Response to Climate Change and GAP Analysis of Thuja koraiensis Nakai
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Sources and Screening
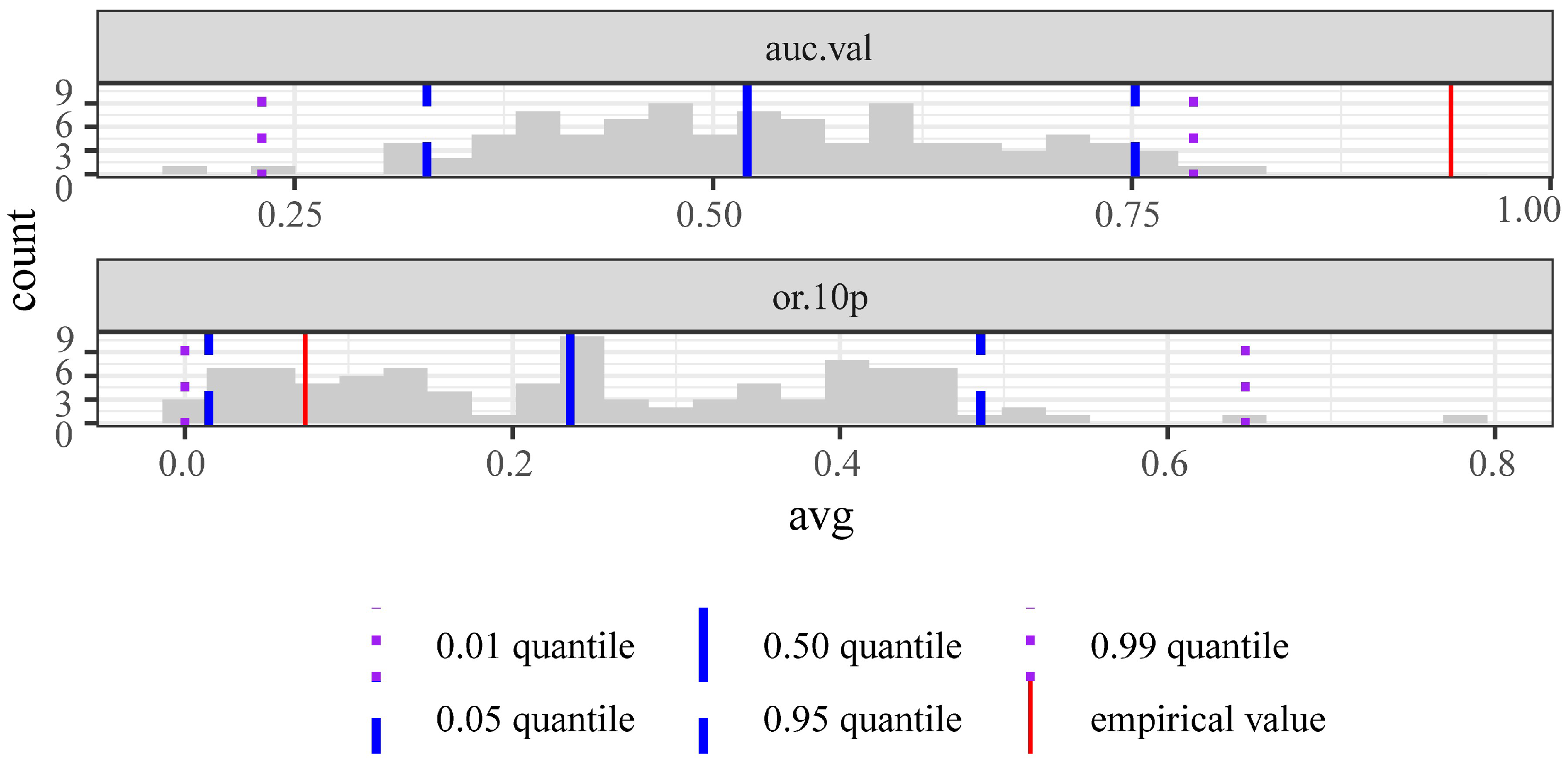
2.2. Maxent Model Construction
2.3. Marxan Model Construction
- = LDI ranking for landscape unit
- = percent of the total area of influence in land use
- = landscape development intensity coefficient for land use .
