Effects of Photoperiod and Light Quality on Germination and Growth of Camellia sinensis ‘HuangKui’
Abstract
1. Introduction
2. Results
2.1. Tea Plant’s Phenological Periods and Growth
2.2. Leaf Photosynthetic Performance under Different Photoperiod Treatments
2.3. Leaf Chlorophyll Content under Different Photoperiod Treatments
2.4. Growth of Tea Plants under Different Photoperiod Treatments
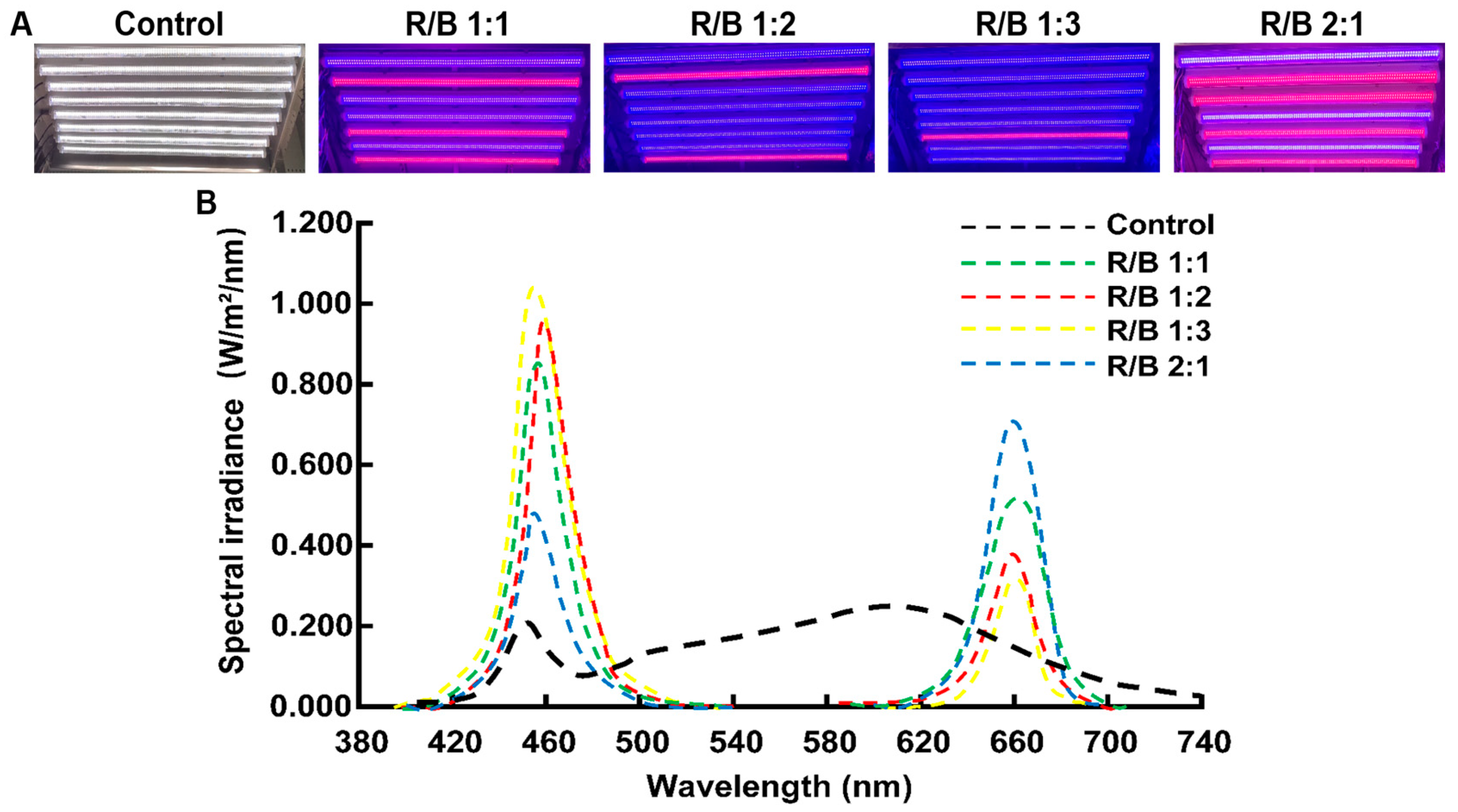
2.5. Tea Plant’s Phenological Periods under Different Light Quality Treatments
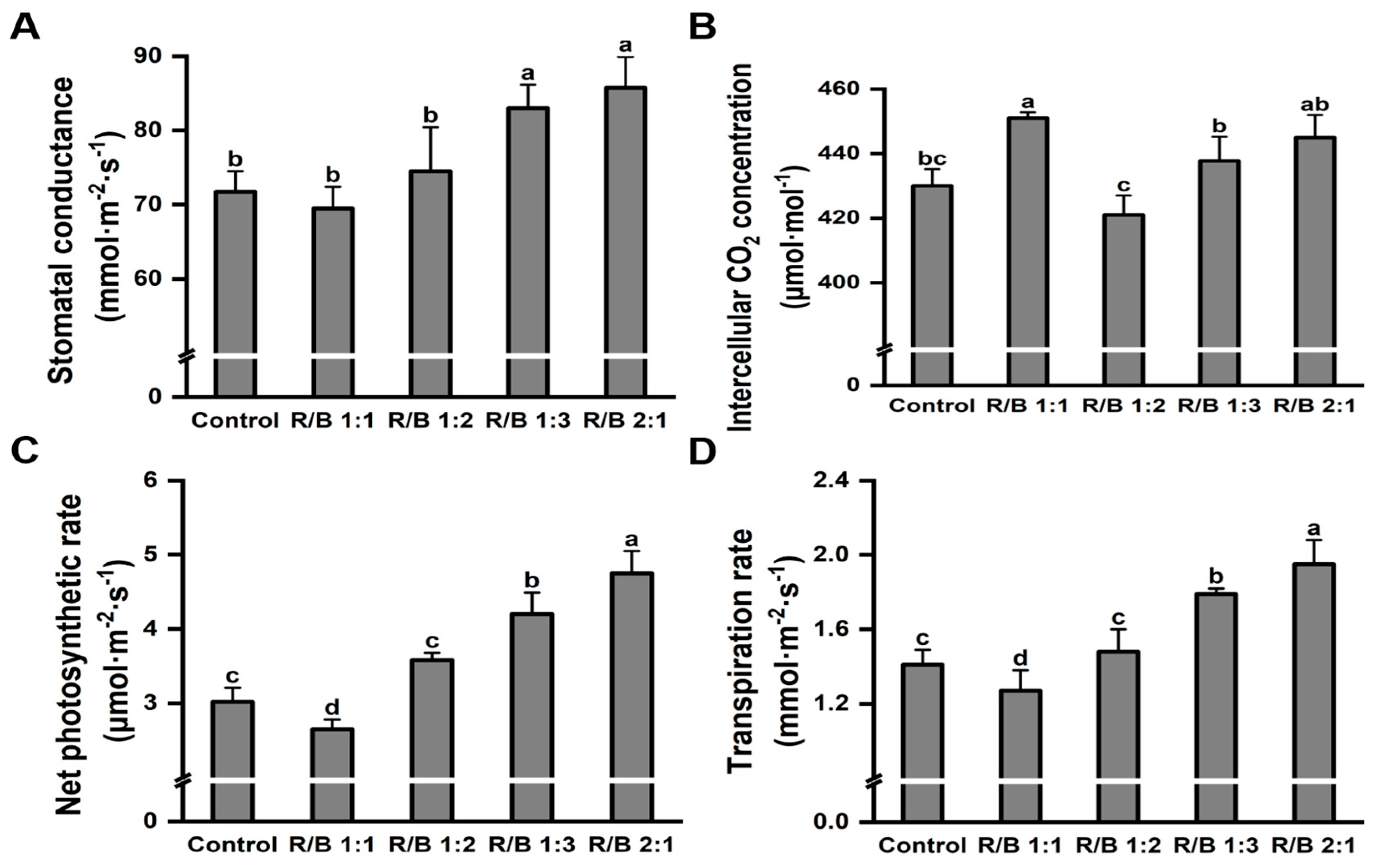
2.6. Leaf Photosynthetic Performance under Different Light Quality Treatments
2.7. Growth of Tea Plants under Different Light Quality Treatments
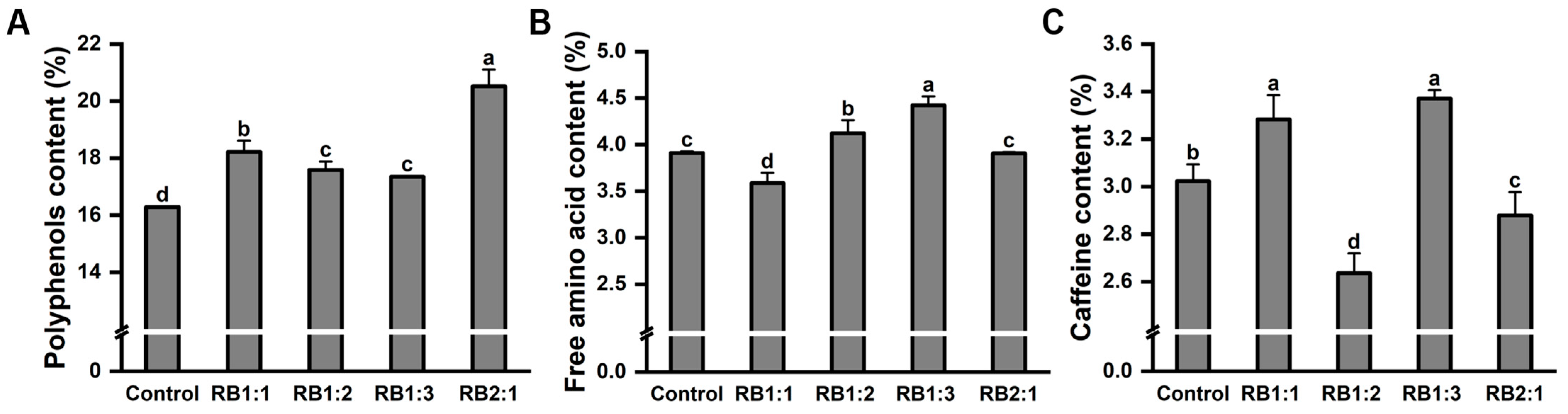
2.8. Tea Plant Quality under Different Light Quality Treatments
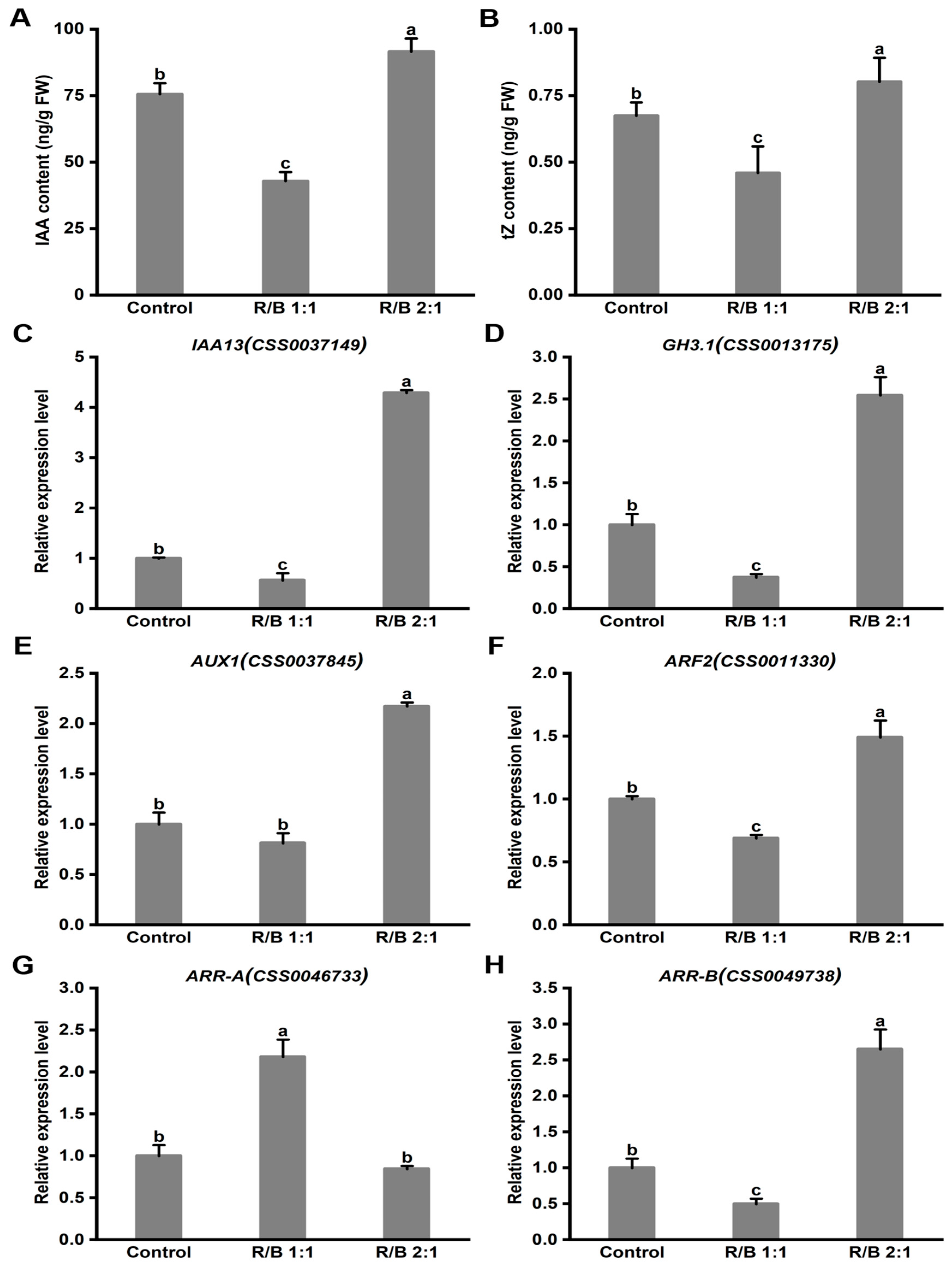
2.9. The Hormone Content and Gene Expression Levels under Different Light Qualities
3. Discussion
4. Materials and Methods
4.1. Plant Material and Experimental Settings
4.2. Determination of Phenological Period Timing
4.3. Determination of Photosynthetic Characteristics
4.4. Chlorophyll Concentration
4.5. Determination of Tea Plant Growth
4.6. Tea Polyphenols, Free Amino Acids, and Caffeine
4.7. Hormone Determination
4.8. Primer Design and Quantitative Real-Time Polymerase Chain Reaction (PCR) (qRT-PCR)
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chang, N.; Zhou, Z.; Li, Y.; Zhang, X. Exogenously applied Spd and Spm enhance drought tolerance in tea plants by increasing fatty acid desaturation and plasma membrane H+-ATPase activity. Plant Physiol. Biochem. 2022, 170, 225–233. [Google Scholar]
- Liu, X.; Cheng, X.; Cao, J.; Zhu, W.; Sun, Y.; Lin, N.; Wan, X.; Liu, L. UV-B regulates seasonal greening of albino leaves by modulating CsHY5-inhibiting chlorophyll biosynthesis in Camellia sinensis cv. Huangkui. Plant Sci. 2023, 328, 111569. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Cao, J.; Cheng, X.; Zhu, W.; Sun, Y.; Wan, X.; Liu, L. CsRVE1 promotes seasonal greening of albino Camellia sinensis cv. Huangkui by activating chlorophyll biosynthesis. Tree Physiol. 2023, 43, 1432–1443. [Google Scholar] [CrossRef] [PubMed]
- Hao, G.; Zheng, Z.; Ma, H.; Ye, Y.; Guo, M.; Cheng, H.; Rua, L. New albino tea variety-Zhongbai 4. China Tea 2019, 41, 11–13. [Google Scholar]
