Genetic Biofortification of Winter Wheat with Selenium (Se)
Abstract
1. Introduction
2. Selenium and Its Role in Human Health
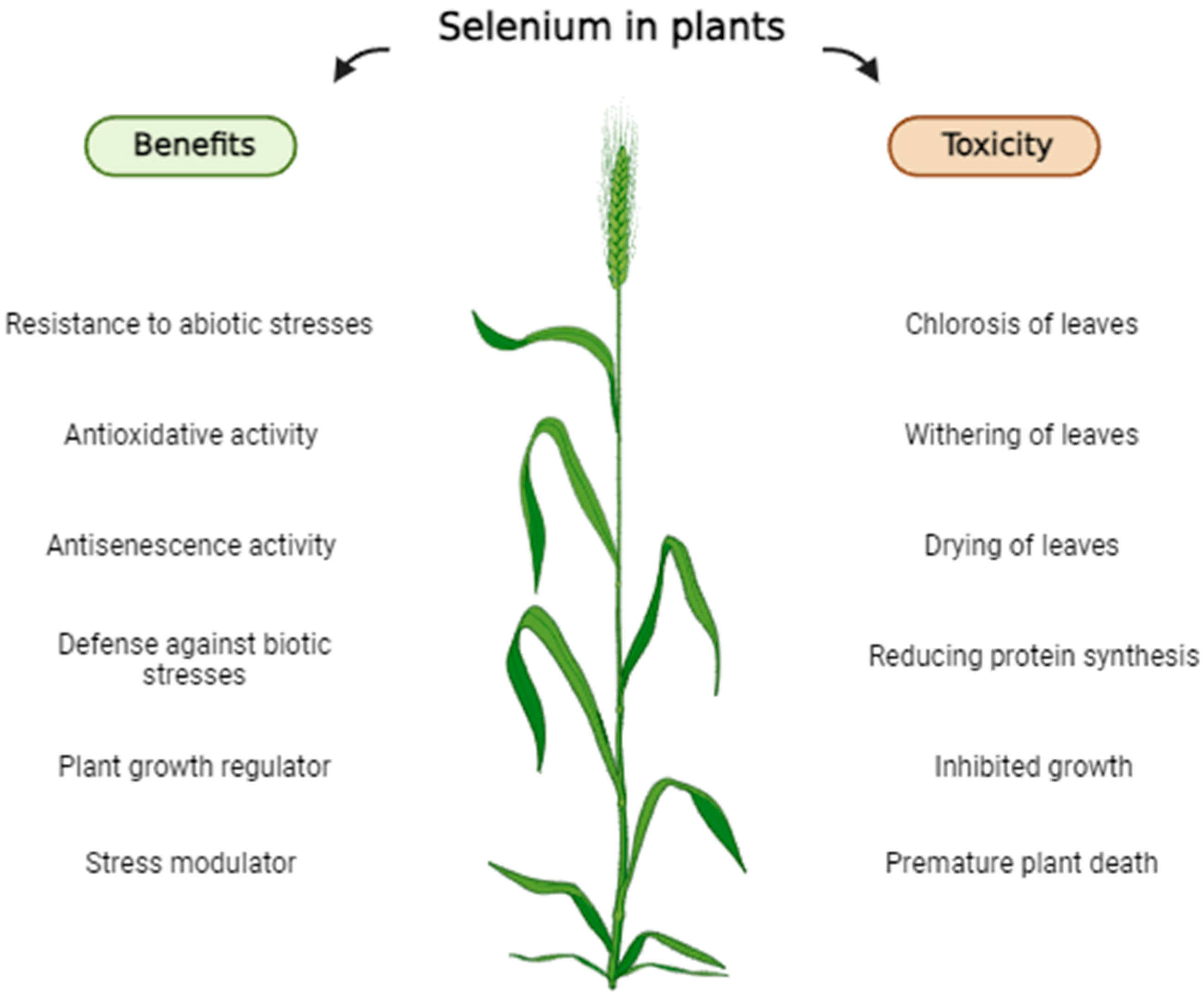
3. Selenium in Plants
4. Biofortification of Wheat with Selenium
4.1. Agronomic Biofortification
4.2. Genetic Biofortification
4.2.1. Breeding Wheat for Increased Uptake and Accumulation of Selenium
4.2.2. Genetic Engineering of Wheat for Increased Uptake and Accumulation of Selenium
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Balfourier, F.; Bouchet, S.; Robert, S.; DeOliveira, R.; Rimbert, H.; Kitt, J.; Choulet, F.; Paux, E. Worldwide Phylogeography and History of Wheat Genetic Diversity. Sci. Adv. 2019, 5, eaav0536. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.W.; Borrill, P. Applying Genomic Resources to Accelerate Wheat Biofortification. Heredity 2020, 125, 386–395. [Google Scholar] [CrossRef] [PubMed]
- Phougat, D.; Sethi, S.K. Wheat Biofortification: Agricultural Investment to Refrain Malnutrition Especially in Developing World. J. Entomol. Zool. Stud. 2019, 7, 506–510. [Google Scholar]
- Wieser, H.; Koehler, P.; Scherf, K.A. The Two Faces of Wheat. Front. Nutr. 2020, 7, 517313. [Google Scholar] [CrossRef] [PubMed]
- International Grains Council. Grain Market Report. 2019. Available online: https://www.igc.int/downloads/gmrsummary/gmrsumme.pdf (accessed on 24 April 2024).
