Variation in Soluble Sugars in Arabica Coffee Cherry Fruits
Abstract
1. Introduction
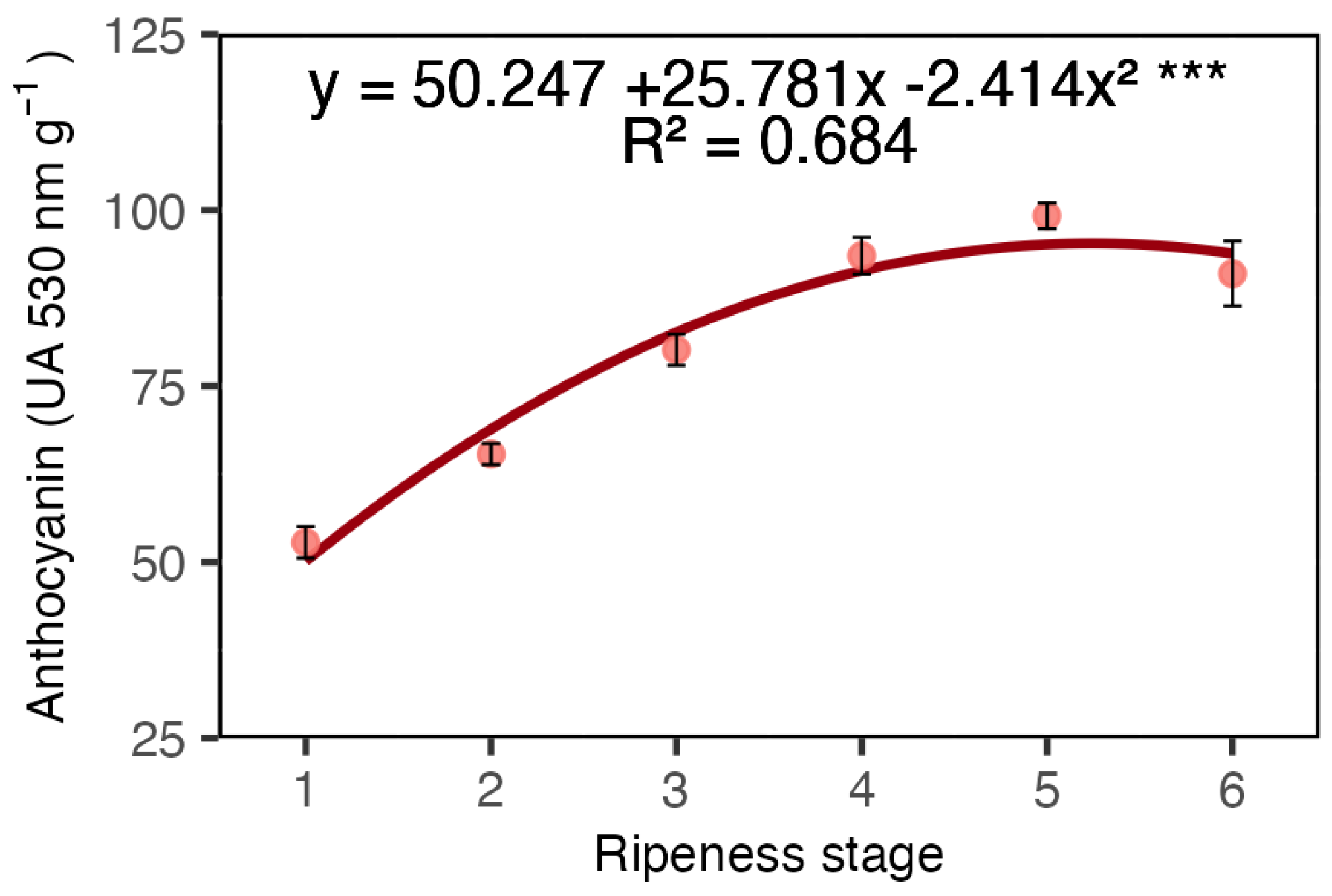
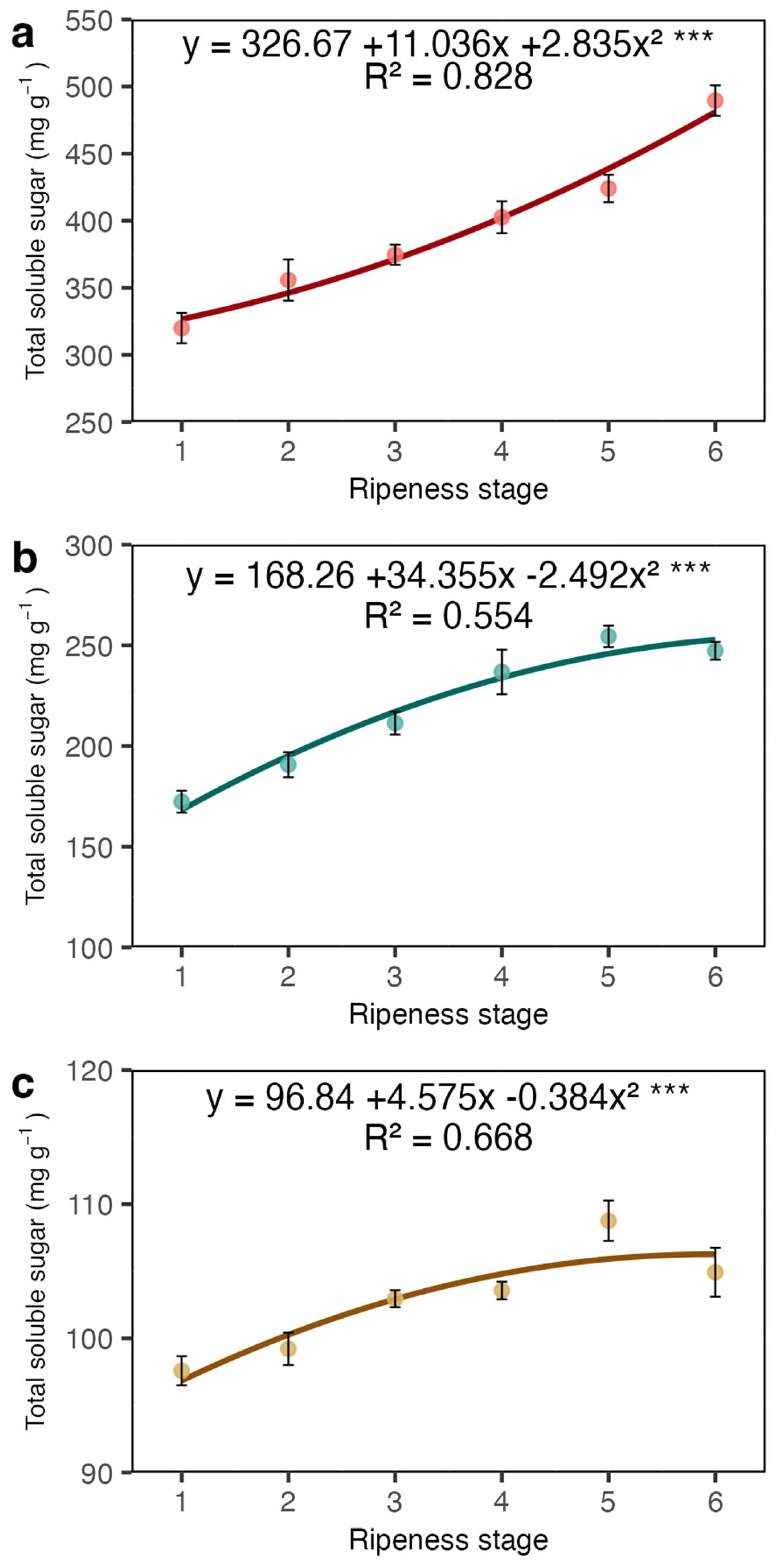
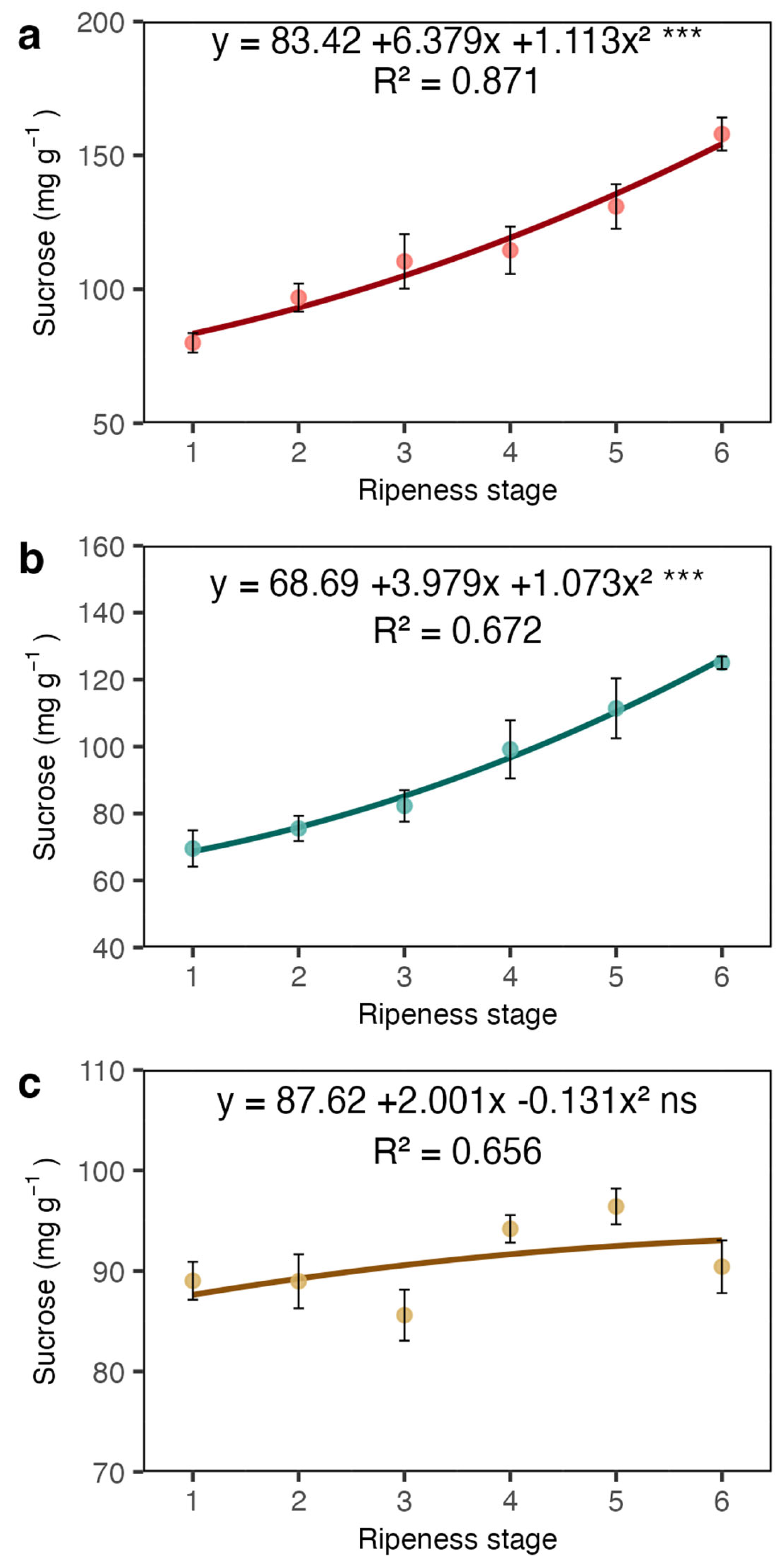
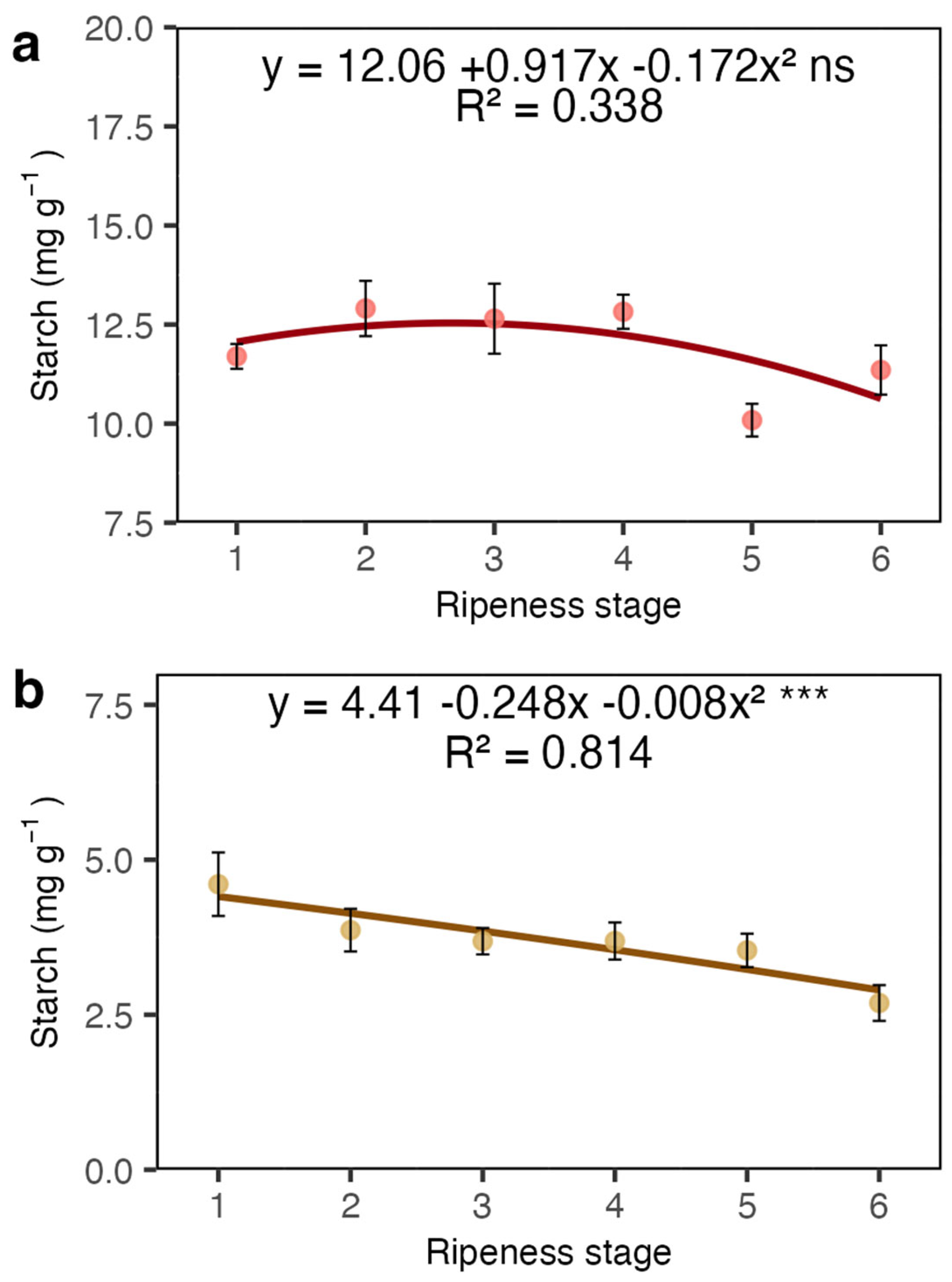
2. Results
3. Discussion
4. Materials and Methods
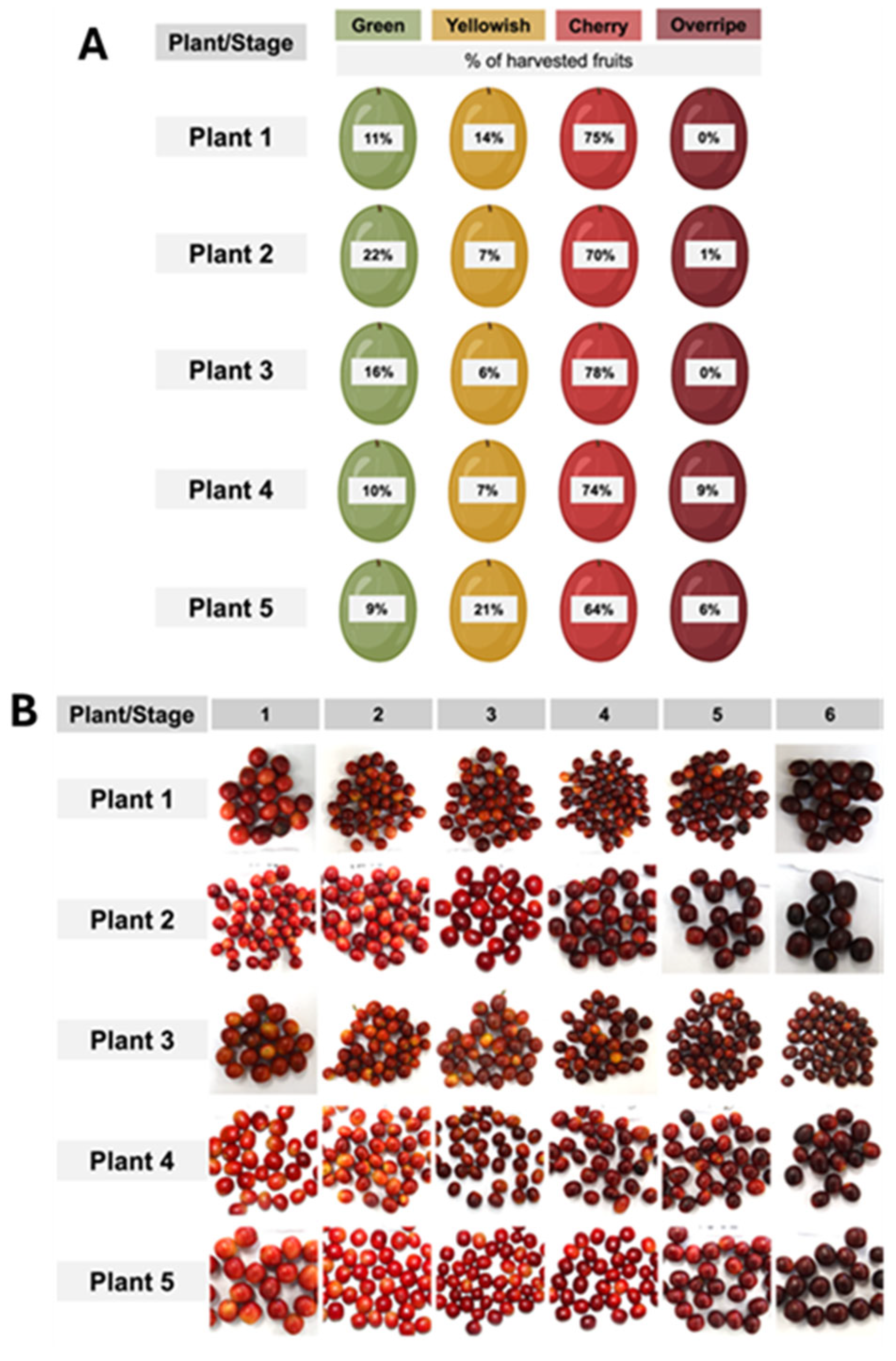
4.1. Experimental Design and Fruit Harvest
4.2. Fruit Classification by Colour
4.3. Sample Preparation and Analysis
4.4. Data Analysis
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Leroy, T.; Ribeyre, F.; Bertrand, B.; Charmetant, P.; Dufour, M.; Montagnon, C.; Marraccini, P.; Pot, D. Genetics of Coffee Quality. Braz. J. Plant Physiol. 2006, 18, 229–242. [Google Scholar] [CrossRef]
- Vaast, P.; Bertrand, B.; Perriot, J.J.; Guyot, B.; Génard, M. Fruit Thinning and Shade Improve Bean Characteristics and Beverage Quality of Coffee (Coffea arabica L.) under Optimal Conditions. J. Sci. Food Agric. 2006, 86, 197–204. [Google Scholar] [CrossRef]
