Clubroot-Induced Changes in the Root and Rhizosphere Microbiome of Susceptible and Resistant Canola
Abstract
1. Introduction
2. Results
2.1. Clubroot Symptoms, Disease Index, and Pathotyping
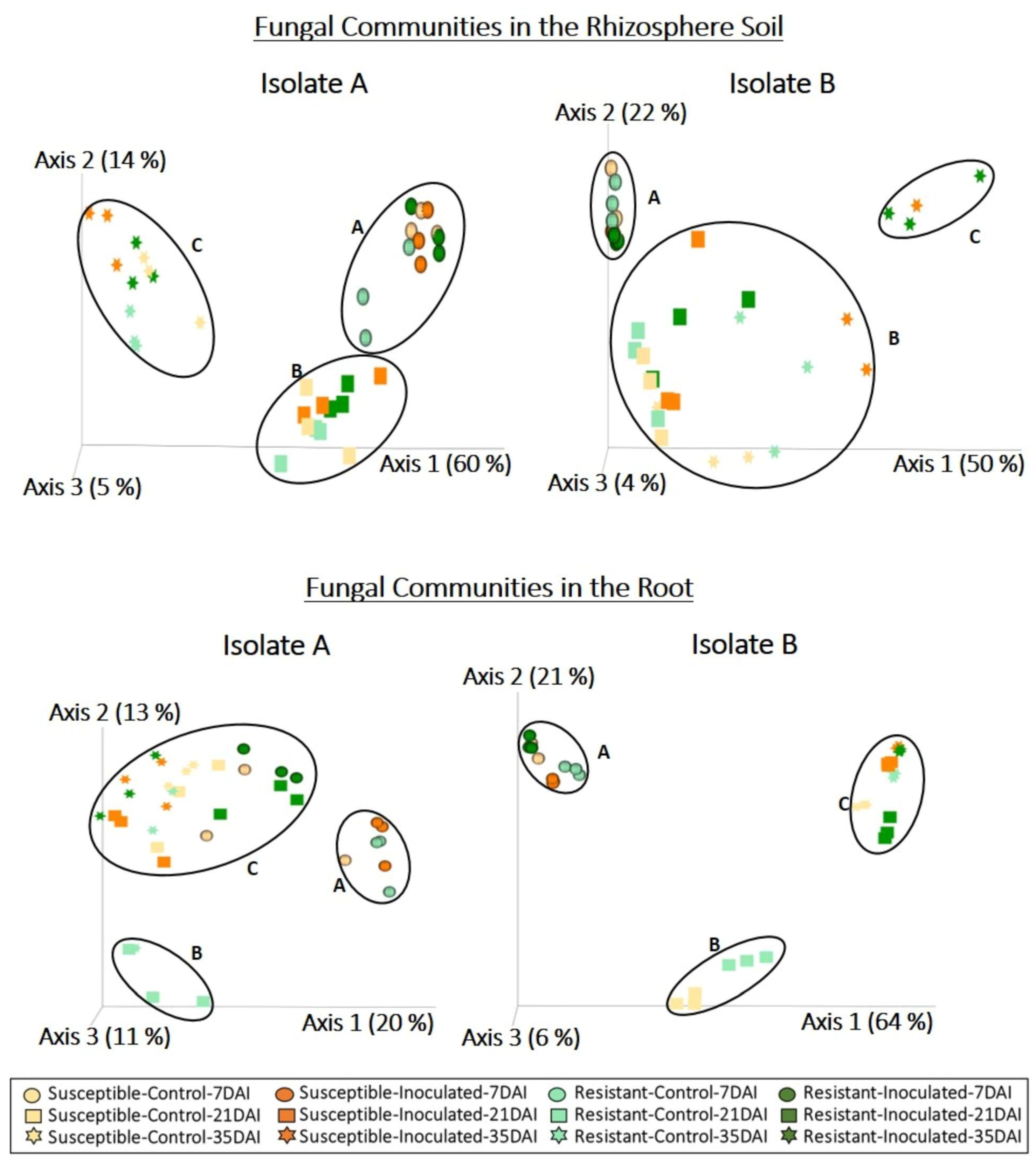
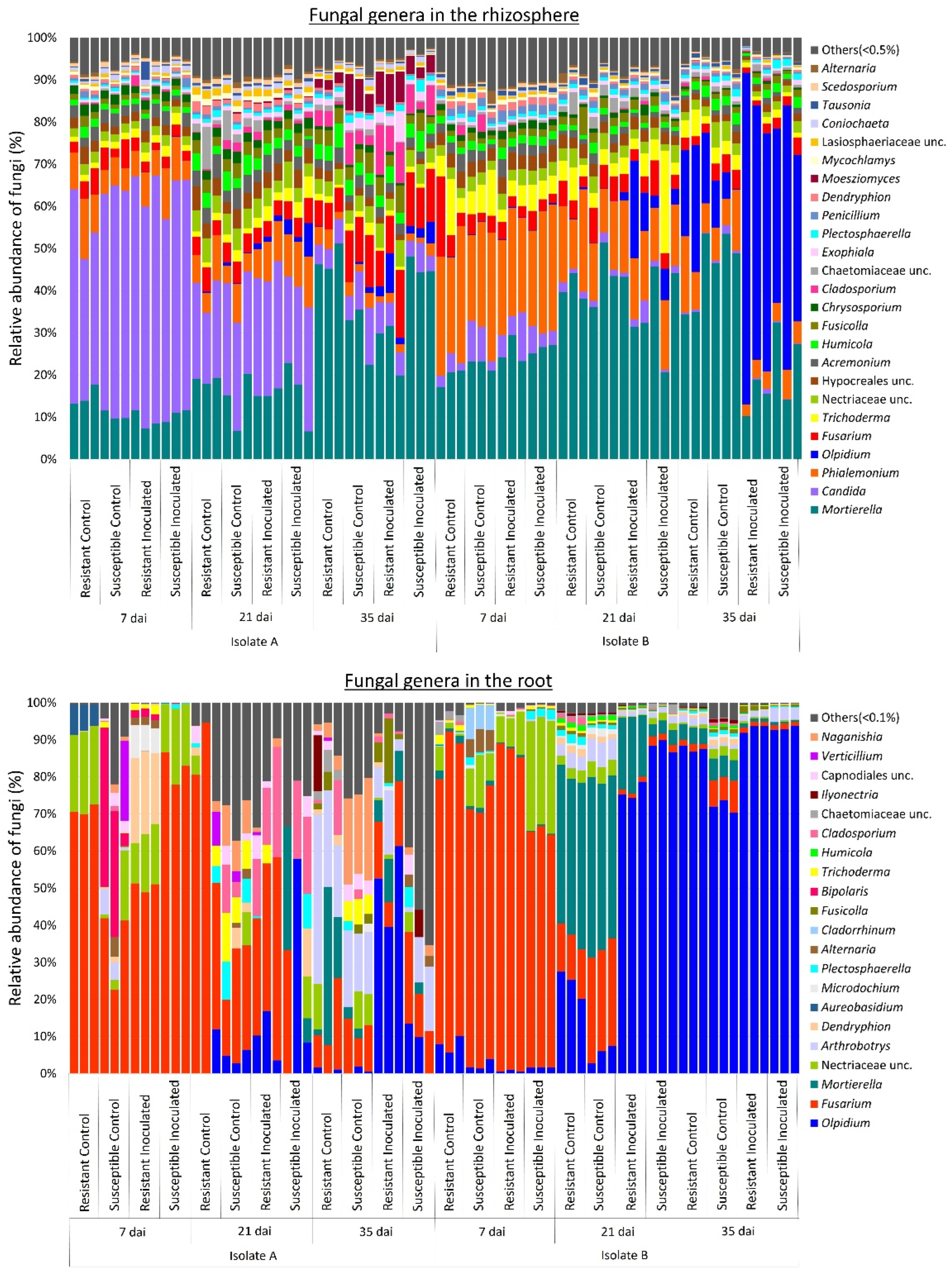
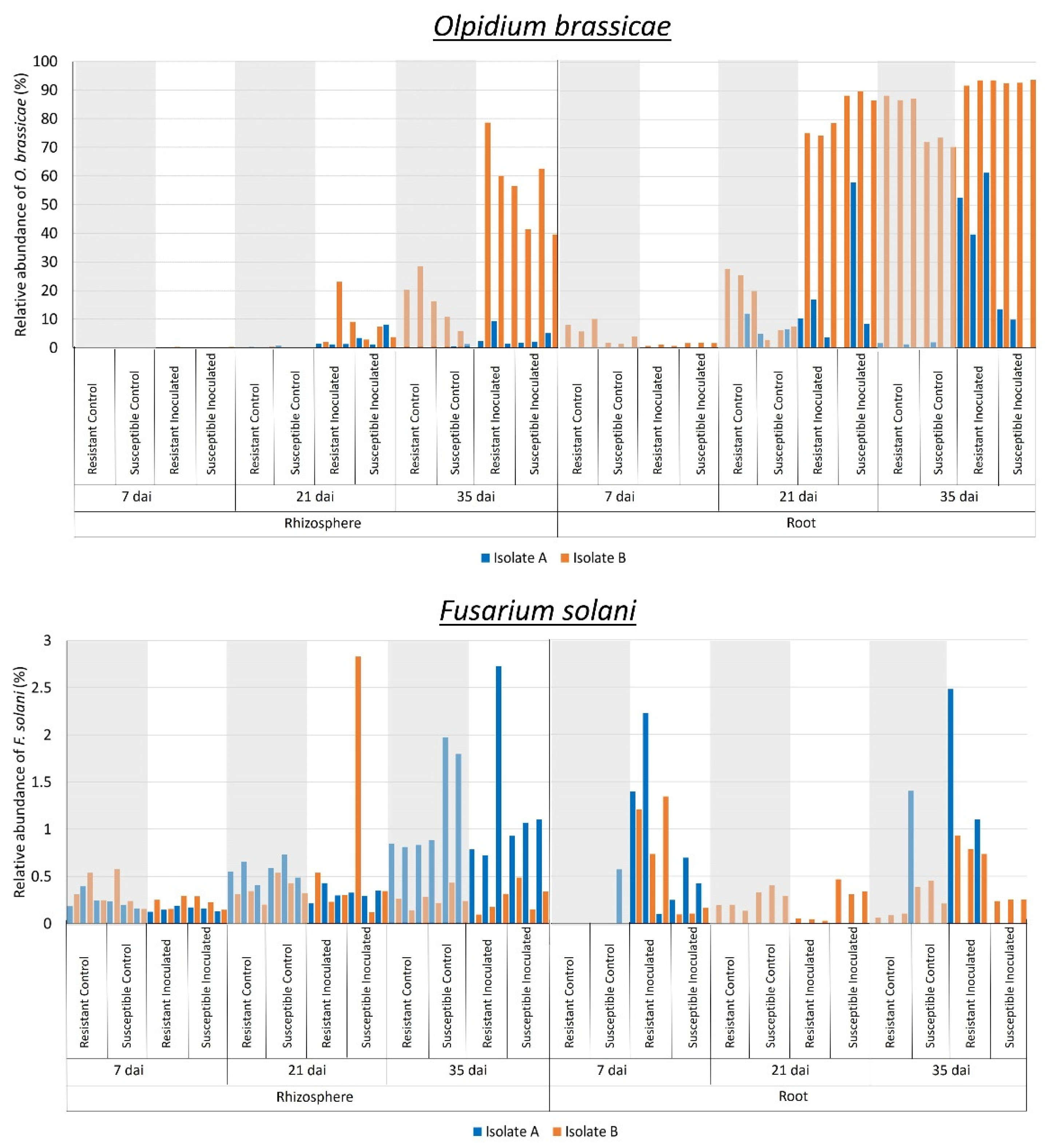
2.2. Effect of P. brassicae Inoculation on Fungal Communities Associated with Canola
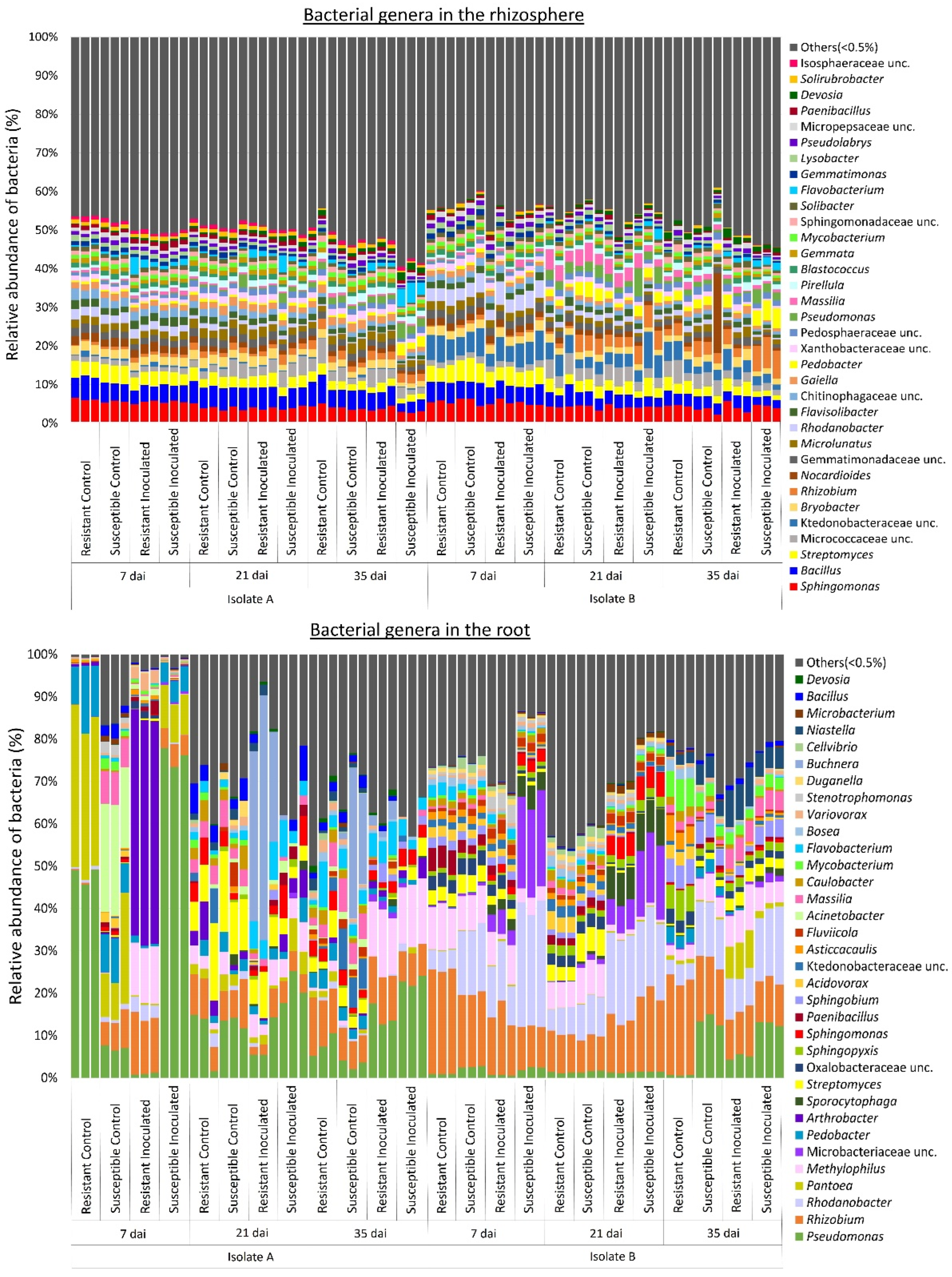
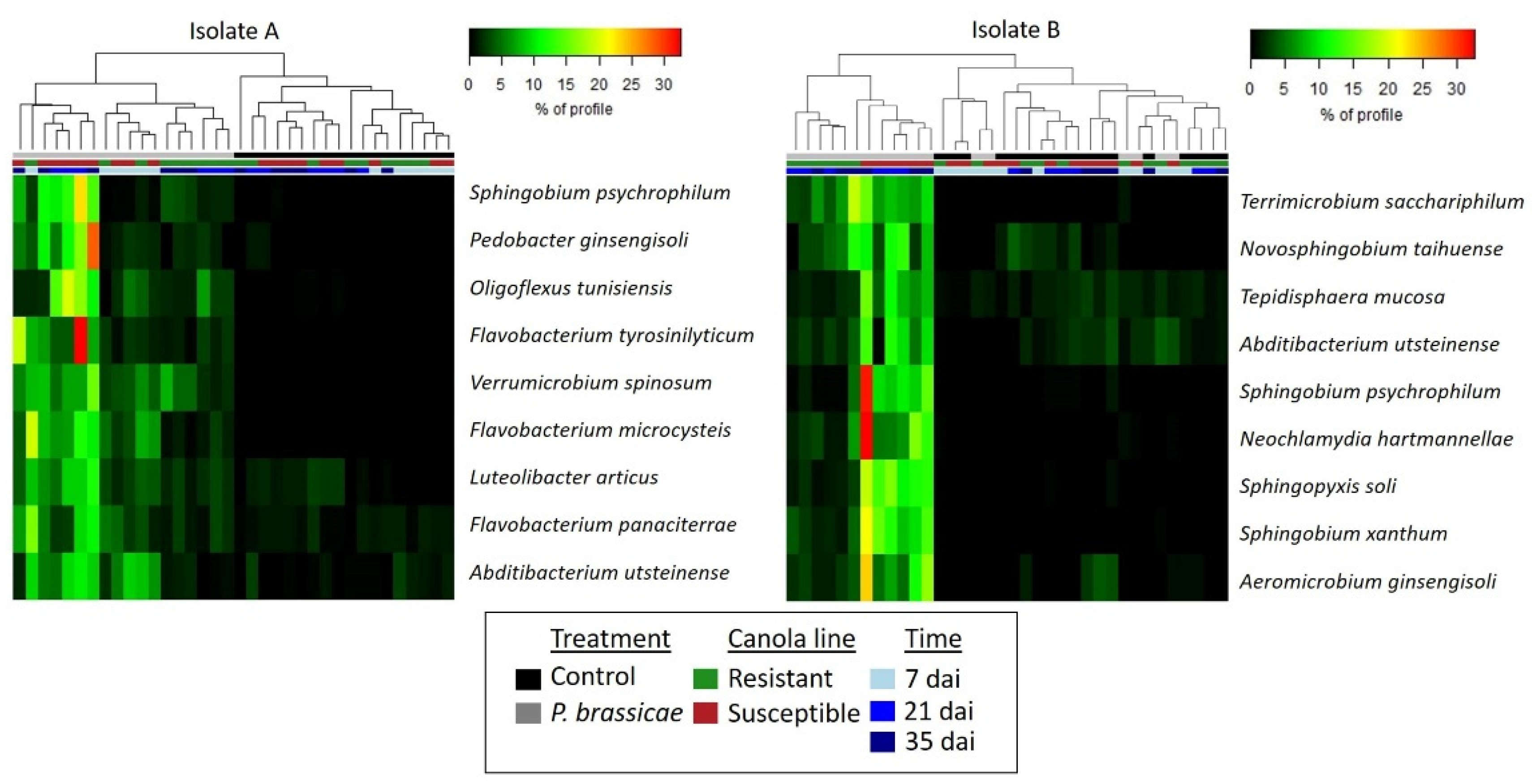
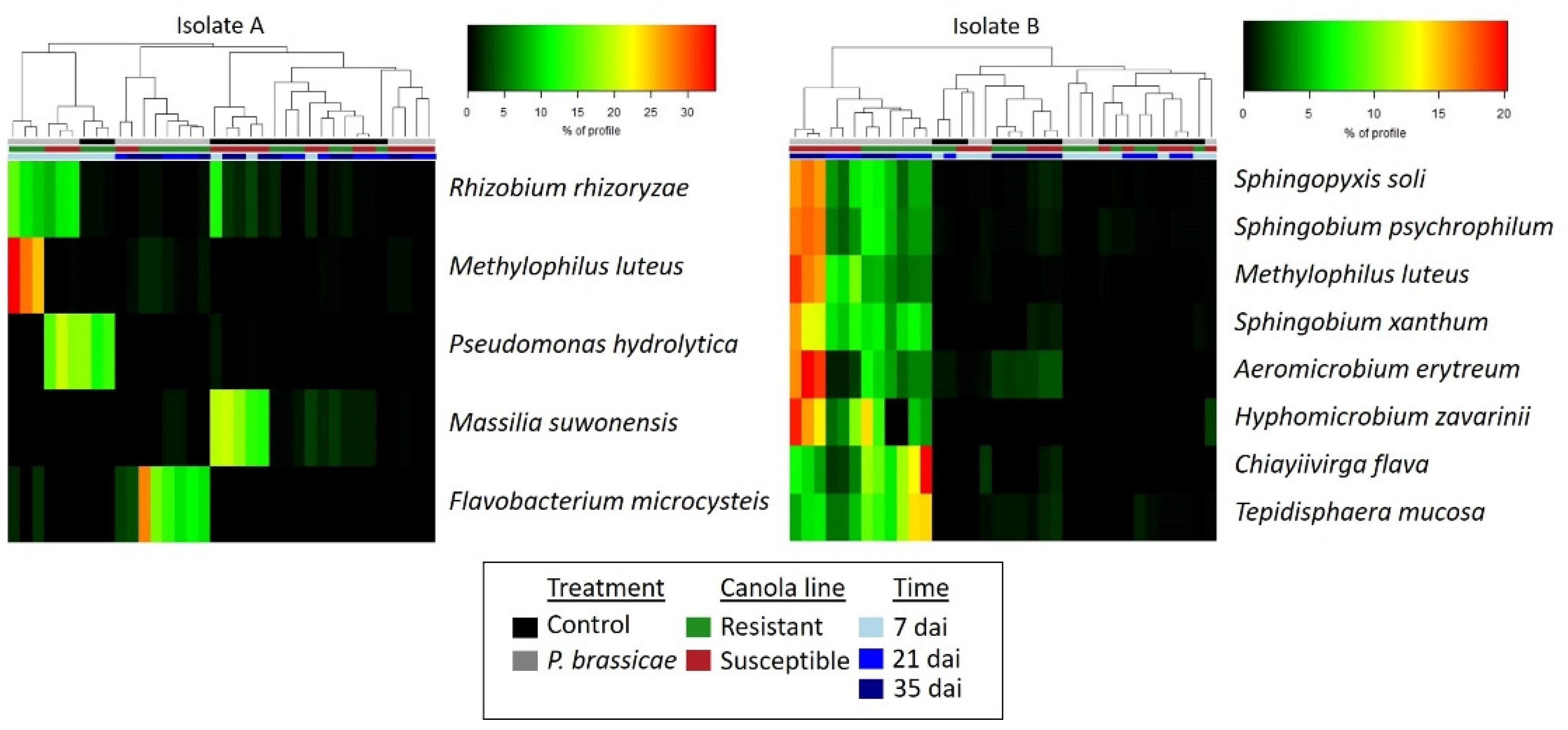
2.3. Effect of P. brassicae Inoculation on Bacterial Communities Associated with Canola
3. Discussion
4. Materials and Methods
4.1. Plant and Pathogen Material
4.2. Inoculation, Greenhouse Conditions, and Experimental Design
4.3. Sample Collection
4.4. DNA Extraction and Microbiome Profiling
4.5. Bioinformatics and Statistic Analyses
4.6. Disease Assessment
4.7. Pathotype Composition
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Strelkov, S.E.; Tewari, J.P.; Smith-Degenhardt, E. Characterization of Plasmodiophora brassicae Populations from Alberta, Canada. Can. J. Plant Pathol. 2006, 28, 467–474. [Google Scholar] [CrossRef]
- Kageyama, K.; Asano, T. Life Cycle of Plasmodiophora brassicae. J. Plant Growth Regul. 2009, 28, 203–211. [Google Scholar] [CrossRef]
- Liu, H.; Brettell, L.E.; Qiu, Z.; Singh, B.K. Microbiome-Mediated Stress Resistance in Plants. Trends Plant Sci. 2020, 25, 733–743. [Google Scholar] [CrossRef] [PubMed]
- Strelkov, S.E.; Hwang, S.-F. Clubroot in the Canadian Canola Crop: 10 Years into the Outbreak. Can. J. Plant Pathol. 2014, 36, 27–36. [Google Scholar] [CrossRef]
- Strelkov, S.E.; Hwang, S.-F.; Manolii, V.P.; Cao, T.; Fredua-Agyeman, R.; Harding, M.W.; Peng, G.; Gossen, B.D.; Mcdonald, M.R.; Feindel, D. Virulence and Pathotype Classification of Plasmodiophora brassicae Populations Collected from Clubroot Resistant Canola (Brassica napus) in Canada. Can. J. Plant Pathol. 2018, 40, 284–298. [Google Scholar] [CrossRef]
- Howard, R.J.; Strelkov, S.E.; Harding, M.W. Clubroot of Cruciferous Crops—New Perspectives on an Old Disease†. Can. J. Plant Pathol. 2010, 32, 43–57. [Google Scholar] [CrossRef]
- Hwang, S.-F.; Strelkov, S.E.; Feng, J.; Gossen, B.D.; Howard, R.J. Plasmodiophora brassicae: A Review of an Emerging Pathogen of the Canadian Canola (Brassica napus) Crop. Mol. Plant Pathol. 2012, 13, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Donald, E.C.; Porter, I.J. Clubroot in Australia: The History and Impact of Plasmodiophora brassicae in Brassica Crops and Research Efforts Directed towards Its Control. Can. J. Plant Pathol. 2014, 36, 66–84. [Google Scholar] [CrossRef]
- Hwang, S.-F.; Howard, R.J.; Strelkov, S.E.; Gossen, B.D.; Peng, G. Management of Clubroot (Plasmodiophora brassicae) on Canola (Brassica napus) in Western Canada. Can. J. Plant Pathol. 2014, 36, 49–65. [Google Scholar] [CrossRef]
- Diederichsen, E.; Frauen, M.; Linders, E.G.A.; Hatakeyama, K.; Hirai, M. Status and Perspectives of Clubroot Resistance Breeding in Crucifer Crops. J. Plant Growth Regul. 2009, 28, 265–281. [Google Scholar] [CrossRef]
- Peng, G.; Lahlali, R.; Hwang, S.-F.; Pageau, D.; Hynes, R.K.; McDonald, M.R.; Gossen, B.D.; Strelkov, S.E. Crop Rotation, Cultivar Resistance, and Fungicides/Biofungicides for Managing Clubroot (Plasmodiophora brassicae) on Canola. Can. J. Plant Pathol. 2014, 36, 99–112. [Google Scholar] [CrossRef]
