Diurnal High Temperatures Affect the Physiological Performance and Fruit Quality of Highbush Blueberry (Vaccinium corymbosum L.) cv. Legacy
Abstract
1. Introduction
2. Results
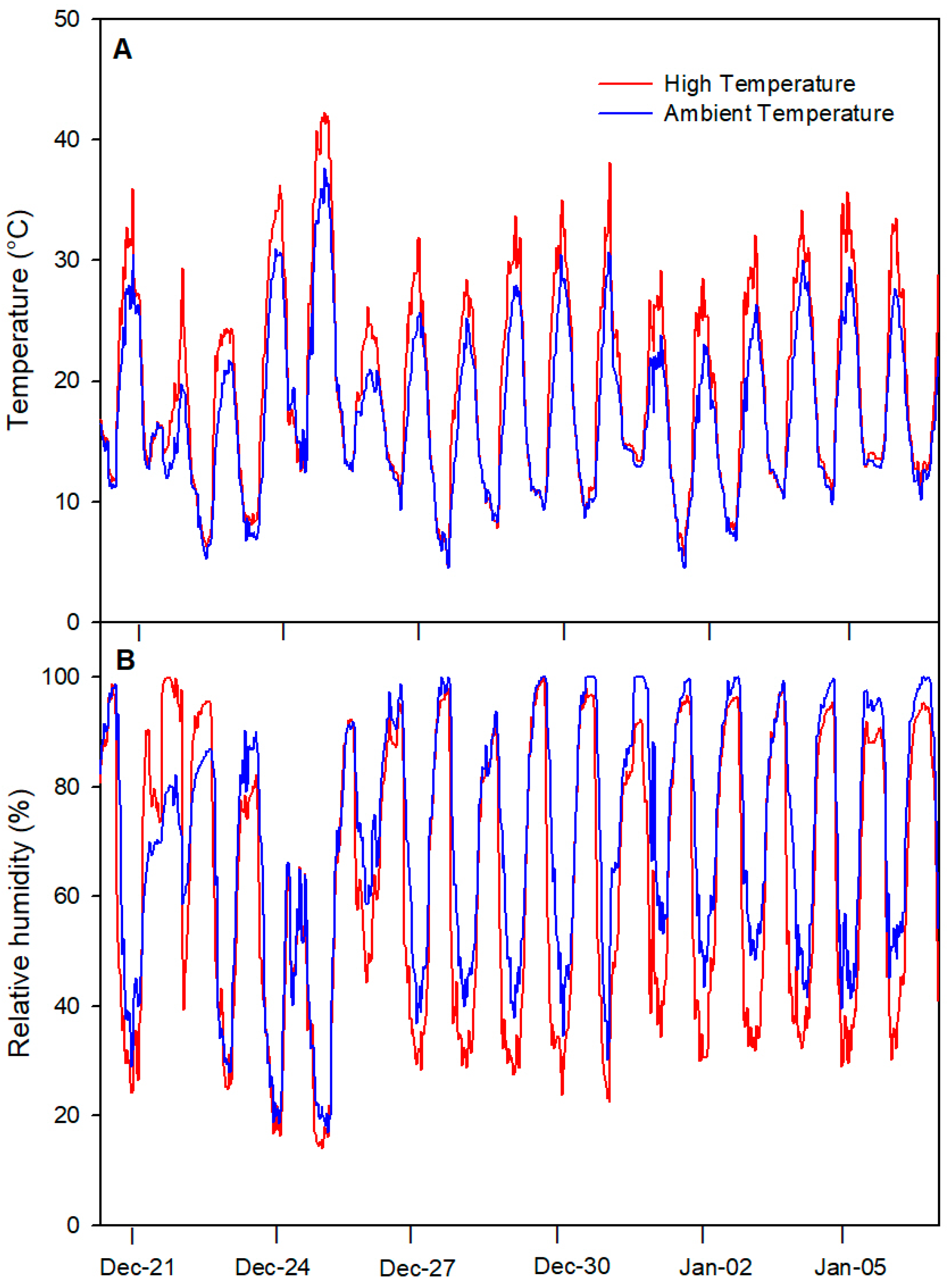
2.1. Environmental Conditions during the Experiment
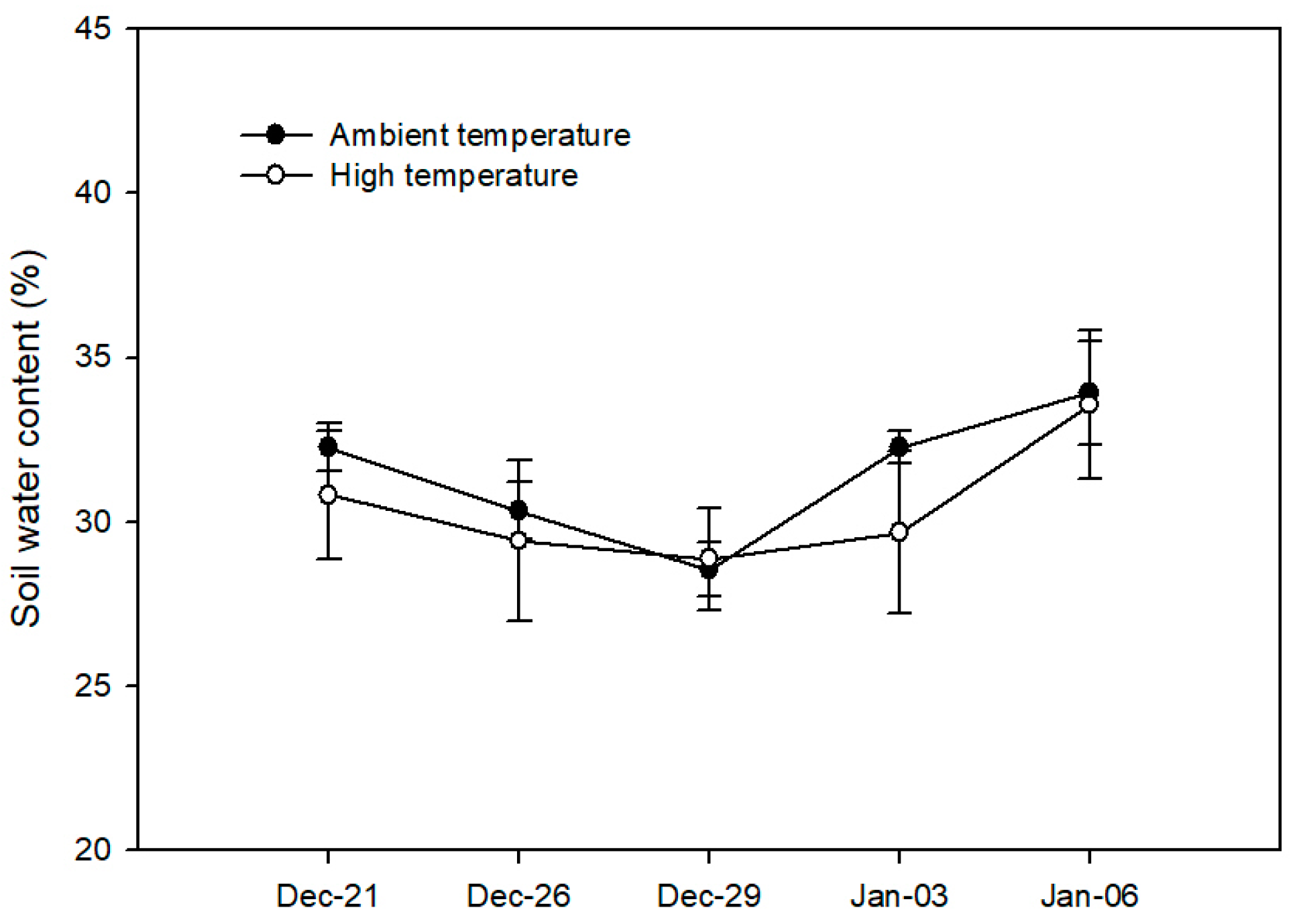
2.2. Soil Water Content, Plant Water Status, and Leaf Temperature
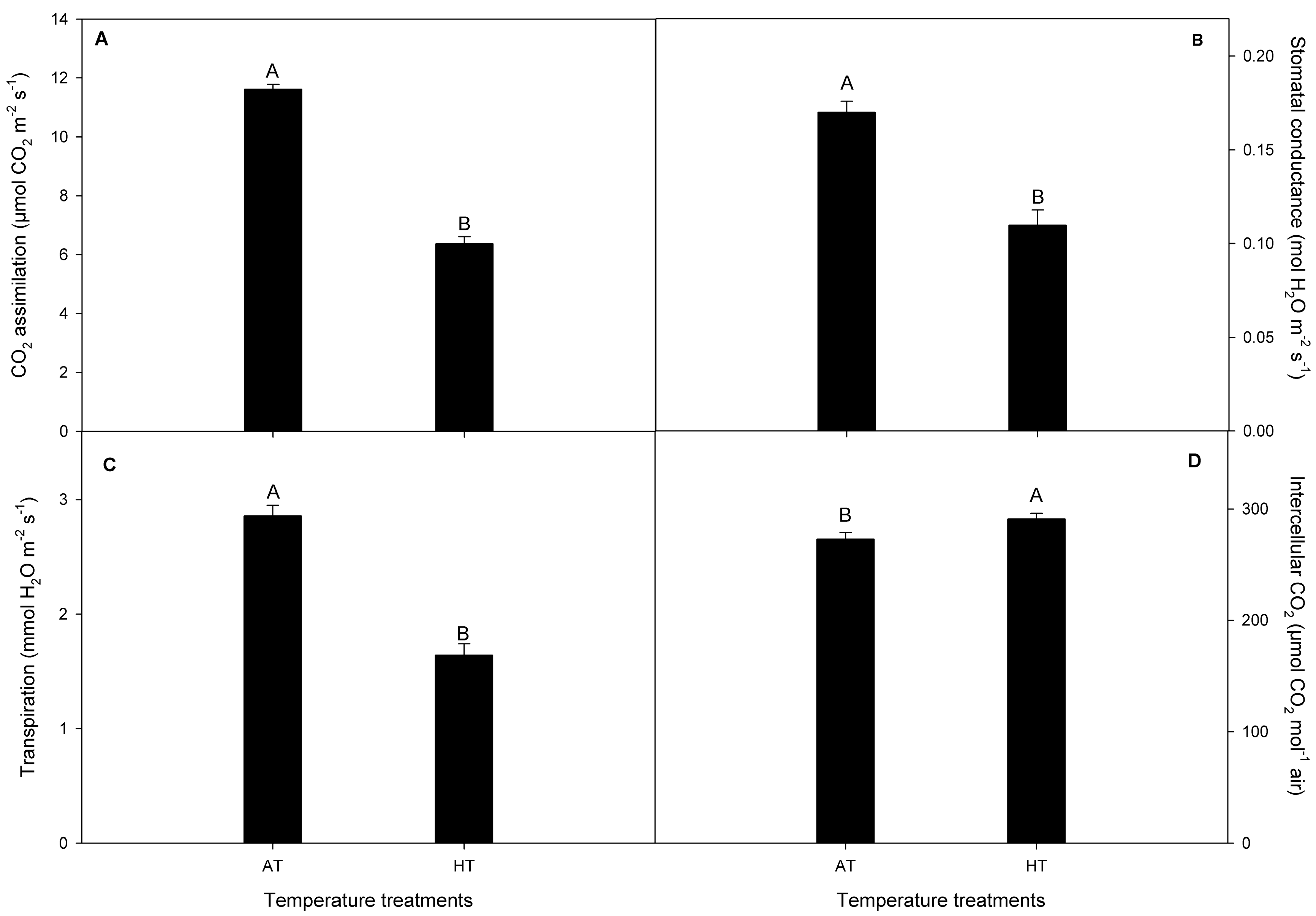
2.3. Gas-Exchange in V. corymbosum Plants
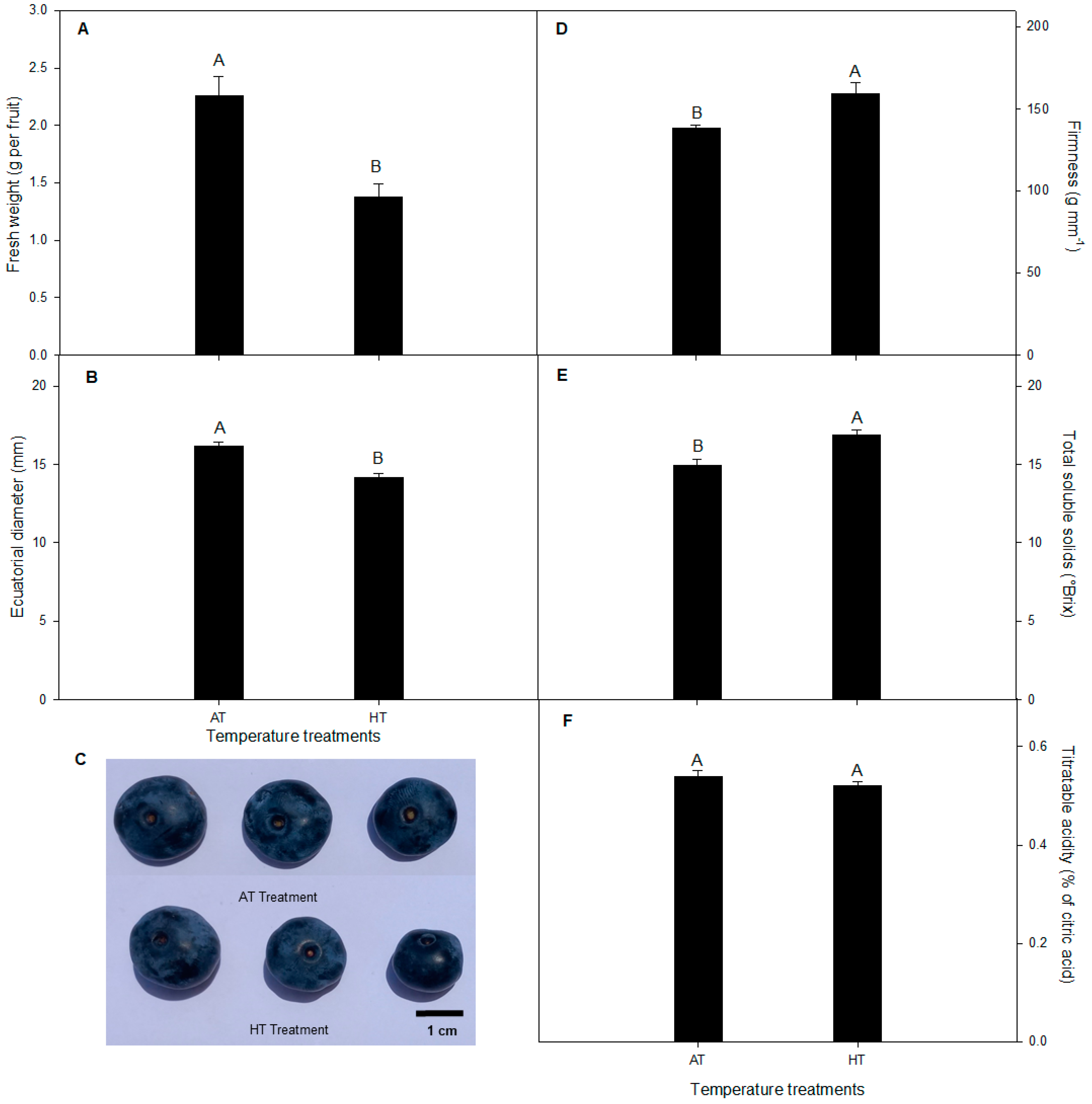
2.4. Fruit Quality
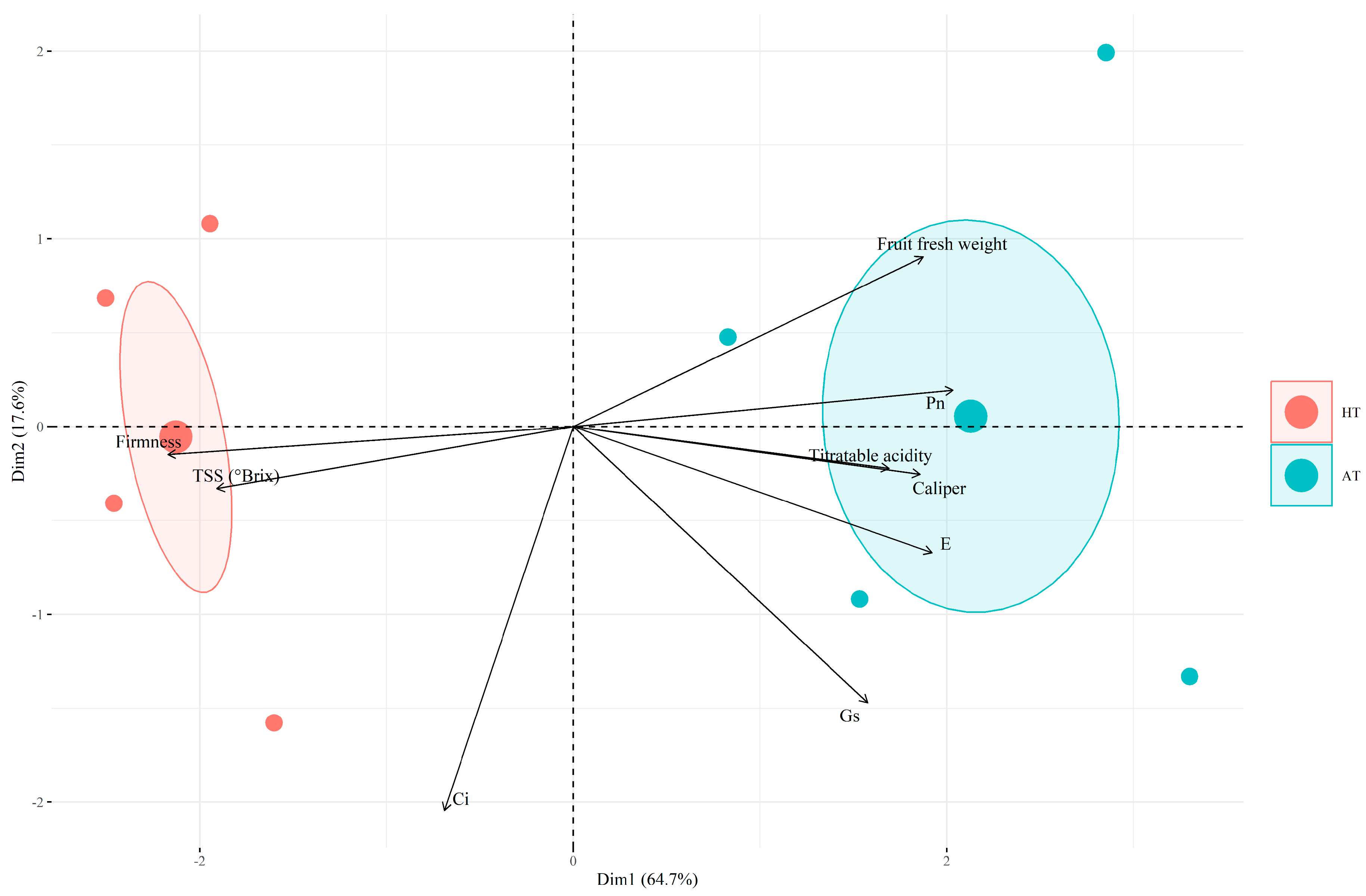
2.5. Multivariate Analysis Based on Physiological Responses and Quality Parameters Measured in V. corymbosum under Different Treatments
3. Discussion
3.1. Physiological Responses of V. corymbosum cv. Legacy Plants
3.2. Fruit Quality Changes in V. corymbosum cv. Legacy Plants
3.3. Principal Component Analysis (PCA)
4. Materials and Methods
4.1. Plant Material and Description of the Study Site
4.2. Treatments and Experimental Conditions
4.3. Soil Water Content and Plant Water Status
4.4. Leaf Temperature
