First Gynogenesis of Vanilla planifolia for Haploid Production and Ploidy Verification Protocol
Abstract
1. Introduction
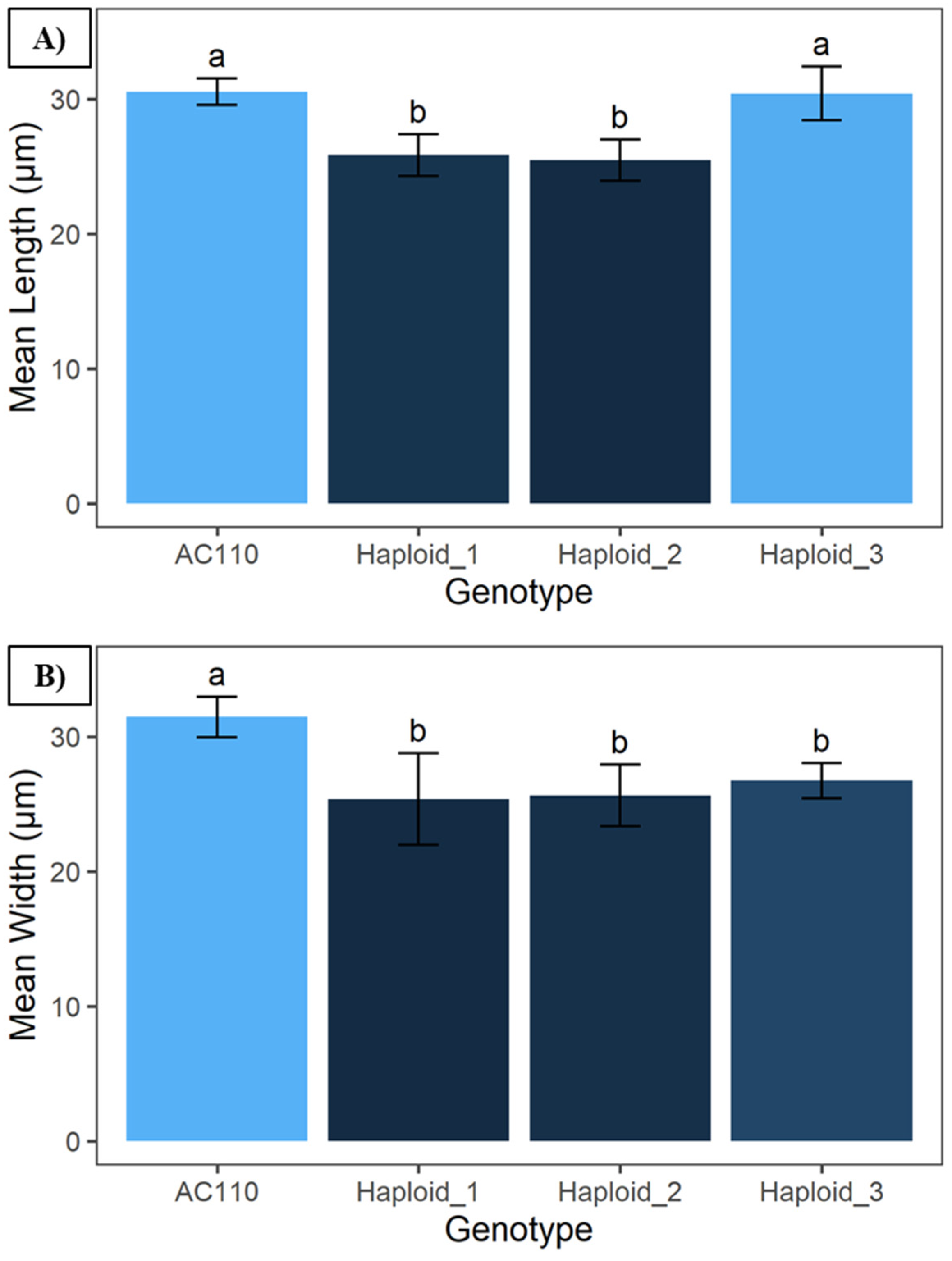
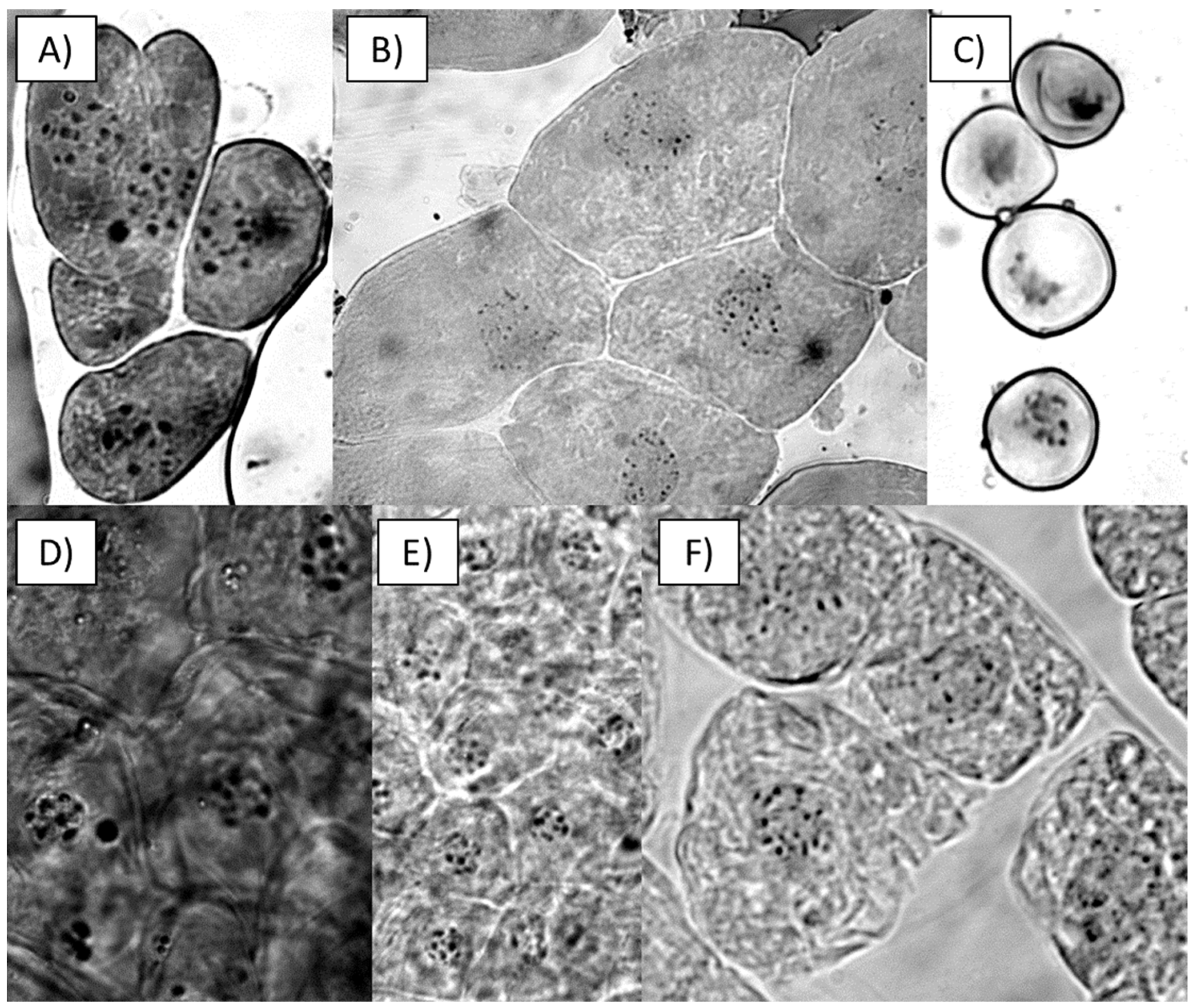
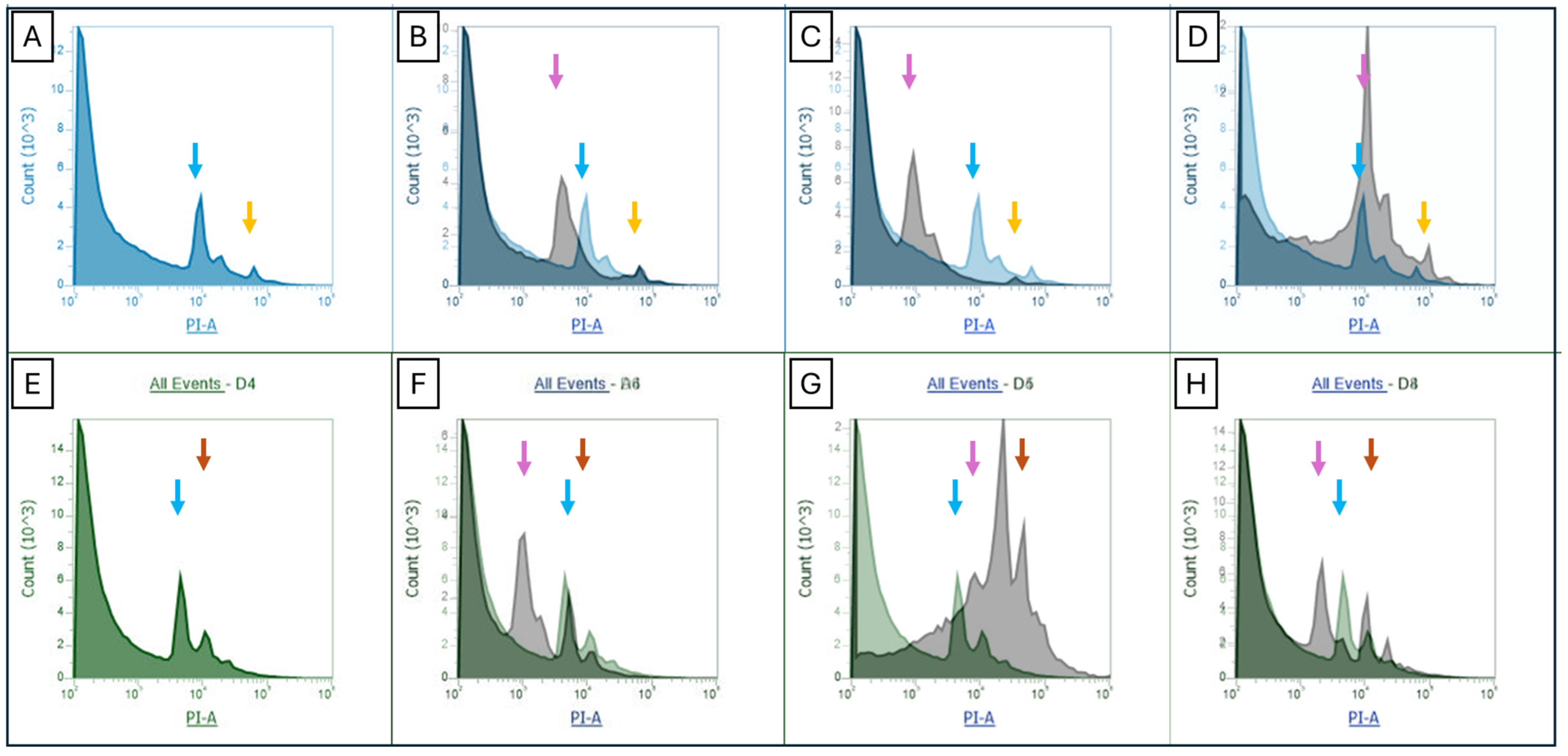
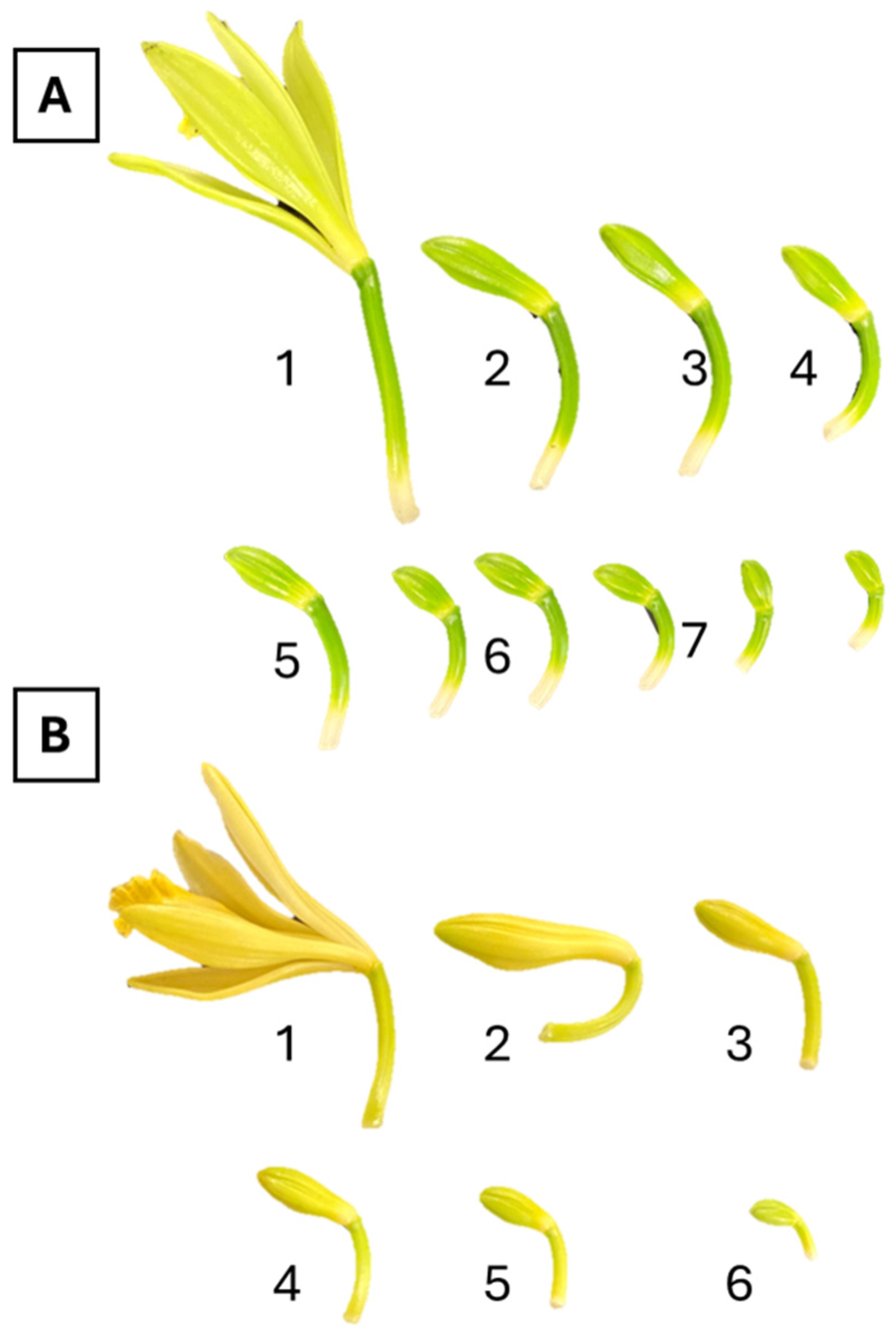
2. Results
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Chromosome Counting
4.3. Stomatal Morphology Comparison
4.4. Flow Cytometry Genome Size Estimation
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Menz, K.M.; Fleming, E.M. Economic Prospects for Vanilla in the South Pacific. In ACIAR Technical Reports; Australian Centre for International Agricultural Research: Canberra, Australia, 1989. [Google Scholar]
- Erawati, D.N.; Wardati, I.; Humaida, S.; Fisdiana, U. Micropropagation of Vanilla (Vanilla planifolia Andrews) with Modification of Cytokinins. IOP Conf. Ser. Earth Environ. Sci. 2020, 411, 012009. [Google Scholar] [CrossRef]
- Chambers, A.; Cibrián-Jaramillo, A.; Karremans, A.P.; Martinez, D.M.; Hernandez-Hernandez, J.; Brym, M.; Resende, M.F.; Moloney, R.; Sierra, S.N.; Hasing, T.; et al. Genotyping-By-Sequencing diversity analysis of international Vanilla collections uncovers hidden diversity and enables plant improvement. Plant Sci. 2021, 311, 111019. [Google Scholar] [CrossRef]
