The Identification of Kabatiella zeae as a Causal Agent of Northern Anthracnose of Sorghum in China and Estimation of Host Resistance
Abstract
1. Introduction
2. Results
2.1. Identification of the Pathogen
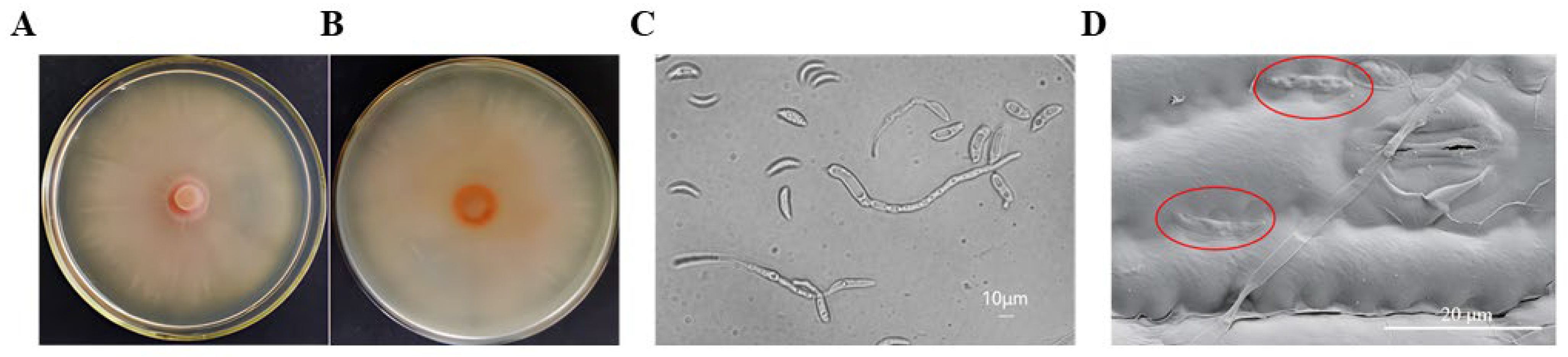
2.1.1. Morphology Identification
2.1.2. Molecular Identification
2.1.3. Pathogenicity
2.2. Biological Characteristics
2.2.1. Effects of Temperature Conditions on Kabatiella zeae Mycelium Growth
2.2.2. Effects of Light Conditions on Kabatiella zeae Mycelium Growth
2.2.3. Effects of Culture Medium on Kabatiella zeae Mycelium Growth
2.2.4. Effects of Carbon and Nitrogen Sources on Kabatiella zeae Mycelium Growth
2.3. Identification of Sorghum Germplasm Resources Resistant to Northern Anthracnose
2.4. Screening of Indoor Pesticides
3. Discussion
4. Materials and Methods
4.1. Sample Collection, Isolation, and Preservation of the Pathogen
4.2. Identification of the Pathogen
4.2.1. Morphological Observation
4.2.2. Molecular Biological Identification
4.2.3. Pathogenicity Determination
4.3. Study on Biological Characteristics
4.3.1. Effects of Temperature Conditions on Kabatiella zeae Mycelium Growth
4.3.2. Effects of Light Conditions on Kabatiella zeae Mycelium Growth
4.3.3. Effects of Culture Medium on Kabatiella zeae Mycelium Growth
4.3.4. Effects of Carbon and Nitrogen Sources on Mycelium Growth
4.4. Evaluation of Disease Resistance of Germplasm Resources
4.4.1. Evaluation of Sorghum Germplasm Resources
4.4.2. Preparation of Inoculum
4.4.3. Inoculation Method
4.5. Indoor Screening of Fungicides
4.5.1. Test Reagent
4.5.2. Screening Method
4.6. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ahn, E.; Fan, F.; Magill, C. Effect of leaf age, leaf segments and surface treatments on pathogenicity levels of Colletotrichum sublineola in sorghum and johnson grass. Crops 2021, 1, 111–117. [Google Scholar] [CrossRef]
- Yan, P.; Song, Y.H.; Zhang, K.Y.; Zhang, F.; Tang, Y.J.; Zhao, X.N.; Wang, N.; Ke, F.L.; Gao, F.J.; Li, J.H.; et al. Interaction of genotype-ecological type-plant spacing configuration in sorghum [Sorghum bicolor (L.) Moench] in China. Front. Plant Sci. 2023, 13, 1076854. [Google Scholar] [CrossRef] [PubMed]
- Narita, T.; Hiratsuka, Y. Studies on Kabatiella zeae n. sp., the causal fungus of a new leaf spot disease of corn. Jpn. J. Phytopathol. 1959, 24, 147–153. [Google Scholar] [CrossRef]
- Qi, P.K.; Bai, J.K.; Zhu, G.X. Fungal Diseases of Cultivated Plants in Jilin Province; Science Publishing House: Beijing, China, 1966. (In Chinese) [Google Scholar]
- Mueller, D.S.; Wise, K.A.; Sisson, A.J.; Allen, T.W.; Bergstrom, G.C.; Bosley, D.B.; Bradley, C.A.; Broders, K.D.; Byamukama, E.; Chilvers, M.I.; et al. Corn Yield Loss Estimates Due to Diseases in the United States and Ontario, Canada from 2012 to 2015. Plant Health Prog. 2016, 17, 211–222. [Google Scholar] [CrossRef]
- Chen, N.; Xiao, S.; Sun, J.; He, L.; Liu, M.; Gao, W.; Xu, J.; Wang, H.; Huang, S.; Xue, C. Virulence and molecular diversity in the Kabatiella zeae population causing maize eyespot in China. Plant Dis. 2020, 104, 3197–3206. [Google Scholar] [CrossRef]
