CrossRef]
- Vittori Antisari, L.; Ferronato, C.; De Feudis, M.; Natali, C.; Bianchini, G.; Falsone, G. Soil biochemical indicators and biological fertility in agricultural soils: A case study from northern Italy. Minerals 2021, 11, 219. [Google Scholar] [CrossRef]
- Jabeen, Z.; Hussain, N.; Irshad, F.; Zeng, J.; Tahir, A.; Zhang, G. Physiological and Antioxidant Responses of Cultivated and Wild Barley under Salt Stress. Plant Soil Environ. 2020, 66, 334–344. [Google Scholar] [CrossRef]
- Hassan, A.; Amjad, S.F.; Saleem, M.H.; Yasmin, H.; Imran, M.; Riaz, M.; Ali, Q.; Joyia, F.A.; Ahmed, S.; Ali, S.; et al. Foliar Application of Ascorbic Acid Enhances Salinity Stress Tolerance in Barley (Hordeum vulgare L.) through Modulation of Morpho-Physio-Biochemical Attributes, Ions Uptake, Osmo-Protectants and Stress Response Genes Expression. Saudi J. Biol. Sci. 2021, 28, 4276–4290. [Google Scholar] [CrossRef] [PubMed]
- Moradbeygi, H.; Jamei, R.; Heidari, R.; Darvishzadeh, R. Investigating the Enzymatic and Non-Enzymatic Antioxidant Defense by Applying Iron Oxide Nanoparticles in Dracocephalum moldavica L. Plant Under Salin. Stress. Sci. Hortic. 2020, 272, 109537. [Google Scholar] [CrossRef]
- Sarker, U.; Oba, S. The Response of Salinity Stress-Induced A. Tricolor to Growth, Anatomy, Physiology, Non-Enzymatic and Enzymatic Antioxidants. Front. Plant Sci. 2020, 11, 559876. [Google Scholar] [CrossRef]
- Rajput, V.D.; Harish; Singh, R.K.; Verma, K.K.; Sharma, L.; Quiroz-Figueroa, F.R.; Meena, M.; Gour, V.S.; Minkina, T.; Sushkova, S.; et al. Recent Developments in Enzymatic Antioxidant Defence Mechanism in Plants with Special Reference to Abiotic Stress. Biology 2021, 10, 267. [Google Scholar] [CrossRef] [PubMed]
- Asadi, M.; Rasouli, F.; Amini, T.; Hassanpouraghdam, M.B.; Souri, S.; Skrovankova, S.; Mlcek, J.; Ercisli, S. Improvement of Photosynthetic Pigment Characteristics, Mineral Content, and Antioxidant Activity of Lettuce (Lactuca sativa L.) by Arbuscular Mycorrhizal Fungus and Seaweed Extract Foliar Application. Agronomy 2022, 12, 1943. [Google Scholar] [CrossRef]
- Skwaryło-Bednarz, B.; Jamiołkowska, A.; Yildirim, I.; Krzepiłko, A.; Kopacki, M.; Patkowska, E. Evaluation of Catalase Activity and Antioxidant Properties of Soils Located in the Buffer Zone of the Roztocze National Park Depending on Their Use. Agron. Sci. 2023, 78, 5–18. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemists (AOAC). Official Methods of Analysis of A.O.A.C. International, 17th ed.; Horwitz, S.W., Ed.; AOAC: Rockville, MD, USA, 2000; Volume 2, pp. 66–68. [Google Scholar]
- Magomya, A.M.; Kubmarawa, D.; Ndahi, J.A.; Yebpella, G.G. Determination of plant proteins via the kjeldahl method and amino acid analysis: A comparative study. Int. J. Sci. Technol. Res. 2014, 3, 68–72. [Google Scholar]
- Chapman, H.D.; Parker, F. Methods of analysis for soil, plant, and water. J. Plant Nutr. 1961, 22, 121–128. [Google Scholar]
- Page, A.L.; Miller, R.H.; Keeney, D.R. Methods of Soil Analysis-Chemical and Microbiology Properties; American Society of Agronomy Inc.: Madison, WI, USA, 1982; 1159p. [Google Scholar]
- Allen, U.N. Experiments in Soil Bacteriology, 1st ed.; Burges Pub. Co.: Statesville, NC, USA, 1959. [Google Scholar]
- Casida, L.E., Jr.; Klein, D.A.; Santoro, T. Soil dehydrogenase activity. Soil Sci. 1964, 98, 371–376. [Google Scholar] [CrossRef]
- Rimmer, D.L. Free Radicals, Antioxidants, and Soil Organic Matter Recalcitrance. Eur. J. Soil Sci. 2005, 57, 91–94. [Google Scholar] [CrossRef]
- Snedecor, G.W.; Cochran, W.G. Statistical Methods, 5th ed.; Iowa State University Press: Ames, IA, USA, 1967. [Google Scholar]
- CoStat Ver. 6.4, Cohort Software 798 Light House Ave. 2005. Available online: https://cohortsoftware.com/index.html (accessed on 11 May 2023).
