Drought Protective Effects of Exogenous ABA and Kinetin on Lettuce: Sugar Content, Antioxidant Enzyme Activity, and Productivity
Abstract
1. Introduction
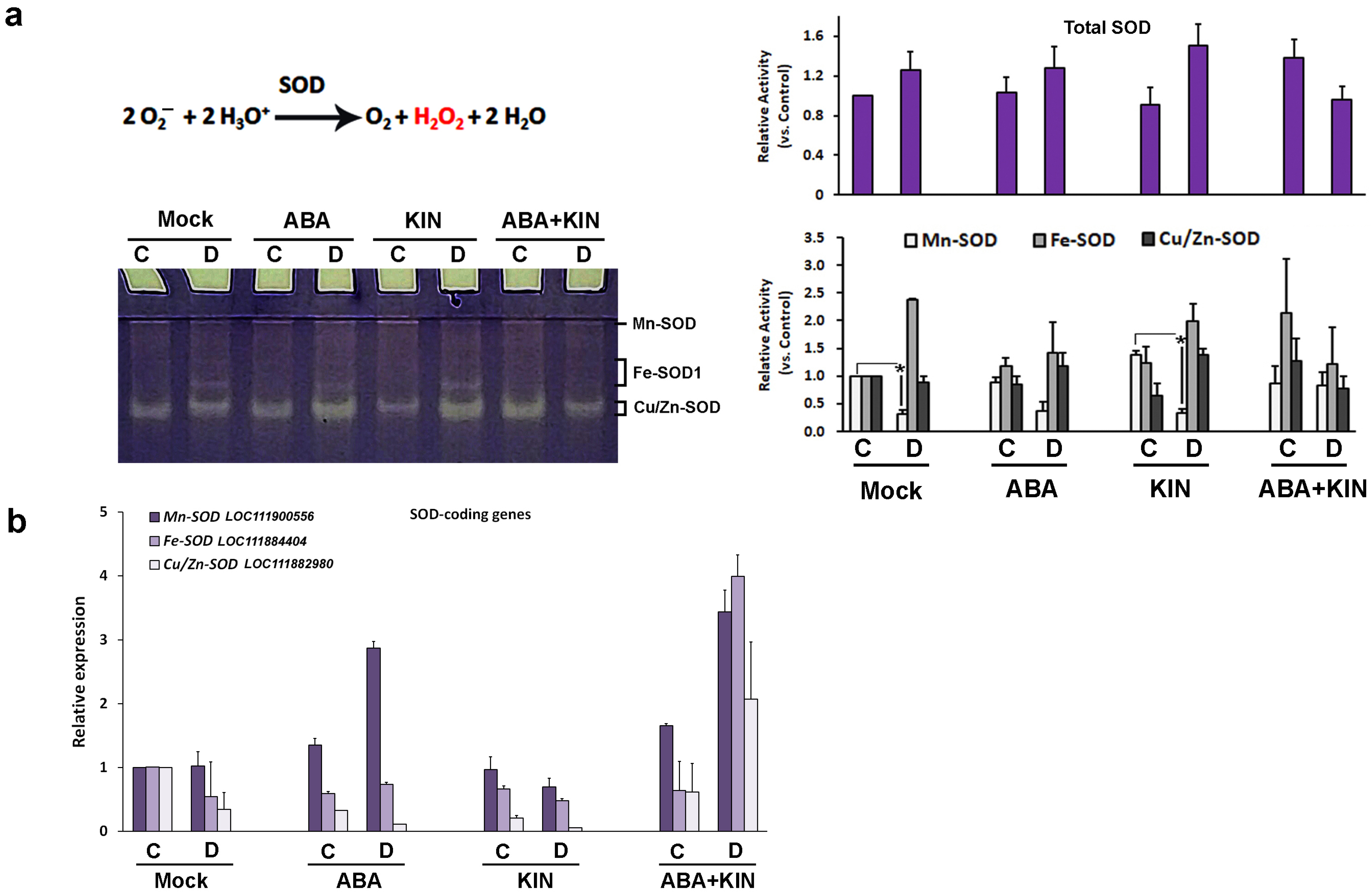
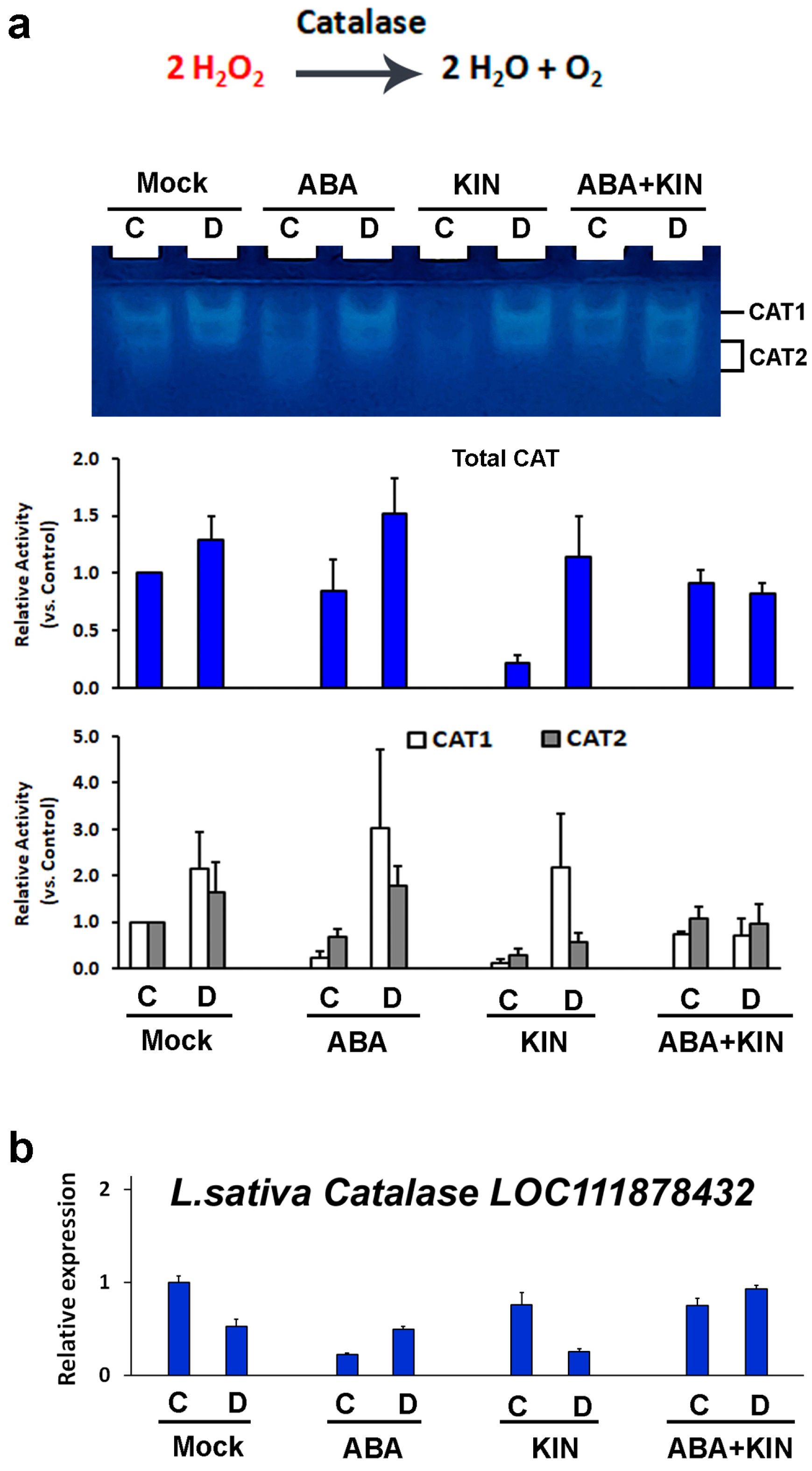
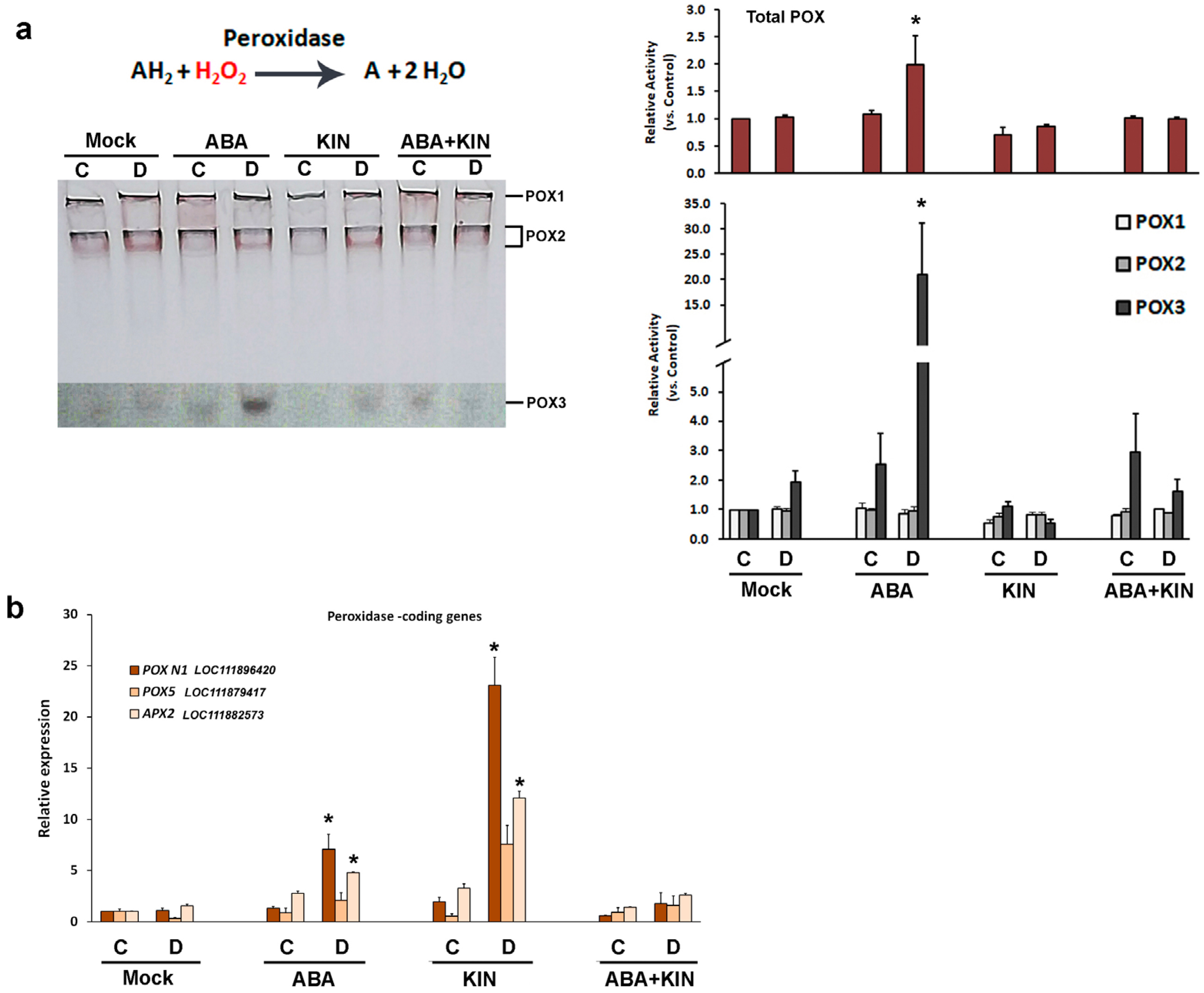
2. Results
3. Discussion
4. Materials and Methods
4.1. Growth Conditions
4.2. Sample Preparation for Determination of Sugars by HPLC
4.3. Determination of Sugars by HPLC
4.4. Non-Denaturing Electrophoresis and In-Gel Activity Staining of Antioxidant Enzymes
4.5. RT-PCR Analysis
4.6. Biometric Measurements
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rosegrant, M.W.; Cline, S.A. Global Food Security: Challenges and Policies. Science 2003, 302, 1917–1919. [Google Scholar] [CrossRef]
- Nguyen, H.N.; Perry, L.; Kisiala, A.; Olechowski, H.; Emery, R.J.N. Cytokinin Activity during Early Kernel Development Corresponds Positively with Yield Potential and Later Stage ABA Accumulation in Field-Grown Wheat (Triticum aestivum L.). Planta 2020, 252, 76. [Google Scholar] [CrossRef]
- Werner, T.; Nehnevajova, E.; Köllmer, I.; Novák, O.; Strnad, M.; Krämer, U.; Schmülling, T. Root-Specific Reduction of Cytokinin Causes Enhanced Root Growth, Drought Tolerance, and Leaf Mineral Enrichment in Arabidopsis and Tobacco. Plant Cell 2011, 22, 3905–3920. [Google Scholar] [CrossRef]
- Bielach, A.; Hrtyan, M.; Tognetti, V. Plants under Stress: Involvement of Auxin and Cytokinin. Int. J. Mol. Sci. 2017, 18, 1427. [Google Scholar] [CrossRef]
- Joshi, S.; Choukimath, A.; Isenegger, D.; Panozzo, J.; Spangenberg, G.; Kant, S. Improved Wheat Growth and Yield by Delayed Leaf Senescence Using Developmentally Regulated Expression of a Cytokinin Biosynthesis Gene. Front. Plant Sci. 2019, 10, 1285. [Google Scholar] [CrossRef]
