Enrichment of Water Bodies with Phenolic Compounds Released from Betula and Pinus Pollen in Surface Water
Abstract
:1. Introduction
2. Results
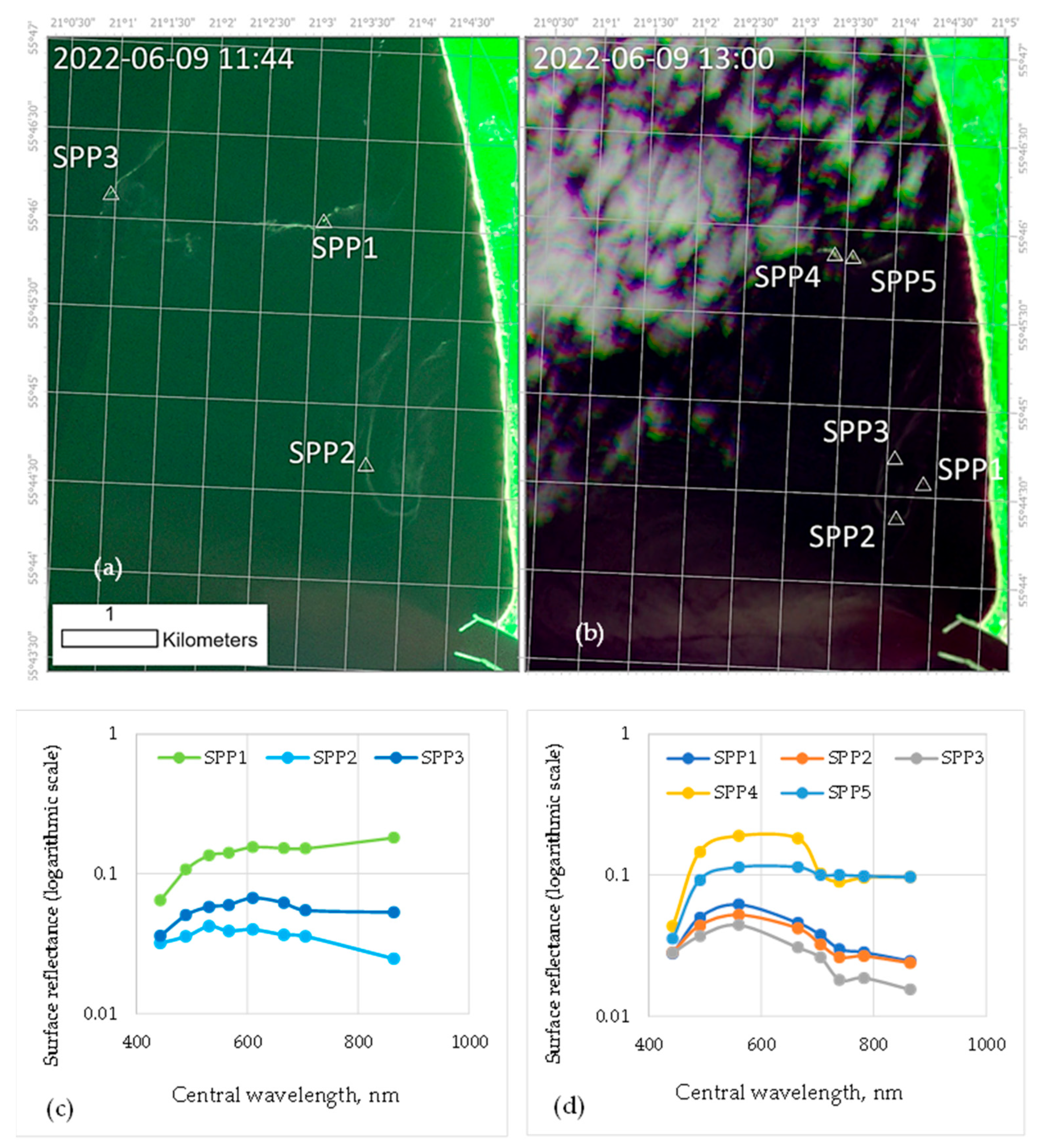
2.1. Pollen-Spreading Intensity and Pollen Coverage of the Water Surface during the Flowering of Betula and Pinus
2.2. Pollen Content and Composition in Water-Suspended Particles
2.3. Total Phenolic Content in Water-Suspended Particles and Surface Water during Betula and Pinus Pollen-Spreading Period
2.4. Analysis of Individual Phenolic Compounds in Water-Suspended Particles and Surface Water during Betula and Pinus Pollen-Spreading Periods
2.5. Release of Phenolic Compounds from Betula and Pinus Pollen into the Water: A Laboratory Experiment
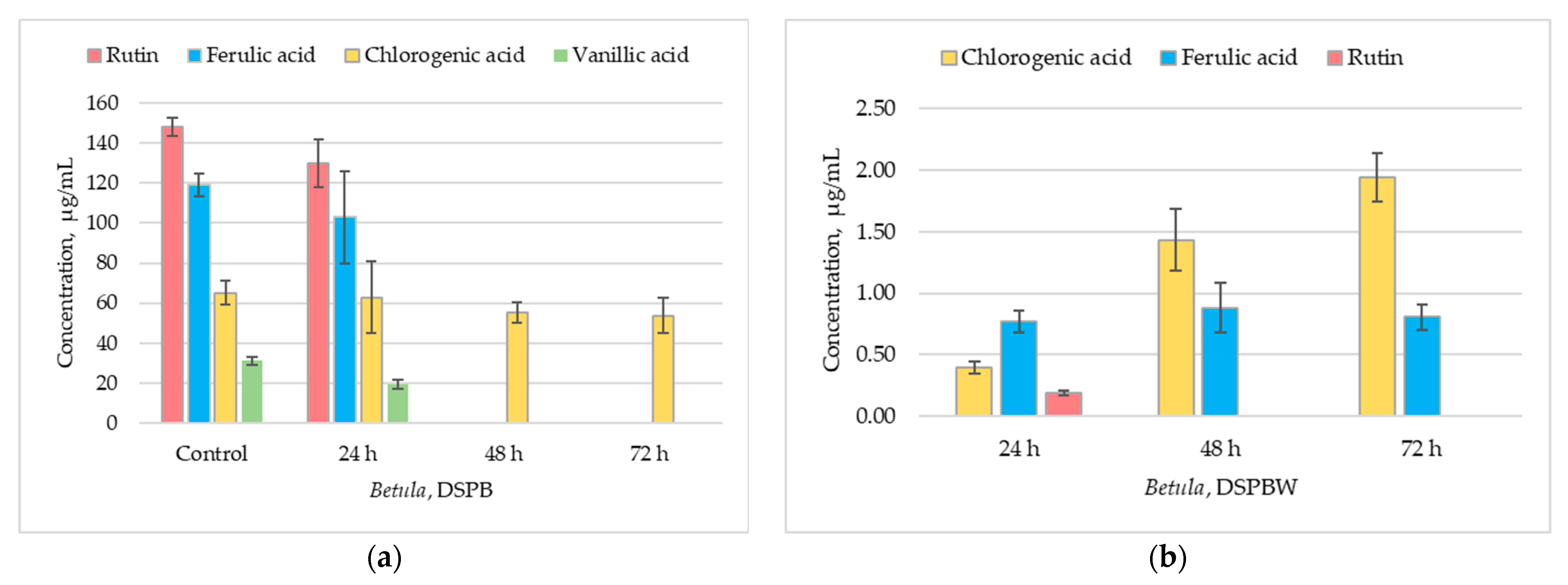
2.5.1. Total Phenolic Content and Antioxidant Activity
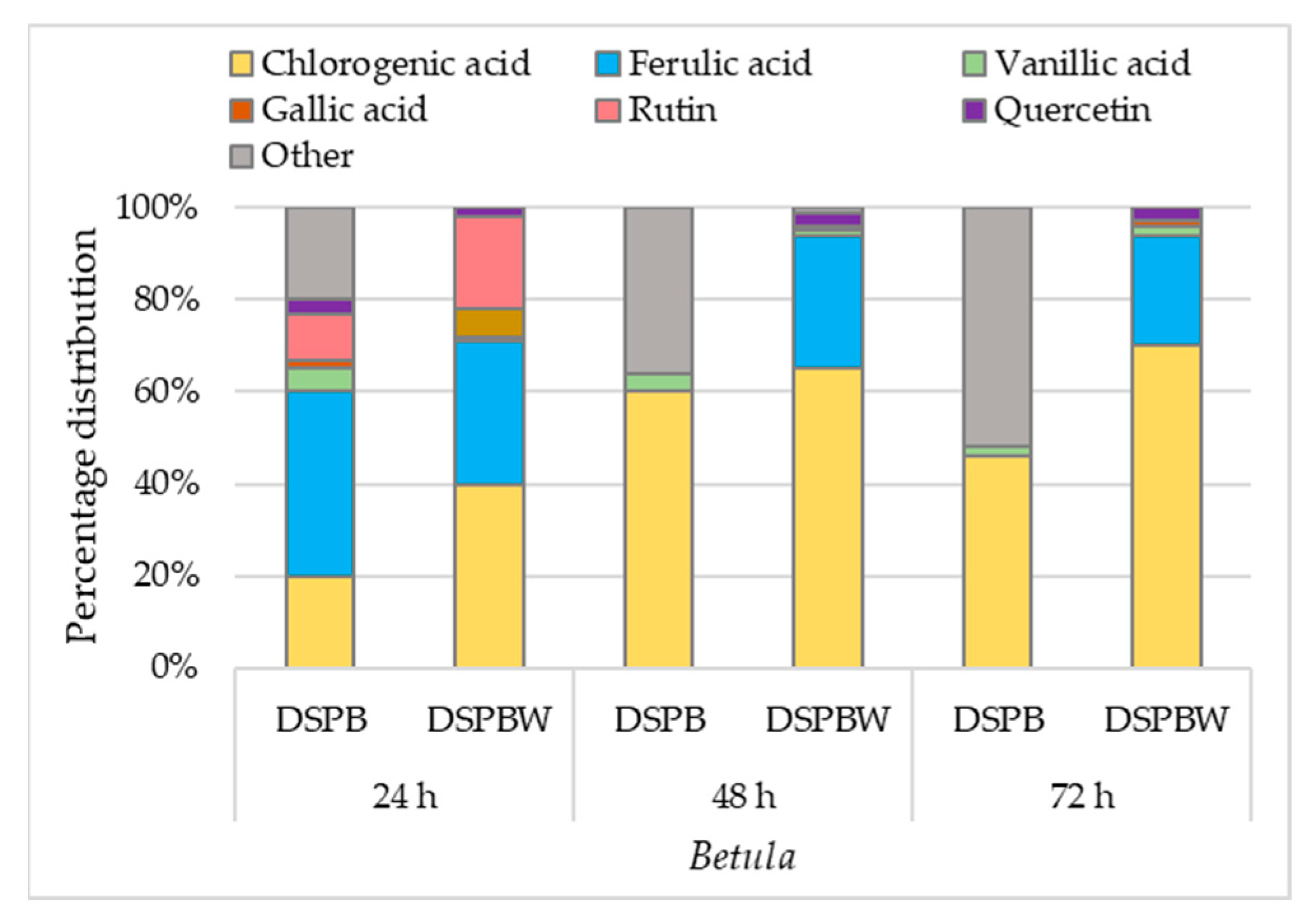
2.5.2. Individual Phenolic Compounds in Betula and Pinus Pollen Exposed to Distilled Water
3. Discussion
4. Materials and Methods
4.1. Analysis of Betula and Pinus Pollen Data and Information Gathered by Satellites
4.2. Natural and Artificial Water Bodies’ Characteristics
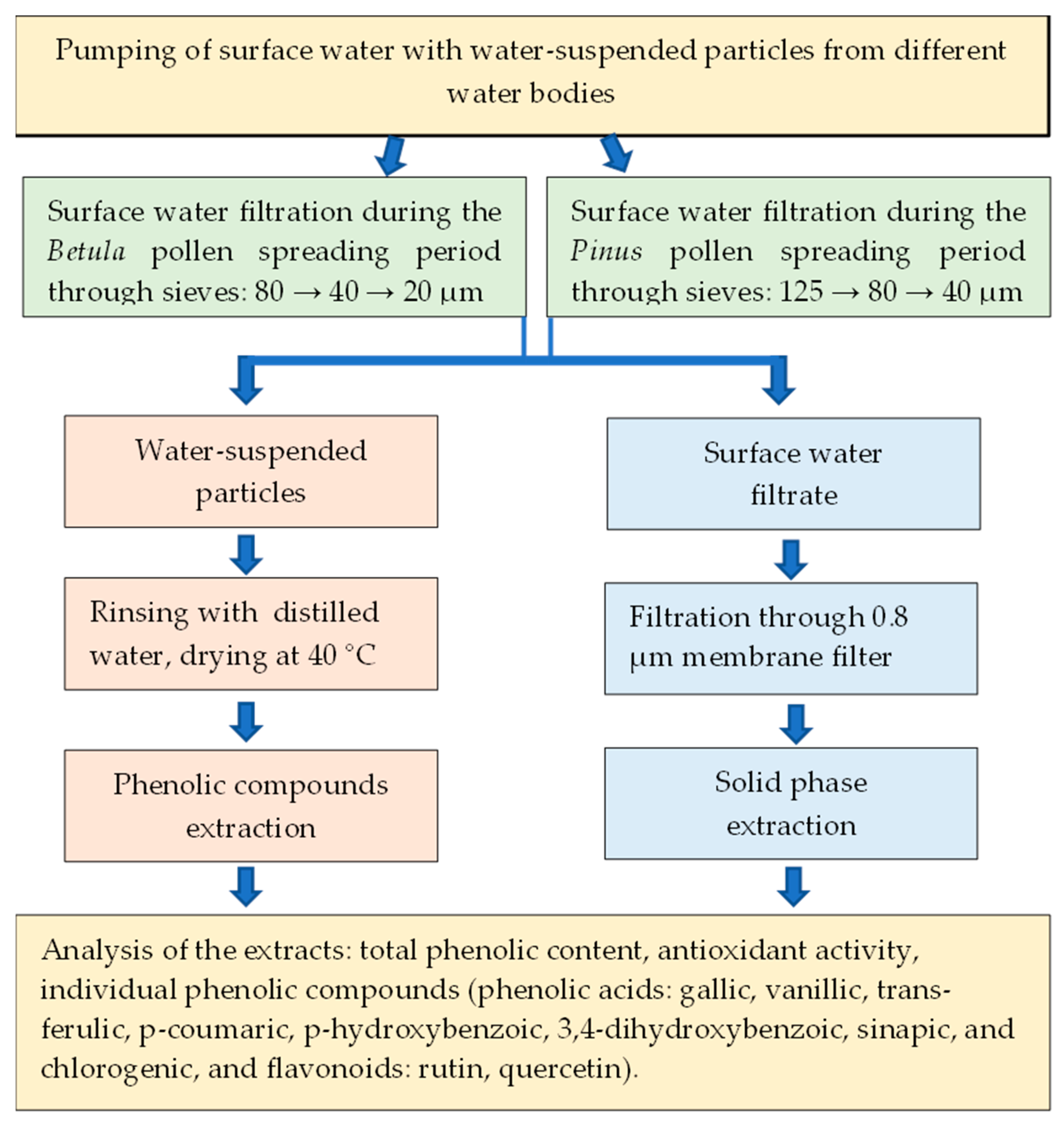
4.3. Sampling of Water-Suspended Particles and Surface Water during Betula and Pinus Pollen-Spreading Periods
- SPB—water-suspended particles collected from the surface water of different water bodies during the Betula pollen-spreading period.
- SPBW—surface water samples filtered and collected in parallel with SPB sampling. SPB and SPDW samples were collected from 29 April to 10 May 2022.
- SPP—water-suspended particles collected from the surface water of different water bodies during the Pinus pollen-spreading period.
- SPPW—surface water samples filtered and collected in parallel with SPP sampling. SPP and SPPW samples were collected from 4 to 8 June 2022.
- PW—clear surface water samples were collected before Betula and Pinus pollen spreading. The surface water samples were collected from 20 to 23 March 2022.