- Ribeiro, D.E.; Borem, F.M.; Cirillo, M.A.; Vilela, M.B.P.; Ferraz, V.P.; Alves, H.M.R.; Taveira, J.H.d.S. Interaction of Genotype, Environment and Processing in the Chemical Composition Expression and Sensorial Quality of Arabica Coffee. Afr. J. Agric. Res. 2016, 11, 2412–2422. [Google Scholar] [CrossRef]
- Mazzafera, P. Chemical Composition of Defective Coffee Beans. Food Chem. 1999, 64, 547–554. [Google Scholar] [CrossRef]
- Osorio Pérez, V.; Matallana Pérez, L.G.; Fernandez-Alduenda, M.R.; Alvarez Barreto, C.I.; Gallego Agudelo, C.P.; Montoya Restrepo, E.C. Chemical Composition and Sensory Quality of Coffee Fruits at Different Stages of Maturity. Agronomy 2023, 13, 341. [Google Scholar] [CrossRef]
- Borém, F.M.; Figueiredo, L.P.; Ribeiro, F.C.; Taveira, J.H.S.; Giomo, G.S.; Salva, T.J.G. The Relationship between Organic Acids, Sucrose and the Quality of Specialty Coffees. Afr. J. Agric. Res. 2016, 11, 709–717. [Google Scholar] [CrossRef]
- Kerler, J.; Winkel, C.; Davidek, T.; Blank, I. Basic Chemistry and Process Conditions for Reaction FLavours with Particular Focus on Maillard-Type Reactions. In Food Flavour Technology; Taylor, A.J., Linforth, R.S.T., Eds.; Blackwell: Hoboken, NJ, USA, 2010; pp. 51–88. ISBN 9781405185431. [Google Scholar]