- Li, W.; Xiang, F.; Su, Y.; Luo, Z.; Luo, W.; Zhou, L.; Liu, H.; Xiao, L. Gibberellin Increases the Bud Yield and Theanine Accumulation in Camellia sinensis (L.) Kuntze. Molecules 2021, 26, 3290. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wu, S.; Mao, K.; Li, J.; Dong, F.; Tang, J.; Zeng, L.; Gu, D. Adverse effects of shading on the tea yield and the restorative effects of exogenously applied brassinolide. Ind. Crops Prod. 2023, 197, 116546. [Google Scholar] [CrossRef]
- Thirugnanasambantham, K.; Prabu, G.; Mandal, A.K.A. Synergistic effect of cytokinin and gibberellins stimulates release of dormancy in tea (Camellia sinensis (L.) O. Kuntze) bud. Physiol. Mol. Biol. Plants 2020, 26, 1035–1045. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wang, H.; Chen, Y.; Li, Y.; Wang, X.; Yue, C. Study on the Regulation Roles of Plant Hormones on the Growth and Development of Tea Shoots inSpring. J. Tea Sci. 2023, 43, 335–348. [Google Scholar]
- Di, T.; Zhao, L.; Chen, H.; Qian, W.; Wang, P.; Zhang, X.; Xia, T. Transcriptomic and Metabolic Insights into the Distinctive Effects of Exogenous Melatonin and Gibberellin on Terpenoid Synthesis and Plant Hormone Signal Transduction Pathway in Camellia sinensis. J. Agric. Food Chem. 2019, 67, 4689–4699. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Lin, Y.; Jiang, S.; Liu, W.; Xie, J. Effects of Foliar Application of Silicon Fertilizer on Yield and Quality of White Tea. China Tea 2021, 43, 52–55. [Google Scholar]
- Zhang, Q.; Shi, Y.; Hu, H.; Shi, Y.; Tang, D.; Ruan, J.; Fernie, A.; Liu, M. Magnesium promotes tea plant growth via enhanced glutamine synthetase-mediated nitrogen assimilation. Plant Physiol. 2023, 192, 1321–1337. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wu, L.; Wei, K.; Wang, L. Tea plant timing of spring bud flush QTL candidate genes. J. Tea Sci. 2023, 6, 10. [Google Scholar]
- Tan, L.; Cui, D.; Wang, L.; Liu, Q.; Zhang, D.; Hu, X.; Fu, Y.; Chen, S.; Zou, Y.; Chen, W.; et al. Genetic analysis of the early bud flush trait of tea plants (Camellia sinensis) in the cultivar ‘Emei Wenchun’ and its open-pollinated offspring. Hortic. Res. 2022, 9, 086. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Gao, X.; Yang, J.; Kong, X. Genome-Wide Association Study to Identify Favorable SNP Allelic Variations and Candidate Genes That Control the Timing of Spring Bud Flush of Tea (Camellia sinensis) Using SLAF-seq. J. Agric. Food Chem. 2019, 67, 10380–10391. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Pham, M.; Cui, M.; Lee, H.; Hwang, H.; Jang, I.; Chun, C. Growth and physiological responses of Panax ginseng seedlings as affected by light intensity and photoperiod. Hortic. Environ. Biotechnol. 2022, 63, 835–846. [Google Scholar] [CrossRef]