4.4. Water-Suspended Particle and Surface Water Extract Preparation
4.5. Water-Suspended Particles’ Composition Microscopy
4.6. Water-Suspended Particle and Surface Water Extract Chemical Analysis
4.7. Preparation of Laboratory Experiment of Betula and Pinus Pollen Phenolic Compounds Exposed to the Distilled Water
4.8. Statistical Data Analysis
5. Conclusions and Outlook
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Veriankaitė, L.; Siljamo, P.; Sofiev, M.; Šaulienė, I.; Kukkonen, J. Modelling analysis of source regions of long-range transported birch pollen that influences allergenic seasons in Lithuania. Aerobiologia 2010, 26, 47–62. [Google Scholar] [CrossRef]
- Jato, V.; Rodríguez-Rajo, F.J.; Aira, M.J. Use of phenological and pollen-production data for interpreting atmospheric birch pollen curves. Ann. Agric. Environ. Med. 2007, 14, 271–280. [Google Scholar] [PubMed]
- Graham, M.D.; Vinebrooke, R.D.; Turner, M.D.M. Coupling of boreal forests and lakes: Effects of conifer pollen on littoral communities. Limnol. Oceanogr. 2006, 51, 1524–1529. [Google Scholar] [CrossRef]
- Hu, C.; Qi, L.; English, D.C.; Wang, M.; Mikelsons, K.; Barnes, B.B.; Pawlik, M.M.; Ficek, F. Pollen in the Baltic Sea as viewed from space. Remote Sens. Environ. 2023, 284, 113337. [Google Scholar] [CrossRef]
- Rösel, S.; Rychła, A.; Wurzbacher, C.; Grossart, H.P. Effects of pollen leaching and microbial degradation on organic carbon and nutrient availability in lake water. Aquat. Sci. 2012, 74, 87–99. [Google Scholar] [CrossRef]
- Kenđel, A.; Zimmermann, B. Chemical analysis of pollen by FT-Raman and FTIR spectroscopies. Front. Plant Sci. 2020, 11, 352. [Google Scholar] [CrossRef] [PubMed]
- Augustin, S.; Wex, H.; Niedermeier, D.; Pummer, B.; Grothe, H.; Hartmann, S.; Tomsche, L.; Clauss, T.; Voigtländer, J.; Ignatius, K.; et al. Immersion freezing of birch pollen washing water. Atmos. Chem. Phys. 2013, 13, 10989–11003. [Google Scholar] [CrossRef]