- Mazzafera, P.; Schimpl, F.; Kiyota, E. Proteins of Coffee Beans: Recent. In Coffee: Production, Quality and Chemistry; Farah, A., Ed.; The Royal Society of Chemistry: London, UK, 2019; pp. 431–444. [Google Scholar]
- Geromel, C.; Ferreira, L.P.; Guerreiro, S.M.C.; Cavalari, A.A.; Pot, D.; Pereira, L.F.P.; Leroy, T.; Vieira, L.G.E.; Mazzafera, P.; Marraccini, P. Biochemical and Genomic Analysis of Sucrose Metabolism during Coffee (Coffea arabica) Fruit Development. J. Exp. Bot. 2006, 57, 3243–3258. [Google Scholar] [CrossRef]
- Clifford, M.N.; Kazi, T. The Influence of Coffee Bean Maturity on the Content of Chlorogenic Acids, Caffeine and Trigonelline. Food Chem. 1987, 26, 59–69. [Google Scholar] [CrossRef]
- Geromel, C.; Ferreira, L.P.; Davrieux, F.; Guyot, B.; Ribeyre, F.; Brígida dos Santos Scholz, M.; Protasio Pereira, L.F.; Vaast, P.; Pot, D.; Leroy, T.; et al. Effects of Shade on the Development and Sugar Metabolism of Coffee (Coffea arabica L.) Fruits. Plant Physiol. Biochem. 2008, 46, 569–579. [Google Scholar] [CrossRef]