4.5. Gas Exchange Measurement
4.6. Fruit Quality
4.7. Experimental Design and Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- IPCC. Climate Change 2014. Fifth Assessment Synthesis Report (Longer Report) of Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2014. [Google Scholar]
- IPCC. Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Masson-Delmotte, V., Zhai, P., Pirani, A., Connors, S.L., Péan, C., Berger, S., Caud, N., Chen, Y., Goldfarb, L., Gomis, M.I., et al., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2021. [Google Scholar]
- Siebert, S.; Ewert, F.; Rezaei, E.; Kage, H.; Grab, R. Impact of heat stress on crop yield-on the importance of considering canopy temperature. Environ. Res. Lett. 2014, 9, 044012. [Google Scholar] [CrossRef]
- Wang, L.; Ma, K.B.; Lu, Z.G.; Ren, S.X.; Jiang, H.R.; Cui, J.W.; Chen, G.; Teng, N.J.; Lam, H.M.; Jin, B. Differential physiological, transcriptomic and metabolomic responses of Arabidopsis leaves under prolonged warming and heat shock. BMC Plant Biol. 2020, 20, 86. [Google Scholar] [CrossRef] [PubMed]
- Sato, H.; Mizoi, J.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Complex plant responses to drought and heat stress under climate change. Plant J. 2024, 177, 1873–1892. [Google Scholar] [CrossRef] [PubMed]
- Ávila-Valdés, A.; Quinet, M.; Lutts, S.; Martínez, J.P.; Lizana, C. Tuber yield and quality responses of potato to moderate temperature increase during Tuber bulking under two water availability scenarios. Field Crops Res. 2020, 251, 107786. [Google Scholar] [CrossRef]
- Kim, J.; Slafer, G.; Savin, R. Are Are portable polyethylene tents reliable for imposing heat treatments in field-grown wheat? Field Crops Res. 2021, 271, 108206. [Google Scholar] [CrossRef]
- Kim, J.; Savin, R.; Slafer, G. Quantifying pre- and post-anthesis heat waves on grain number and grain weight of contrasting wheat cultivars. Field Crops Res. 2024, 307, 109264. [Google Scholar] [CrossRef]
- Rivero, R.; Mestre, T.; Mittler, R.; Rubio, F.; Garcia-Sánchez, F.; Martínez, V. The combined effect of salinity and heat reveales a specific physiological, biochemical and molecular response in tomato plants. Plant Cell Environ. 2014, 38, 1037–1258. [Google Scholar]