- Koyyappurath, S.; Atuahiva, T.; Le Guen, R.; Batina, H.; Le Squin, S.; Gautheron, N.; Hermann, V.E.; Peribe, J.; Jahiel, M.; Steinberg, C.; et al. Fusarium oxysporum f. sp. radicis-vanillae is the causal agent of root and stem rot of vanilla. Plant Pathol. 2015, 65, 612–625. [Google Scholar] [CrossRef]
- Ramos-Castellá, A.; Iglesias-Andreu, L.G.; Bello-Bello, J.; Lee-Espinosa, H. Improved propagation of vanilla (Vanilla planifolia Jacks. ex Andrews) using a temporary immersion system. Vitr. Cell. Dev. Biol.-Plant 2014, 50, 576–581. [Google Scholar] [CrossRef]
- Dwivedi, S.L.; Britt, A.B.; Tripathi, L.; Sharma, S.; Upadhyaya, H.D.; Ortiz, R. Haploids: Constraints and opportunities in plant breeding. Biotechnol. Adv. 2015, 33, 812–829. [Google Scholar] [CrossRef]
- Cobb, J.N.; Juma, R.U.; Biswas, P.S.; Arbelaez, J.D.; Rutkoski, J.; Atlin, G.; Hagen, T.; Quinn, M.; Ng, E.H. Enhancing the rate of genetic gain in public-sector plant breeding programs: Lessons from the breeder’s equation. Theor. Appl. Genet. 2019, 132, 627–645. [Google Scholar] [CrossRef] [PubMed]
- Indrianto, A.; Siregar, C.; Linuhung, S.; Sartikoningsih, T. Induction of sporophytic division in orchids microspores by stress. KnE Life Sci. 2015, 2, 390. [Google Scholar] [CrossRef]
- Jaswal, T.; Kaur, S. A Review on In-vitro Haploid Production in Orchids. J. Pharm. Res. Int. 2021, 33, 15–19. [Google Scholar] [CrossRef]