- Ramireddy, E.; Hosseini, S.A.; Eggert, K.; Gillandt, S.; Gnad, H.; Von Wirén, N.; Schmülling, T. Root Engineering in Barley: Increasing Cytokinin Degradation Produces a Larger Root System, Mineral Enrichment in the Shoot and Improved Drought Tolerance. Plant Physiol. 2018, 177, 1078–1095. [Google Scholar] [CrossRef]
- Basu, S.; Ramegowda, V.; Kumar, A.; Pereira, A. Plant Adaptation to Drought Stress. F1000Research 2016, 5, F1000 Faculty Rev-1554. [Google Scholar] [CrossRef]
- Cortleven, A.; Schmülling, T. Regulation of Chloroplast Development and Function by Cytokinin. J. Exp. Bot. 2015, 66, 4999–5013. [Google Scholar] [CrossRef]
- Rivero, R.M.; Gimeno, J.; Van Deynze, A.; Walia, H.; Blumwald, E. Enhanced Cytokinin Synthesis in Tobacco Plants Expressing PSARK::IPT Prevents the Degradation of Photosynthetic Protein Complexes During Drought. Plant Cell Physiol. 2010, 51, 1929–1941. [Google Scholar] [CrossRef]
- Piñol, R.; Simón, E. Effect of 24-Epibrassinolide on Chlorophyll Fluorescence and Photosynthetic CO2 Assimilation in Vicia faba Plants Treated with the Photosynthesis-Inhibiting Herbicide Terbutryn. J. Plant Growth Regul. 2009, 28, 97–105. [Google Scholar] [CrossRef]
- Hönig, M.; Plíhalová, L.; Husičková, A.; Nisler, J.; Doležal, K. Role of Cytokinins in Senescence, Antioxidant Defence and Photosynthesis. Int. J. Mol. Sci. 2018, 19, 4045. [Google Scholar] [CrossRef]
- Jiang, M.; Zhang, J. Effect of Abscisic Acid on Active Oxygen Species, Antioxidative Defence System and Oxidative Damage in Leaves of Maize Seedlings. Plant Cell Physiol. 2001, 42, 1265–1273. [Google Scholar] [CrossRef]