- Chen, J.; Chen, Z.; Li, Z.; Zhao, Y.; Chen, X.; Guo, R. Effect of Photoperiod on Chinese Kale (Brassica alboglabra) Sprouts Under White or Combined Red and Blue Light. Front. Plant Sci. 2021, 11, 589746. [Google Scholar] [CrossRef] [PubMed]
- Nabipour, S.; Hir, P.; Chamani, E.; Estaji, A. LED Lighting Influences Germination, Growth, and Biochemical Indices of Snapdragon. Russ. J. Plant Physiol. 2023, 70, 121. [Google Scholar] [CrossRef]
- Vatistas, C.; Avgoustaki, D.D.; Monedas, G.; Bartzanas, T. The effect of different light wavelengths on the germination of lettuce, cabbage, spinach and arugula seeds in a controlled environment chamber. Sci. Hortic. 2024, 331, 113118. [Google Scholar] [CrossRef]
- Paik, I.; Huq, E. Plant photoreceptors: Multi-functional sensory proteins and their signaling networks. Semin. Cell Dev. Biol. 2019, 92, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Cheng, G.; Shu, X.; Wang, N.; Wang, Z. Transcriptome Analysis of Lycoris chinensis Bulbs Reveals Flowering in the Age-Mediated Pathway. Biomolecules 2022, 12, 899. [Google Scholar] [CrossRef] [PubMed]
- Varinder, S.; Valérie, B.; Marianne, L.; Valérie, G. Effect of light quality and extended photoperiod on flower bud induction during transplant production of day-neutral strawberry cultivars. Can. J. Plant Sci. 2022, 102, 356–367. [Google Scholar]
- Pérez, V.; Castro, P.; Valladares, F. Differential and interactive effects of temperature and photoperiod on budburst and carbon reserves in two co-occurring Mediterranean oaks. Plant Biol. 2009, 11, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Elkins, C.; van Iersel, M.W. Longer Photoperiods with the Same Daily Light Integral Improve Growth of Rudbeckia Seedlings in a Greenhouse. HortScience 2020, 55, 1676–1682. [Google Scholar] [CrossRef]
- Chu, Q.; Qin, Y.; Li, C.; Cheng, S.; Su, L.; He, Z.; Zhou, X.; Shao, D.; Guo, X. Effects of Different Photoperiods on the Growth and Nutritional Characteristics of Two Celery Cultivars in Plant Factory. Agronomy 2023, 13, 3039. [Google Scholar] [CrossRef]