| Land Use Type | LDI Coefficient |
|---|---|
| Cultivated land | 4.54 |
| Forest | 1.58 |
| Grass land | 2.77 |
| Shrubland | 1.58 |
| Wetland | 1 |
| Water body | 1 |
| Artificial surfaces | 8.66 |
| Bareland | 6.92 |
| Permanent snow and Ice | 1 |
2.4. Data Analysis and Processing
3. Results
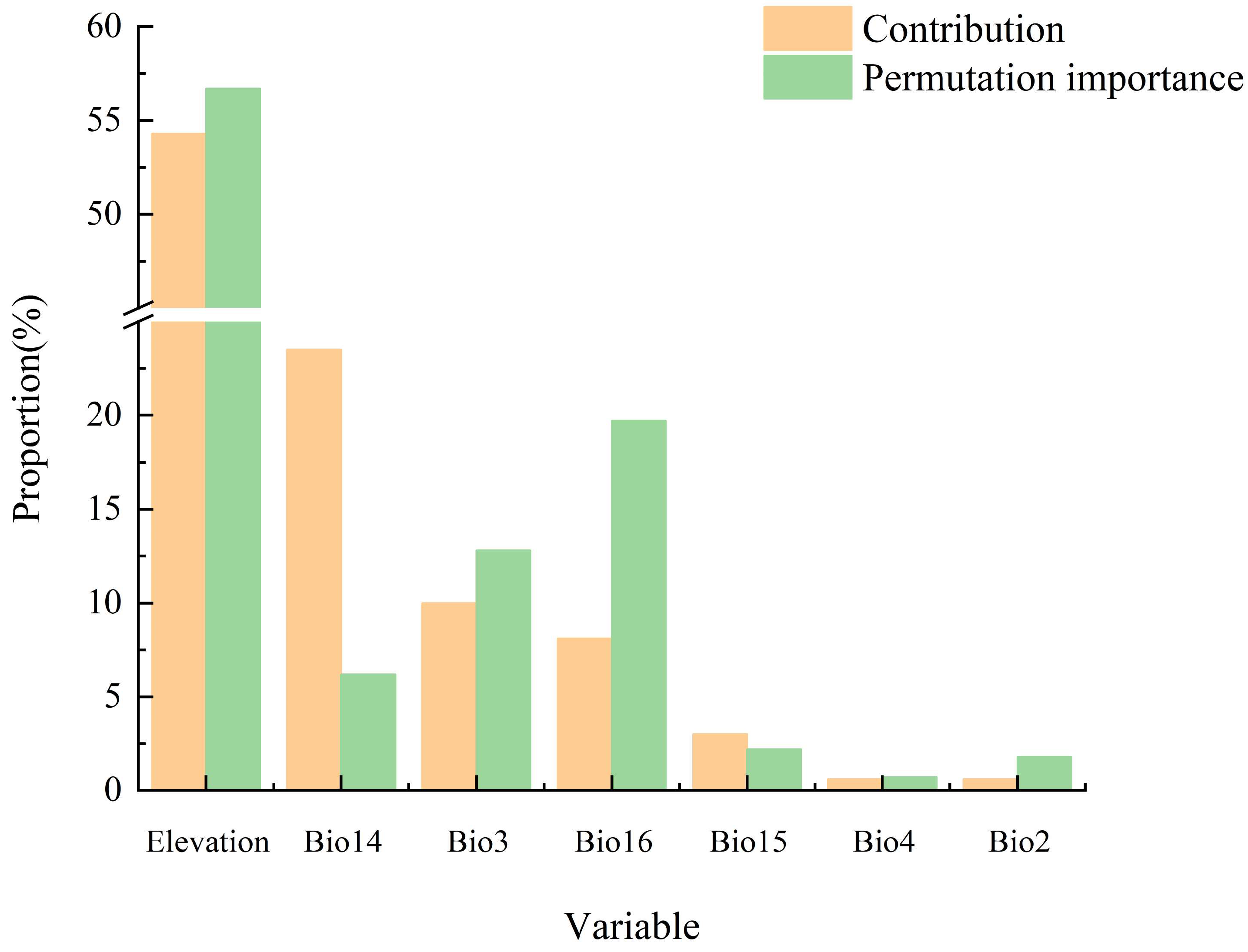
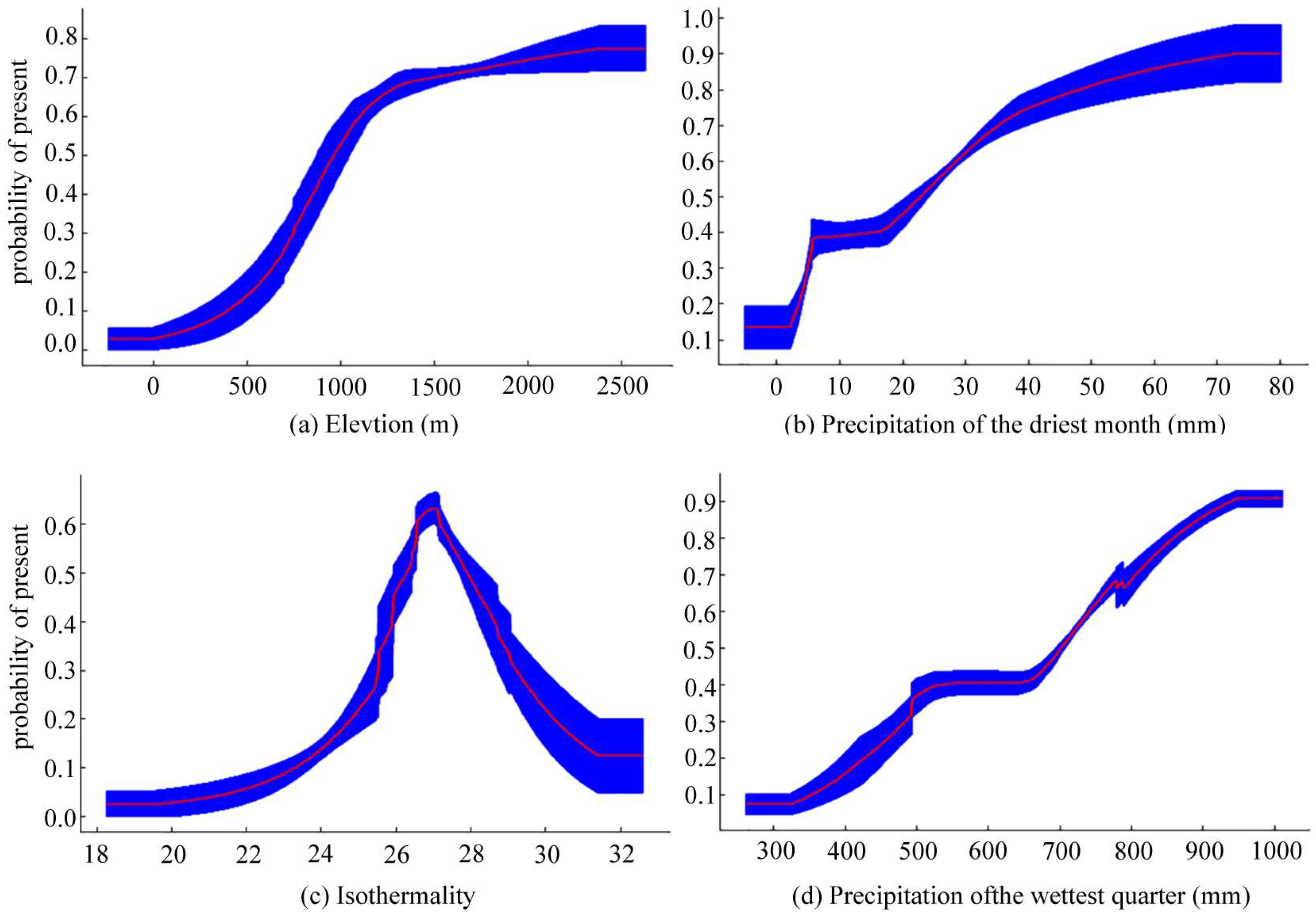
3.1. Importance Analysis on Environmental Factors
3.2. Prediction of Potentially Suitable Areas for T. koraiensis under Current Climatic Conditions
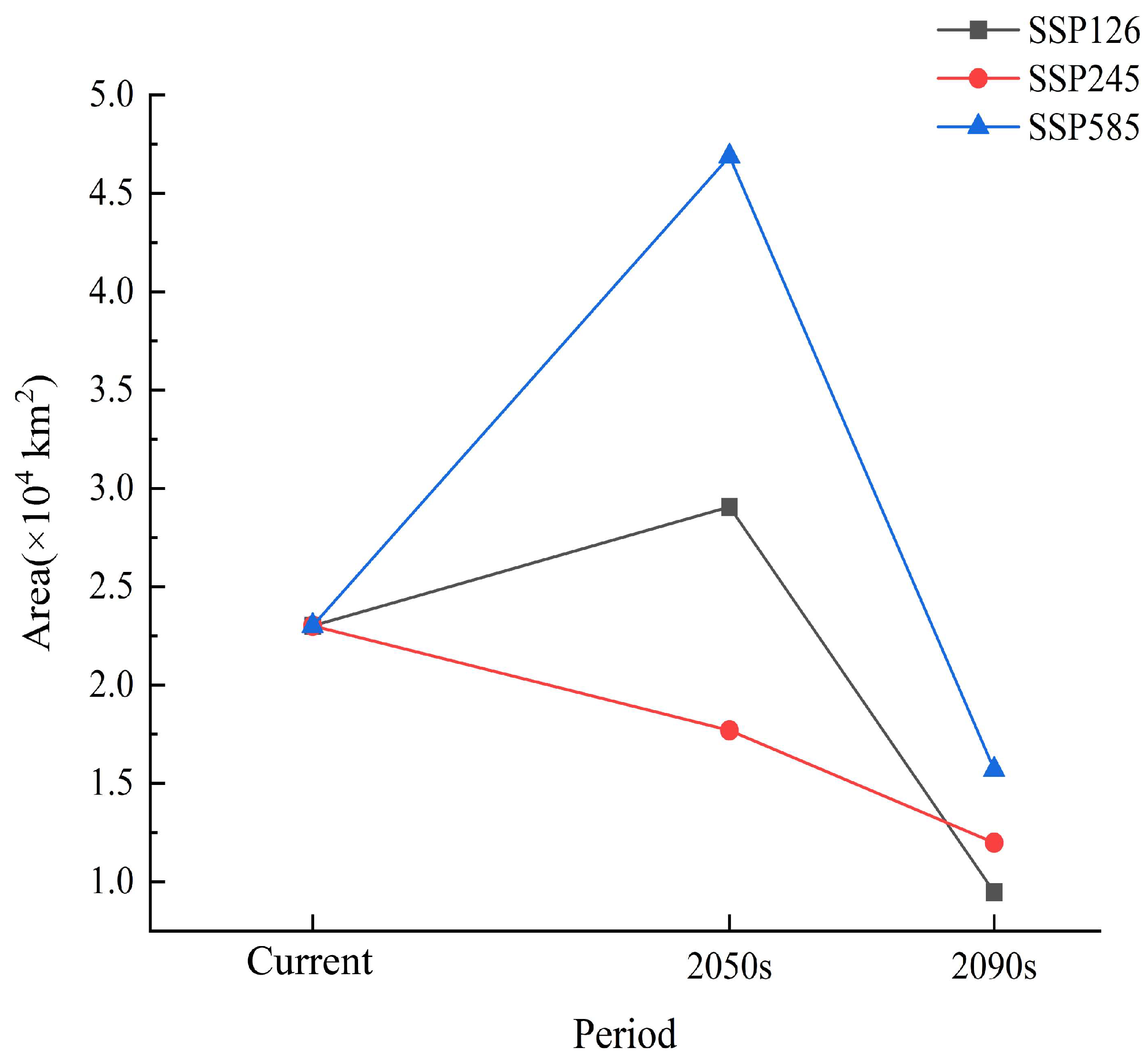
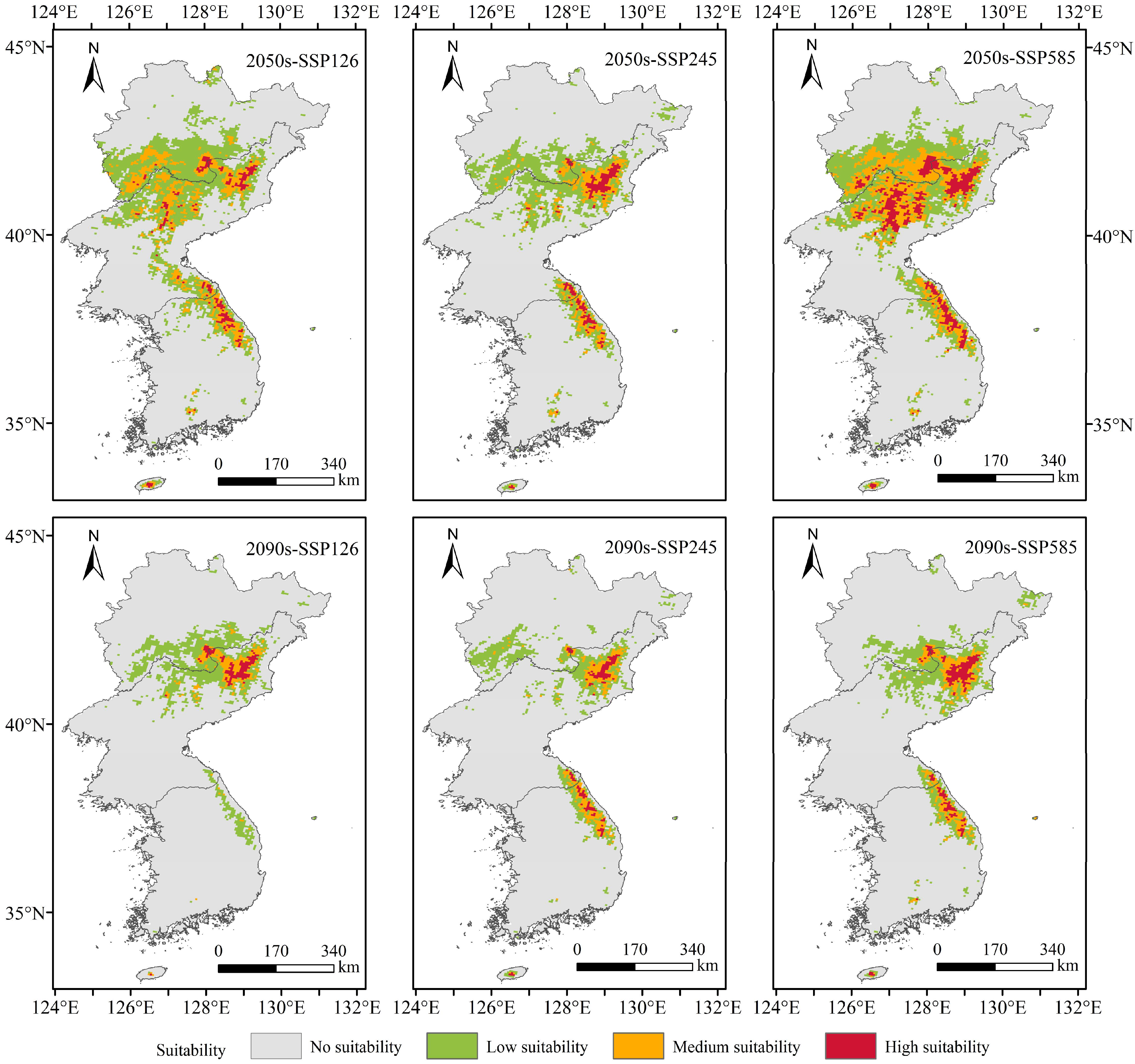
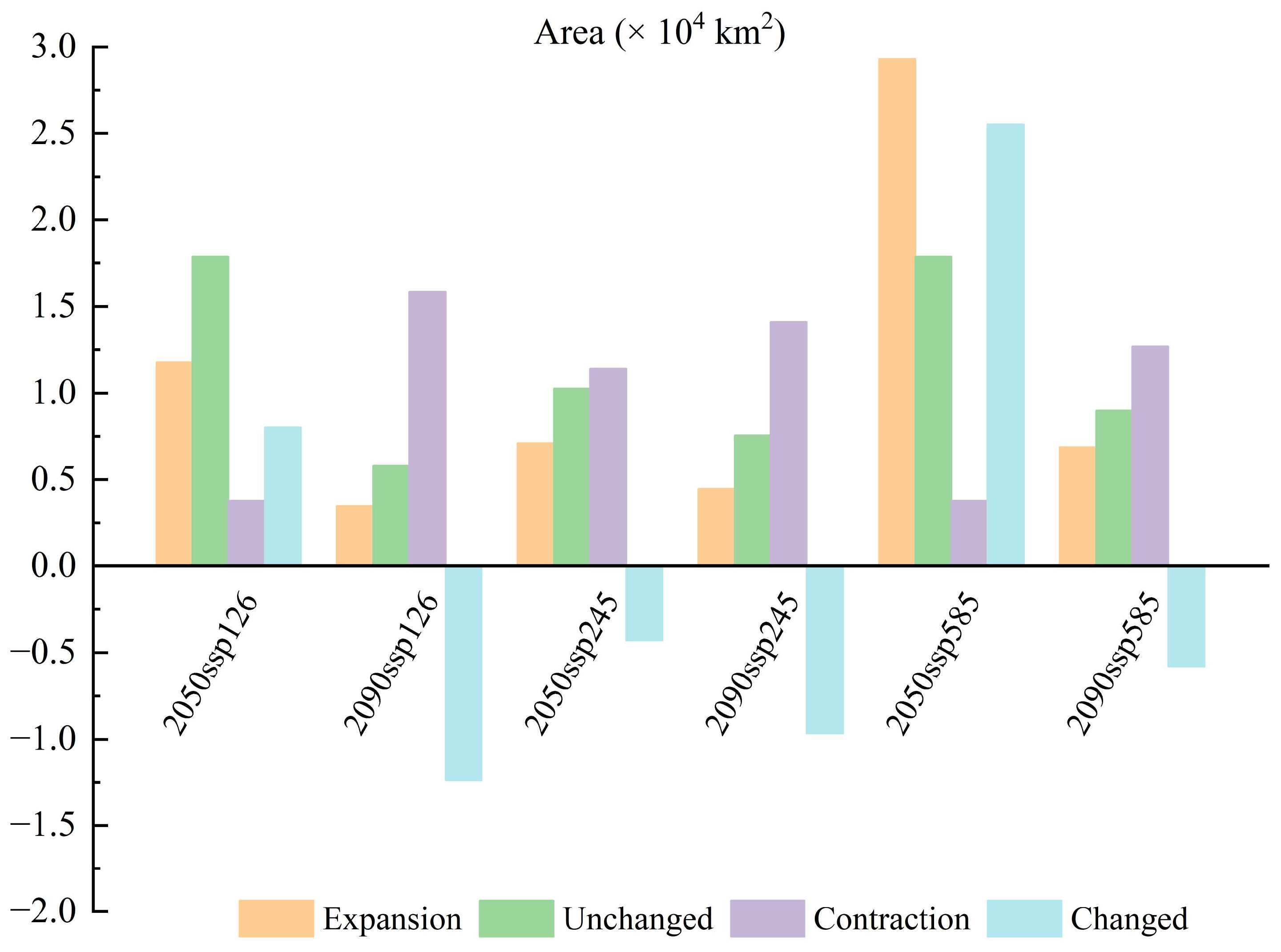
3.3. Potentially Suitable Areas of T. koraiensis and Change in Spatial Patterns under Future Climate Scenarios
3.4. GAP Analysis
4. Discussion
4.1. The Dominant Environmental Factors Influencing Potential Distribution of T. koraiensis
4.2. Influence of Climate Changes on the Geographical Distribution of T. koraiensis
4.3. Suggestions for the Protection of T. koraiensis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gallardo, B.; Aldridge, D.C. Evaluating the combined threat of climate change and biological invasions on endangered species. Biol. Conserv. 2013, 160, 225–233. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2021: The Physical Science Basis; Cambridge University Press: Cambridge, UK, 2021. [Google Scholar]
- Root, T.L.; Price, J.T.; Hall, K.R.; Schneider, S.H.; Rosenzweig, C.; Pounds, J.A. Fingerprints of global warming on wild animals and plants. Nature 2003, 421, 57–60. [Google Scholar] [CrossRef] [PubMed]
- Fischer, M.; Matthies, D.; Schmid, B. Responses of rare calcareous grassland plants to elevated CO2: A field experiment with Gentianella germanica and Gentiana cruciata. J. Ecol. 1997, 85, 681–691. [Google Scholar] [CrossRef]
- Ye, L.; Zhang, W.; Ye, X.; Liu, Y.; Zhang, G.; Liu, B.; Ruan, S. Prediction of Potential Distribution Area and Analysis of Dominant Environmental Variables of Davidia involucrate Based on Maxent. J. Sichuan Agric. Univ. 2021, 39, 604–612. [Google Scholar] [CrossRef]
- Zhu, G.; Liu, G.; Bu, W.; Gao, Y. Ecological niche modeling and its applications in biodiversity conservation. Biodivers. Sci. 2013, 21, 90–98. [Google Scholar]