- Ozfidan, C.; Turkan, I.; Sekmen, A.H.; Seckin, B. Abscisic Acid-regulated Responses of aba2-1 under Osmotic Stress: The Abscisic Acid-inducible Antioxidant Defence System and Reactive Oxygen Species Production. Plant Biol. 2012, 14, 337–346. [Google Scholar] [CrossRef]
- Castro-Cegrí, A.; Sierra, S.; Hidalgo-Santiago, L.; Esteban-Muñoz, A.; Jamilena, M.; Garrido, D.; Palma, F. Postharvest Treatment with Abscisic Acid Alleviates Chilling Injury in Zucchini Fruit by Regulating Phenolic Metabolism and Non-Enzymatic Antioxidant System. Antioxidants 2023, 12, 211. [Google Scholar] [CrossRef]
- Jiang, Z.; Zhu, H.; Zhu, H.; Tao, Y.; Liu, C.; Liu, J.; Yang, F.; Li, M. Exogenous ABA Enhances the Antioxidant Defense System of Maize by Regulating the AsA-GSH Cycle under Drought Stress. Sustainability 2022, 14, 3071. [Google Scholar] [CrossRef]
- Mou, W.; Li, D.; Luo, Z.; Mao, L.; Ying, T. Transcriptomic Analysis Reveals Possible Influences of ABA on Secondary Metabolism of Pigments, Flavonoids and Antioxidants in Tomato Fruit during Ripening. PLoS ONE 2015, 10, e0129598. [Google Scholar] [CrossRef]
- Kuromori, T.; Seo, M.; Shinozaki, K. ABA Transport and Plant Water Stress Responses. Trends Plant Sci. 2018, 23, 513–522. [Google Scholar] [CrossRef]
- Wilkinson, S.; Davies, W.J. Drought, Ozone, ABA and Ethylene: New Insights from Cell to Plant to Community. Plant Cell Environ. 2010, 33, 510–525. [Google Scholar] [CrossRef]
- Zhang, K.; Gan, S.-S. An Abscisic Acid-AtNAP Transcription Factor-SAG113 Protein Phosphatase 2C Regulatory Chain for Controlling Dehydration in Senescing Arabidopsis Leaves. Plant Physiol. 2012, 158, 961–969. [Google Scholar] [CrossRef]