- Fahad, S.; Bajwa, A.A.; Nazir, U.; Anjum, S.A.; Farooq, A.; Zohaib, A.; Sadia, S.; Nasim, W.; Adkins, S.; Saud, S.; et al. Crop Production under Drought and Heat Stress: Plant Responses and Management Options. Front. Plant Sci. 2017, 8, 1147. [Google Scholar] [CrossRef]
- Djalovic, I.; Kundu, S.; Bahuguna, R.; Pareek, A.; Raza, A.; Singla-Pareek, S.; Prasad, P.; Varshney, R. Maize and heat stress: Physiological, genetic, and molecular insights. Plant Genome 2024, 17, e20378. [Google Scholar] [CrossRef]
- Cai, Y.; Tarin, M.; Fan, L.; Xie, D.; Rong, J.; He, T.; Chen, L.; Zheng, Y. Responses of photosynthesis, chloroplast ultrastructure, and antioxidant system of Morinda officinalis how. to exogenous 2, 4-epibrassinolide treatments under high temperature stress. Appl. Ecol. Environ. Res. 2020, 18, 3981–4004. [Google Scholar] [CrossRef]
- Lal, M.K.; Tiwari, R.K.; Gahlaut, V. Physiological and molecular insights on wheat responses to heat stress. Plant Cell Rep. 2022, 41, 501–518. [Google Scholar] [CrossRef] [PubMed]