- Anderson, R.P. A framework for using niche models to estimate impacts of climate change on species distributions. Ann. N. Y. Acad. Sci. 2013, 1297, 8–28. [Google Scholar] [CrossRef]
- Wang, Z.; Yin, J.; Wang, X.; Chen, X.; Mao, Z.; Lin, F.; Gong, Z.; Wang, X. Habitat suitability evaluation of invasive plant species Datura stramonium in Liaoning Province: Based on Biomod2 combination model. Chin. J. Appl. Ecol. 2023, 34, 1272–1280. [Google Scholar] [CrossRef]
- Du, Z.; Liu, W.; Cao, X.; Nie, X.; Wang, B.; Zhou, Y.; Liu, W.; Xu, X. Suitability analysis of wheat blast in the world and China under climate change scenarios based on MaxEnt. Plant Prot. 2022, 48, 158–166. [Google Scholar] [CrossRef]
- Bosso, L.; Rebelo, H.; Garonna, A.P.; Russo, D. Modelling geographic distribution and detecting conservation gaps in Italy for the threatened beetle Rosalia alpina. J. Nat. Conserv. 2013, 21, 72–80. [Google Scholar] [CrossRef]
- Xu, J.; Cao, B.; Bai, C. Prediction of potential suitable distribution of endangered plant Kingdonia uniflora in China with MaxEnt. Chin. J. Ecol. 2015, 34, 3354–3359. [Google Scholar] [CrossRef]
- Xiong, Z.; Zhang, X.; Zhou, X.; Zhao, Y.; Chen, X. Prediction of the suitable distribution and responses to climate change of Sorbus tianschanica in China. Ecol. Sci. 2019, 38, 44–51. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, Y.; Lu, X.; Shen, Z.; Wang, M.; Li, Q.; Wang, R. Climatic suitable area analysis and response to climate change of Actinidia arguta in China. Chin. J. Eco-Agric. 2020, 28, 1523–1532. [Google Scholar] [CrossRef]
- Guo, Y.; Zhao, Z.; Qiao, H.; Wang, R.; Wei, H.; Wang, L.; Gu, W.; Li, X. Challenges and Development Trend of Species Distribution Model. Adv. Earth Sci. 2020, 35, 1292–1305. [Google Scholar] [CrossRef]
- Chen, H.; Zheng, D.; Huang, C.; Mei, L.; Li, C.; Chen, Y.; Yin, H.; Peng, X.; Zhou, G.; Qin, L.; et al. Humanistic Geography; Science Press: Beijing, China, 2013. [Google Scholar]
- Yu, J.; Ma, X.; Feng, L. Urban bird diversity conservation plan based on the systematic conservation planning approach—A case study of Beijing Ecological Cultivation Area. Ecol. Indic. 2023, 156, 111082. [Google Scholar] [CrossRef]
- He, P.; Li, J.; Li, Y.; Xu, N.; Gao, Y.; Guo, Y.; Huo, T.; Peng, C.; Meng, F. Habitat protection and planning for three Ephedra using the MaxEnt and Marxan models. Ecol. Indic. 2021, 133, 108399. [Google Scholar] [CrossRef]