- Pummer, B.G.; Bauer, H.; Bernardi, J.; Bleicher, S.; Grothe, H. Suspendable macromolecules are responsible for ice nucleation activity of birch and conifer pollen. Atmos. Chem. Phys. 2012, 12, 2541–2550. [Google Scholar] [CrossRef]
- Božič, A.; Šiber, A. Mechanics of inactive swelling and bursting of porate pollen grains. Biophys. J. 2022, 121, 782–792. [Google Scholar] [CrossRef]
- Bağcıoğlu, M.; Zimmermann, B.; Kohler, A. A multiscale vibrational spectroscopic approach for identification and biochemical characterization of pollen. PLoS ONE 2015, 10, e0137899. [Google Scholar] [CrossRef]
- Rozema, J.; Broekman, R.A.; Blokker, P.; Meijkamp, B.B.; de Bakker, N.; van de Staaij, J.A.; van Beema, F.; Ariese, F.; Kars, S.M. UV-B absorbance and UV-B absorbing compounds (para-coumaric acid) in pollen and sporopollenin: The perspective to track historic UV-B levels. J. Photochem. Photobiol. B Biol. 2001, 62, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Osthoff, K.S.; Wiermann, R. Phenols as integrated compounds of sporopollenin from Pinus pollen. J. Plant Physiol. 1987, 131, 5–15. [Google Scholar] [CrossRef]
- Rho, S.J.; Mun, S.; Park, J.; Kim, Y.R. Retarding oxidative and enzymatic degradation of phenolic compounds using large-ring cycloamylose. Foods 2021, 10, 1457. [Google Scholar] [CrossRef] [PubMed]
- Skroza, D.; Šimat, V.; Vrdoljak, L.; Jolić, N.; Skelin, A.; Čagalj, M.; Frleta, R.; Generalić Mekinić, I. Investigation of Antioxidant Synergisms and Antagonisms among Phenolic Acids in the Model Matrices Using FRAP and ORAC Methods. Antioxidants 2022, 11, 1784. [Google Scholar] [CrossRef]
- Bajkacz, S.; Baranowska, I.; Buszewski, B.; Kowalski, B.; Ligor, M. Determination of flavonoids and phenolic acids in plant materials using SLE-SPE-UHPLC-MS/MS method. Food Anal. Methods 2018, 11, 3563–3575. [Google Scholar] [CrossRef]
- Cheynier, V.; Comte, G.; Davies, K.M.; Lattanzio, V.; Martens, S. Plant phenolics: Recent advances on their biosynthesis, genetics, and ecophysiology. Plant Physiol. Biochem. 2013, 72, 1–20. [Google Scholar] [CrossRef]