- Šrajer Gajdošik, M.; Vicić, A.; Gvozdić, V.; Galić, V.; Begović, L.; Mlinarić, S. Effect of Prolonged Photoperiod on Light-Dependent Photosynthetic Reactions in Cannabis. Int. J. Mol. Sci. 2022, 23, 9702. [Google Scholar] [CrossRef] [PubMed]
- Shibaeva, T.G.; Mamaev, A.V.; Titov, A.F. Possible Physiological Mechanisms of Leaf Photodamage in Plants Grown under Continuous Lighting. Russ. J. Plant Physiol. 2023, 70, 15. [Google Scholar] [CrossRef]
- Peng, Y.; Fan, M.; Wang, Q.; Lan, W.; Long, Y. Best hyperspectral indices for assessing leaf chlorophyll content in a degraded temperate vegetation. Ecol. Evol. 2018, 8, 7068–7078. [Google Scholar] [CrossRef] [PubMed]
- Haijie, D.; Genhua, N.; Mengmeng, G.; Joseph, M.J.H. Effects of Light Quality on Growth and Phytonutrient Accumulation of Herbs under Controlled Environments. Horticulturae 2017, 3, 36. [Google Scholar] [CrossRef]
- Li, K.; Ye, Q.; Li, Q.; Xia, R.; Guo, W.; Cheng, J. Effects of the spatial and spectral distribution of red and blue light on Haematococcus pluvialis growth. Algal Res. 2020, 51, 102045. [Google Scholar] [CrossRef]
- Li, Q.; Yi, Z.; Yang, G.; Xu, Y.; Jin, Y.; Tan, L.; Du, A.; He, K.; Zhao, H.; Fang, Y. Effects of various spectral compositions on micro-polluted water purification and biofuel feedstock production using duckweed. Environ. Sci. Pollut. Res. 2022, 29, 52003–52012. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, M.; Gulandaz, A.; Islam, S.; Reza, N.; Ali, M.; Islam, N.; Park, S.; Chung, S. Lighting conditions affect the growth and glucosinolate contents of Chinese kale leaves grown in an aeroponic plant factory. Hortic. Environ. Biotechnol. 2023, 64, 97–113. [Google Scholar] [CrossRef]
- Lv, B.; Zhu, J.; Kong, X.; Ding, Z. Light participates in the auxin-dependent regulation of plant growth. J. Integr. Plant Biol. 2021, 63, 819–822. [Google Scholar] [CrossRef] [PubMed]
- Roman, H.; Girault, T.; Barbier, F.; Péron, T.; Brouard, N.; Pěnčík, A.; Novák, O.; Vian, A.; Sakr, S.; Lothier, J.; et al. Cytokinins Are Initial Targets of Light in the Control of Bud Outgrowth. Plant Physiol. 2016, 172, 489–509. [Google Scholar] [CrossRef] [PubMed]