- Shewry, P.R.; Hey, S.J. The Contribution of Wheat to Human Diet and Health. Food Energy Secur. 2015, 4, 178–202. [Google Scholar] [CrossRef]
- Khan, M.K.; Pandey, A.; Akkaya, M.S.; Gezgin, S.; Hamurcu, M.; Hakki, E.E. Wheat Biofortification—A Potential Key to Human Malnutrition. J. Elem. 2017, 22, 937–944. [Google Scholar] [CrossRef]
- White, P.J.; Broadley, M.R. Biofortification of Crops with Seven Mineral Elements Often Lacking in Human Diets—Iron, Zinc, Copper, Calcium, Magnesium, Selenium and Iodine. New Phytol. 2009, 182, 49–84. [Google Scholar] [CrossRef] [PubMed]
- Wakeel, A.; Farooq, M.; Bashir, K.; Ozturk, L. Micronutrient Malnutrition and Biofortification: Recent Advances and Future Perspectives. In Plant Micronutrient Use Efficiency: Molecular and Genomic Perspectives in Crop Plants; Hossain, M.A., Kamiya, T., Burritt, D.J., Tran, L.P., Fujiwara, T., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 225–243. [Google Scholar]
- Kiran, A.; Wakeel, A.; Mahmood, K.; Mubaraka, R.; Hafsa; Haefele, S.M. Biofortification of Staple Crops to Alleviate Human Malnutrition: Contributions and Potential in Developing Countries. Agronomy 2022, 12, 452. [Google Scholar] [CrossRef]
- Beal, T.; Massiot, E.; Arsenault, J.E.; Smith, M.R.; Hijmans, R.J. Global Trends in Dietary Micronutrient Supplies and Estimated Prevalence of Inadequate Intakes. PLoS ONE 2017, 12, e0175554. [Google Scholar] [CrossRef]
- Sarwar, N.; Akhtar, M.; Kamran, M.A.; Imran, M.; Riaz, M.A.; Kamran, K.; Hussain, S. Selenium Biofortification in Food Crops: Key Mechanisms and Future Perspectives. J. Food Compos. Anal. 2020, 93, 103615. [Google Scholar] [CrossRef]
- Fairweather-Tait, S.J.; Bao, Y.; Broadley, M.R.; Collings, R.; Ford, D.; Hesketh, J.E.; Hurst, R. Selenium in Human Health and Disease. Antioxid. Redox Signal. 2011, 14, 1337–1383. [Google Scholar] [CrossRef] [PubMed]
- Schrauzer, G.N.; Surai, P.F. Selenium in Human and Animal Nutrition: Resolved and Unresolved Issues. A Partly Historical Treatise in Commemoration of the Fiftieth Anniversary of the Discovery of the Biological Essentiality of Selenium, Dedicated to the Memory of Klaus Schwarz (1914–1978). Crit. Rev. Biotechnol. 2009, 29, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Radawiec, A.; Szulc, W.; Rutkowska, B. Selenium Biofortification of Wheat as a Strategy to Improve Human Nutrition. Agriculture 2021, 11, 144. [Google Scholar] [CrossRef]
- Fordyce, F.M. Selenium Deficiency and Toxicity in the Environment. In Essentials of Medical Geology: Revised Edition; Selinus, O., Ed.; Springer: Cham, The Netherlands, 2013; pp. 375–416. [Google Scholar]
- Hartikainen, H. Biogeochemistry of Selenium and Its Impact on Food Chain Quality and Human Health. J. Trace Elem. Med. Biol. 2005, 18, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.C.; Fordyce, F.M.; Rayman, M.P. Symposium on “Geographical and Geological Influences on Nutrition”: Factors Controlling the Distribution of Selenium in the Environment and Their Impact on Health and Nutrition. In Proceedings of the Nutrition Society; Cambridge University Press: Cambridge, UK, 2010; pp. 119–132. [Google Scholar]