- Vilcherrez-Atoche, J.A.; Iiyama, C.M.; Cardoso, J.C. Polyploidization in Orchids: From Cellular Changes to Breeding Applications. Plants 2022, 11, 469. [Google Scholar] [CrossRef]
- Kunakh, V.A.; Adonin, V.I.; Ozheredov, S.P.; Blyum, Y.B. Mixoploidy in wild and cultivated species of Cruciferae capable of hybridizing with rapeseed Brassica napus. Cytol. Genet. 2008, 42, 204–209. [Google Scholar] [CrossRef]
- Mochizuki, A.; Sueoka, N. Genetic Studies on the Number of Plastid in Stomata I. Effects of autopolyploidy in sugar beets. Cytologia 1955, 20, 358–365. [Google Scholar] [CrossRef]
- Lawson, T. Guard cell photosynthesis and stomatal function. New Phytol. 2009, 181, 13–34. [Google Scholar] [CrossRef] [PubMed]
- Kurtar, E.S.; Balkaya, A. Production of in vitro haploid plants from in situ induced haploid embryos in winter squash (Cucurbita maxima Duchesne ex Lam.) via irradiated pollen. Plant Cell Tissue Organ Cult. 2010, 102, 267–277. [Google Scholar] [CrossRef]
- Parsons, J.L.; Martin, S.L.; James, T.; Golenia, G.; Boudko, E.A.; Hepworth, S.R. Polyploidization for the Genetic Improvement of Cannabis sativa. Front. Plant Sci. 2019, 10, 476. [Google Scholar] [CrossRef] [PubMed]
- Watts, A.; Bondada, R.; Maruthachalam, R. Identification of Arabidopsis thaliana haploid plants by counting the chloroplast numbers in stomatal guard cells. Plant Physiol. Rep. 2023, 28, 180–184. [Google Scholar] [CrossRef]