- Swarup, R.; Bhosale, R. Developmental Roles of AUX1/LAX Auxin Influx Carriers in Plants. Front. Plant Sci. 2019, 10, 1306. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Cheng, L.; Hu, G.; Guo, X.; Lan, Y. Auxin and CmAP1 regulate the reproductive development of axillary buds in Chinese chestnut (Castanea mollissima). Plant Cell Rep. 2023, 42, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Sherif, M.S. Hormonal Orchestration of Bud Dormancy Cycle in Deciduous Woody Perennials. Front. Plant Sci. 2019, 10, 01136. [Google Scholar] [CrossRef] [PubMed]
- Hao, X.; Tang, H.; Wang, B.; Wang, L.; Cao, H.; Wang, Y.; Zeng, J.; Fang, S.; Chu, J.; Yang, Y.; et al. Gene Characterization and Expression Analysis Reveal the Importance of Auxin Signaling in Bud Dormancy Regulation in Tea Plant. J. Plant Growth Regul. 2019, 38, 225–240. [Google Scholar] [CrossRef]
- Wang, X.; Jia, C.; An, L.; Zeng, J.; Ren, A.; Han, X.; Wang, Y.; Wu, S. Genome-wide identification and expression characterization of the GH3 gene family of tea plant (Camellia sinensis). BMC Genom. 2024, 25, 120. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Li, H.; Yu, J.; Zhao, H.; Zhang, K.; Ge, W. Regulatory Mechanisms of ArAux/IAA13 and ArAux/IAA16 in the Rooting Process of Acer rubrum. Genes 2023, 14, 1206. [Google Scholar] [CrossRef] [PubMed]
- Vert, G.; Walcher, C.; Chory, J.; Nemhauser, J. Integration of auxin and brassinosteroid pathways by Auxin Response Factor 2. Proc. Natl. Acad. Sci. USA 2008, 105, 9829–9834. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zhang, K.; Li, L. Identification and Expression Analysis of AUX1/LAX Gene Family in Moso Bamboo (Phyllostachys edulis). J. Northwest For. Univ. 2022, 37, 89–95. [Google Scholar]
- Peng, L.; Li, H.; Song, J.; Xie, W.; Zhang, L.; Li, S.; Cai, Y.; Zhao, Z. Morphological and transcriptome analyses reveal mechanism for efficient regeneration of adventitious buds from in vitro leaves of Rhododendron delavayi regulated by exogenous TDZ. In Vitro Cell. Dev. Biol. Plant 2022, 58, 1025–1037. [Google Scholar] [CrossRef]
- Ren, Z.; Liu, R.; Gu, W.; Dong, X. The Solanum lycopersicum auxin response factor SlARF2 participates in regulating lateral root formation and flower organ senescence. Plant Sci. Int. J. Exp. Plant Biol. 2017, 256, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Sun, H.; Li, J.; Gong, X.; Huang, S.; Zhu, X.; Zhang, Y.; Xu, G. Auxin distribution is differentially affected by nitrate in roots of two rice cultivars differing in responsiveness to nitrogen. Ann. Bot. 2013, 112, 1383–1393. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zheng, H.; Zhang, X.; Sui, N. Cytokinins as central regulators during plant growth and stress response. Plant Cell Rep. 2021, 40, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Dai, X.; Li, J.; Liu, N.; Liu, X.; Li, S.; Xiang, F. The Type-B Cytokinin Response Regulator ARR1 Inhibits Shoot Regeneration in an ARR12-Dependent Manner in Arabidopsis. Plant Cell 2020, 32, 2271–2291. [Google Scholar] [CrossRef] [PubMed]
- Ou, X.; Wang, Y.; Zhang, J.; Xie, Z.; He, B.; Jiang, Z.; Wang, Y.; Su, W.; Song, S.; Hao, Y.; et al. Identification of BcARR Genes and CTK Effects on Stalk Development of Flowering Chinese Cabbage. Int. J. Mol. Sci. 2022, 23, 7412. [Google Scholar] [CrossRef] [PubMed]
- To, J.P.; Haberer, G.; Ferreira, F.J.; Deruère, J.; Mason, M.G.; Schaller, G.E.; Alonso, J.M.; Ecker, J.R.; Kieber, J.J. Type-A Arabidopsis response regulators are partially redundant negative regulators of cytokinin signaling. Plant Cell 2004, 16, 658–671. [Google Scholar] [CrossRef] [PubMed]
- Yi, X.; Zhao, Y.; Huang, C.; Wang, H. Phenological Observation and Meteorological Conditions Analysis during 2019 Spring Tea growth in Nanjian. Agric. Technol. Equip. 2019, 4, 37–38+40. [Google Scholar]
- Yang, N.; Han, M.; Teng, R.; Yang, Y.; Wang, Y.; Xiong, A.; Zhuang, J. Exogenous Melatonin Enhances Photosynthetic Capacity and Related Gene Expression in A Dose-Dependent Manner in the Tea Plant (Camellia sinensis (L.) Kuntze). Int. J. Mol. Sci. 2022, 23, 6694. [Google Scholar] [CrossRef]
- Tian, Y.; Wang, H.; Sun, P.; Fan, Y.; Qiao, M.; Zhang, L.; Zhang, Z. Response of leaf color and the expression of photoreceptor genes of Camellia sinensis cv. Huangjinya to different light quality conditions. Sci. Hortic. 2019, 251, 225–232. [Google Scholar] [CrossRef]
- Sun, M.; Jiang, C.; Kong, Y.; Luo, J.; Yin, P.; Guo, G. Recent Advances in Analytical Methods for Determination of Polyphenols in Tea: A Comprehensive Review. Foods 2022, 11, 1425. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, K.; Zhao, Z.; Li, S.; Li, Y. A Novel LED Light Radiation Approach Enhances Growth in Green and Albino Tea Varieties. Plants 2023, 12, 988. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Wang, Y.; Wang, Y.; Hong, L.; Jia, X.; Kang, J.; Lin, S.; Wu, Z.; Wang, H. Improvement of soil acidification in tea plantations by long-term use of organic fertilizers and its effect on tea yield and quality. Front. Plant Sci. 2022, 13, 1055900. [Google Scholar] [CrossRef] [PubMed]