- Zahra, N.; Hafeez, M.; Ghaffar, A.; Kausar, A.; Zeidi, M.; Siddique, K.; Farooq, M. Plant photosynthesis under heat stress: Effects and management. Environ. Exp. Bot. 2023, 206, 105178. [Google Scholar] [CrossRef]
- Pospíšil, P. Production of Reactive Oxygen Species by Photosystem II as a Response to Light and Temperature Stress. Front. Plant Sci. 2016, 7, 1950. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.-L.; Chen, J.-H.; He, N.-Y.; Guo, F.-Q. Metabolic reprogramming in chloroplasts under heat stress in plants. Int. J. Mol. Sci. 2018, 19, 849. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Min, L.; Ma, Y.; Zeeshan, M.; Jin, S.; Zhang, X. High-temperature stress in crops: Male sterility, yield loss and potential remedy approaches. Plant Biotechnol. J. 2023, 21, 680–697. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Jiang, F.; Niu, L.; Wang, G.; Yin, J.; Song, X.; Ottosen, C.; Rosenqvist, E.; Mittler, R.; Wu, Z.; et al. Synergistic regulation at physiological, transcriptional and metabolic levels in tomato plants subjected to a combination of salt and heat stress. Plant J. 2024, 177, 1656–1675. [Google Scholar] [CrossRef]