- Rahman, H.; Peng, G.; Yu, F.; Falk, K.C.; Kulkarni, M.; Selvaraj, G. Genetics and Breeding for Clubroot Resistance in Canadian Spring Canola (Brassica napus L.). Can. J. Plant Pathol. 2014, 36, 122–134. [Google Scholar] [CrossRef]
- Strelkov, S.E.; Hwang, S.-F.; Manolii, V.P.; Cao, T.; Feindel, D. Emergence of New Virulence Phenotypes of Plasmodiophora brassicae on Canola (Brassica napus) in Alberta, Canada. Eur. J. Plant Pathol. 2016, 145, 517–529. [Google Scholar] [CrossRef]
- Compant, S.; Clément, C.; Sessitsch, A. Plant Growth-Promoting Bacteria in the Rhizo- and Endosphere of Plants: Their Role, Colonization, Mechanisms Involved and Prospects for Utilization. Soil Biol. Biochem. 2010, 42, 669–678. [Google Scholar] [CrossRef]
- Glick, B. Stress Control and ACC Deaminase. Principles of Plant-Microbe Interactions; Lugtenberg, B., Ed.; Springer: Cham, Switzerland, 2015. [Google Scholar] [CrossRef]
- Niu, B.; Wang, W.; Yuan, Z.; Sederoff, R.R.; Sederoff, H.; Chiang, V.L.; Borriss, R. Microbial Interactions Within Multiple-Strain Biological Control Agents Impact Soil-Borne Plant Disease. Front. Microbiol. 2020, 11, 585404. [Google Scholar] [CrossRef] [PubMed]
- Conrath, U.; Beckers, G.J.M.; Langenbach, C.J.G.; Jaskiewicz, M.R. Priming for Enhanced Defense. Annu. Rev. Phytopathol. 2015, 53, 97–119. [Google Scholar] [CrossRef]
- Ray, P.; Lakshmanan, V.; Labbé, J.L.; Craven, K.D. Microbe to Microbiome: A Paradigm Shift in the Application of Microorganisms for Sustainable Agriculture. Front. Microbiol. 2020, 11, 622926. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, S.; Gaurav, A.K.; Srivastava, S.; Verma, J.P. Plant Growth-Promoting Bacteria: Biological Tools for the Mitigation of Salinity Stress in Plants. Front. Microbiol. 2020, 11, 1216. [Google Scholar] [CrossRef]
- Bakker, P.A.; Berendsen, R.L.; Van Pelt, J.A.; Vismans, G.; Yu, K.; Li, E.; Van Bentum, S.; Poppeliers, S.W.; Gil, J.J.S.; Zhang, H. The Soil-Borne Identity and Microbiome-Assisted Agriculture: Looking Back to the Future. Mol. Plant 2020, 13, 1394–1401. [Google Scholar] [CrossRef]
- Wang, D.; Sun, T.; Zhao, S.; Pan, L.; Liu, H.; Tian, X. Physiological Change Alters Endophytic Bacterial Community in Clubroot of Tumorous Stem Mustard Infected by Plasmodiophora brassicae. BMC Microbiol. 2020, 20, 244. [Google Scholar] [CrossRef]
- Peng, G.; Mcgregor, L.; Lahlali, R.; Gossen, B.D.; Hwang, S.F.; Adhikari, K.K.; Strelkov, S.E.; Mcdonald, M.R. Potential Biological Control of Clubroot on Canola and Crucifer Vegetable Crops. Plant Pathol. 2011, 60, 566–574. [Google Scholar] [CrossRef]
- Lahlali, R.; Peng, G.; Gossen, B.D.; McGregor, L.; Yu, F.Q.; Hynes, R.K.; Hwang, S.F.; McDonald, M.R.; Boyetchko, S.M. Evidence That the Biofungicide Serenade (Bacillus subtilis) Suppresses Clubroot on Canola via Antibiosis and Induced Host Resistance. Phytopathology 2013, 103, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Dong, D.; Gou, Z.; Wang, X.; Jiang, H.; Yan, Y.; Wu, C.; Zhou, C. Isolation and Characterization of Zhihengliuella aestuarii B18 Suppressing Clubroot on Brassica juncea Var. Tumida Tsen. Eur. J. Plant Pathol. 2018, 150, 213–222. [Google Scholar] [CrossRef]