- Song, Y.; Xiang, F.; Zhang, G.; Miao, Y.; Miao, C.; Song, C.-P. Abscisic Acid as an Internal Integrator of Multiple Physiological Processes Modulates Leaf Senescence Onset in Arabidopsis thaliana. Front. Plant Sci. 2016, 7, 181. [Google Scholar] [CrossRef]
- Tahaei, A.; Soleymani, A.; Shams, M. Seed Germination of Medicinal Plant, Fennel (Foeniculum vulgare Mill), as Affected by Different Priming Techniques. Appl. Biochem. Biotechnol. 2016, 180, 26–40. [Google Scholar] [CrossRef]
- Wang, Y.; Li, L.; Ye, T.; Zhao, S.; Liu, Z.; Feng, Y.; Wu, Y. Cytokinin Antagonizes ABA Suppression to Seed Germination of Arabidopsis by Downregulating ABI5 Expression. Plant J. 2011, 68, 249–261. [Google Scholar] [CrossRef]
- Davies, P.J. Plant Hormones: Biosythesis, Signal Transduction, Action! 3rd ed.; Davies, P.J., Ed.; Kluwer Academic: Dordrecht, The Netherlands; Boston, UK, 2004; ISBN 9781402026850. [Google Scholar]
- Rivero, R.M.; Shulaev, V.; Blumwald, E. Cytokinin-Dependent Photorespiration and the Protection of Photosynthesis during Water Deficit. Plant Physiol. 2009, 150, 1530–1540. [Google Scholar] [CrossRef]
- Lalarukh, I.; Ashraf, M.A.; Azeem, M.; Hussain, M.; Akbar, M.; Ashraf, M.Y.; Javed, M.T.; Iqbal, N. Growth Stage-Based Response of Wheat (Triticum aestivum L.) to Kinetin under Water-Deficit Environment: Pigments and Gas Exchange Attributes. Acta Agric. Scand. Sect. B—Soil Plant Sci. 2014, 64, 501–510. [Google Scholar] [CrossRef]
- Ahanger, M.A.; Alyemeni, M.N.; Wijaya, L.; Alamri, S.A.; Alam, P.; Ashraf, M.; Ahmad, P. Potential of Exogenously Sourced Kinetin in Protecting Solanum lycopersicum from NaCl-Induced Oxidative Stress through up-Regulation of the Antioxidant System, Ascorbate-Glutathione Cycle and Glyoxalase System. PLoS ONE 2018, 13, e0202175. [Google Scholar] [CrossRef]