- Cannell, M.G.R. Physiology of the Coffee Crop. In Coffee: Botany, Biochemistry and Production of Beans and Beverage; Springer: Boston, MA, USA, 1985; pp. 108–134. [Google Scholar]
- Trugo, L.C. Carbohydrates. In Coffee: Volume 1—Chemistry; Clarke, R.J., Macrae, R., Eds.; Springer: Dordrecht, The Netherlands, 1985; pp. 83–114. ISBN 978-94-010-8693-6. [Google Scholar]
- Rogers, W.J.; Michaux, S.; Bastin, M.; Bucheli, P. Changes to the Content of Sugars, Sugar Alcohols, Myo-Inositol, Carboxylic Acids and Inorganic Anions in Developing Grains from Different Varieties of Robusta (Coffea canephora) and Arabica (C. arabica) Coffees. Plant Sci. 1999, 149, 115–123. [Google Scholar] [CrossRef]
- Morais, H.; Caramori, P.H.; Koguishi, M.S.; de Arruda Ribeiro, A.M. Escala Fenológica Da Fase Reprodutiva de Coffea arabica. Bragantia 2008, 67, 257–260. [Google Scholar] [CrossRef]
- Pezzopane, J.R.M.; Pedro Júnior, M.J.; Thomaziello, R.A.; de Camargo, M.B.P. Coffee Phenological Stages Evaluation Scale. Bragantia 2003, 62, 499–505. [Google Scholar] [CrossRef]
- Li, Z.; Zhou, B.; Zheng, T.; Zhao, C.; Shen, X.; Wang, X.; Qiu, M.; Fan, J. Integrating Metabolomics and Proteomics Technologies Provides Insights into the Flavor Precursor Changes at Different Maturity Stages of Arabica Coffee Cherries. Foods 2023, 12, 1432. [Google Scholar] [CrossRef] [PubMed]