- Broadley, M.R.; White, P.J.; Bryson, R.J.; Meacham, M.C.; Bowen, H.C.; Johnson, S.E.; Hawkesford, M.J.; McGrath, S.P.; Zhao, F.-J.; Breward, N.; et al. Biofortification of UK Food Crops with Selenium. Proc. Nutr. Soc. 2006, 65, 169–181. [Google Scholar] [CrossRef] [PubMed]
- Mrština, T.; Praus, L.; Kaplan, L.; Száková, J.; Tlustoš, P. Efficiency of Selenium Biofortification of Spring Wheat: The Role of Soil Properties and Organic Matter Amendment. Plant Soil Environ. 2022, 68, 572–579. [Google Scholar] [CrossRef]
- Santos, S.; Ungureanu, G.; Boaventura, R.; Botelho, C. Selenium Contaminated Waters: An Overview of Analytical Methods, Treatment Options and Recent Advances in Sorption Methods. Sci. Total Environ. 2015, 521–522, 246–260. [Google Scholar] [CrossRef] [PubMed]
- Institute of Medicine. Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids; The National Academies Press: Washington, DC, USA, 2000; pp. 284–324. [Google Scholar]
- Jones, G.D.; Droz, B.; Greve, P.; Gottschalk, P.; Poffet, D.; McGrath, S.P.; Seneviratne, S.I.; Smith, P.; Winkel, L.H.E. Selenium Deficiency Risk Predicted to Increase under Future Climate Change. Proc. Natl. Acad. Sci. USA 2017, 114, 2848–2853. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Zhao, C.; Zhang, T. Selenium Transformation and Selenium-Rich Foods. Food Biosci. 2021, 40, 100875. [Google Scholar] [CrossRef]
- Lyons, G.; Stangoulis, J.; Graham, R. High-Selenium Wheat: Biofortification for Better Health. Nutr. Res. Rev. 2003, 16, 45. [Google Scholar] [CrossRef]
- Vinceti, M.; Burlingame, B.; Filippini, T.; Naska, A.; Bargellini, A.; Borella, P. The Epidemiology of Selenium and Human Health. In Selenium: Its Molecular Biology and Role in Human Health, 4th ed.; Hatfield, D., Schweizer, U., Tsuji, P., Gladyshev, V., Eds.; Springer International Publishing: Berlin/Heidelberg, Germany, 2016; pp. 365–376. [Google Scholar]
- Combs, G.F. Selenium in Global Food Systems. Br. J. Nutr. 2001, 85, 517–547. [Google Scholar] [CrossRef] [PubMed]
- Flynn, A.; Hirvonen, T.; Mensink, G.B.M.; Ocké, M.C.; Serra-Majem, L.; Stos, K.; Szponar, L.; Tetens, I.; Turrini, A.; Fletcher, R.; et al. Intake of Selected Nutrients from Foods, from Fortification and from Supplements in Various European Countries. Food Nutr. Res. 2009, 53, 1–51. [Google Scholar] [CrossRef] [PubMed]
- Genchi, G.; Lauria, G.; Catalano, A.; Sinicropi, M.S.; Carocci, A. Biological Activity of Selenium and Its Impact on Human Health. Int. J. Mol. Sci. 2023, 24, 2633. [Google Scholar] [CrossRef] [PubMed]
- Schomburg, L.; Köhrle, J. On the Importance of Selenium and Iodine Metabolism for Thyroid Hormone Biosynthesis and Human Health. Mol. Nutr. Food Res. 2008, 52, 1235–1246. [Google Scholar] [CrossRef] [PubMed]
- Razaghi, A.; Poorebrahim, M.; Sarhan, D.; Björnstedt, M. Selenium Stimulates the Antitumour Immunity: Insights to Future Research. Eur. J. Cancer 2021, 155, 256–267. [Google Scholar] [CrossRef] [PubMed]
- Rayman, M.P. Selenium and Human Health. Lancet 2012, 379, 1256–1268. [Google Scholar] [CrossRef]