- Duncan, S., Jr. Nonverbal Communication. Psychol. Bull. 1969, 72, 118–137. [Google Scholar] [CrossRef]



| Treatments | Plant Height (cm) | Spike Length (cm) | Spike Weight (g) | |||
|---|---|---|---|---|---|---|
| 2018/2019 | 2019/2020 | 2018/2019 | 2019/2020 | 2018/2019 | 2019/2020 | |
| T1 | 68.1 ± 0.4 f | 72.4 ± 1.3 f | 8 ± 0.4 e | 8.1 ± 0.2 g | 1.6 ± 0.1 d | 1.6 ± 0.1 d |
| T2 | 82.1 ± 1.6 e | 87.4 ± 1.6 e | 9.6 ± 0.2 d | 9.8 ± 0.2 f | 2.2 ± 0.1 c | 2.3 ± 0.1 c |
| T3 | 92.4 ± 0.5 d | 98.6 ± 1.8 cd | 10.8 ± 0.2 bcd | 11.1 ± 0.5 def | 2.4 ± 0.1 c | 2.4 ± 0.1 c |
| T4 | 96.9 ± 0.4 cd | 113.2 ± 1.6 ab | 10.2 ± 0.3 cd | 12.7 ± 0.4 abc | 2.4 ± 0.1 c | 2.5 ± 0.1 c |
| T5 | 83.4 ± 1.5 e | 89 ± 0.8 e | 9.8 ± 0.3 d | 10 ± 0.5 ef | 2.3 ± 0.1 c | 2.3 ± 0.1 c |
| T6 | 97.5 ± 1.7 cd | 105.3 ± 1.7 c | 11.4 ± 0.5 bc | 11.8 ± 0.4 bcd | 2.4 ± 0.1 c | 2.6 ± 0.1 c |
| T7 | 104 ± 0.8 b | 111.8 ± 2 b | 12.2 ± 0.6 ab | 12.6 ± 0.6 abc | 3.8 ± 0.2 a | 4 ± 0.2 a |
| T8 | 112 ± 1.8 a | 119.1 ± 1.6 a | 13.1 ± 0.6 a | 13.4 ± 0.6 a | 4 ± 0.2 a | 4.1 ± 0.2 a |
| T9 | 84.4 ± 2.1 e | 92.5 ± 1.4 de | 9.9 ± 0.5 d | 10.4 ± 0.5 def | 3.1 ± 0.1 b | 3.1 ± 0.1 b |
| T10 | 93.3 ± 2 cd | 101.3 ± 1.8 c | 10.9 ± 0.5 bcd | 11.4 ± 0.5 cde | 3.7 ± 0.2 a | 3.9 ± 0.2 a |
| T11 | 98.2 ± 1.9 c | 103.1 ± 1.8 c | 11.5 ± 0.5 bc | 11.6 ± 0.5 ef | 4.1 ± 0.2 a | 4.2 ± 0.2 a |
| T12 | 110.3 ± 1.7 a | 115.4 ± 1.6 ab | 12.9 ± 0.6 a | 13 ± 0.6 ab | 4.1 ± 0.2 a | 4.2 ± 0.2 a |
| Treatments | Number of Grains Spike−1 | No. of Spikes/m2 | 1000-Grain Weight (g) | |||
| 2018/2019 | 2019/2020 | 2018/2019 | 2019/2020 | 2018/2019 | 2019/2020 | |
| T1 | 25.7 ± 1.2 d | 27.5 ± 1.2 d | 320.7 ± 7.1 e | 303.6 ± 5.4 g | 25.2 ± 0.6 f | 27.4 ± 0.5 e |
| T2 | 36.4 ± 1.7 c | 38.8 ± 1.8 c | 323.5 ± 8.7 e | 317.8 ± 5.3 fg | 35.8 ± 1 e | 38.8 ± 0.7 d |
| T3 | 39.2 ± 1.9 c | 41.2 ± 1.9 c | 337.4 ± 7.2 de | 349.4 ± 11.8 ef | 38.6 ± 0.8 de | 41.2 ± 1.4 d |
| T4 | 40.2 ± 1.9 c | 42.7 ± 1.9 c | 344.4 ± 10 cde | 361 ± 7.3 e | 39.6 ± 1.8 d | 42.7 ± 0.9 d |
| T5 | 37.7 ± 1.8 c | 39.1 ± 1.8 c | 343.4 ± 6.7 cde | 320.1 ± 9.2 fg | 37.1 ± 1.8 de | 39.1 ± 1.1 d |