- Ardron, J.A.; Possingham, H.P.; Klein, C.J. Marxan Good Practices Handbook; Pacific Marine Analysis and Research Association: Vancouver, BC, Canada, 2008; 149p. [Google Scholar]
- Li, D.; Song, Y. Review on hot spot and GAP analysis. Biodivers. Sci. 2000, 8, 208–214. [Google Scholar]
- Yang, N.; Wang, Z.; Zhang, X.; Zhang, H. The Method and Advance of GAP Analysis. Biotechnol. Bull. 2008, 1, 100–107. [Google Scholar]
- Yang, Z.; Bai, Y.; Alatalo, J.M.; Huang, Z.; Yang, F.; Pu, X.; Wang, R.; Guo, X. Spatio-temporal variation in potential habitats for rare and endangered plants and habitat conservation based on the maximum entropy model. Sci. Total Environ. 2021, 784, 147080. [Google Scholar] [CrossRef]
- Burgess, N.; KÜper, W.; Mutke, J.; Brown, J.; Westaway, S.; Turpie, S.; Meshack, C.; Taplin, J.; McClean, C.; Lovett, J.C. Major gaps in the distribution of protected areas for threatened and narrow range Afrotropical plants. Biodivers. Conserv. 2005, 14, 1877–1894. [Google Scholar] [CrossRef]
- Allen, C.R.; Pearlstine, L.G.; Kitchens, W.M. Modeling viable mammal populations in gap analyses. Biol. Conserv. 2001, 99, 135–144. [Google Scholar] [CrossRef]
- Merchant, D.; Lathrop, R.G.; Santos, C.D.; Paludo, D.; Niles, L.; Smith, J.A.; Feigin, S.; Dey, A. Distribution modeling and gap analysis of shorebird conservation in northern Brazil. Remote Sens. 2023, 15, 452. [Google Scholar] [CrossRef]
- Sowa, S.P.; Annis, G.; Morey, M.E.; Diamond, D.D. A gap analysis and comprehensive conservation strategy for riverine ecosystems of Missouri. Ecol. Monogr. 2007, 77, 301–334. [Google Scholar] [CrossRef]
- Ni, Y.; Yang, W.; Liu, J.; Jang, Z. Karyomorphology of five Thuja species. For. Res. 2017, 30, 189–193. [Google Scholar] [CrossRef]
- Kong, W.S. Selection of vulnerable indicator plants by global warming. Asia-Pac. J. Atmos. Sci. 2005, 41, 263–273. [Google Scholar]
- Yuan, J.; Yu, Z.; Li, C.; Wang, M.; Zhou, M.; Du, F. Research Status and Conservation Strategy of Endangered Species Thuja koraiensis Nakai. J. Anhui Agric. Sci. 2019, 47, 135–137. [Google Scholar]
- Yuan, J.; Yu, Z.; Lan, X.; Li, C.; Tian, J.; Du, F. Effects of shading treatments on photosynthetic characteristics of endangered plant Thuja koraiensis. J. Nanjing For. Univ. Nat. Sci. Ed. 2022, 46, 58–66. [Google Scholar] [CrossRef]