- Ribera, A.E.; Reyes-Diaz, M.; Alberdi, M.; Zuñiga, G.E.; Mora, M.L. Antioxidant compounds in skin and pulp of fruits change among genotypes and maturity stages in highbush blueberry (Vaccinium corymbosum L.) grown in southern Chile. J. Soil Sci. Plant Nutr. 2010, 10, 509–536. [Google Scholar] [CrossRef]
- Onuh, J.O.; Dawkins, N.L.; Aluko, R.E. Cardiovascular disease protective properties of blueberry polyphenols (Vaccinium corymbosum): A concise review. Food Prod. Process Nutr. 2023, 5, 27. [Google Scholar] [CrossRef]
- Shi, J.; Xiao, Y.; Jia, C.; Zhang, H.; Gan, Z.; Li, X.; Yang, M.; Yin, Y.; Zhang, G.; Hao, J.; et al. Physiological and biochemical changes during fruit maturation and ripening in highbush blueberry (Vaccinium corymbosum L.). Food Chem. 2023, 410, 135299. [Google Scholar]
- FAS-USDA. Blueberries around the Globe—Past, Present, and Future. 2021. Available online: https://www.fas.usda.gov/sites/default/files/2021-10/GlobalBlueberriesFinal_1.pdf (accessed on 8 April 2024).