- Krysiak, R.; Szkróbka, W.; Okopień, B. The Effect of Gluten-Free Diet on Thyroid Autoimmunity in Drug-Naïve Women with Hashimoto’s Thyroiditis: A Pilot Study. Exp. Clin. Endocrinol. Diabetes 2019, 127, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Conner, T.S.; Richardson, A.C.; Miller, J.C. Optimal Serum Selenium Concentrations Are Associated with Lower Depressive Symptoms and Negative Mood among Young Adults. J. Nutr. 2015, 145, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Schiavon, M.; Nardi, S.; dalla Vecchia, F.; Ertani, A. Selenium Biofortification in the 21st Century: Status and Challenges for Healthy Human Nutrition. Plant Soil 2020, 453, 245–270. [Google Scholar] [CrossRef]
- Gui, J.Y.; Rao, S.; Huang, X.; Liu, X.; Cheng, S.; Xu, F. Interaction between Selenium and Essential Micronutrient Elements in Plants: A Systematic Review. Sci. Total Environ. 2022, 853, 158673. [Google Scholar] [CrossRef]
- Zhang, L.; Hu, B.; Li, W.; Che, R.; Deng, K.; Li, H.; Yu, F.; Ling, H.; Li, Y.; Chu, C. OsPT2, a Phosphate Transporter, Is Involved in the Active Uptake of Selenite in Rice. New Phytol. 2014, 201, 1183–1191. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Wang, Y.; Li, K.; Wan, Y.; Wang, Q.; Zhuang, Z.; Guo, Y.; Li, H. Uptake, Translocation and Biotransformation of Selenium Nanoparticles in Rice Seedlings (Oryza sativa L.). J. Nanobiotechnol. 2020, 18, 103. [Google Scholar] [CrossRef] [PubMed]
- Kikkert, J.; Berkelaar, E. Plant Uptake and Translocation of Inorganic and Organic Forms of Selenium. Arch. Environ. Contam. Toxicol. 2013, 65, 458–465. [Google Scholar] [CrossRef] [PubMed]
- Pilon-Smits, E.A.; LeDuc, D.L. Phytoremediation of Selenium Using Transgenic Plants. Curr. Opin. Biotechnol. 2009, 20, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Pilon, M.; Owen, J.D.; Garifullina, G.F.; Kurihara, T.; Mihara, H.; Esaki, N.; Pilon-Smits, E.A.H. Erratum: Enhanced Selenium Tolerance and Accumulation in Transgenic Arabidopsis Expressing a Mouse Selenocysteine Lyase. Plant Physiol. 2003, 132, 400. [Google Scholar] [CrossRef]
- Eiche, E.; Bardelli, F.; Nothstein, A.K.; Charlet, L.; Göttlicher, J.; Steininger, R.; Dhillon, K.S.; Sadana, U.S. Selenium Distribution and Speciation in Plant Parts of Wheat (Triticum aestivum) and Indian Mustard (Brassica juncea) from a Seleniferous Area of Punjab, India. Sci. Total Environ. 2015, 505, 952–961. [Google Scholar] [CrossRef] [PubMed]
- Van Hoewyk, D. A Tale of Two Toxicities: Malformed Selenoproteins and Oxidative Stress Both Contribute to Selenium Stress in Plants. Ann. Bot. 2013, 112, 965–972. [Google Scholar] [CrossRef] [PubMed]
- Li, H.F.; McGrath, S.P.; Zhao, F.J. Selenium Uptake, Translocation and Speciation in Wheat Supplied with Selenate or Selenite. New Phytol. 2008, 178, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Pickering, I.J.; Prince, R.C.; Salt, D.E.; George, G.N. Quantitative, Chemically Specific Imaging of Selenium Transformation in Plants. Proc. Natl. Acad. Sci. USA 2000, 97, 10717–10722. [Google Scholar] [CrossRef]
- Whanger, P.D. Selenium and Its Relationship to Cancer: An Update. Br. J. Nutr. 2004, 91, 11–28. [Google Scholar] [CrossRef]
- Zhang, X.; He, H.; Xiang, J.; Yin, H.; Hou, T. Selenium-Containing Proteins/Peptides from Plants: A Review on the Structures and Functions. J. Agric. Food Chem. 2020, 68, 15061–15073. [Google Scholar] [CrossRef] [PubMed]