- Freile-Pelegrín, Y.; Robledo, D. Bioactive phenolic compounds from algae. In Bioactive Compounds from Marine Foods: Plant and Animal Sources; Hernández-Ledesma, B., Miguel Herrero, M., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2013; pp. 113–129. [Google Scholar] [CrossRef]
- Max, B.; Tugores, F.; Cortés–Diéguez, S.; Domínguez, J.M. Bioprocess design for the microbial production of natural phenolic compounds by Debaryomyces hansenii. Appl. Biochem. Biotechnol. 2012, 168, 2268–2284. [Google Scholar] [CrossRef]
- Vladimirov, M.S.; Nikolić, V.D.; Stanojević, L.P.; Nikolić, L.B.; Tačić, A.D. Common birch (Betula pendula Roth.): Chemical composition and biological activity of isolates. Adv. Technol. 2019, 8, 65–77. [Google Scholar] [CrossRef]
- Dróżdż, P.; Pyrzynska, K. Extracts from pine and oak barks: Phenolics, minerals and antioxidant potential. Int. J. Environ. Anal. Chem. 2021, 101, 464–472. [Google Scholar] [CrossRef]
- Kerienė, I.; Šaulienė, I.; Šukienė, L.; Judžentienė, A.; Ligor, M.; Buszewski, B. Patterns of Phenolic Compounds in Betula and Pinus Pollen. Plants 2023, 12, 356. [Google Scholar] [CrossRef]
- Lithuanian Hydrometeorological Service. Naujos Vidutinės Lietuvos Klimato Sąlygos (New Average Climate Conditions for Lithuania). Available online: http://www.meteo.lt/lt/skn (accessed on 30 April 2023).
- Gailiušis, B.; Jablonskis, J.; Kovalenkovienė, A. Lietuvos Upės. Nuotėkis ir Hidrografija (The Lithuanian Rivers. Hydrography and Runoff); Lietuvos Energetikos Institutas (Lithuanian Energy Institute): Vilnius, Lithuania, 2001; p. 790. [Google Scholar]
- Jablonskis, J.; Jurgelėnaitė, A. Lietuvos ežerų statistika (Statistics of Lithuanian lakes). Geography 2007, 43, 16–26. [Google Scholar]
- Ministry of Environment of the Republic of Lithuania. UETK (Upių, Ežerų ir Tvenkinių Kadastras) (UETK (Cadastre of Rivers, Lakes and Ponds)). Available online: https://uetk.biip.lt/zemelapis/ (accessed on 24 April 2023).