- Zhou, L.; Zhang, L.; He, Y.; Liu, F.; Li, M.; Wang, Z.; Ji, G. Isolation and Characterization of Bacterial Isolates for Biological Control of Clubroot on Chinese Cabbage. Eur. J. Plant Pathol. 2014, 140, 159–168. [Google Scholar] [CrossRef]
- Zhu, M.; He, Y.; Li, Y.; Ren, T.; Liu, H.; Huang, J.; Jiang, D.; Hsiang, T.; Zheng, L. Two New Biocontrol Agents Against Clubroot Caused by Plasmodiophora brassicae. Front. Microbiol. 2020, 10, 3099. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Ahmed, W.; Dai, Z.; Zhou, X.; He, Z.; Wei, L.; Ji, G. Microbial Consortia: An Engineering Tool to Suppress Clubroot of Chinese Cabbage by Changing the Rhizosphere Bacterial Community Composition. Biology 2022, 11, 918. [Google Scholar] [CrossRef]
- Li, J.; Philp, J.; Li, J.; Wei, Y.; Li, H.; Yang, K.; Ryder, M.; Toh, R.; Zhou, Y.; Denton, M.D.; et al. Trichoderma harzianum Inoculation Reduces the Incidence of Clubroot Disease in Chinese Cabbage by Regulating the Rhizosphere Microbial Community. Microorganisms 2020, 8, 1325. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Chen, X.; Cheng, J.; Xie, J.; Lin, Y.; Jiang, D.; Fu, Y.; Chen, T. Application of Trichoderma Hz36 and Hk37 as Biocontrol Agents against Clubroot Caused by Plasmodiophora brassicae. J. Fungi 2022, 8, 777. [Google Scholar] [CrossRef] [PubMed]
- Caulier, S.; Nannan, C.; Gillis, A.; Licciardi, F.; Bragard, C.; Mahillon, J. Overview of the Antimicrobial Compounds Produced by Members of the Bacillus subtilis Group. Front. Microbiol. 2019, 10, 302. [Google Scholar] [CrossRef]
- Miljaković, D.; Marinković, J.; Balešević-Tubić, S. The Significance of Bacillus Spp. in Disease Suppression and Growth Promotion of Field and Vegetable Crops. Microorganisms 2020, 8, 1037. [Google Scholar] [CrossRef]
- Bulgarelli, D.; Schlaeppi, K.; Spaepen, S.; van Themaat, E.V.L.; Schulze-Lefert, P. Structure and Functions of the Bacterial Microbiota of Plants. Annu. Rev. Plant Biol. 2013, 64, 807–838. [Google Scholar] [CrossRef]
- Gaiero, J.R.; McCall, C.A.; Thompson, K.A.; Day, N.J.; Best, A.S.; Dunfield, K.E. Inside the Root Microbiome: Bacterial Root Endophytes and Plant Growth Promotion. Am. J. Bot. 2013, 100, 1738–1750. [Google Scholar] [CrossRef] [PubMed]
- Daval, S.; Gazengel, K.; Belcour, A.; Linglin, J.; Guillerm-Erckelboudt, A.-Y.; Sarniguet, A.; Manzanares-Dauleux, M.J.; Lebreton, L.; Mougel, C. Soil Microbiota Influences Clubroot Disease by Modulating Plasmodiophora brassicae and Brassica napus Transcriptomes. Microb. Biotechnol. 2020, 13, 1648–1672. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Gao, Z.; Tian, B.; Bi, K.; Chen, T.; Liu, H.; Xie, J.; Cheng, J.; Fu, Y.; Jiang, D. Endosphere Microbiome Comparison between Symptomatic and Asymptomatic Roots of Brassica napus Infected with Plasmodiophora brassicae. PLoS ONE 2017, 12, e0185907. [Google Scholar] [CrossRef] [PubMed]
- Garbeva, P.; van Veen, J.A.; van Elsas, J.D. Microbial Diversity in Soil: Selection of Microbial Populations by Plant and Soil Type and Implications for Disease Suppressiveness. Annu. Rev. Phytopathol. 2004, 42, 243–270. [Google Scholar] [CrossRef] [PubMed]
- Williams, P.H. A System for the Determination of Races of Plasmodiophora brassicae That Infect Cabbage and Rutabaga. Phytopathology 1966, 56, 624–626. [Google Scholar]