- Rogers, W.J.; Bézard, G.; Deshayes, A.; Meyer, I.; Pétiard, V.; Marraccini, P. Biochemical and Molecular Characterization and Expression of the 11S-Type Storage Protein from Coffea arabica Endosperm. Plant Physiol. Biochem. 1999, 37, 261–272. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, C.; Zhang, Y.; Zeng, W.; Cesarino, I. Coffee Cell Walls—Composition, Influence on Cup Quality and Opportunities for Coffee Improvements. Food Qual. Saf. 2021, 5, fyab012. [Google Scholar] [CrossRef]
- Cheng, B.; Furtado, A.; Henry, R.J. The Coffee Bean Transcriptome Explains the Accumulation of the Major Bean Components through Ripening. Sci. Rep. 2018, 8, 11414. [Google Scholar] [CrossRef] [PubMed]
- Cannell, M.G.R. Changes in the Respiration and Growth Rates of Developing Fruits of Coffea arabica L. J. Hortic. Sci. 1971, 46, 263–272. [Google Scholar] [CrossRef]
- Avallone, S.; Guiraud, J.P.; Guyot, B.; Olguin, E.; Brillouet, J.M. Polysaccharide Constituents of Coffee-Bean Mucilage. J. Food Sci. 2000, 65, 1308–1311. [Google Scholar] [CrossRef]