- Kijidani, Y.; Tsuyama, T.; Tokumoto, Y. Distribution of Plant Hormones and Their Precursors in Cambial Region Tissues of Quercus myrsinifolia and Castanopsis cuspidata var. sieboldii after Bending Stems or Applying Ethylene precursor. Forests 2023, 14, 813. [Google Scholar] [CrossRef]
- Zeng, L.; Wang, X.; Liao, Y.; Gu, D.; Dong, F.; Yang, Z. Formation of and changes in phytohormone levels in response to stress during the manufacturing process of oolong tea (Camellia sinensis). Postharvest Biol. Technol. 2019, 157, 110974. [Google Scholar] [CrossRef]
- Wang, P.; Zheng, Y.; Guo, Y.; Chen, X.; Sun, Y.; Yang, J.; Ye, N. Identification, expression, and putative target gene analysis of nuclear factor-Y (NF-Y) transcription factors in tea plant (Camellia sinensis). Planta 2019, 250, 1671–1686. [Google Scholar] [CrossRef] [PubMed]
- Pi, X.; Chang, N.; Zhou, Z.; Li, Y.; Zhang, X. CsFAD2 and CsFAD5 are key genes for C18:2 fatty acid pathway-mediated cold tolerance in tea (Camellia sinensis). Environ. Exp. Bot. 2023, 210, 105317. [Google Scholar] [CrossRef]





| Photoperiod Treatments | Budding | One Bud and One Leaf | One Bud and Two Leaves | One Bud and Three Leaves |
|---|---|---|---|---|
| Days | Days | Days | Days | |
| 12L/12D | 10 d | 16 d | 21 d | 27 d |
| 14L/10D | 9 d | 14 d | 19 d | 25 d |
| 16L/8D | 8 d | 12 d | 17 d | 24 d |
| 18L/6D | 8 d | 13 d | 18 d | 25 d |
| Photoperiod Treatments | Chlorophyll A (mg/g FW) | Chlorophyll B (mg/g FW) | Chlorophyll (A + B) (mg/g FW) |
|---|---|---|---|
| 12L/12D | 0.563 ± 0.011 b | 0.136 ± 0.020 b | 0.699 ± 0.013 d |
| 14L/10D | 0.575 ± 0.004 b | 0.159 ± 0.009 ab | 0.734 ± 0.011 c |
| 16L/8D | 0.649 ± 0.022 a | 0.189 ± 0.034 a | 0.838 ± 0.013 a |
| 18L/6D | 0.642 ± 0.015 a | 0.142 ± 0.009 b | 0.784 ± 0.010 b |
| Light Quality Treatments | Budding | One Bud and One Leaf | One Bud and Two Leaves | One Bud and Three Leaves |
|---|---|---|---|---|
| Days | Days | Days | Days | |
| Control | 8 d | 12 d | 17 d | 24 d |
| R/B 1:1 | 10 d | 14 d | 17 d | 26 d |
| R/B 1:2 | 7 d | 12 d | 16 d | 22 d |
| R/B 1:3 | 6 d | 10 d | 15 d | 21 d |
| R/B 2:1 | 5 d | 9 d | 14 d | 18 d |
| Light Quality Treatments | Control | R/B 1:1 | R/B 1:2 | R/B 1:3 | R/B 2:1 |
|---|---|---|---|---|---|