- Wang, G.; Xia, F.; Liu, B.; Sun, Y.; Mu, H. Habitat and Height Growth Rhythm of Thuja koraiensis. J. Beihua Univ. (Nat. Sci.) 2017, 18, 312–314. [Google Scholar]
- Jin, H.; Zhao, Y.; Liu, L.; Jia, X.; Dai, Y.; Qin, L.; Wang, C.; Yin, H. Quantitative characteristics and population dynamics of the endangered plant Thuja koraiensis in Changbai Mountain, China. Chin. J. Appl. Ecol. 2019, 30, 1563–1570. [Google Scholar] [CrossRef]
- Yang, C.; Zhang, H.; Bai, Y.; Yang, T. The complete chloroplast genome sequence of Thuja koraiensis from Changbai Mountain in China. Mitochondrial DNA Part B 2020, 5, 947–948. [Google Scholar] [CrossRef]
- Yin, H.; Zhao, Y.; Cui, K.; Liu, L.; Yu, C.; Chen, Q.; Dai, Y.; Zhao, W. Asexual Reproducition Technique of Thuja koraiensis Nakai. Chin. Wild Plant Resour. 2013, 32, 68–69. [Google Scholar] [CrossRef]
- Wang, H.; Deng, Z.; Huang, Z.; Li, R. Structural Research on Secondary Xylem of Stem of Thuja koraiensis Nakai under SEM. J. Tonghua Norm. Univ. 2007, 145, 22–24. [Google Scholar]
- Wang, J.; Lv, W.; Wang, R.; Mao, D.; Wang, H.; Du, F. Function and Application of Essential Oil of Thuja koraiensis. J. Temp. For. Res. 2022, 5, 67–69. [Google Scholar] [CrossRef]
- Lan, X.; Fu, C.; Li, L.; Yuan, M.; Tan, T.; Wang, M.; Wang, Y.; Du, F. Prediction of Potential Suitable Distribution Areas in China for Thuja koraiensis Nakai Based on MaxEnt Mode. J. Beihua Univ. Nat. Sci. 2021, 22, 292–298. [Google Scholar]
- Lee, S.; Jung, H.; Choi, J. Projecting the impact of climate change on the spatial distribution of six subalpine tree species in South Korea using a multi-model ensemble approach. Forests 2020, 12, 37. [Google Scholar] [CrossRef]
- Xu, J.; Ou, G.; Xiao, J.; Wang, B.; Sun, R. The Potential Suitable Area of The Relic Plant Craigia yunnanensis in Yunnan Province. J. West China For. Sci. 2024, 53, 29–37. [Google Scholar] [CrossRef]
- Sorbe, F.; Gränzig, T.; Förster, M. Evaluating sampling bias correction methods for invasive species distribution modeling in maxent. Ecol. Inform. 2023, 76, 102124. [Google Scholar] [CrossRef]