- Kilkus, K. Lietuvos Ežerų Hidrologija (Hydrology of Lithuanian Lakes); Mokslas (Science): Vilnius, Lithuania, 1989; p. 153. [Google Scholar]
- Valiuškevičius, G. Mažieji Lietuvos Ežerai: Ištekliai, Genezė, Hidrologija (The Small Lithuanian Lakes: Resources, Genesis and Hydrology); Vilnius University Press: Vilnius, Lithuania, 2007; p. 274. [Google Scholar]
- Linkevičienė, R.; Taminskas, J.; Šimanauskienė, R. Ežero baseino ir apyežerio įtaka organogeninio atabrado raidai (The influence of the lake basin and the lakeside area on the evolution of organogenic littoral zone). Geogr. Yearb. 2004, 37, 35–46. [Google Scholar]
- Galkus, A. Vandens cirkuliacija ir erdvinė drumstumo dinamika vasarą Kuršių marių ir Baltijos jūros Lietuvos akvatorijose (Summer water circulation and spatial turbidity dynamics in the Lithuanian water of Curonian Lagoon and Baltic Sea). Geogr. Yearb. 2003, 36, 48–60. [Google Scholar]
- Oteros, J.; Sofiev, M.; Smith, M.; Clot, B.; Damialis, A.; Prank, M.; Werchan, M.; Wachter, R.; Weber, A.; Kutzora, S.; et al. Building an automatic pollen monitoring network (ePIN): Selection of optimal sites by clustering pollen stations. Sci. Total Environ. 2019, 688, 1263–1274. [Google Scholar] [CrossRef] [PubMed]
- Matthias, I.; Giesecke, T. Insights into pollen source area, transport and deposition from modern pollen accumulation rates in lake sediments. Quat. Sci. Rev. 2014, 87, 12–23. [Google Scholar] [CrossRef]
- Pawlik, M.; Ficek, D. Pine pollen grains in coastal waters of the Baltic Sea. Oceanol. Hydrobiol. Stud. 2016, 45, 35–41. [Google Scholar] [CrossRef]
- Lee, E.; Booth, T. Macronutrient input from pollen in two regenerating pine stands in southeast Korea. Ecol. Res. 2003, 18, 423–430. [Google Scholar] [CrossRef]
- Greenfield, L. Weight loss and release of mineral nitrogen from decomposing pollen. Soil Biol. Biochem. 1999, 31, 353–361. [Google Scholar] [CrossRef]
- Masclaux, H.; Perga, M.E.; Kagami, M.; Desvilettes, C.; Bourdier, G.; Bec, A. How pollen organic matter enters freshwater food webs. Limnol. Oceanogr. 2013, 58, 1185–1195. [Google Scholar] [CrossRef]
- Anku, W.W.; Mamo, M.A.; Govender, P.P. Phenolic compounds in water: Sources, reactivity, toxicity and treatment methods. In Phenolic Compounds-Natural Sources, Importance and Applications; Soto-Hernández, M., Tenango, M.P., García-Mateos, R., Eds.; IntechOpen: London, UK, 2017; pp. 419–443. [Google Scholar] [CrossRef]
- Mateos, R.; Pérez-Correa, J.R.; Domínguez, H. Bioactive properties of marine phenolics. Mar. Drugs 2020, 18, 501. [Google Scholar] [CrossRef]
- Giesecke, T.; Fontana, S.L. Revisiting pollen accumulation rates from Swedish lake sediments. Holocene 2008, 18, 293–305. [Google Scholar] [CrossRef]
- Taylor, P.E.; Flagan, R.C.; Miguel, A.G.; Valenta, R.; Glovsky, M.M. Birch pollen rupture and the release of aerosols of respirable allergens. Clin. Exp. Allergy 2004, 34, 1591–1596. [Google Scholar] [CrossRef] [PubMed]
- Frenguelli, G. Pollen structure and morphology. Adv. Dermatol. Allergol./Postępy Dermatol. I Alergol. 2004, 20, 200–204. [Google Scholar]
- Shi, J.; Cui, M.; Yang, L.; Kim, Y.J.; Zhang, D. Genetic and biochemical mechanisms of pollen wall development. Trends Plant Sci. 2015, 20, 741–753. [Google Scholar] [CrossRef]
- Liénart, C.; Cirtwill, A.R.; Hedgespeth, M.L.; Bradshaw, C. A sprinkling of gold dust: Pine pollen as a carbon source in Baltic Sea coastal food webs. Limnol. Oceanogr. 2022, 67, 53–65. [Google Scholar] [CrossRef]
- Apak, R.; Gorinstein, S.; Böhm, V.; Schaich, K.M.; Özyürek, M.; Güçlü, K. Methods of measurement and evaluation of natural antioxidant capacity/activity (IUPAC Technical Report). Pure Appl. Chem. 2013, 85, 957–998. [Google Scholar] [CrossRef]
- Katana, B.; Kókai, K.P.; Sáringer, S.; Szerlauth, A.; Takács, D.; Szilágyi, I. The Influence of Solvents and Colloidal Particles on the Efficiency of Molecular Antioxidants. Antioxidants 2022, 12, 99. [Google Scholar] [CrossRef]
- Romera-Castillo, C.; Jaffé, R. Free radical scavenging (antioxidant activity) of natural dissolved organic matter. Mar. Chem. 2015, 177, 668–676. [Google Scholar] [CrossRef]
- Judzentiene, A.; Budiene, J.; Nedveckyte, I.; Garjonyte, R. Antioxidant and toxic activity of Helichrysum arenarium (L.) Moench and Helichrysum italicum (Roth) G. Don essential oils and extracts. Molecules 2022, 27, 1311. [Google Scholar] [CrossRef]
- Lizárraga-Velázquez, C.E.; Hernández, C.; González-Aguilar, G.A.; Heredia, J.B. Effect of dietary intake of phenolic compounds from mango peel extract on growth, lipid peroxidation and antioxidant enzyme activities in zebrafish (Danio rerio). Lat. Am. J. Aquat. 2019, 47, 602–611. [Google Scholar] [CrossRef]