- Some, A.; Manzanares, M.J.; Laurens, F.; Baron, F.; Thomas, G.; Rouxel, F. Variation for Virulence on Brassica napus L. amongst Plasmodiophora brassicae Collections from France and Derived Single-Spore Isolates. Plant Pathol. 1996, 45, 432–439. [Google Scholar] [CrossRef]
- LeBoldus, J.M.; Manolii, V.P.; Turkington, T.K.; Strelkov, S.E. Adaptation to Brassica Host Genotypes by a Single-Spore Isolate and Population of Plasmodiophora brassicae (Clubroot). Plant Dis. 2012, 96, 833–838. [Google Scholar] [CrossRef]
- Tanaka, S.; Ito, S. Pathogenic and Genetic Diversity in Plasmodiophora brassicae (Clubroot) from Japan. J. Gen. Plant Pathol. 2013, 79, 297–306. [Google Scholar] [CrossRef]
- Hwang, S.F.; Strelkov, S.E.; Ahmed, H.U.; Manolii, V.P.; Zhou, Q.; Fu, H.; Turnbull, G.; Fredua-Agyeman, R.; Feindel, D. Virulence and Inoculum Density-dependent Interactions between Clubroot Resistant Canola (Brassica napus) and Plasmodiophora brassicae. Plant Pathol. 2017, 66, 1318–1328. [Google Scholar] [CrossRef]
- Smalla, K.; Wieland, G.; Buchner, A.; Zock, A.; Parzy, J.; Kaiser, S.; Roskot, N.; Heuer, H.; Berg, G. Bulk and Rhizosphere Soil Bacterial Communities Studied by Denaturing Gradient Gel Electrophoresis: Plant-Dependent Enrichment and Seasonal Shifts Revealed. Appl. Environ. Microbiol. 2001, 67, 4742–4751. [Google Scholar] [CrossRef] [PubMed]
- Dunfield, K.E.; Germida, J.J. Seasonal Changes in the Rhizosphere Microbial Communities Associated with Field-Grown Genetically Modified Canola (Brassica napus). Appl. Environ. Microbiol. 2003, 69, 7310–7318. [Google Scholar] [CrossRef] [PubMed]
- Farina, R.; Beneduzi, A.; Ambrosini, A.; de Campos, S.B.; Lisboa, B.B.; Wendisch, V.; Vargas, L.K.; Passaglia, L.M.P. Diversity of Plant Growth-Promoting Rhizobacteria Communities Associated with the Stages of Canola Growth. Appl. Soil Ecol. 2012, 55, 44–52. [Google Scholar] [CrossRef]
- de Campos, S.B.; Youn, J.-W.; Farina, R.; Jaenicke, S.; Jünemann, S.; Szczepanowski, R.; Beneduzi, A.; Vargas, L.K.; Goesmann, A.; Wendisch, V.F.; et al. Changes in Root Bacterial Communities Associated to Two Different Development Stages of Canola (Brassica napus L. Var Oleifera) Evaluated through Next-Generation Sequencing Technology. Microb. Ecol. 2013, 65, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Copeland, J.K.; Yuan, L.; Layeghifard, M.; Wang, P.W.; Guttman, D.S. Seasonal Community Succession of the Phyllosphere Microbiome. Mol. Plant-Microbe Interact. 2015, 28, 274–285. [Google Scholar] [CrossRef] [PubMed]
- Bell, J.K.; Mamet, S.D.; Helgason, B.; Siciliano, S.D. Brassica Napus Bacterial Assembly Processes Vary with Plant Compartment and Growth Stage but Not between Lines. Appl. Environ. Microbiol. 2022, 88, e00273-22. [Google Scholar] [CrossRef] [PubMed]
- Gdanetz, K.; Trail, F. The Wheat Microbiome Under Four Management Strategies, and Potential for Endophytes in Disease Protection. Phytobiomes 2017, 1, 158–168. [Google Scholar] [CrossRef]
- Chiarini, L.; Bevivino, A.; Dalmastri, C.; Nacamulli, C.; Tabacchioni, S. Influence of Plant Development, Cultivar and Soil Type on Microbial Colonization of Maize Roots. Appl. Soil Ecol. 1998, 8, 11–18. [Google Scholar] [CrossRef]
- Fierer, N.; Bradford, M.A.; Jackson, R.B. Toward an Ecological Classification of Soil Bacteria. Ecology 2007, 88, 1354–1364. [Google Scholar] [CrossRef]