- Reifschneider, F.J.B.; Arny, D.C. Host range of Kabatiella zeae, causal agent of eyespot of maize. Phytopathology 1979, 70, 485–487. [Google Scholar] [CrossRef]
- Reifschneider, F.J.B.; Arny, D.C. Yield loss of maize caused by Kabatiella zeae. Phytopathology 1983, 73, 607–609. [Google Scholar] [CrossRef]
- Chinchilla-Lopez, C.M. Pathogen Survival and Host Resistance in the Eyespot Disease of Maize Caused by Kabatiella zeae Narita and Hiratsuka. Ph.D. Thesis, Iowa State University, Ames, IA, USA, 1985. [Google Scholar]
- Dingley, J.M. ‘Eye spot’ disease of maize in New Zealand. N. Z. J. Agric. Res. 1973, 16, 325–328. [Google Scholar] [CrossRef]
- Arny, D.C.; Smalley, E.B.; Ullstrup, A.J.; Worf, G.L.; Ahrens, R.W. Eyespot of Maize, a disease New to North America. Phytopathology 1970, 61, 54–57. [Google Scholar] [CrossRef]
- Koima, I.N.; Kilalo, D.C.; Orek, C.O.; Wagacha, J.M.; Nyaboga, E.N. Identification and characterization of Colletotrichum species causing sorghum anthracnose in Kenya and screening of sorghum germplasm for resistance to anthracnose. J. Fungi 2023, 9, 100. [Google Scholar] [CrossRef]
- Wei, G.; Zhao, W.; Hu, A.; Ren, M.; Huang, Y.; Xu, H. Identification of a New Pathogenic fungi Causing Sorghum Leaf Spot Disease and Its Management Using Natural Product and Microorganisms. Microorganisms 2023, 11, 1431. [Google Scholar] [CrossRef] [PubMed]
- Tong, L.; Liu, Z.; Cui, J.; Hu, J.; Zhang, R.Y. First report of leaf blight caused by Setosphaeria turcica on sweet sorghum (Sorghum bicolor) in China. Plant Dis. 2017, 101, 1952. [Google Scholar] [CrossRef]
- Meng, L.M.; Su, Q.F.; Jia, J.; Zhang, W.; Li, H.; Jin, Q.M. Identification of the pathogen of corn northern anthrax and its growth affecting factors. J. Maize Sci. 2016, 24, 160–165. [Google Scholar]
- Xu, J.; Qin, P.W.; Jiang, Y.; Hu, L.; Liu, K.J.; Xu, X.D. Evaluation of sorghum germplasm resistance to anthracnose by Colletotrichum sublineolum in China. Crop Prot. 2020, 134, 105173. [Google Scholar] [CrossRef]
- Sierra-Orozco, E.; Sandoya, G.; Lee, S.; Vallad, G.; Hutton, S. Need for disease resistance breeding against Corynespora cassiicola in crops. Front. Agron. 2023, 5, 1275906. [Google Scholar] [CrossRef]
- Williams, R.J.; Frederiksen, R.A.; Girard, J.C. Sorghum and Pearl Millet Disease Identification Handbook; International Crops Research Institute: College Station, TX, USA, 1978. [Google Scholar]
- Das, I.K.; Rakshit, S.; Patil, J.V. Assessment of artificial inoculation methods for development of sorghum pokkah boeng caused by Fusarium subglutinans. Crop Prot. 2015, 77, 94–101. [Google Scholar] [CrossRef]
- Birr, T.; Tillessen, A.; Verreet, J.A.; Hasler, M.; Klink, H. Efficacy of different fungicide spraying techniques on the infestation with Kabatiella zeae and formation of Fusarium Mycotoxins in Forage Maize. Agriculture 2023, 13, 1269. [Google Scholar] [CrossRef]
- Xu, J.; Hu, L.; Jiang, Y.; Jiang, Y.; Liu, K.; Yan, J.; Xu, X. Pathogenic variability of isolates of Colletotrichum sublineola on Sorghum in China. Physiol. Mol. Plant P 2023, 126, 102046. [Google Scholar] [CrossRef]