- Cação, S.M.B.; Leite, T.F.; Budzinski, I.G.F.; dos Santos, T.B.; Scholz, M.B.S.; Carpentieri-Pipolo, V.; Domingues, D.S.; Vieira, L.G.E.; Pereira, L.F.P. Gene Expression and Enzymatic Activity of Pectin Methylesterase during Fruit Development and Ripening in Coffea arabica L. Genet. Mol. Res. 2012, 11, 3186–3197. [Google Scholar] [CrossRef]
- Redgwell, R.; Fischer, M. Coffee Carbohydrates. Braz. J. Plant Physiol. 2006, 18, 165–174. [Google Scholar] [CrossRef]
- Munõz, M.; Roa, M.; Correa, R. Endocarp Analysis of a Traditional Variety of Colombian Coffee. Coffee Sci. 2019, 14, 206. [Google Scholar] [CrossRef]
- Ramírez-Martínez, A.; Salgado-Cervantes, M.A.; Rodríguez-Jimenes, G.C.; García-Alvarado, M.A.; Cherblanc, F.; Bénet, J.C. Water Transport in Parchment and Endosperm of Coffee Bean. J. Food Eng. 2013, 114, 375–383. [Google Scholar] [CrossRef]
- Cocking, E.C.; Yemm, E.W. Estimation of Amino Acids by Ninhydrin. Biochem. J. 1954, 58, xii. [Google Scholar]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Van Handel, E. Direct Microdetermination of Sucrose. Anal. Biochem. 1968, 22, 280–283. [Google Scholar] [CrossRef]