- Arriaga-Flores, J.C.; Rodríguez-Moreno, A.; Correa-Sandoval, A.; Horta-Vega, J.V.; Castro-Arellano, I.; Vázquez-Reyes, C.J.; Venegas-Barrera, C.S. Spatial and environmental variation in phyllostomid bat (Chiroptera, Phyllostomidae) distribution in Mexico. Anim. Biodivers. Conserv. 2018, 41, 141–159. [Google Scholar] [CrossRef]
- Ye, X.; Zhang, M.; Lai, W.; Yang, M.; Fan, H.; Zhang, G.; Chen, S.; Liu, B. Prediction of potential suitable distribution of Phoebe bournei based on MaxEnt optimization model. Acta Ecol. Sin. 2021, 41, 8135–8144. [Google Scholar] [CrossRef]
- Bohl, C.L.; Kass, J.M.; Anderson, R.P. A new null model approach to quantify performance and significance for ecological niche models of species distributions. J. Biogeogr. 2019, 46, 1101–1111. [Google Scholar] [CrossRef]
- Zuo, X. Identifying and Protection Efficiency Analysis of Species and Ecosystem Priority Conservation Areas in Yunnan Province. Master’s Thesis, Yunnan University, Kunming, China, 2020. [Google Scholar]
- Xu, Y.; Xu, X.; Tang, Q. Human activity intensity of land surface: Concept, methods and application in China. J. Geogr. Sci. 2016, 26, 1349–1361. [Google Scholar] [CrossRef]
- Yuan, C. Discussion on Boundary Optimizationstrategy of Jinfo Mountain Nature Reservebased on Systematic Protection Planning. Master’s Thesis, Southwest University, Chongqing, China, 2024. [Google Scholar]
- Yongxiu, S.; Shiliang, L.; Fangning, S.; Yi, A.; Mingqi, L.; Yixuan, L. Spatio-temporal variations and coupling of human activity intensity and ecosystem services based on the four-quadrant model on the Qinghai-Tibet Plateau. Sci. Total Environ. 2020, 743, 140721. [Google Scholar] [CrossRef]
- Brown, M.T.; Vivas, M.B. Landscape development intensity index. Environ. Monit. Assess. 2005, 101, 289–309. [Google Scholar] [CrossRef]
- Nematollahi, S.; Fakheran, S.; Jafari, A.; Pourmanafi, S.; Kienast, F. Applying a systematic conservation planning tool and ecological risk index for spatial prioritization and optimization of protected area networks in Iran. J. Nat. Conserv. 2022, 66, 126144. [Google Scholar] [CrossRef]
- Mou, X.; Rao, S.; Zhang, X. Evaluation of Biodiversity Conservation Priority Area and Nature Reserve System Optimization in County Level Area: A Case Study of Wuyishan City. J. Ecol. Rural. Environ. 2021, 37, 769–777. [Google Scholar] [CrossRef]