- Lagos, L.O.; Souto, C.; Lillo-Saavedra, M. Daily crop evapotranspiration and diurnal dynamics of the surface energy balance of a drip-irrigated blueberry (Vaccinium corymbosum) orchard. Irrig. Sci. 2024, 42, 1–13. [Google Scholar] [CrossRef]
- INE. Censo Agropecuario y Forestal 2021; Instituto Nacional de Estadística (INE): Santiago, Chile, 2021; Available online: https://www.ine.cl/estadisticas/economia/agricultura-agroindustria-y-pesca/censos-agropecuarios (accessed on 5 March 2024).
- Hancock, J. Highbush blueberry breeding. Latvian J. Agron. 2009, 12, 35–38. [Google Scholar]
- Hao, L.; Guo, L.; Li, R.; Cheng, Y.; Huang, L.; Zhou, H.; Xu, M.; Li, F.; Zhang, X.; Zheng, Y. Responses of phosotynthesis to high temperature stress associated with changes in leaf structure and biochemistry of blueberry (Vaccinium corymbosum L.). Sci. Hortic. 2019, 246, 251–264. [Google Scholar] [CrossRef]
- Chen, W.; Cen, W.; Chen, L.; Di, L.; Li, Y.; Guo, W. Differential sensitivity of four highbush blueberry (Vaccinium corymbosum) cultivars to heat stress. Pak. J. Boyt. 2012, 44, 853–860. [Google Scholar]
- Estrada, F.; Escobar, A.; Romero-Bravo, S.; González-Talice, J.; Poblete-Echeverría, C.; Caligari, P.; Lobos, G.A. Fluorescence phenotyping in blueberrry breeding for genotype selection under drought conditions, with or with heat stress. Sci. Hortic. 2015, 181, 147–161. [Google Scholar] [CrossRef]
- Yang, F.-H.; Bryla, D.; Strik, B. Critical Temperatures and Heating Times for Fruit Damage in Northern Highbush Blueberry. HortScience 2019, 54, 2231–2239. [Google Scholar] [CrossRef]
- Flexas, J.; Medrano, H. Drought-inhibition of Photosynthesis in C3 Plants: Stomatal and Non-stomatal Limitations Revisited. Ann. Bot. 2002, 89, 183–189. [Google Scholar] [CrossRef]
- Zhou, J.; Jiang, X.; Agathokleous, E.; Lu, X.; Yang, Z.; Li, R. High temperature inhibits photosynthesis of chrysanthemum (Chrysanthemum morifolium Ramat.) seedlings more than relative humidity. Front. Plant Sci. 2023, 14, 1272013. [Google Scholar] [CrossRef]
- Chen, J.H.; Tang, M.; Jin, X.-Q.; Li, H.; Chen, L.-S.; Wang, Q.-L.; Sun, A.-Z.; Yi, Y.; Guo, F.-Q. Regulation of Calvin–Benson cycle enzymes under high temperature stress. aBIOTECH 2022, 3, 65–77. [Google Scholar] [CrossRef]
- Bryla, D.; Yorgey, B.; Shireman, A. Irrigation management effects on yield and fruit quality of highbush blueberry. Acta Hortic. 2009, 810, 649–656. [Google Scholar] [CrossRef]
- Faghih, S.; Zamani, Z.; Fatahi, R.; Omidi, M. Influence of kaolin application on most important fruit and leaf characteristics of two apple cultivars under sustained deficit irrigation. Biol. Res. 2021, 54, 1. [Google Scholar] [CrossRef]
- CIREN. Estudio Agrológico. Descripciones de suelos, materiales y símbolos IX Región. Centro de Información de Recursos Naturales. Publicación 2022, 122, 343. [Google Scholar]