- Hughes, N.M.; Neufeld, H.S.; Burkey, K.O. Functional Role of Anthocyanins in High-light Winter Leaves of the Evergreen Herb Galax Urceolata. New Phytol. 2005, 168, 575–587. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baptistella, J.L.C.; Assoni, G.; da Silva, M.S.; Mazzafera, P. Variation in Soluble Sugars in Arabica Coffee Cherry Fruits. Plants 2024, 13, 1853. https://doi.org/10.3390/plants13131853
Baptistella JLC, Assoni G, da Silva MS, Mazzafera P. Variation in Soluble Sugars in Arabica Coffee Cherry Fruits. Plants. 2024; 13(13):1853. https://doi.org/10.3390/plants13131853
Chicago/Turabian StyleBaptistella, João Leonardo Corte, Giovane Assoni, Marcio Souza da Silva, and Paulo Mazzafera. 2024. "Variation in Soluble Sugars in Arabica Coffee Cherry Fruits" Plants 13, no. 13: 1853. https://doi.org/10.3390/plants13131853
APA StyleBaptistella, J. L. C., Assoni, G., da Silva, M. S., & Mazzafera, P. (2024). Variation in Soluble Sugars in Arabica Coffee Cherry Fruits. Plants, 13(13), 1853. https://doi.org/10.3390/plants13131853