- Liu, Y.; Qian, J.; Li, J.; Xing, M.; Grierson, D.; Sun, C.; Cu, X.; Li, X.; Chen, K. Hydroxylation decoration patterns of flavonoids in horticultural crops: Chemistry, bioactivity, and biosynthesis. Hortic. Res. 2022, 9, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Deladino, L.; Scneider Teixeira, A.; Reta, M.R.; Molina Garcia, A.D.; Navarro, A.S.D.R.; Martino, M.N. Major phenolics in yerba mate extracts (Ilex paraguariensis) and their contribution to the total antioxidant capacity. Food Nutr. Sci. 2013, 4, 154–162. [Google Scholar] [CrossRef]
- Biela, M.; Kleinová, A.; Klein, E. Phenolic acids and their carboxylate anions: Thermodynamics of primary antioxidant action. Phytochemistry 2022, 200, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kroon, P.A.; Garcia-Conesa, M.T.; Fillingham, I.J.; Hazlewood, G.P.; Williamson, G. Release of ferulic acid dehydrodimers from plant cell walls by feruloyl esterases. J. Sci. Food Agric. 1999, 79, 428–434. [Google Scholar] [CrossRef]
- Kumar, N.; Pruthi, V. Potential applications of ferulic acid from natural sources. Biotechnol. Rep. 2014, 4, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Mathew, S.; Abraham, T.M. Bioconversions of ferulic acid, an hydroxycinnamic acid. Crit. Rev. Microbiol. 2006, 32, 115–125. [Google Scholar] [CrossRef]
- Ghosh, S.; Sachan, A.; Mitra, A. Formation of vanillic acid from ferulic acid by Paecilomyces variotii MTCC 6581. Curr. Sci. 2006, 6, 825–829. [Google Scholar]
- Yang, J.; Lee, H.; Sung, J.; Kim, Y.; Jeong, H.S.; Lee, J. Conversion of rutin to quercetin by acid treatment in relation to biological activities. Prev. Nutr. Food Sci. 2019, 24, 313–320. [Google Scholar] [CrossRef]
- Suzuki, T.; Morishita, T.; Kim, S.J.; Park, S.U.; Woo, S.H.; Noda, T.; Takigawa, S. Physiological roles of rutin in the buckwheat plant. Jpn. Agric. Res. Q. 2015, 9, 37–43. [Google Scholar] [CrossRef]
- Taniguchi, M.; LaRocca, C.A.; Bernat, J.D.; Lindsey, J.S. Digital database of absorption spectra of diverse flavonoids enables structural comparisons and quantitative evaluations. J. Nat. Prod. 2023, 86, 1087–1119. [Google Scholar] [CrossRef]
- Cheng, Y.; Wang, Z.; Quan, W.; Xue, C.; Qu, T.; Wang, T.; Chen, Q.; Wang, Z.; Zeng, M.; Qin, F.; et al. Pine pollen: A review of its chemical composition, health effects, processing, and food applications. Trends Food Sci. Technol. 2023, 138, 599–614. [Google Scholar] [CrossRef]
- Carpenter, S.R.; Pace, M.P. Dystrophy and eutrophy in lake ecosystems: Implications of fluctuating inputs. Oikos 1997, 78, 3–14. [Google Scholar] [CrossRef]
- Kostkevičienė, J.; Laučiūtė, R. Contribution to the Lithuanian freshwater red algae. Bot. Lith. 2009, 15, 93–104. [Google Scholar]
- Davis, M.B.; Brubaker, L.B. Differential sedimentation of pollen grains in lakes. Limnol. Oceanogr. 1973, 18, 635–646. [Google Scholar] [CrossRef]
- Kabailienė, M. Lietuvos Holocenas (Holocene of Lithuania); Mokslas (Science): Vilnius, Lithuania, 1990; p. 176. [Google Scholar]
- Kabailienė, M. Ozera Yugo-Vostochnoj Litvy i ikh okruzhayushchaya sreda v pozdnem lednikovje i golocene po dannym diatomovogo i pyljcevogo analiza otlozhenij (Lakes of South-Eastern Lithuania and their environment in the Late Glacial and Holocene according to diatom and pollen sediment analysis). Appl. Limnol. 2002, 3, 123–132. [Google Scholar]
- Garunkštis, A. Sedimentacionnyje Process v Oziorakh Litvy (Sedimentation Processes in Lakes of Lithuania); Mokslas (Science): Vilnius, Lithuania, 1975; p. 296. [Google Scholar]
- Odgaard, B.V. Wind-determined sediment distribution and Holocene sediment yield in a small, Danish, kettle lake. J. Paleolimnol. 1993, 8, 3–13. [Google Scholar] [CrossRef]
- Gorelick, N.; Matt Hancher, M.; Dixon, M.; Ilyushchenko, S.; Thau, D.; Moore, R. Google earth engine: Planetary-scale geospatial analysis for everyone. Remote Sens. Environ. 2017, 202, 18–27. [Google Scholar] [CrossRef]
- PBC, Planet Labs. Planet Application Program Interface: In Space for Life on Earth. 2023. Available online: https://api.planet.com (accessed on 15 May 2023).
- Aleksandrov, S.V. Long-Term Variability of the Trophic Status of the Curonian and Vistula Lagoons of Baltic Sea. Inland. Water Biol. 2009, 2, 319–326. [Google Scholar] [CrossRef]
- Wasmund, N.; Andrushaitis, A.; Lysiak-Pastuszak, E.; Müller-Karulis, B.; Nausch, G.; Neuman, T.; Ojaveer, H.; Olenina, I.; Postel, L.; Witek, Z. Trophic Status of the South-Eastern Baltic Sea: A Comparison of Coastal and Open areas. Estuar. Coast. Shelf Sci. 2001, 53, 849–864. [Google Scholar] [CrossRef]
- Balevičienė, J.; Balevičius, A.; Bukelskis, E.; Ciūnys, A.; Stanevičius, V.; Vaitkus, G.; Valiuškevičius, G.; Kalytytė, D.; Ūselytė, R.; Šalčiūnienė, K. Restauruotinų Lietuvos Ežerų Nustatymas ir Preliminarus Restauravimo Priemonių Parinkimas Šiems Ežerams, Siekiant Pagerinti jų Būklę. Galutinė Ataskaita, I Dalis (Identification of Lithuanian Lakes to Be Restored and Preliminary Selection of Restoration Means for These Lakes to Improve Their Condition. Final Report, Part I) 2009. pp. 1–265. Available online: https://vanduo.old.gamta.lt/files/REST%20EZERU%20GALUTINE%20ATASKAITA%20I%20dalis.pdf (accessed on 30 April 2023).