- Begg, J.E.; Turner, N.C. Water potential gradients in field tobacco. Plant Physiol. 1970, 46, 343–346. [Google Scholar] [CrossRef] [PubMed]
- Barai, K.; Calderwood, L.; Wallhead, M.; Vanhanen, H.; Hall, B.; Drummond, F.; Zhang, Y.-J. High Variation in Yield among Wild Blueberry Genotypes: Can Yield Be Predicted by Leaf and Stem Functional Traits? Agronomy 2022, 12, 617. [Google Scholar] [CrossRef]
- Reyes-Díaz, M.; Meriño-Gergichevich, C.; Alarcón, E.; Alberdi, M.; Horst, W.J. Calcium sulfate ameliorates the effect of aluminum toxicity differentially in genotypes of highbush blueberry (Vaccinium corymbosum L.). J. Soil Sci. Plant Nutr. 2011, 11, 59–78. [Google Scholar] [CrossRef]
- Retamal-Salgado, J.; Batías, R.; Wilckens, R.; Paulino, L. Influence of microclimatic conditions under high tunnels on the physiological and productive responses in blueberry ‘O’Neal’. Chil. J. Agric. Res. 2015, 75, 291–297. [Google Scholar] [CrossRef]
- Mazzoni, L.; Balducci, F.; Di Vittori, L.; Scalzo, J.; Capocasa, F.; Zhong, C.; Forbes-Hernández, T.; Giampieri, F.; Battino, M.; Mezzetti, B. Yield and nutritional quality of highbush blueberry genotypes trialled in a Mediterranean hot summer climate. J. Sci. Food Agric. 2020, 100, 3675–3686. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Villagra, J.; Ávila, K.; Gajardo, H.A.; Bravo, L.A.; Ribera-Fonseca, A.; Jorquera-Fontena, E.; Curaqueo, G.; Roldán, C.; Falquetto-Gomes, P.; Nunes-Nesi, A.; et al. Diurnal High Temperatures Affect the Physiological Performance and Fruit Quality of Highbush Blueberry (Vaccinium corymbosum L.) cv. Legacy. Plants 2024, 13, 1846. https://doi.org/10.3390/plants13131846
González-Villagra J, Ávila K, Gajardo HA, Bravo LA, Ribera-Fonseca A, Jorquera-Fontena E, Curaqueo G, Roldán C, Falquetto-Gomes P, Nunes-Nesi A, et al. Diurnal High Temperatures Affect the Physiological Performance and Fruit Quality of Highbush Blueberry (Vaccinium corymbosum L.) cv. Legacy. Plants. 2024; 13(13):1846. https://doi.org/10.3390/plants13131846
Chicago/Turabian StyleGonzález-Villagra, Jorge, Kevin Ávila, Humberto A. Gajardo, León A. Bravo, Alejandra Ribera-Fonseca, Emilio Jorquera-Fontena, Gustavo Curaqueo, Cecilia Roldán, Priscilla Falquetto-Gomes, Adriano Nunes-Nesi, and et al. 2024. "Diurnal High Temperatures Affect the Physiological Performance and Fruit Quality of Highbush Blueberry (Vaccinium corymbosum L.) cv. Legacy" Plants 13, no. 13: 1846. https://doi.org/10.3390/plants13131846
APA StyleGonzález-Villagra, J., Ávila, K., Gajardo, H. A., Bravo, L. A., Ribera-Fonseca, A., Jorquera-Fontena, E., Curaqueo, G., Roldán, C., Falquetto-Gomes, P., Nunes-Nesi, A., & Reyes-Díaz, M. M. (2024). Diurnal High Temperatures Affect the Physiological Performance and Fruit Quality of Highbush Blueberry (Vaccinium corymbosum L.) cv. Legacy. Plants, 13(13), 1846. https://doi.org/10.3390/plants13131846