- Lin, Y.-J.; Chen, Y.-C.; Tseng, K.-C.; Chang, W.-C.; Ko, S.-S. Phototropins Mediate Chloroplast Movement in Phalaenopsis aphrodite (Moth Orchid). Plant Cell Physiol. 2019, 60, 2243–2254. [Google Scholar] [CrossRef] [PubMed]
- Bory, S.; Catrice, O.; Brown, S.; Leitch, I.J.; Gigant, R.; Chiroleu, F.; Grisoni, M.; Duval, M.-F.; Besse, P. Natural polyploidy in Vanilla planifolia (Orchidaceae). Genome 2008, 51, 816–826. [Google Scholar] [CrossRef]
- Piet, Q.; Droc, G.; Marande, W.; Sarah, G.; Bocs, S.; Klopp, C.; Bourge, M.; Siljak-Yakovlev, S.; Bouchez, O.; Lopez-Roques, C.; et al. A chromosome-level, haplotype-phased Vanilla planifolia genome highlights the challenge of partial endoreplication for accurate whole-genome assembly. Plant Commun. 2022, 3, 100330. [Google Scholar] [CrossRef]
- Piosik, Ł.; Zenkteler, E.; Zenkteler, M. Development of haploid embryos and plants of Lactuca sativa induced by distant pollination with Helianthus annuus and H. tuberosus. Euphytica 2016, 208, 439–451. [Google Scholar] [CrossRef]
- Ochatt, S.J.; Patat-Ochatt, E.M.; Moessner, A. Ploidy level determination within the context of in vitro breeding. Plant Cell Tissue Organ Cult. 2011, 104, 329–341. [Google Scholar] [CrossRef]
- Hasing, T.; Tang, H.; Brym, M.; Khazi, F.; Huang, T.; Chambers, A.H. A phased Vanilla planifolia genome enables genetic improvement of flavour and production. Nat. Food 2020, 1, 811–819. [Google Scholar] [CrossRef] [PubMed]