- Rayman, M.P. Food-Chain Selenium and Human Health: Emphasis on Intake. Br. J. Nutr. 2008, 100, 254–268. [Google Scholar] [CrossRef] [PubMed]
- Lanza, M.G.D.B.; Reis, A.R. dos Roles of Selenium in Mineral Plant Nutrition: ROS Scavenging Responses against Abiotic Stresses. Plant Physiol. Biochem. 2021, 164, 27–43. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Chu, J.; Wang, G. Effects of Selenium on Wheat Seedlings under Drought Stress. Biol. Trace Elem. Res. 2009, 130, 283–290. [Google Scholar] [CrossRef]
- Sieprawska, A.; Kornaś, A.; Filek, M. Involvement of Selenium in Protective Mechanisms of Plants under Environmental Stress Conditions—Review. Acta Biol. Cracoviensia Ser. Bot. 2015, 57, 9–20. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Raza, A.; Hawrylak-Nowak, B.; Matraszek-Gawron, R.; Al Mahmud, J.; Nahar, K.; Fujita, M. Selenium in Plants: Boon or Bane? Environ. Exp. Bot. 2020, 178, 104170. [Google Scholar] [CrossRef]
- Khan, F.; Hussain, S.; Tanveer, M.; Khan, S.; Hussain, H.A.; Iqbal, B.; Geng, M. Coordinated Effects of Lead Toxicity and Nutrient Deprivation on Growth, Oxidative Status, and Elemental Composition of Primed and Non-Primed Rice Seedlings. Environ. Sci. Pollut. Res. 2018, 25, 21185–21194. [Google Scholar] [CrossRef]
- Tiwari, S.; Lata, C. Heavy Metal Stress, Signaling, and Tolerance Due to Plant-Associated Microbes: An Overview. Front. Plant Sci. 2018, 9, 452. [Google Scholar] [CrossRef]
- Khan, Z.; Thounaojam, T.C.; Chowdhury, D.; Upadhyaya, H. The Role of Selenium and Nano Selenium on Physiological Responses in Plant: A Review. Plant Growth Regul. 2023, 100, 409–433. [Google Scholar] [CrossRef]
- Germ, M.; Kreft, I.; Osvald, J. Influence of UV-B Exclusion and Selenium Treatment on Photochemical Efficiency of Photosystem II, Yield and Respiratory Potential in Pumpkins (Cucurbita pepo L.). Plant Physiol. Biochem. 2005, 43, 445–448. [Google Scholar] [CrossRef]
- Shahbaz, M.; Akram, A.; Mehak, A.; Haq, E.U.; Fatima, N.; Wareen, G.; Fitriatin, B.N.; Sayyed, R.Z.; Ilyas, N.; Sabullah, M.K. Evaluation of Selenium Nanoparticles in Inducing Disease Resistance against Spot Blotch Disease and Promoting Growth in Wheat under Biotic Stress. Plants 2023, 12, 761. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.; Gupta, S. An Overview of Selenium Uptake, Metabolism, and Toxicity in Plants. Front. Plant Sci. 2017, 7, 2074. [Google Scholar] [CrossRef] [PubMed]
- Hondal, R.J.; Marino, S.M.; Gladyshev, V.N. Selenocysteine in Thiol/Disulfide-like Exchange Reactions. Antioxid. Redox Signal. 2013, 18, 1675–1689. [Google Scholar] [CrossRef] [PubMed]
- Hugouvieux, V.; Dutilleul, C.; Jourdain, A.; Reynaud, F.; Lopez, V.; Bourguignon, J. Arabidopsis Putative Selenium-Binding Protein1 Expression Is Tightly Linked to Cellular Sulfur Demand and Can Reduce Sensitivity to Stresses Requiring Glutathione for Tolerance. Plant Physiol. 2009, 151, 768–781. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.K.; Balyan, H.S.; Sharma, S.; Kumar, R. Biofortification and Bioavailability of Zn, Fe and Se in Wheat: Present Status and Future Prospects. Theor. Appl. Genet. 2021, 134, 1–35. [Google Scholar] [CrossRef]