- Kilkus, K. Lietuvos Draustinių Ežerai (Lakes in Lithuanian Nature Reserves); Mokslas (Science): Vilnius, Lithuania, 1986; p. 142. [Google Scholar]
- Koreivienė, J.; Kasperovičienė, J.; Karosienė, J. Morphological variability of raphidophycean algae in the lakes of Lithuania. In Current Advances in Algal Taxonomy and Its Aplications: Phylogenetic, Ecological and Applied Perspective; Wolowski, K., Kaczmarska, I., Ehrman, L.M., Wojtal, Z.A., Eds.; Polish Academy of Sciences, W Szafer Institute of Botany: Krakow, Poland, 2012; pp. 153–164. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.R-project.org/ (accessed on 15 May 2023).
- RStudio Team. RStudio: Integrated Development for R; Rstudio: Boston, MA, USA, 2020; Available online: http://www.rstudio.com/ (accessed on 15 May 2023).
- Ogle, D.H.; Doll, J.C.; Wheeler, A.P.; Dinno, A. FSA: Simple Fisheries Stock Assessment Methods. R Package Version 0.9.5. 2023. Available online: https://CRAN.R-project.org/package=FSA (accessed on 25 October 2023).





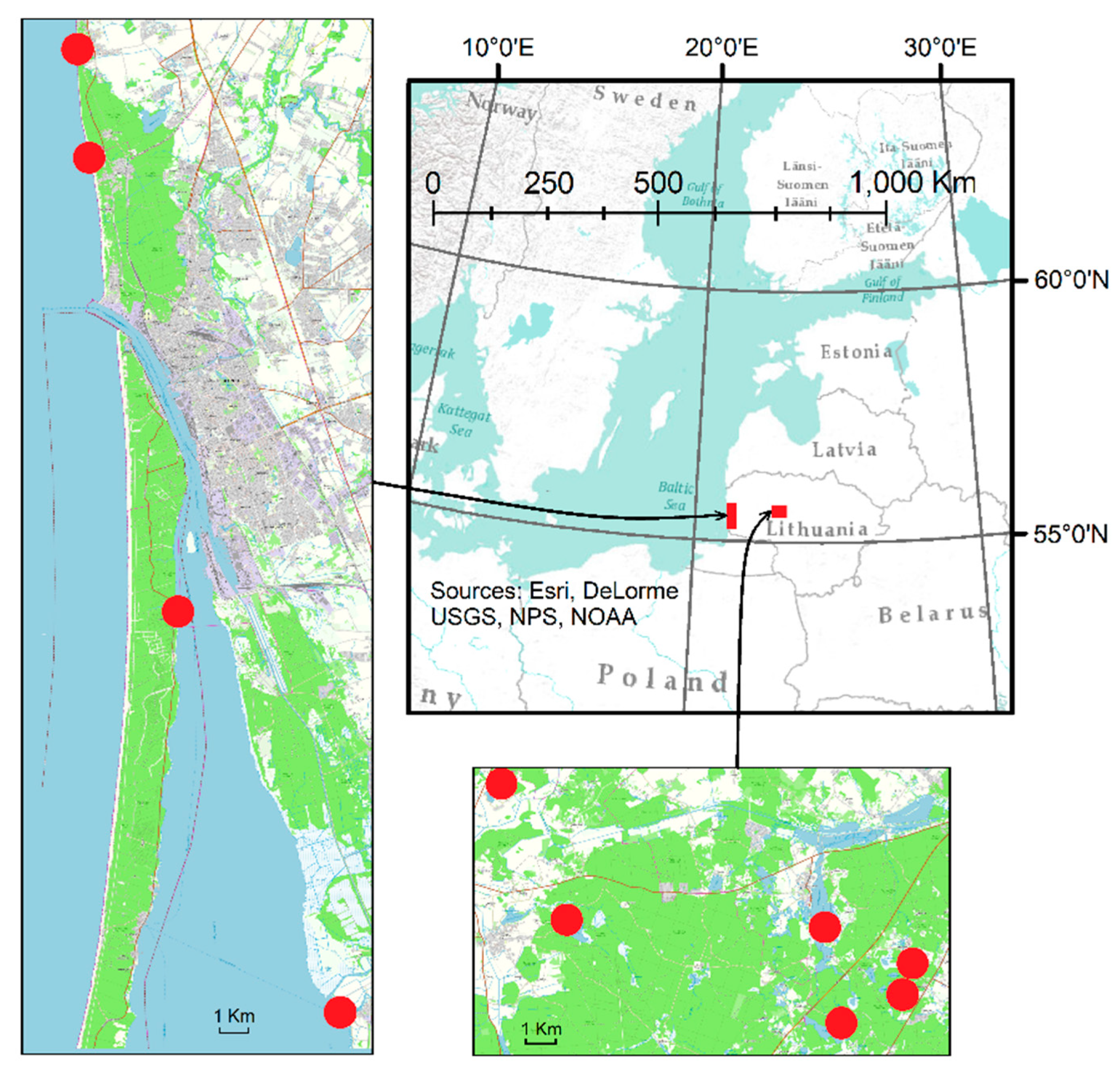
| Trophicity of Water Bodies | Code of Water Bodies | I Stage (29 April–10 May 2022), SPB | II Stage (4 June–8 June 2022), SPP | ||
|---|---|---|---|---|---|
| Pollen Content, % | Pollen Composition | Pollen Content, % | Pollen Composition | ||
| Dystrophic | NLS | 3 | 98% Betula, 2% Pinus | 50 | 99.5% Pinus, 0.5% Picea |
| Eutrophic with indications of dystrophy | NLG | - | - | 95 | 98% Pinus, 2% Picea |
| Mesotrophic-eutrophic | DNLB | 10 | 90% Betula, 10% Pinus, Alnus, Corylus | 80 | 100% Pinus |
| Mesotrophic | FQK | 21 | 99% Betula, 1% Pinus, Fraxinus, Alnus | 80 | 100% Pinus |
| Eutrophic | BSC | 5 | 52% Corylus, 34% Alnus, 10% Betula, 4% Pinus, Carpinus | 90 | 100% Pinus |
| Eutrophic-hypetrophic | DNLJ | 57 | 99% Betula, 1% Alnus, Corylus | 95 | 99.8% Pinus, 0.2% Picea |
| Hypertrophic | CLC | 6 | 83% Betula, 7% Salix, Fraxinus | 80 | 100% Pinus |
| Hypertrophic | DFD | 6 | 91% Betula, 9% Pinus, Alnus | 80 | 99.5% Pinus, 0.5% Picea |
| Water Body | SPB, mg/g (n = 4) | SPP, mg/g (n = 4) | |||
|---|---|---|---|---|---|
| Trophicity | Code | Bound PC | Free PC | Bound PC | Free PC |
| Dystrophic | NLS | 6.5 ± 0.2 | n/d | 9.0 ± 0.1 | n/d |
| Eutrophic (with indications of dystrophy) | NLG | n/d | n/d | 10.6 ± 0.7 | 7.8 ± 0.7 |
| Mesotrophic | FQK | 10.9 ± 0.2 | n/d | 4.1 ± 0.6 | 4.0 ± 0.3 |
| Mesotrophic-eutrophic | DNLB | 3.0 ± 0.0 | n/d | 11.3 ± 0.1 | 11.4 ± 0.1 |
| Eutrophic | BSC | 3.4 ± 0.5 | 1.0 ± 0.1 | 6.8 ± 1.4 | 6.4 ± 0.6 |
| Eutrophic-hypereutrophic | DNLJ | 7.3 ± 0.3 | n/d | 9.7 ± 2.0 | 10.2 ± 0.1 |
| Hypereutrophic | CLC | 9.1 ± 0.2 | 2.2 ± 0.1 | 5.4 ± 0.1 | 1.7 ± 0.2 |
| Hypereutrophic | DFD | 9.1 ± 0.7 | 4.2 ± 0.2 | 6.4 ± 0.0 | n/d |
| Phenolic Compounds | PW | SPB | SPBW | SPP | SPPW |
|---|---|---|---|---|---|
| trans-Ferulic acid | + | + | + | + | + |
| Vanillic acid | + | + | + | + | + |
| Gallic acid | + | + | + | ||
| Chlorogenic acid | + | ||||
| 3,4-DHB | + | + | |||
| p-Coumaric acid | + | ||||
| Quercetin | + | + |