- Almejo Vázquez, L.; Iglesias Andreu, L.; Escobedo Gracia-Medrano, R.M. Comportamiento meiótico en vainilla (V. planifolia jacks. orchidaceae). Acta Biol. Colomb. 2022, 27, 458–463. [Google Scholar] [CrossRef]
- Brown, S.C.; Bourge, M.; Maunoury, N.; Wong, M.; Bianchi, M.W.; Lepers-Andrzejewski, S.; Besse, P.; Siljak-Yakovlev, S.; Dron, M.; Satiat-Jeunemaître, B. DNA Remodeling by Strict Partial Endoreplication in Orchids, an Original Process in the Plant Kingdom. Genome Biol. Evol. 2017, 9, 1051–1071. [Google Scholar] [CrossRef] [PubMed]
- Joubès, J.; Chevalier, C. Endoreduplication in Higher Plants. In The Plant Cell Cycle; Springer Science & Business Media: Berlin, Germany, 2000; pp. 191–201. [Google Scholar] [CrossRef]
- Manzoor, A.; Ahmad, T.; Bashir, M.; Hafiz, I.; Silvestri, C. Studies on Colchicine Induced Chromosome Doubling for Enhancement of Quality Traits in Ornamental Plants. Plants 2019, 8, 194. [Google Scholar] [CrossRef] [PubMed]
- Kermani, M.J.; Sarasan, V.; Roberts, A.V.; Yokoya, K.; Wentworth, J.; Sieber, V.K. Oryzalin-induced chromosome doubling in Rosa and its effect on plant morphology and pollen viability. Theor. Appl. Genet. 2003, 107, 1195–1200. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Dong, B.; Jiang, J.; Fang, W.; Guan, Z.; Liao, Y.; Chen, S.; Chen, F. Characterization of in vitro haploid and doubled haploid Chrysanthemum morifolium plants via unfertilized ovule culture for phenotypical traits and DNA methylation pattern. Front. Plant Sci. 2014, 5, 738. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, B.; Ebrahimzadeh, H. In vitro androgenesis: Spontaneous vs. artificial genome doubling and characterization of regenerants. Plant Cell Rep. 2020, 39, 299–316. [Google Scholar] [CrossRef] [PubMed]
- Campion, B.; Alloni, C. Induction of haploid plants in onion (Allium cepa L.) by in vitro culture of unpollinated ovules. Plant Cell Tissueand Organ Cult. 1990, 20, 1–6. [Google Scholar] [CrossRef]
- Ouyang, J.-W.; Liang, H.; Jia, S.-E.; Zhang, C.; Zhao, T.-H.; He, L.-Z.; Jia, X. Studies on the chromosome doubling of wheat pollen plants. Plant Sci. 1994, 98, 209–214. [Google Scholar] [CrossRef]
- Chen, W.H.; Tang, C.Y.; Kao, Y.L. Ploidy doubling by in vitro culture of excised protocorms or protocorm-like bodies in Phalaenopsis species. Plant Cell Tissue Organ Cult. 2009, 98, 229–238. [Google Scholar] [CrossRef]
- Vilcherrez-Atoche, J.A.; Silva, J.C.; Clarindo, W.R.; Mondin, M.; Cardoso, J.C. In vitro Polyploidization of Brassolaeliocattleya Hybrid Orchid. Plants 2023, 12, 281. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.K. Chromosome Techniques; Taylor & Francis Group: Boca Raton, FL, USA, 1994. [Google Scholar]