- Evans, S.E.; Wallenstein, M.D. Climate Change Alters Ecological Strategies of Soil Bacteria. Ecol. Lett. 2014, 17, 155–164. [Google Scholar] [CrossRef]
- Peng, J.; Liu, H.; Hu, Y.; Sun, Y.; Liu, Q.; Li, J.; Dong, Y. Shift in Soil Bacterial Communities from K- to r-Strategists Facilitates Adaptation to Grassland Degradation. Land Degrad. Dev. 2022, 33, 2076–2091. [Google Scholar] [CrossRef]
- Liu, T.; Wu, X.; Li, H.; Ning, C.; Li, Y.; Zhang, X.; He, J.; Filimonenko, E.; Chen, S.; Chen, X.; et al. Soil Quality and r—K Fungal Communities in Plantations after Conversion from Subtropical Forest. Catena 2022, 219, 106584. [Google Scholar] [CrossRef]
- Yao, F.; Yang, S.; Wang, Z.; Wang, X.; Ye, J.; Wang, X.; DeBruyn, J.M.; Feng, X.; Jiang, Y.; Li, H. Microbial Taxa Distribution Is Associated with Ecological Trophic Cascades along an Elevation Gradient. Front. Microbiol. 2017, 8, 2071. [Google Scholar] [CrossRef] [PubMed]
- Leplat, J.; Friberg, H.; Abid, M.; Steinberg, C. Survival of Fusarium graminearum, the Causal Agent of Fusarium Head Blight. A Review. Agron. Sustain. Dev. 2013, 33, 97–111. [Google Scholar] [CrossRef]
- Phalip, V.; Delalande, F.; Carapito, C.; Goubet, F.; Hatsch, D.; Leize-Wagner, E.; Dupree, P.; Dorsselaer, A.V.; Jeltsch, J.-M. Diversity of the Exoproteome of Fusarium graminearum Grown on Plant Cell Wall. Curr. Genet. 2005, 48, 366–379. [Google Scholar] [CrossRef]
- Wolf, K. Nonconventional Yeasts in Biotechnology: A Handbook; Springer Science & Business Media: Berlin, Germany, 2012; ISBN 978-3-642-79856-6. [Google Scholar]
- Zamani, A.I.; Barig, S.; Ibrahim, S.; Mohd. Yusof, H.; Ibrahim, J.; Low, J.Y.S.; Kua, S.F.; Baharum, S.N.; Stahmann, K.-P.; Ng, C.L. Comparative Metabolomics of Phialemonium curvatum as an Omnipotent Fungus Cultivated on Crude Palm Oil versus Glucose. Microb. Cell Factories 2020, 19, 179. [Google Scholar] [CrossRef] [PubMed]
- Lay, C.-Y.; Hamel, C.; St-Arnaud, M. Taxonomy and Pathogenicity of Olpidium brassicae and Its Allied Species. Fungal Biol. 2018, 122, 837–846. [Google Scholar] [CrossRef]
- Moreno, A.; Fereres, A. Virus Diseases in Lettuce in the Mediterranean Basin. In Advances in Virus Research; Elsevier: Amsterdam, The Netherlands, 2012; Volume 84, pp. 247–288. ISBN 978-0-12-394314-9. [Google Scholar]
- Ni, H.; Zong, R.; Sun, J.; Wu, Y.; Yu, L.; Liu, Y.; Liu, J.; Ju, R.; Sun, X.; Zheng, Y.; et al. Response of Bacterial Community to the Occurrence of Clubroot Disease in Chinese Cabbage. Front. Microbiol. 2022, 13, 922660. [Google Scholar] [CrossRef]
- Saraiva, A.L.D.R.; Bhering, A.D.S.; do Carmo, M.G.; Andreote, F.D.; Dias, A.C.; Coelho, I.D.S. Bacterial Composition in Brassica-Cultivated Soils with Low and High Severity of Clubroot. J. Phytopathol. 2023, 168, 613–619. [Google Scholar] [CrossRef]
- Ludwig-Müller, J.; Prinsen, E.; Rolfe, S.A.; Scholes, J.D. Metabolism and Plant Hormone Action During Clubroot Disease. J. Plant Growth Regul. 2009, 28, 229–244. [Google Scholar] [CrossRef]
- Kamilova, F.; Kravchenko, L.V.; Shaposhnikov, A.I.; Makarova, N.; Lugtenberg, B. Effects of the Tomato Pathogen Fusarium oxysporum f. sp. Radicis-Lycopersici and of the Biocontrol Bacterium Pseudomonas fluorescens WCS365 on the Composition of Organic Acids and Sugars in Tomato Root Exudate. Mol. Plant-Microbe Interact. 2006, 19, 1121–1126. [Google Scholar] [CrossRef] [PubMed]