| Trophicity | Eutrophic (with Indications of Dystrophy) | Eutrophic | Mesotrophic-Eutrophic | Eutrophic-Hyper-Eutrophic | Hyper-Eutrophic | Mesotrophic | Dystrophic |
|---|---|---|---|---|---|---|---|
| Eutrophic (with indications of dystrophy) | n/d | n/d | n/d | n/d | n/d | n/d | |
| Eutrophic | 5.3 ** | 1.1 | 0.2 | 0.5 | 1.9 | 0.5 | |
| Mesotrophic-eutrophic | 1.9 | 1.0 | 1.2 | 0.9 | 1.0 | 1.0 | |
| Eutrophic-hypereutrophic | 3.1 * | 2.3 | 0.4 | 0.8 | 2.0 | 1.1 | |
| Hypereutrophic | 7.8 ** | 0.7 | 1.4 | 3.6 * | 1.8 | 0.1 | |
| Mesotrophic | 8.7 ** | 1.6 | 1.9 | 4.6 ** | 1.3 | 1.8 | |
| Dystrophic | 0.4 | 3.1* | 1.7 | 1.8 | 3.6 * | 4.1 * |
| Water Bodies and Code | Origin of the Water Body | Water Level Altitude, m | Area of Water Body, km2 | Mean Depth, m * | Watershed Area, km2 | Trophic Status | Coastal Landscape ** |
|---|---|---|---|---|---|---|---|
| Lake Geluva, NLG | Natural | 134 | 0.179 | 3–5 | 0.61 | Eutrophic (with indications of dystrophy) | Forests, swamps |
| Lake Bijotė, DNLB | Natural (dammed) | 128 | 0.665 | 4.3 | 4.32 | Mesotrophic–eutrophic | Forests |
| Lake Juodlė, DNLJ | Natural (dammed) | 114 | 0.340 | 1–3 | 2.24 | Eutrophic–hypertrophic | Forests, swamps |
| Lake Šermukšnynas, NLS | Natural | 134 | 0.035 | 1–3 | 0.33 | Dystrophic | Forests, swamps, bushes |
| Lake Damba, DFD | Artificial (fishpond) | 111 | 0.545 | 1–3 | 1.91 | Hypertrophic | Forests, meadows |
| Lake Kalniškiai, FQK | Artificial (flooded quarry) | 99 | 0.214 | 1–3 | 0.35 | Mesotrophic | Cultivated fields, meadows, bushes |
| Curonian Lagoon, CLC | Natural | 0 | 1584 | 3.8 | 100,500 | Hypertrophic | Forests, swamps, bushes, sandy beaches |
| Baltic Sea, BSC | Natural | 0 | 412,500 | 55 | 2.13 mill. | Eutrophic | Sandy beaches, forests |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kerienė, I.; Šaulienė, I.; Šukienė, L.; Judžentienė, A.; Ligor, M.; Valiuškevičius, G.; Grendaitė, D.; Buszewski, B. Enrichment of Water Bodies with Phenolic Compounds Released from Betula and Pinus Pollen in Surface Water. Plants 2024, 13, 99. https://doi.org/10.3390/plants13010099
Kerienė I, Šaulienė I, Šukienė L, Judžentienė A, Ligor M, Valiuškevičius G, Grendaitė D, Buszewski B. Enrichment of Water Bodies with Phenolic Compounds Released from Betula and Pinus Pollen in Surface Water. Plants. 2024; 13(1):99. https://doi.org/10.3390/plants13010099
Chicago/Turabian StyleKerienė, Ilona, Ingrida Šaulienė, Laura Šukienė, Asta Judžentienė, Magdalena Ligor, Gintaras Valiuškevičius, Dalia Grendaitė, and Bogusław Buszewski. 2024. "Enrichment of Water Bodies with Phenolic Compounds Released from Betula and Pinus Pollen in Surface Water" Plants 13, no. 1: 99. https://doi.org/10.3390/plants13010099
APA StyleKerienė, I., Šaulienė, I., Šukienė, L., Judžentienė, A., Ligor, M., Valiuškevičius, G., Grendaitė, D., & Buszewski, B. (2024). Enrichment of Water Bodies with Phenolic Compounds Released from Betula and Pinus Pollen in Surface Water. Plants, 13(1), 99. https://doi.org/10.3390/plants13010099