- Cox, A.V.; Abdelnour, G.J.; Bennett, M.D.; Leitch, I.J. Genome size and karyotype evolution in the slipper orchids (Cypripedioideae: Orchidaceae). Am. J. Bot. 1998, 85, 681–687. [Google Scholar] [CrossRef] [PubMed]
- Preeda, N.; Yanagi, T.; Sone, K.; Taketa, S.; Okuda, N. Chromosome observation method at metaphase and pro-metaphase stages in diploid and octoploid strawberries. Sci. Hortic. 2007, 114, 133–137. [Google Scholar] [CrossRef]
- Windham, M.D.; Pryer, K.M.; Poindexter, D.B.; Li, F.; Rothfels, C.J.; Beck, J.B. A step-by-step protocol for meiotic chromosome counts in flowering plants: A powerful and economical technique revisited. Appl. Plant Sci. 2020, 8, e11342. [Google Scholar] [CrossRef]
- Loureiro, J.; Rodriguez, E.; Dolezel, J.; Santos, C. Two New Nuclear Isolation Buffers for Plant DNA Flow Cytometry: A Test with 37 Species. Ann. Bot. 2007, 100, 875–888. [Google Scholar] [CrossRef]




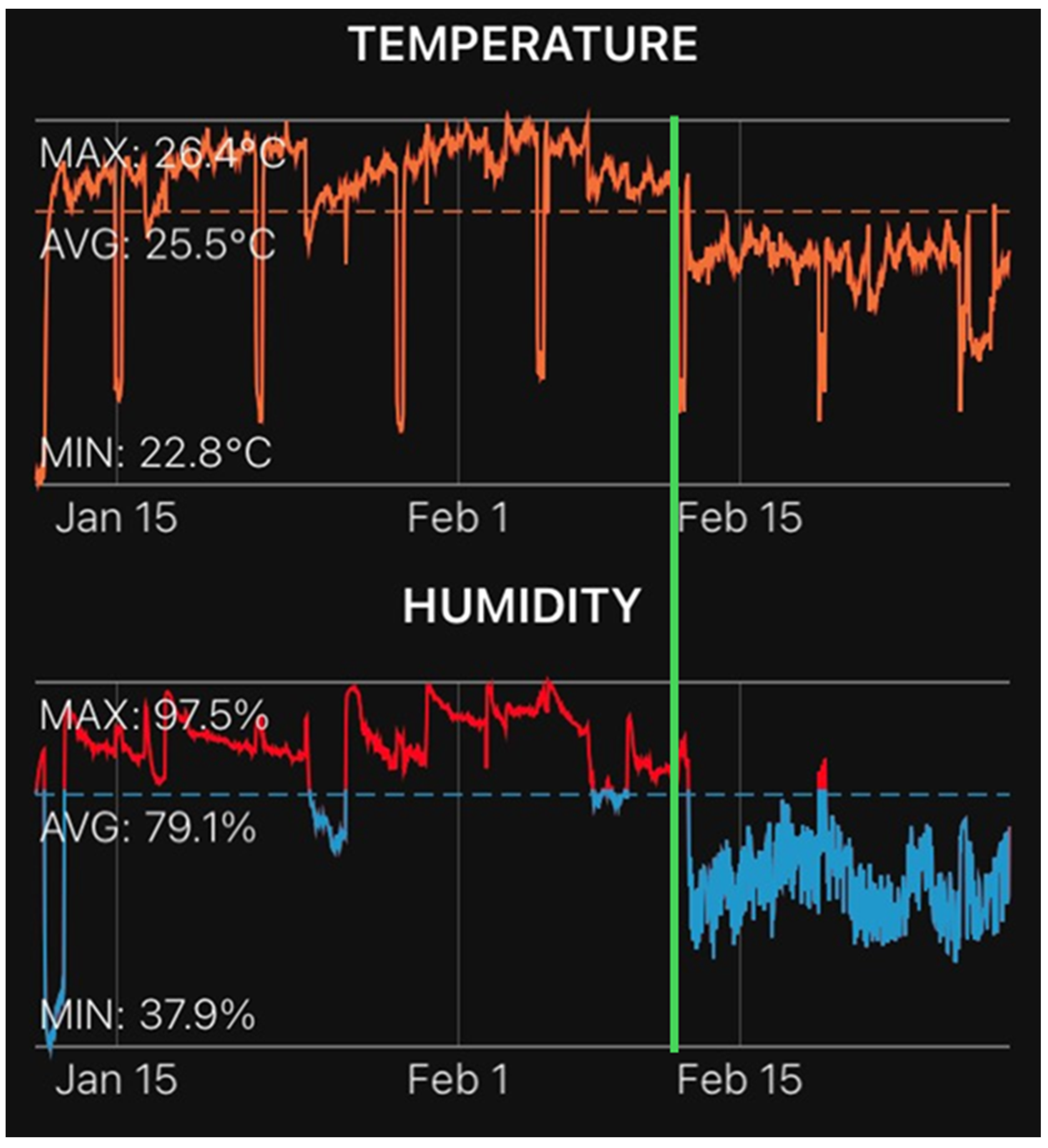
| Genotype | Start Hardening | End Hardening | Hardening Time (Months) | Initial Number of Plants | Final Number of Plants | Survival Rate (%) |
|---|---|---|---|---|---|---|
| PHaploid#1 | 3 January 24 | 28 February 24 | 1.8 | 6 | 6 | 100 |
| PHaploid#2 | 3 January 24 | 28 February 24 | 1.8 | 12 | 10 | 83 |
| PHaploid#3 | 3 January 24 | 28 February 24 | 1.8 | 10 | 10 | 100 |
| Sample | Number of Chromosomes | Ploidy |
|---|---|---|
| PHaploid#1 root tips | 14 | n |
| PHaploid#2 root tips | 14 | n |