- Neumann, G.; Römheld, V. Rhizosphere Chemistry in Relation to Plant Nutrition. In Marschner’s Mineral Nutrition of Higher Plants; Academic Press: Cambridge, MA, USA, 2012. [Google Scholar]
- Phillips, D.A.; Fox, T.C.; King, M.D.; Bhuvaneswari, T.V.; Teuber, L.R. Microbial Products Trigger Amino Acid Exudation from Plant Roots. Plant Physiol. 2004, 136, 2887–2894. [Google Scholar] [CrossRef] [PubMed]
- Mitter, B.; Petric, A.; Shin, M.; Chain, P.; Hauberg-Lotte, L.; Reinhold-Hurek, B.; Nowak, J.; Sessitsch, A. Comparative Genome Analysis of Burkholderia phytofirmans PsJN Reveals a Wide Spectrum of Endophytic Lifestyles Based on Interaction Strategies with Host Plants. Front. Plant Sci. 2013, 4, 120. [Google Scholar] [CrossRef] [PubMed]
- Gkarmiri, K.; Mahmood, S.; Ekblad, A.; Alström, S.; Högberg, N.; Finlay, R. Identifying the Active Microbiome Associated with Roots and Rhizosphere Soil of Oilseed Rape. Appl. Environ. Microbiol. 2017, 83, e01938-17. [Google Scholar] [CrossRef]
- Floc’h, J.-B.; Hamel, C.; Harker, K.N.; St-Arnaud, M. Fungal Communities of the Canola Rhizosphere: Keystone Species and Substantial Between-Year Variation of the Rhizosphere Microbiome. Microb. Ecol. 2020, 80, 762–777. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Bazghaleh, N.; Vail, S.; Mamet, S.D.; Siciliano, S.D.; Helgason, B. Root and Rhizosphere Fungi Associated with the Yield of Diverse Brassica napus Genotypes. Rhizosphere 2023, 25, 100677. [Google Scholar] [CrossRef]
- Chen, Y.; Zhou, Q.; Strelkov, S.E.; Hwang, S.-F. Genetic Diversity and Aggressiveness of Fusarium spp. Isolated from Canola in Alberta, Canada. Plant Dis. 2014, 98, 727–738. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, M. Fusarium Populations in Roots of Oilseed and Pulse Crops Grown in Eastern Saskatchewan. Can. J. Plant Sci. 2023, 87, 945–952. [Google Scholar] [CrossRef]
- Lay, C.-Y.; Bell, T.H.; Hamel, C.; Harker, K.N.; Mohr, R.; Greer, C.W.; Yergeau, É.; St-Arnaud, M. Canola Root–Associated Microbiomes in the Canadian Prairies. Front. Microbiol. 2018, 9, 1188. [Google Scholar] [CrossRef]
- Town, J.R.; Dumonceaux, T.; Tidemann, B.; Helgason, B.L. Crop Rotation Significantly Influences the Composition of Soil, Rhizosphere, and Root Microbiota in Canola (Brassica napus L.). Environ. Microbiome 2023, 18, 40. [Google Scholar] [CrossRef]
- Boller, T.; He, S.Y. Innate Immunity in Plants: An Arms Race Between Pattern Recognition Receptors in Plants and Effectors in Microbial Pathogens. Science 2009, 324, 742–744. [Google Scholar] [CrossRef] [PubMed]
- Cordero-Elvia, J.; de Freitas, J.R.; Germida, J.J. Bacterial Microbiome Associated with the Rhizosphere and Root Interior of Crops in Saskatchewan, Canada. Can. J. Microbiol. 2020, 66, 71–85. [Google Scholar] [CrossRef] [PubMed]
- Cordero-Elvia, J.; de Freitas, J.R.; Germida, J.J. Bacterial Microbiomes Associated with the Rhizosphere, Root Interior, and Aboveground Plant Organs of Wheat and Canola at Different Growth Stages. Phytobiomes J. 2021, 5, 442–451. [Google Scholar] [CrossRef]
- Taye, Z.M.; Helgason, B.L.; Bell, J.K.; Norris, C.E.; Vail, S.; Robinson, S.J.; Parkin, I.A.P.; Arcand, M.; Mamet, S.; Links, M.G.; et al. Core and Differentially Abundant Bacterial Taxa in the Rhizosphere of Field Grown Brassica Napus Genotypes: Implications for Canola Breeding. Front. Microbiol. 2020, 10, 3007. [Google Scholar] [CrossRef] [PubMed]