- Singh, M.; Bashri, G.; Prasad, S.M.; Singh, V.P. Kinetin Alleviates UV-B-Induced Damage in Solanum lycopersicum: Implications of Phenolics and Antioxidants. J. Plant Growth Regul. 2019, 38, 831–841. [Google Scholar] [CrossRef]
- Acidri, R.; Sawai, Y.; Sugimoto, Y.; Handa, T.; Sasagawa, D.; Masunaga, T.; Yamamoto, S.; Nishihara, E. Exogenous Kinetin Promotes the Nonenzymatic Antioxidant System and Photosynthetic Activity of Coffee (Coffea arabica L.) Plants Under Cold Stress Conditions. Plants 2020, 9, 281. [Google Scholar] [CrossRef]
- Bozsó, Z.; Barna, B. Diverse Effect of Two Cytokinins, Kinetin and Benzyladenine, on Plant Development, Biotic Stress Tolerance, and Gene Expression. Life 2021, 11, 1404. [Google Scholar] [CrossRef]
- Hamayun, M.; Hussain, A.; Khan, S.A.; Irshad, M.; Khan, A.L.; Waqas, M.; Shahzad, R.; Iqbal, A.; Ullah, N.; Rehman, G.; et al. Kinetin Modulates Physio-Hormonal Attributes and Isoflavone Contents of Soybean Grown under Salinity Stress. Front. Plant Sci. 2015, 6, 377. [Google Scholar] [CrossRef]
- Alscher, R.G. Role of Superoxide Dismutases (SODs) in Controlling Oxidative Stress in Plants. J. Exp. Bot. 2002, 53, 1331–1341. [Google Scholar] [CrossRef]
- Yang, P.; Guo, Y.; Qiu, L. Effects of Ozone-Treated Domestic Sludge on Hydroponic Lettuce Growth and Nutrition. J. Integr. Agric. 2018, 17, 593–602. [Google Scholar] [CrossRef]
- Liang, Y.-C.; Reid, M.S.; Jiang, C.-Z. Controlling Plant Architecture by Manipulation of Gibberellic Acid Signalling in Petunia. Hortic. Res. 2014, 1, 14061. [Google Scholar] [CrossRef]