- Galić, L.; Vinković, T.; Ravnjak, B.; Lončarić, Z. Agronomic Biofortification of Significant Cereal Crops with Selenium—A Review. Agronomy 2021, 11, 1015. [Google Scholar] [CrossRef]
- Ludwig, Y.; Slamet-Loedin, I.H. Genetic Biofortification to Enrich Rice and Wheat Grain Iron: From Genes to Product. Front. Plant Sci. 2019, 10, 833. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.F.; Zhao, Z.Q.; Han, Z.Y.; Huang, L.Q.; Lv, C.H.; Zhang, Z.H.; Zhang, H.Q.; Liu, X.W. Selenium Uptake and Fruit Quality of Pear (Pyrus communis L.) Treated with Foliar Se Application. J. Plant Nutr. Soil Sci. 2019, 182, 637–646. [Google Scholar] [CrossRef]
- Mackowiak, C.L.; Amacher, M.C. Soil Sulfur Amendments Suppress Selenium Uptake by Alfalfa and Western Wheatgrass. J. Environ. Qual. 2008, 37, 772–779. [Google Scholar] [CrossRef]
- Johnsson, L. Selenium Uptake by Plants as a Function of Soil Type, Organic Matter Content and PH. Plant Soil 1991, 133, 57–64. [Google Scholar] [CrossRef]
- White, P.J. Selenium Metabolism in Plants. Biochim. Biophys. Acta-Gen. Subj. 2018, 1862, 2333–2342. [Google Scholar] [CrossRef] [PubMed]
- Reeves, P.G.; Gregoire, B.R.; Garvin, D.F.; Hareland, G.A.; Lindlauf, J.E.; Johnson, L.K.; Finley, J.W. Determination of Selenium Bioavailability from Wheat Mill Fractions in Rats by Using the Slope-Ratio Assay and a Modified Torula Yeast-Based Diet. J. Agric. Food Chem. 2007, 55, 516–522. [Google Scholar] [CrossRef] [PubMed]
- Danso, O.P.; Asante-Badu, B.; Zhang, Z.; Song, J.; Wang, Z.; Yin, X.; Zhu, R. Selenium Biofortification: Strategies, Progress and Challenges. Agriculture 2023, 13, 416. [Google Scholar] [CrossRef]
- Cakmak, I.; Kutman, U.B. Agronomic Biofortification of Cereals with Zinc: A Review. Eur. J. Soil Sci. 2018, 69, 172–180. [Google Scholar] [CrossRef]
- Garg, M.; Sharma, N.; Sharma, S.; Kapoor, P.; Kumar, A.; Chunduri, V.; Arora, P. Biofortified Crops Generated by Breeding, Agronomy, and Transgenic Approaches Are Improving Lives of Millions of People around the World. Front. Nutr. 2018, 5, 12. [Google Scholar] [CrossRef] [PubMed]
- Ramkissoon, C.; Degryse, F.; da Silva, R.C.; Baird, R.; Young, S.D.; Bailey, E.H.; McLaughlin, M.J. Improving the Efficacy of Selenium Fertilizers for Wheat Biofortification. Sci. Rep. 2019, 9, 19520. [Google Scholar] [CrossRef]
- Ros, G.H.; van Rotterdam, A.M.D.; Bussink, D.W.; Bindraban, P.S. Selenium Fertilization Strategies for Bio-Fortification of Food: An Agro-Ecosystem Approach. Plant Soil 2016, 404, 99–112. [Google Scholar] [CrossRef]
- Peak, D.; Sparks, D.L. Mechanisms of Selenate Adsorption on Iron Oxides and Hydroxides. Environ. Sci. Technol. 2002, 36, 1460–1466. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Kim, M.; Ro, H.M. Factors Modifying the Structural Configuration of Oxyanions and Organic Acids Adsorbed on Iron (Hydr)Oxides in Soils. A Review. Environ. Chem. Lett. 2020, 18, 631–662. [Google Scholar] [CrossRef]
- Ku, Y.S.; Rehman, H.M.; Lam, H.M. Possible Roles of Rhizospheric and Endophytic Microbes to Provide a Safe and Affordable Means of Crop Biofortification. Agronomy 2019, 9, 764. [Google Scholar] [CrossRef]