- Misko, A.L.; Germida, J.J. Taxonomic and Functional Diversity of Pseudomonads Isolated from the Roots of Field-Grown Canola. FEMS Microbiol. Ecol. 2002, 42, 399–407. [Google Scholar] [CrossRef]
- Belimov, A.A.; Safronova, V.I.; Sergeyeva, T.A.; Egorova, T.N.; Matveyeva, V.A.; Tsyganov, V.E.; Borisov, A.Y.; Tikhonovich, I.A.; Kluge, C.; Preisfeld, A.; et al. Characterization of Plant Growth Promoting Rhizobacteria Isolated from Polluted Soils and Containing 1-Aminocyclopropane-1-Carboxylate Deaminase. Can. J. Microbiol. 2001, 47, 642–652. [Google Scholar] [CrossRef]
- Walitang, D.I.; Kim, K.; Madhaiyan, M.; Kim, Y.K.; Kang, Y.; Sa, T. Characterizing Endophytic Competence and Plant Growth Promotion of Bacterial Endophytes Inhabiting the Seed Endosphere of Rice. BMC Microbiol. 2017, 17, 209. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Kour, D.; Yadav, A.N.; Saxena, R.; Rai, P.K.; Jyoti, A.; Tomar, R.S. Biodiversity of Methylotrophic Microbial Communities and Their Potential Role in Mitigation of Abiotic Stresses in Plants. Biologia 2019, 74, 287–308. [Google Scholar] [CrossRef]
- Rangjaroen, C.; Sungthong, R.; Rerkasem, B.; Teaumroong, N.; Noisangiam, R.; Lumyong, S. Untapped Endophytic Colonization and Plant Growth-Promoting Potential of the Genus Novosphingobium to Optimize Rice Cultivation. Microbes Environ. 2017, 32, 84–87. [Google Scholar] [CrossRef]
- Saghafi, D.; Ghorbanpour, M.; Lajayer, B.A. Efficiency of Rhizobium Strains as Plant Growth Promoting Rhizobacteria on Morpho-Physiological Properties of Brassica napus L. under Salinity Stress. J. Soil Sci. Plant Nutr. 2018, 18, 253–268. [Google Scholar] [CrossRef]
- Noel, T.C.; Sheng, C.; Yost, C.K.; Pharis, R.P.; Hynes, M.F. Rhizobium leguminosarum as a Plant Growth-Promoting Rhizobacterium: Direct Growth Promotion of Canola and Lettuce. Can. J. Microbiol. 1996, 42, 279–283. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Qiu, L.; Zhang, Z.; Liu, K.; Xia, X.; Xiong, S.; Zhao, S.; Zhao, Z.; Hu, Y.; Liang, Y. Control of Streptomyces alfalfae XY25T Over Clubroot Disease and Its Effect on Rhizosphere Microbial Community in Chinese Cabbage Field Trials. Front. Microbiol. 2023, 12, 641556. [Google Scholar] [CrossRef] [PubMed]
- Shakeel, Q.; Lyu, A.; Zhang, J.; Wu, M.; Chen, S.; Chen, W.; Li, G.; Yang, L. Optimization of the Cultural Medium and Conditions for Production of Antifungal Substances by Streptomyces platensis 3-10 and Evaluation of Its Efficacy in Suppression of Clubroot Disease (Plasmodiophora brassicae) of Oilseed Rape. Biol. Control 2016, 101, 59–68. [Google Scholar] [CrossRef]
- Soil Classification Working Group. The Canadian System of Soil Classification; Agriculture and Agri-Food Canada Publication: Ottawa, ON, Canada, 1998; ISBN 0-660-17404-9. [Google Scholar]
- Kwak, M.-J.; Kong, H.G.; Choi, K.; Kwon, S.-K.; Song, J.Y.; Lee, J.; Lee, P.A.; Choi, S.Y.; Seo, M.; Lee, H.J.; et al. Rhizosphere Microbiome Structure Alters to Enable Wilt Resistance in Tomato. Nat. Biotechnol. 2018, 36, 1100–1109. [Google Scholar] [CrossRef] [PubMed]
- Siciliano, S.D.; Theoret, C.M.; de Freitas, J.R.; Hucl, P.J.; Germida, J.J. Differences in the Microbial Communities Associated with the Roots of Different Cultivars of Canola and Wheat. Can. J. Microbiol. 1998, 44, 844–851. [Google Scholar] [CrossRef]