- Miyashima, S.; Roszak, P.; Sevilem, I.; Toyokura, K.; Blob, B.; Heo, J.; Mellor, N.; Help-Rinta-Rahko, H.; Otero, S.; Smet, W.; et al. Mobile PEAR Transcription Factors Integrate Positional Cues to Prime Cambial Growth. Nature 2019, 565, 490–494. [Google Scholar] [CrossRef]
- Dörffling, K.; Böttger, M. Steigerung der keimungshemmenden Wirkung von Abscisinsäure auf Lactuca-Früchte durch Saccharose und Glucose. Planta 1972, 103, 340–347. [Google Scholar] [CrossRef]
- Zhou, W.; Liang, X.; Zhang, Y.; Dai, P.; Liang, B.; Li, J.; Sun, C.; Lin, X. Role of Sucrose in Modulating the Low-nitrogen-induced Accumulation of Phenolic Compounds in Lettuce (Lactuca sativa L.). J. Sci. Food Agric. 2020, 100, 5412–5421. [Google Scholar] [CrossRef]
- Ibrahim, H.A.; Abdellatif, Y.M.R. Effect of Maltose and Trehalose on Growth, Yield and Some Biochemical Components of Wheat Plant under Water Stress. Ann. Agric. Sci. 2016, 61, 267–274. [Google Scholar] [CrossRef]
- Zhang, W.; Du, T. Fresh/Brackish Watering at Growth Period Provided a Trade-off between Lettuce Growth and Resistance to NaCl-Induced Damage. Sci. Hortic. 2022, 304, 111283. [Google Scholar] [CrossRef]
- Xie, Y.; Sun, G.; Wang, L.; Tang, Y. Effects of Spraying Abscisic Acid on Growth and Antioxidant Enzyme of Lettuce Seedlings under Salt Stress. IOP Conf. Ser. Earth Environ. Sci. 2018, 199, 032015. [Google Scholar] [CrossRef]
- Vaseva, I.; Akiscan, Y.; Simova-Stoilova, L.; Kostadinova, A.; Nenkova, R.; Anders, I.; Feller, U.; Demirevska, K. Antioxidant Response to Drought in Red and White Clover. Acta Physiol. Plant 2012, 34, 1689–1699. [Google Scholar] [CrossRef]