| GC | 5.16 ± 0.21 a | 3.99 ± 0.40 b | 3.60 ± 0.43 b | 3.43 ± 0.78 b | 3.68 ± 0.48 b |
| EGC | 34.47 ± 0.42 ab | 33.22 ± 1.64 bc | 32.20 ± 0.23 c | 29.86 ± 0.39 d | 35.00 ± 0.31 a |
| C | 0.96 ± 0.02 c | 0.95 ± 0.00 c | 1.78 ± 0.06 b | 2.21 ± 0.04 a | 1.80 ± 0.01 b |
| EC | 13.85 ± 0.10 ab | 13.37 ± 0.54 b | 12.00 ± 0.13 c | 12.35 ± 0.36 c | 14.40 ± 0.30 a |
| EGCG | 66.18 ± 0.18 b | 57.83 ± 0.37 e | 64.73 ± 0.87 c | 62.86 ± 0.49 d | 69.65 ± 1.39 a |
| GCG | 1.45 ± 0.40 c | 2.38 ± 0.28 b | 2.47 ± 0.04 b | 3.06 ± 0.03 a | 2.98 ± 0.05 a |
| ECG | 8.56 ± 0.46 bc | 10.52 ± 0.36 a | 9.16 ± 0.10 b | 8.28 ± 0.29 c | 10.76 ± 0.55 a |
| TC | 130.64 ± 0.74 b | 122.27 ± 0.62 d | 125.92 ± 0.85 c | 122.06 ± 1.20 d | 138.26 ± 2.15 a |
| Light Quality Treatments | Control | R/B 1:1 | R/B 1:2 | R/B 1:3 | R/B 2:1 |
|---|---|---|---|---|---|
| Number of R/B LED Tube Assemblies | / | 4/2 | 3/3 | 3/2 | 3/2 |
| Half-width HW/nm | 170.6 | 22.5 | 22.5 | 22.7 | 24.6 |
| Irradiance Ee (W/m2) | 53.31 | 42.0 | 33.8 | 36.1 | 34.4 |
| Light intensity range μmol−1 m−2 s−1 | 201~223 | 192~229 | 204~247 | 193~233 | 207~238 |
| Red light band share (R) | LED plant full spectrum light | 50% | 33% | 25% | 66% |
| Lue light band share (B) | LED plant full spectrum light | 50% | 66% | 75% | 33% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, G.; Li, X.; Yang, J.; Yuan, Q.; Yang, S.; Fu, W.; Zhang, X.; Li, Y.; Shen, Z.; Jiang, J. Effects of Photoperiod and Light Quality on Germination and Growth of Camellia sinensis ‘HuangKui’. Plants 2024, 13, 1782. https://doi.org/10.3390/plants13131782
Hu G, Li X, Yang J, Yuan Q, Yang S, Fu W, Zhang X, Li Y, Shen Z, Jiang J. Effects of Photoperiod and Light Quality on Germination and Growth of Camellia sinensis ‘HuangKui’. Plants. 2024; 13(13):1782. https://doi.org/10.3390/plants13131782
Chicago/Turabian StyleHu, Gan, Xingchen Li, Junlong Yang, Qingqing Yuan, Shijun Yang, Wenjun Fu, Xianchen Zhang, Yeyun Li, Zhougao Shen, and Jiayue Jiang. 2024. "Effects of Photoperiod and Light Quality on Germination and Growth of Camellia sinensis ‘HuangKui’" Plants 13, no. 13: 1782. https://doi.org/10.3390/plants13131782
APA StyleHu, G., Li, X., Yang, J., Yuan, Q., Yang, S., Fu, W., Zhang, X., Li, Y., Shen, Z., & Jiang, J. (2024). Effects of Photoperiod and Light Quality on Germination and Growth of Camellia sinensis ‘HuangKui’. Plants, 13(13), 1782. https://doi.org/10.3390/plants13131782