| T6 | 40.1 ± 1.9 c | 43.1 ± 2 c | 376.1 ± 7.5 abc | 436 ± 8.7 cd | 39.5 ± 0.8 de | 43 ± 0.9 d |
| T7 | 63.9 ± 1.5 a | 67.3 ± 1.6 a | 388.4 ± 13 ab | 463.7 ± 9.6 cd | 63.1 ± 1.5 b | 67.4 ± 1.4 b |
| T8 | 65.7 ± 1.8 a | 69.2 ± 1.8 a | 410.4 ± 12.6 ab | 509.1 ± 9.3 bc | 64.9 ± 1.4 ab | 69.3 ± 1.3 ab |
| T9 | 50.9 ± 2 b | 52.6 ± 1.6 b | 332.6 ± 11.5 e | 339.4 ± 6.8 ef | 50.3 ± 1.5 c | 52.6 ± 1 c |
| T10 | 61.8 ± 1.9 a | 65.3 ± 1.5 a | 369.5 ± 12.4 bcd | 419.4 ± 9.4 d | 61 ± 1.5 ab | 65.4 ± 1.6 ab |
| T11 | 68.5 ± 1.9 a | 71.5 ± 1.3 a | 384.5 ± 5.4 ab | 444.8 ± 8.2 bcd | 67.3 ± 0.9 a | 71.6 ± 1.3 ab |
| T12 | 68.7 ± 2.3 a | 71.6 ± 1.4 a | 395.4 ± 10.3 ab | 473.3 ± 11.7 b | 67.8 ± 1.4 a | 71.7 ± 1.4 a |
| Treatments | N/Straw (kg ha−1) | K/Straw (kg ha−1) | P/Straw (kg ha−1) | |||
|---|---|---|---|---|---|---|
| 2018/2019 | 2019/2020 | 2018/2019 | 2019/2020 | 2018/2019 | 2019/2020 | |
| T1 | 30.9 ± 1.5 f | 33.3 ± 1 f | 20.1 ± 1 d | 20.9 ± 1 e | 8.6 ± 0.4 e | 8.9 ± 0.4 f |
| T2 | 43.3 ± 2.1 e | 46.6 ± 0.8 e | 25.6 ± 1.2 c | 26.6 ± 1.2 d | 12.2 ± 0.6 cd | 12.6 ± 0.6 de |
| T3 | 46.6 ± 2.2 e | 49.4 ± 0.6 e | 25.6 ± 1.2 c | 26.8 ± 1.2 d | 11.2 ± 0.5 d | 12.1 ± 0.6 de |
| T4 | 47.1 ± 2.2 e | 50.6 ± 0.8 e | 31.9 ± 1.5 a | 33.2 ± 1.5 a | 13.4 ± 0.6 bc | 13.7 ± 0.6 cd |
| T5 | 44.9 ± 2.1 e | 47 ± 1.2 e | 24.9 ± 1.2 c | 26 ± 1.2 d | 10.5 ± 0.5 de | 11 ± 0.5 e |
| T6 | 47.7 ± 2.3 e | 51.6 ± 1.6 e | 25.8 ± 1.2 c | 26.9 ± 1.3 d | 16.7 ± 0.8 a | 16.8 ± 0.8 ab |
| T7 | 75.3 ± 3.5 bc | 80 ± 2.9 c | 26.8 ± 1.3 c | 27.9 ± 1.3 cd | 15.6 ± 0.7 a | 15.8 ± 0.7 ab |
| T8 | 85.7 ± 2.9 a | 86.9 ± 2.8 a | 32.1 ± 1.6 a | 32.8 ± 1.5 ab | 17.1 ± 0.8 a | 17.4 ± 0.8 a |
| T9 | 60.3 ± 2.9 d | 62.8 ± 2.9 d | 26.6 ± 1.3 c | 27.7 ± 1.3 cd | 11.2 ± 0.5 d | 11.6 ± 0.5 e |
| T10 | 72.9 ± 3.5 c | 77.7 ± 3.6 c | 28.3 ± 1.4 abc | 29.5 ± 1.4 bcd | 16 ± 0.8 a | 15.8 ± 0.7 a |
| T11 | 82.5 ± 3.9 ab | 85.5 ± 3.2 ab | 27.5 ± 1.3 bc | 28.6 ± 1.3 cd | 15.3 ± 0.7 ab | 15.1 ± 0.7 bc |
| T12 | 83.9 ± 4 a | 85.6 ± 2.9 ab | 31.3 ± 1.5 ab | 31.3 ± 0.9 abc | 16.8 ± 0.8 a | 16.8 ± 0.8 ab |
| Sample Depth (cm) | Physical Properties % | Soil Texture | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0–30 | Sand | Silt | Clay | Sandy Silt Loam | ||||||||