- Yildiztugay, E.; Ozfidan-Konakci, C.; Arikan, B.; Alp, F.N.; Elbasan, F.; Zengin, G.; Cavusoglu, H.; Sakalak, H. The Hormetic Dose-Risks of Polymethyl Methacrylate Nanoplastics on Chlorophyll a Fluorescence Transient, Lipid Composition and Antioxidant System in Lactuca sativa. Environ. Pollut. 2022, 308, 119651. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- De Vylder, J.; Vandenbussche, F.; Hu, Y.; Philips, W.; Van Der Straeten, D. Rosette Tracker: An Open-Source Image Analysis Tool for Automatic Quantification of Genotype Effects. Plant Physiol. 2012, 160, 1149–1159. [Google Scholar] [CrossRef]
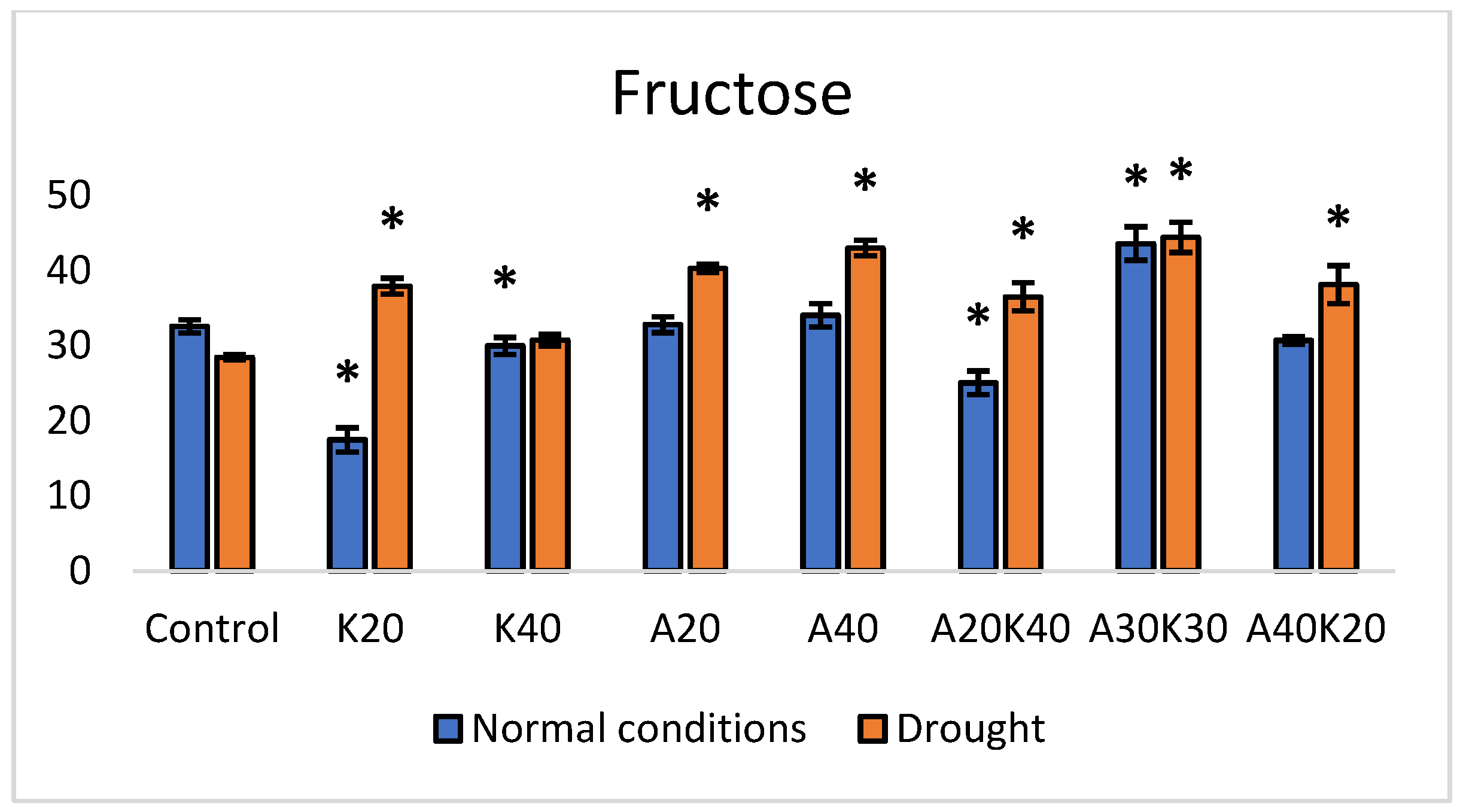
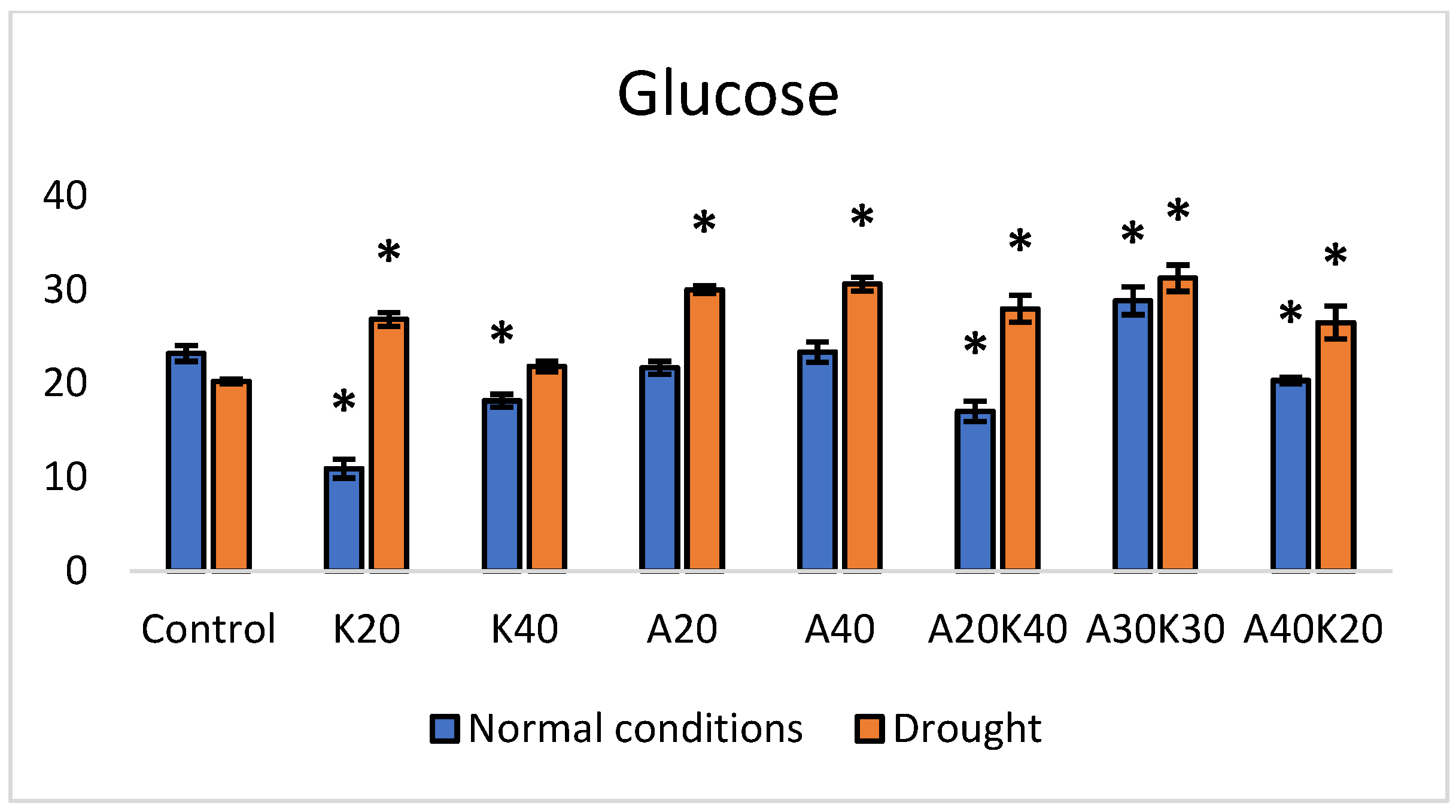
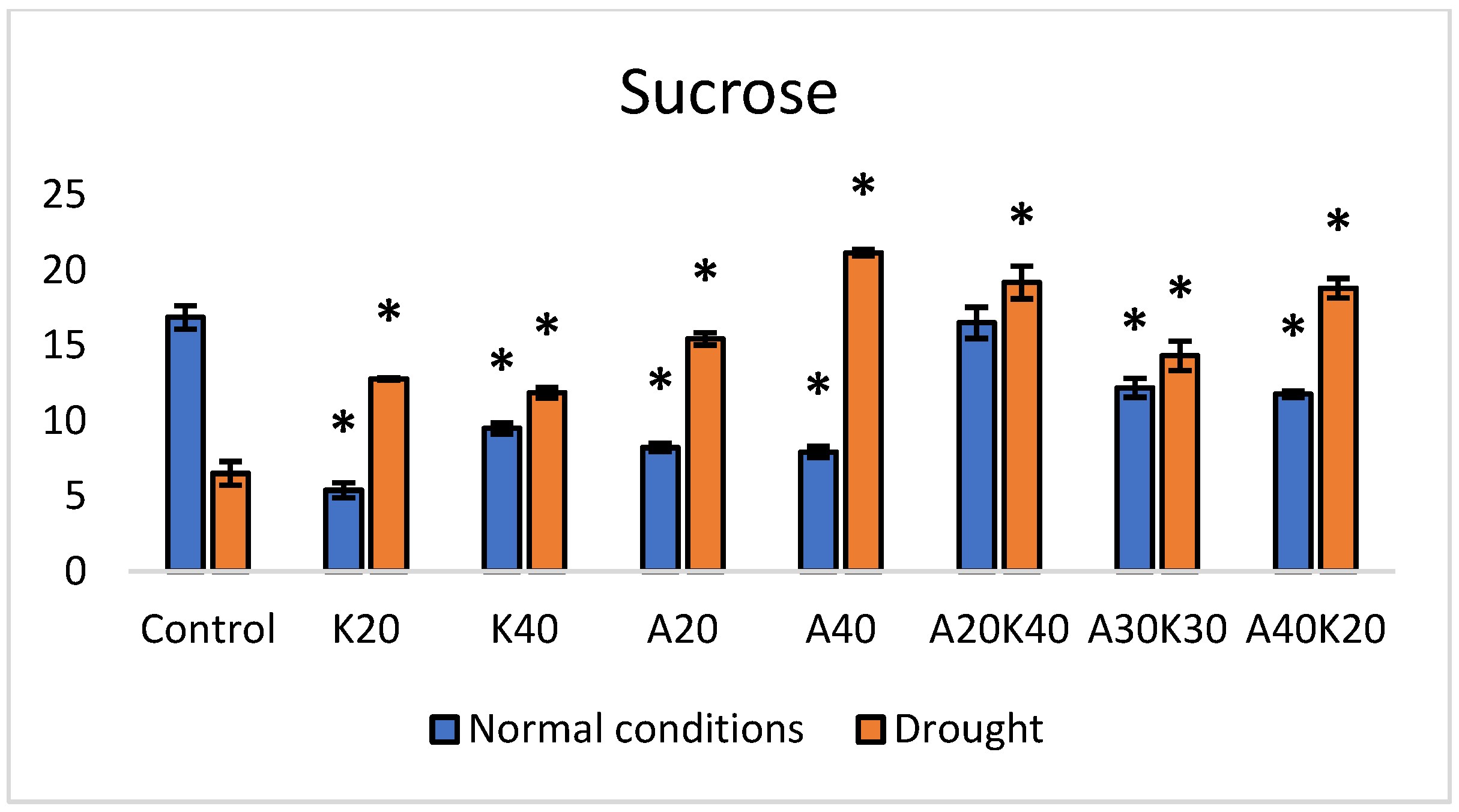
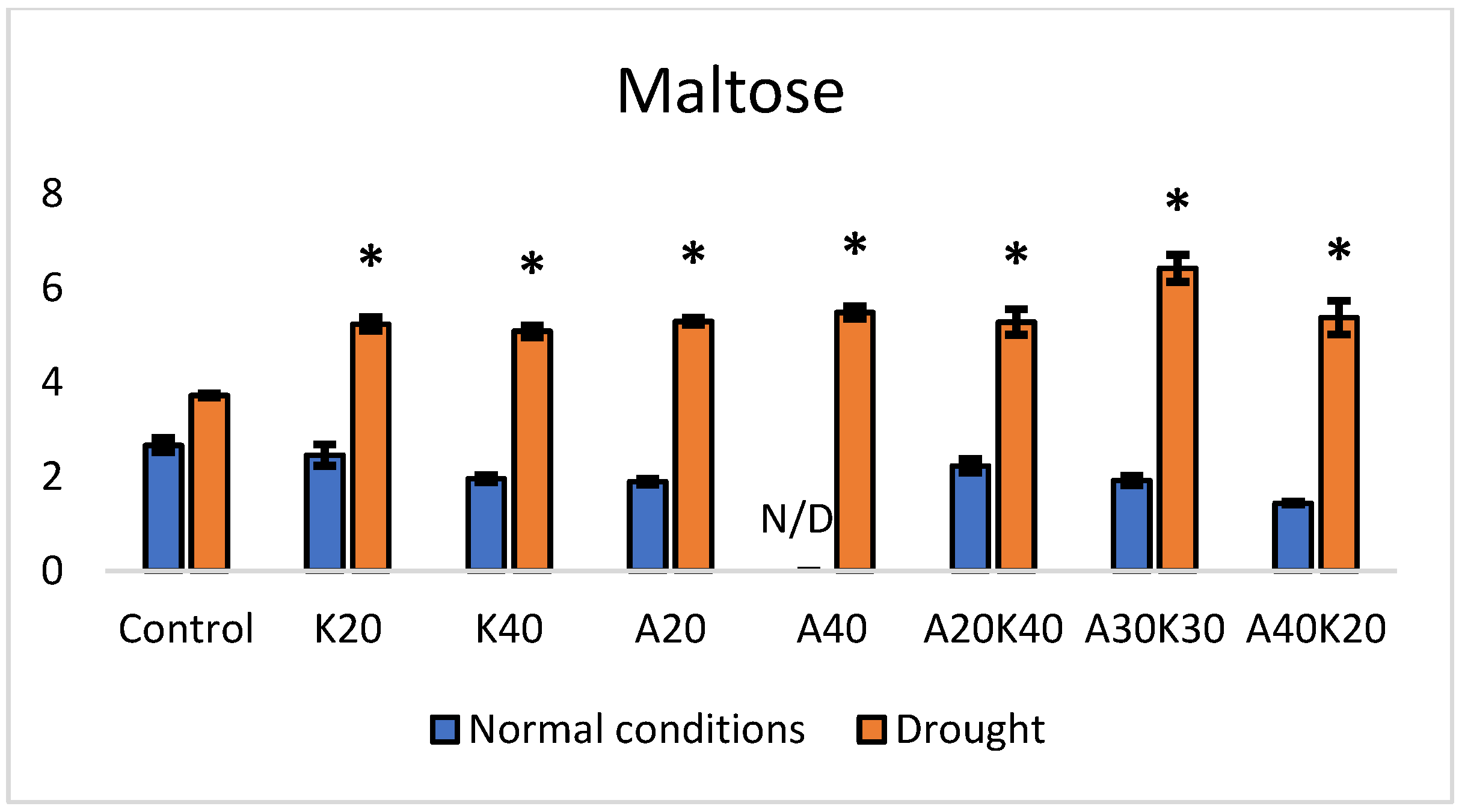
| Fresh Weight [g] | Dry Weight [g] | Leaf Area [mm2] | ||||
|---|---|---|---|---|---|---|
| Normal Conditions | Drought | Normal Conditions | Drought | Normal Conditions | Drought | |
| Control | 2.50 | 1.43 | 0.16 | 0.11 | 6237 | 2828 |
| K20 | 1.67 | 1.20 * | 0.10 * | 0.09 * | 4844 * | 3662 * |
| K40 | 1.54 * | 1.28 * | 0.09 * | 0.10 | 4885 * | 3301 * |
| A20 | 1.86 | 1.31 * | 0.11 | 0.10 | 5740 * | 2806 * |
| A40 | 2.40 | 1.42 | 0.13 | 0.10 | 5612 * | 3561 * |
| A20K40 | 1.90 | 1.27 * | 0.10 | 0.09 * | 5778 * | 3490 * |
| A30K30 | 1.96 | 1.49 * | 0.11 | 0.11 | 5002 * | 3558 * |
| A40K20 | 2.09 | 1.36 * | 0.11 | 0.10 | 6001 | 3600 * |
| Gene Name | Locus | Forward Primer (5′-3′) | Reverse Primer (5′-3′) |
|---|---|---|---|
| Ls18S RNA | AH001680 | CGGGTGACGGAGAATTAGGG | TACCTCCCCGTGTCAGGATT |
| Ls Actin-7 | LOC111882438 | CTGGTGATGGTGTCTCCCAC | GGCGAGCTTCTCCTTCATGT |
| Ls Mn-SOD | LOC111900556 | CACCCCAGTATTTGGATGGCT | CTCCCTCCCCCTATGTGCTA |