- Yang, D.; Hu, C.; Wang, X.; Shi, G.; Li, Y.; Fei, Y.; Song, Y.; Zhao, X. Microbes: A Potential Tool for Selenium Biofortification. Metallomics 2021, 13, mfab054. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, S.; Bahuguna, R.N.; Shrivastava, N.; Singh, S.; Chatterjee, A.; Varma, A.; Jagadish, S.K. Microbial Biofortification: A Sustainable Route to Grow Nutrient-Rich Crops under Changing Climate. Field Crop. Res. 2022, 287, 108662. [Google Scholar] [CrossRef]
- Durán, P.; Acuña, J.J.; Jorquera, M.A.; Azcón, R.; Borie, F.; Cornejo, P.; Mora, M.L. Enhanced Selenium Content in Wheat Grain by Co-Inoculation of Selenobacteria and Arbuscular Mycorrhizal Fungi: A Preliminary Study as a Potential Se Biofortification Strategy. J. Cereal Sci. 2013, 57, 275–280. [Google Scholar] [CrossRef]
- Durán, P.; Acuña, J.J.; Jorquera, M.A.; Azcón, R.; Paredes, C.; Rengel, Z.; de la Luz Mora, M. Endophytic Bacteria from Selenium-Supplemented Wheat Plants Could Be Useful for Plant-Growth Promotion, Biofortification and Gaeumannomyces Graminis Biocontrol in Wheat Production. Biol. Fertil. Soils 2014, 50, 983–990. [Google Scholar] [CrossRef]
- Goulding, K.; Jarvis, S.; Whitmore, A. Optimizing Nutrient Management for Farm Systems. Philos. Trans. R. Soc. B Biol. Sci. 2008, 363, 667–680. [Google Scholar] [CrossRef]
- Saini, D.K.; Devi, P.; Kaushik, P. Advances in Genomic Interventions for Wheat Biofortification: A Review. Agronomy 2020, 10, 62. [Google Scholar] [CrossRef]
- Singh, U.; Praharaj, C.S.; Singh, S.S.; Singh, N.P. Biofortification Food Crops, 1st ed.; Springer New Delhi: New Delhi, India, 2016; pp. 1–490. [Google Scholar]
- Kamaral, C.; Neate, S.M.; Gunasinghe, N.; Milham, P.J.; Paterson, D.J.; Kopittke, P.M.; Seneweera, S. Genetic Biofortification of Wheat with Zinc: Opportunities to Fine-Tune Zinc Uptake, Transport and Grain Loading. Physiol. Plant. 2022, 174, e13612. [Google Scholar] [CrossRef] [PubMed]
- Dempewolf, H.; Baute, G.; Anderson, J.; Kilian, B.; Smith, C.; Guarino, L. Past and Future Use of Wild Relatives in Crop Breeding. Crop. Sci. 2017, 57, 1070–1082. [Google Scholar] [CrossRef]
- Meuwissen, T.H.E.; Hayes, B.J.; Goddard, M.E. Prediction of Total Genetic Value Using Genome-Wide Dense Marker Maps. Genetics 2001, 157, 1819–1829. [Google Scholar] [CrossRef]
- Garvin, D.F.; Welch, R.M.; Finley, J.W. Historical Shifts in the Seed Mineral Micronutrient Concentration of US Hard Red Winter Wheat Germplasm. J. Sci. Food Agric. 2006, 86, 2213–2220. [Google Scholar] [CrossRef]
- Wu, Z.; Bañuelos, G.S.; Lin, Z.Q.; Liu, Y.; Yuan, L.; Yin, X.; Li, M. Biofortification and Phytoremediation of Selenium in China. Front. Plant Sci. 2015, 6, 136. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Xue, W.T.; Yang, R.Z.; Qin, H.B.; Zhao, G.; Tzion, F.; Cheng, J.P. Quantitative Trait Loci Conferring Grain Selenium Nutrient in Durum Wheat × Wild Emmer Wheat RIL Population. Czech J. Genet. Plant Breed. 2018, 54, 52–58. [Google Scholar] [CrossRef]
- Uauy, C.; Distelfeld, A.; Fahima, T.; Blechl, A.; Dubcovsky, J. A NAC Gene Regulating Senescence Improves Grain Protein, Zinc, and Iron Content in Wheat. Science 2006, 314, 1298–1301. [Google Scholar] [CrossRef] [PubMed]
- Yan, J. Grain Mineral and Protein Concentrations of Wild Emmer Wheat (Triticum dicoccoides) and Wild Barley (Hordeum spontaneum) and Their Potential for Crop Improvement. Ph.D. Thesis, University of Haifa, Haifa, Israel, 2010. [Google Scholar]