| PHaploid#3 root tips | 32 | 2n |
| AC110 root tips 1 | 28 | 2n |
| AC110 root tips 2 | 32 | 2n |
| AC110 pollen | 16 | n |
| Daphna root tip | 28 | 2n |
| Daphna pollen | 14 | n |
| Sample | Mean Estimated Genome Size | SD | Ploidy |
|---|---|---|---|
| AC110 | 4.468 | 0.127 | 2x |
| PHaploid#1 | 1.843 | 0.116 | x |
| PHaploid#2 | 1.882 | 0.115 | x |
| PHaploid#3 | 3.030 | 0.929 | Mixoploid |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gastelbondo, M.; Nicholls, U.; Chen, S.; Chambers, A.; Wu, X. First Gynogenesis of Vanilla planifolia for Haploid Production and Ploidy Verification Protocol. Plants 2024, 13, 1733. https://doi.org/10.3390/plants13131733
Gastelbondo M, Nicholls U, Chen S, Chambers A, Wu X. First Gynogenesis of Vanilla planifolia for Haploid Production and Ploidy Verification Protocol. Plants. 2024; 13(13):1733. https://doi.org/10.3390/plants13131733
Chicago/Turabian StyleGastelbondo, Manuel, Ursula Nicholls, Sisi Chen, Alan Chambers, and Xingbo Wu. 2024. "First Gynogenesis of Vanilla planifolia for Haploid Production and Ploidy Verification Protocol" Plants 13, no. 13: 1733. https://doi.org/10.3390/plants13131733
APA StyleGastelbondo, M., Nicholls, U., Chen, S., Chambers, A., & Wu, X. (2024). First Gynogenesis of Vanilla planifolia for Haploid Production and Ploidy Verification Protocol. Plants, 13(13), 1733. https://doi.org/10.3390/plants13131733