- He, Y.; Ma, J.; Chen, G. Potential geographical distribution and its multi-factor analysis of Pinus massoniana in China based on the maxent model. Ecol. Indic. 2023, 154, 110790. [Google Scholar] [CrossRef]
- Wang, X.; Ren, Y.; Huang, Q.; Deng, H. Habitat suitability assessment of endangered plant Alsophila spinulosa in Chishui River area based on GlS and Maxent model. Acta Ecol. Sin. 2021, 41, 6123–6133. [Google Scholar] [CrossRef]
- Lee, S.J.; Byeon, J.G.; Kim, J.S.; Cho, J.H.; Oh, S.H. Modeling Habitat Suitability of the Climate-vulnerable Plant Thuja koraiensis in Response to Climate Change. Sens. Mater. 2024, 36, 1511–1523. [Google Scholar] [CrossRef]
- Zhang, P. Protection of Wild Resources of Thuja koraiensis sutchuen. Agric. Dev. Equip. 2019, 215, 49. [Google Scholar]
- Wang, R.; Li, Q.; Feng, C.; Shi, Z. Predicting potential ecological distribution of Locusta migratoria tibetensis in China using MaxEnt ecological niche modeling. Acta Ecol. Sin. 2017, 37, 8556–8566. [Google Scholar] [CrossRef]
- Wu, Z.; Jin, Y.; Liu, J.; Shang, L.; Zhao, D. Response of Vegetation Distribution to Global Climate Change in Northeast China. Sci. Geogr. Sin. 2003, 5, 564–570. [Google Scholar]
- Yuan, J.; Yu, Z.; Gao, C.; Li, C.; Zhou, B.; Wang, M.; Li, M.; Sun, H.; Zhou, M.; Du, F. Preliminary Study on the Morphology Quality and Germination Characteristics of Thuja koraiensis Seed. J. Jilin For. Sci. Technol. 2019, 48, 1–3+48. [Google Scholar] [CrossRef]
- Liao, J.; Yang, C.; Shao, Q.; Sun, Q.; Han, Y. Construction of an ecological model of Sambucus javanica blume in China under different climate scenarios based on maxent model. Plant Ecol. 2023, 224, 221–237. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, R.; Zhou, X.; Zhang, X.; Zhao, G.; Zhang, F. Prediction of potential distribution areas and priority protected areas of Agastache rugosa based on Maxent model and Marxan model. Front. Plant Sci. 2023, 14, 1200796. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Shao, W.; Jiang, J. Predicting the potential global distribution of Sapindus mukorossi under climate change based on MaxEnt modelling. Environ. Sci. Pollut. Res. 2022, 29, 21751–21768. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Lai, W.; Wen, G.; Jiang, T.; Zhu, X.; Lv, Z.; Zhang, G. Preciction of the Potentia y Suitaple Area of Fraxinus mandshurica Based on Maxent Model. J. Northwest For. Univ. 2022, 37, 149–156. [Google Scholar] [CrossRef]