- Pu, Z.; Pei, Y.; Yang, J.; Ma, J.; Li, W.; Liu, D.; Wang, J.; Wei, Y.; Zheng, Y. A QTL Located on Chromosome 3D Enhances the Selenium Concentration of Wheat Grain by Improving Phytoavailability and Root Structure. Plant Soil 2018, 425, 287–296. [Google Scholar] [CrossRef]
- Ortiz-Monasterio, J.I.; Palacios-Rojas, N.; Meng, E.; Pixley, K.; Trethowan, R.; Peña, R.J. Enhancing the Mineral and Vitamin Content of Wheat and Maize through Plant Breeding. J. Cereal Sci. 2007, 46, 293–307. [Google Scholar] [CrossRef]
- Tveitnes, S.; Singh, B.R.; Ruud, L. Selenium Concentration in Spring Wheat as Influenced by Basal Application and Top Dressing of Selenium-Enriched Fertilizers. Fertil. Res. 1995, 45, 163–167. [Google Scholar] [CrossRef]
- Piergiovanni, A.R.; Rizzi, R.; Pannacciulli, E.; Della Gatta, C. Mineral Composition in Hulled Wheat Grains: A Comparison between Emmer (Triticum dicoccon Schrank) and Spelt (T. spelta L.) Accessions. Int. J. Food Sci. Nutr. 1997, 48, 381–386. [Google Scholar] [CrossRef]
- Lyons, G.; Ortiz-Monasterio, I.; Stangoulis, J.; Graham, R. Selenium Concentration in Wheat Grain: Is There Sufficient Genotypic Variation to Use in Breeding? Plant Soil 2005, 269, 369–380. [Google Scholar] [CrossRef]
- Wang, P.; Wang, H.; Liu, Q.; Tian, X.; Shi, Y.; Zhang, X. QTL Mapping of Selenium Content Using a RIL Population in Wheat. PLoS ONE 2017, 12, e0184351. [Google Scholar] [CrossRef]
- Pu, Z.E.; Yu, M.; He, Q.Y.; Chen, G.Y.; Wang, J.R.; Liu, Y.X.; Jiang, Q.T.; Li, W.; Dai, S.F.; Wei, Y.M.; et al. Quantitative Trait Loci Associated with Micronutrient Concentrations in Two Recombinant Inbred Wheat Lines. J. Integr. Agric. 2014, 13, 2322–2329. [Google Scholar] [CrossRef]


| Age Class | Females | Males | Pregnancy | Lactation |
|---|---|---|---|---|
| <6 months | 15 | 15 | ||
| 7–12 months | 20 | 20 | ||
| 1–3 years | 20 | 20 | ||
| 4–8 years | 30 | 30 | ||
| 9–13 years | 40 | 40 | ||
| 14–18 years | 55 | 55 | 60 | 70 |
| 19–50 years | 55 | 55 | 60 | 70 |
| 51–70 years | 55 | 55 | ||
| >71 years | 55 | 55 |
| Reference | Cross | Mapping Population | No. of QTLs for Se Content |
|---|---|---|---|
| Hexaploid wheat | |||
| [98] | SHW-L1 × Chuanmai 32 | 171 RILs | 39 |
| Chuanmai 42 × Chuannong 16 | 127 RILs | ||
| [97] | Tianong 18 × Limma i6 | 184 RILs | 16 |
| [92] | SHW-L1 × Chuanmai 32 | 171 RILs | 24 |
| Tetraploid wheat | |||
| [89] | LDN × G18-16 | 152 RILs | 15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sunic, K.; Spanic, V. Genetic Biofortification of Winter Wheat with Selenium (Se). Plants 2024, 13, 1816. https://doi.org/10.3390/plants13131816
Sunic K, Spanic V. Genetic Biofortification of Winter Wheat with Selenium (Se). Plants. 2024; 13(13):1816. https://doi.org/10.3390/plants13131816
Chicago/Turabian StyleSunic, Katarina, and Valentina Spanic. 2024. "Genetic Biofortification of Winter Wheat with Selenium (Se)" Plants 13, no. 13: 1816. https://doi.org/10.3390/plants13131816
APA StyleSunic, K., & Spanic, V. (2024). Genetic Biofortification of Winter Wheat with Selenium (Se). Plants, 13(13), 1816. https://doi.org/10.3390/plants13131816