| Ls Fe-SOD | LOC111884404 | GGGAATCCATGCAACCAGGA | AAAACAAGCCAAACCCAGCC |
| Ls Cu/Zn-SOD | LOC111882980 | CACTCTTACAGACGCTTTGCG | ATGGTGCCACTAACACCCTC |
| Ls Catalase | LOC111878432 | GCCATGCTGAACAGTACCCT | TCTCTCTCCTGGCTGCTTGA |
| Ls APX2 | LOC111882573 | GACATCGGCGATCTTCTGGT | TCTCGAAGCTTCCTCTTCGC |
| Ls POX N1 | LOC111896420 | CTATGGTTGATATTGGCGTCGT | ACAAAGTCGGCCATTGGAGAT |
| Ls POX5 | LOC111879417 | GGTCGCTAAAGCCTACTCCC | ACTTGGGTTGTTTGCTGGTG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Urbutis, M.; Vaseva, I.I.; Simova-Stoilova, L.; Todorova, D.; Pukalskas, A.; Samuolienė, G. Drought Protective Effects of Exogenous ABA and Kinetin on Lettuce: Sugar Content, Antioxidant Enzyme Activity, and Productivity. Plants 2024, 13, 1641. https://doi.org/10.3390/plants13121641
Urbutis M, Vaseva II, Simova-Stoilova L, Todorova D, Pukalskas A, Samuolienė G. Drought Protective Effects of Exogenous ABA and Kinetin on Lettuce: Sugar Content, Antioxidant Enzyme Activity, and Productivity. Plants. 2024; 13(12):1641. https://doi.org/10.3390/plants13121641
Chicago/Turabian StyleUrbutis, Martynas, Irina I. Vaseva, Lyudmila Simova-Stoilova, Dessislava Todorova, Audrius Pukalskas, and Giedrė Samuolienė. 2024. "Drought Protective Effects of Exogenous ABA and Kinetin on Lettuce: Sugar Content, Antioxidant Enzyme Activity, and Productivity" Plants 13, no. 12: 1641. https://doi.org/10.3390/plants13121641
APA StyleUrbutis, M., Vaseva, I. I., Simova-Stoilova, L., Todorova, D., Pukalskas, A., & Samuolienė, G. (2024). Drought Protective Effects of Exogenous ABA and Kinetin on Lettuce: Sugar Content, Antioxidant Enzyme Activity, and Productivity. Plants, 13(12), 1641. https://doi.org/10.3390/plants13121641