- Wen, G.; Ye, X.; Shi, C.; Lai, W.; Liu, B.; Jiang, T.; Zhu, X.; Zhang, G. Potential suitable area of Altingia multinervis predicted by optimizated MaxEnt mode. Guihaia 2022, 42, 363–372. [Google Scholar] [CrossRef]
- Ouyang, Z.; Xu, W. Integrating nature protection system and establishing national parks under leqislation. Biodivers. Sci. 2014, 22, 425–427. [Google Scholar]
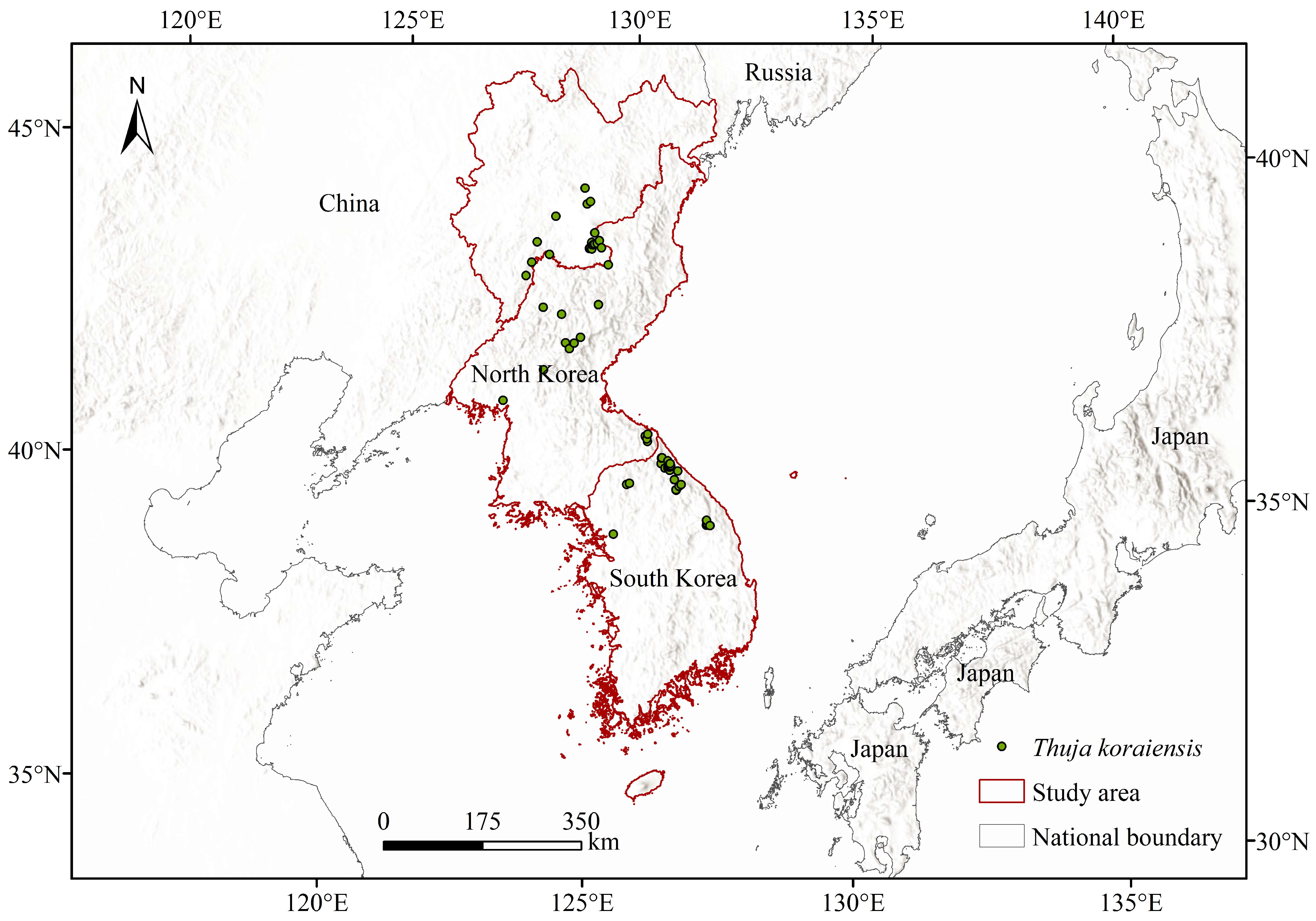



| Data Name | Resolution | Data Source Website |
|---|---|---|
| Distribution data | / | Chinese Virtual Herbarium database (https://www.cvh.ac.cn/), Global Biodiversity Information Facility (https://www.gbif.org/), Documentation |
| Historical climate data | 1 km × 1 km | Worldclim (https://worldclim.org/) |
| Future climate data | 5 km × 5 km | Worldclim (https://worldclim.org/) |
| Elevation | 1 km × 1 km | Worldclim (https://worldclim.org/) |
| Protected areas | / | Protected Planet (https://www.protectedplanet.net/) |
| Population density | 1 km × 1 km | Worldpop (https://hub.worldpop.org/) |
| Road density | / | OpenStreetMap (https://www.openstreetmap.org/) |
| Global Impervious Surface | 1 km × 1 km | Zenodo (https://zenodo.org/record/5220816, accessed on 15 December 2022) |
| Land utilization | 30 m × 30 m | National Catalogue Service For Geographic Information (https://www.webmap.cn/mapDataAction.do?method=globalLandCover, accessed on 15 December 2022) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, X.; Li, X.; Cui, J.; Liu, R.; Li, J.; Yang, C. Response to Climate Change and GAP Analysis of Thuja koraiensis Nakai. Plants 2024, 13, 1750. https://doi.org/10.3390/plants13131750
Yang X, Li X, Cui J, Liu R, Li J, Yang C. Response to Climate Change and GAP Analysis of Thuja koraiensis Nakai. Plants. 2024; 13(13):1750. https://doi.org/10.3390/plants13131750
Chicago/Turabian StyleYang, Xiuhua, Xiaoyu Li, Jiaqi Cui, Ruiqi Liu, Jitong Li, and Chengjun Yang. 2024. "Response to Climate Change and GAP Analysis of Thuja koraiensis Nakai" Plants 13, no. 13: 1750. https://doi.org/10.3390/plants13131750
APA StyleYang, X., Li, X., Cui, J., Liu, R., Li, J., & Yang, C. (2024). Response to Climate Change and GAP Analysis of Thuja koraiensis Nakai. Plants, 13(13), 1750. https://doi.org/10.3390/plants13131750