- Healey, A.; Furtado, A.; Cooper, T.; Henry, R.J. Protocol: A simple method for extracting next-generation sequencing quality genomic DNA from recalcitrant plant species. Plant Methods 2014, 10, 21. [Google Scholar] [CrossRef]
- Yang, R.H.; Su, J.H.; Shang, J.J.; Wu, Y.Y.; Li, Y.; Bao, D.P.; Yao, Y.J. Evaluation of the ribosomal DNA internal transcribed spacer (ITS), specifically ITS1 and ITS2, for the analysis of fungal diversity by deep sequencing. PLoS ONE 2018, 13, e0206428. [Google Scholar] [CrossRef]
- Xu, X.D.; Dong, H.Y.; Jiang, Y.; Qiao, Y.; Liu, Z.H.; Sun, D.J. Preliminary studies on the northern anthracnose disease in maize. J. Shenyang Agric. Univ. 2000, 31, 507–510. [Google Scholar]
- Youssef, K.; Roberto, S.R. Salt strategies to control Botrytis mold of ‘Benitaka’ table grapes and to maintain fruit quality during storage. Postharvest Biol. Technol. 2014, 95, 95–102. [Google Scholar] [CrossRef]







| Rating Scale | Resistance Evaluation | Description of Disease Symptom |
|---|---|---|
| 1 | HR | Only a few chlorotic flecks were present on the inoculated leaves, but no acervuli were visible. Lesions covered less than 5% of the leaf surface. |
| 3 | R | Some circular to oval spots and acervuli development were observed. Lesions covered 5.1–20% of the leaf surface. |
| 5 | MR | Abundant circular to oval spots on inoculated leaves and black acervuli development were observed. Lesions covered 20.1–40% of the leaf surface. |
| 7 | S | The leaf area was found to be covered with coalescing lesions with black acervuli. Lesions covered 40.1–70% of the leaf surface. |
| 9 | HS | Lesions joined to cover a large proportion of the leaf surface with abundant black acervuli. The whole plants were almost dead. Lesions covered more than 70.1% of the leaf surface. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, W.; Wang, Y.; Hu, L.; Xu, J.; Yan, J.; Cao, P.; Liu, C.; Shi, X.; Liu, C.; Jiang, Y.; et al. The Identification of Kabatiella zeae as a Causal Agent of Northern Anthracnose of Sorghum in China and Estimation of Host Resistance. Plants 2024, 13, 1857. https://doi.org/10.3390/plants13131857
Yu W, Wang Y, Hu L, Xu J, Yan J, Cao P, Liu C, Shi X, Liu C, Jiang Y, et al. The Identification of Kabatiella zeae as a Causal Agent of Northern Anthracnose of Sorghum in China and Estimation of Host Resistance. Plants. 2024; 13(13):1857. https://doi.org/10.3390/plants13131857
Chicago/Turabian StyleYu, Wenbo, Yu Wang, Lan Hu, Jing Xu, Jichen Yan, Peng Cao, Chunjuan Liu, Xiaolong Shi, Chang Liu, Yu Jiang, and et al. 2024. "The Identification of Kabatiella zeae as a Causal Agent of Northern Anthracnose of Sorghum in China and Estimation of Host Resistance" Plants 13, no. 13: 1857. https://doi.org/10.3390/plants13131857
APA StyleYu, W., Wang, Y., Hu, L., Xu, J., Yan, J., Cao, P., Liu, C., Shi, X., Liu, C., Jiang, Y., & Zhou, Y. (2024). The Identification of Kabatiella zeae as a Causal Agent of Northern Anthracnose of Sorghum in China and Estimation of Host Resistance. Plants, 13(13), 1857. https://doi.org/10.3390/plants13131857