| 51.95 | 25.73 | 22.32 | ||||||||||
| Chemical analysis | ||||||||||||
| Cation meq/L | Anion meq/L | |||||||||||
| pH | E.C ds.m−1 | CaCo3% | O.M % | T.N % | Ca+2 | Mg+2 | Na+ | K+ | Co3−2 | HCo3 | Cl− | So4−2 |
| 8.67 | 8.24 | 4.29 | 0.56 | 0.09 | 25.2 | 7.83 | 44.35 | 5.34 | 0.92 | 15.67 | 48.9 | 5.92 |
| Chemical analysis of irrigation water | ||||||||||||
| Cation meq./L | Anion meq./L | |||||||||||
| pH | E.C ds.m−1 | Ca+2 | Mg+2 | Na+ | K+ | CO3−2 | HCO3− | Cl− | SO4−2 | |||
| 7.62 | 2.34 | 5.37 | 3.12 | 5.43 | 0.25 | 1.63 | 12.37 | 4.23 | 6.18 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alotaibi, M.M.; Aljuaid, A.; Alsudays, I.M.; Aloufi, A.S.; AlBalawi, A.N.; Alasmari, A.; Alghanem, S.M.S.; Albalawi, B.F.; Alwutayd, K.M.; Gharib, H.S.; et al. Effect of Bio-Fertilizer Application on Agronomic Traits, Yield, and Nutrient Uptake of Barley (Hordeum vulgare) in Saline Soil. Plants 2024, 13, 951. https://doi.org/10.3390/plants13070951
Alotaibi MM, Aljuaid A, Alsudays IM, Aloufi AS, AlBalawi AN, Alasmari A, Alghanem SMS, Albalawi BF, Alwutayd KM, Gharib HS, et al. Effect of Bio-Fertilizer Application on Agronomic Traits, Yield, and Nutrient Uptake of Barley (Hordeum vulgare) in Saline Soil. Plants. 2024; 13(7):951. https://doi.org/10.3390/plants13070951
Chicago/Turabian StyleAlotaibi, Mashael M., Alya Aljuaid, Ibtisam Mohammed Alsudays, Abeer S. Aloufi, Aisha Nawaf AlBalawi, Abdulrahman Alasmari, Suliman Mohammed Suliman Alghanem, Bedur Faleh Albalawi, Khairiah Mubarak Alwutayd, Hany S. Gharib, and et al. 2024. "Effect of Bio-Fertilizer Application on Agronomic Traits, Yield, and Nutrient Uptake of Barley (Hordeum vulgare) in Saline Soil" Plants 13, no. 7: 951. https://doi.org/10.3390/plants13070951
APA StyleAlotaibi, M. M., Aljuaid, A., Alsudays, I. M., Aloufi, A. S., AlBalawi, A. N., Alasmari, A., Alghanem, S. M. S., Albalawi, B. F., Alwutayd, K. M., Gharib, H. S., & Awad-Allah, M. M. A. (2024). Effect of Bio-Fertilizer Application on Agronomic Traits, Yield, and Nutrient Uptake of Barley (Hordeum vulgare) in Saline Soil. Plants, 13(7), 951. https://doi.org/10.3390/plants13070951