- Herlemann, D.P.; Labrenz, M.; Jürgens, K.; Bertilsson, S.; Waniek, J.J.; Andersson, A.F. Transitions in Bacterial Communities along the 2000 Km Salinity Gradient of the Baltic Sea. ISME J. 2011, 5, 1571–1579. [Google Scholar] [CrossRef] [PubMed]
- Manter, D.K.; Vivanco, J.M. Use of the ITS Primers, ITS1F and ITS4, to Characterize Fungal Abundance and Diversity in Mixed-Template Samples by qPCR and Length Heterogeneity Analysis. J. Microbiol. Methods 2007, 71, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, Interactive, Scalable and Extensible Microbiome Data Science Using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef] [PubMed]
- RStudio Team. RStudio: Integrated Development for R; RStudio, PBC: Boston, MA, USA, 2020; Available online: http://www.rstudio.com/ (accessed on 21 March 2022).
- Zhou, Q. Comparative Transcriptome Analysis of Rutabaga (Brassica napus) Cultivars in Response to Plasmodiophora brassicae. Available online: https://era.library.ualberta.ca/items/6ec878a1-5585-46b4-a75d-b3092e4a7589 (accessed on 19 October 2023).
- Kuginuki, Y.; Yoshikawa, H.; Hirai, M. Variation in Virulence of Plasmodiophora brassicae in Japan Tested with Clubroot-Resistant Cultivars of Chinese Cabbage (Brassica rapa L. ssp. Pekinensis). Eur. J. Plant Pathol. 1999, 105, 327–332. [Google Scholar] [CrossRef]


| Factors | Fungi | Bacteria | ||||||
|---|---|---|---|---|---|---|---|---|
| Rhizosphere | Root | Rhizosphere | Root | |||||
| Isolate A | Isolate B | Isolate A | Isolate B | Isolate A | Isolate B | Isolate A | Isolate B | |
| Canola line | 0.49 | 0.54 | 0.36 | 0.33 | 0.06 | 0.22 | 0.15 | 0.06 |
| Clubroot inoculation | 0.17 | 0.05 | 0.03 | 0.01 | 0.08 | 0.09 | 0.0002 | 0.0002 |
| Sampling time | 0.0002 | 0.0002 | 0.0002 | 0.0002 | 0.0002 | 0.0002 | 0.0002 | 0.0002 |
| Canola line × Clubroot inoculation | 0.36 | 0.78 | 0.08 | 0.2 | 0.29 | 0.48 | 0.0004 | 0.01 |
| Canola line × Sampling time | 0.37 | 0.18 | 0.08 | 0.06 | 0.03 | 0.04 | 0.1 | 0.1 |
| Clubroot inoculation × Sampling time | 0.08 | 0.006 | 0.001 | 0.0002 | 0.04 | 0.04 | 0.0002 | 0.0002 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cordero-Elvia, J.; Galindo-González, L.; Fredua-Agyeman, R.; Hwang, S.-F.; Strelkov, S.E. Clubroot-Induced Changes in the Root and Rhizosphere Microbiome of Susceptible and Resistant Canola. Plants 2024, 13, 1880. https://doi.org/10.3390/plants13131880
Cordero-Elvia J, Galindo-González L, Fredua-Agyeman R, Hwang S-F, Strelkov SE. Clubroot-Induced Changes in the Root and Rhizosphere Microbiome of Susceptible and Resistant Canola. Plants. 2024; 13(13):1880. https://doi.org/10.3390/plants13131880
Chicago/Turabian StyleCordero-Elvia, Jorge, Leonardo Galindo-González, Rudolph Fredua-Agyeman, Sheau-Fang Hwang, and Stephen E. Strelkov. 2024. "Clubroot-Induced Changes in the Root and Rhizosphere Microbiome of Susceptible and Resistant Canola" Plants 13, no. 13: 1880. https://doi.org/10.3390/plants13131880
APA StyleCordero-Elvia, J., Galindo-González, L., Fredua-Agyeman, R., Hwang, S.-F., & Strelkov, S. E. (2024). Clubroot-Induced Changes in the Root and Rhizosphere Microbiome of Susceptible and Resistant Canola. Plants, 13(13), 1880. https://doi.org/10.3390/plants13131880





