Antiquorum and Antibiofilm Activities of Piper bogotense C. DC. against Pseudomonas aeruginosa and Identification of Bioactive Compounds
Abstract
:1. Introduction
2. Results and Discussion
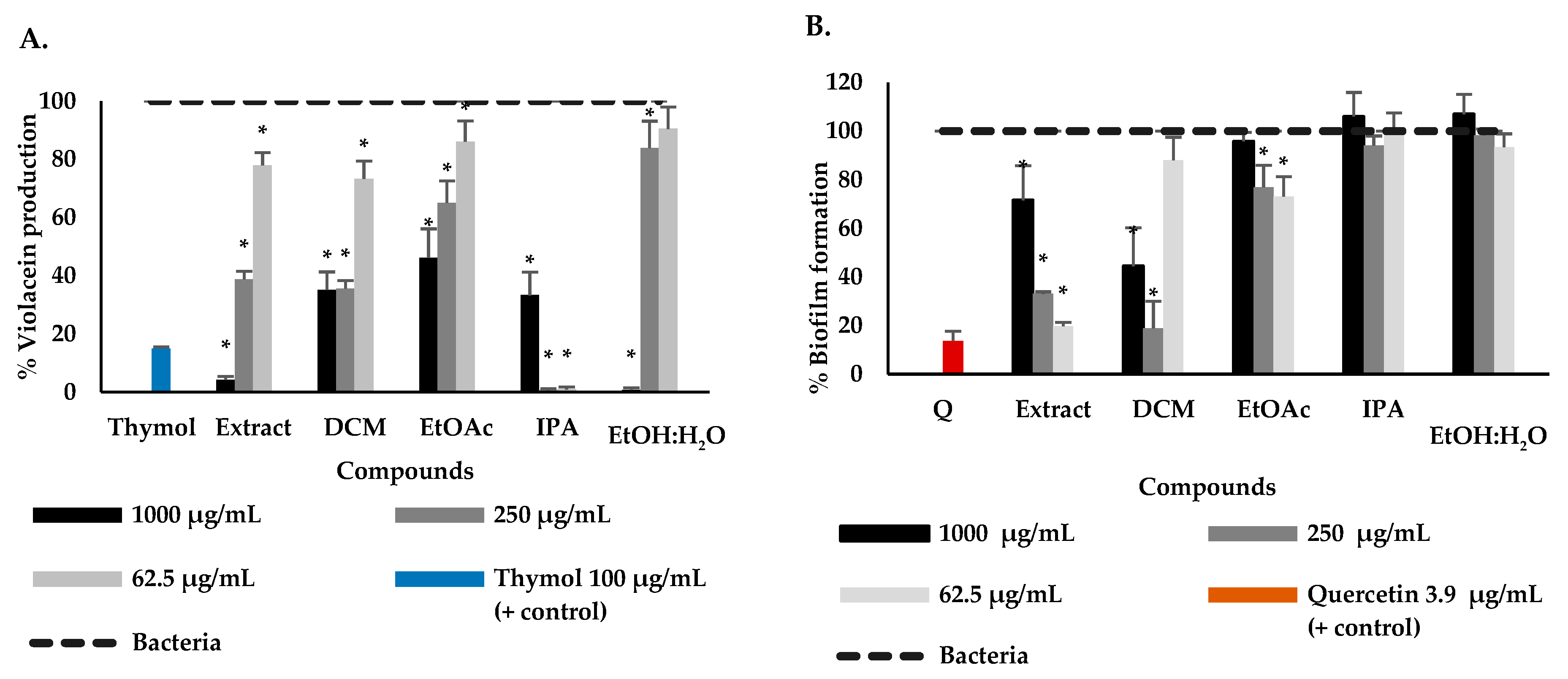
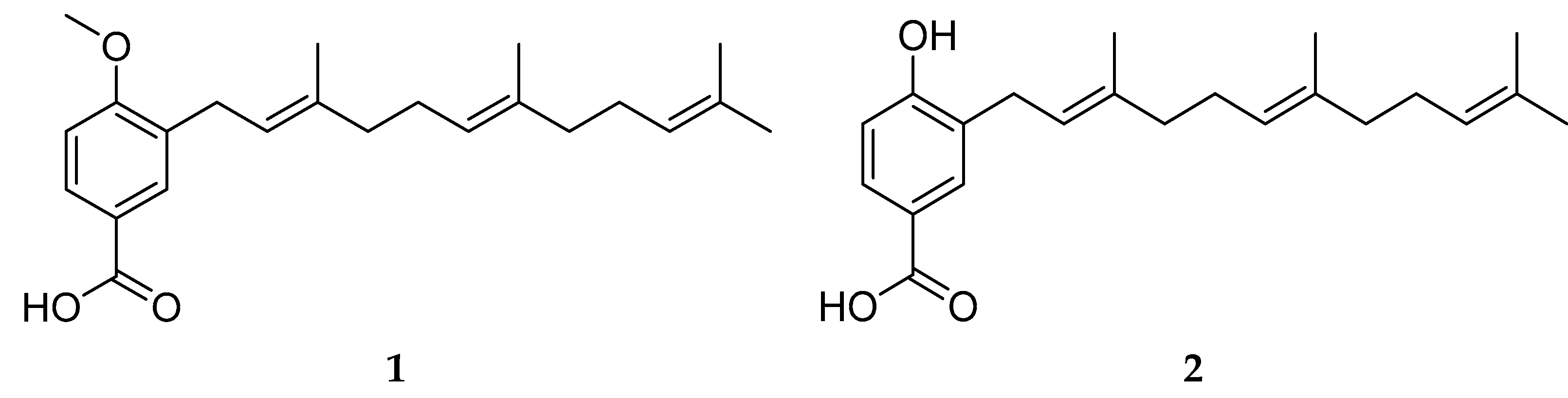
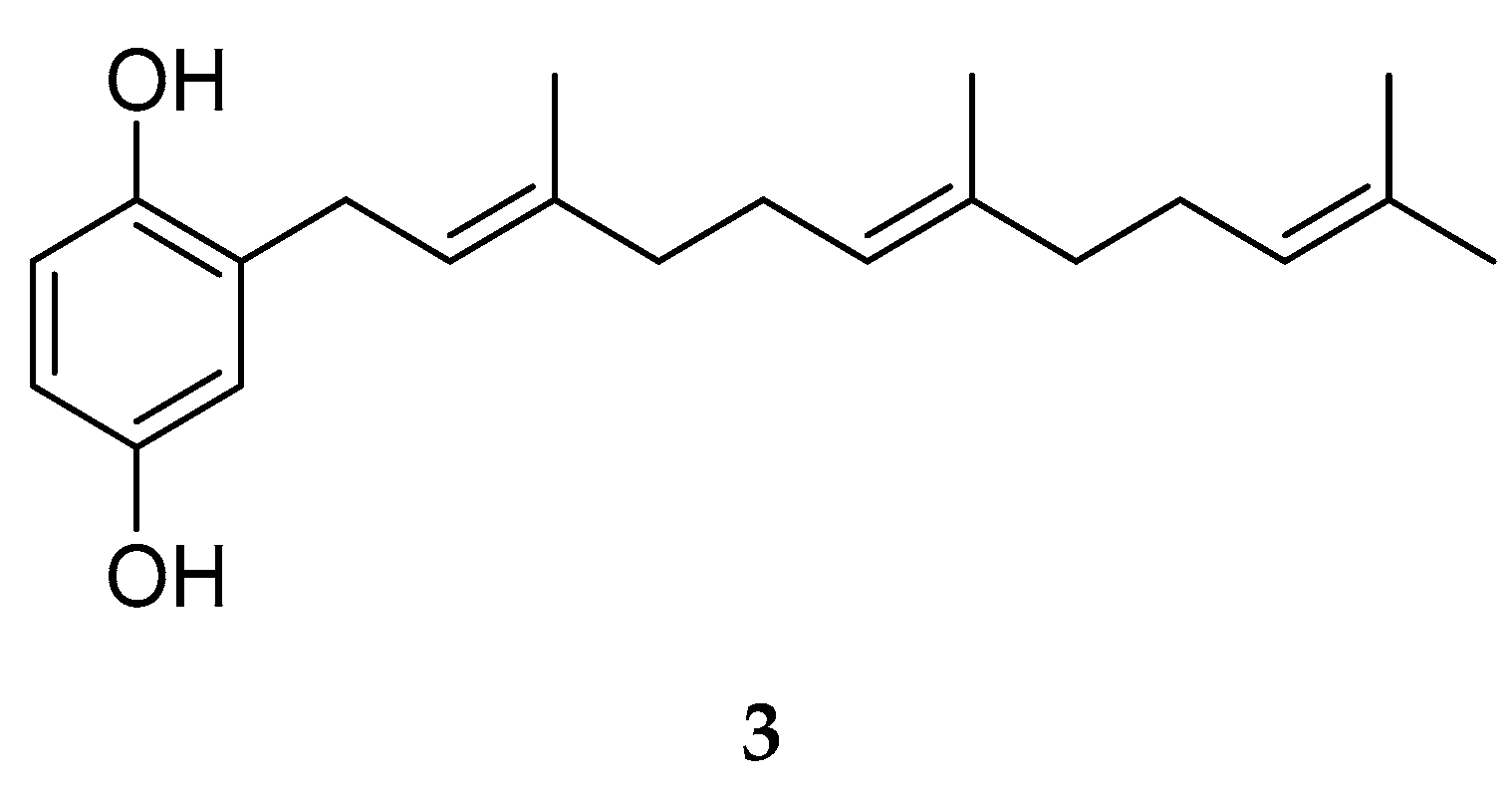
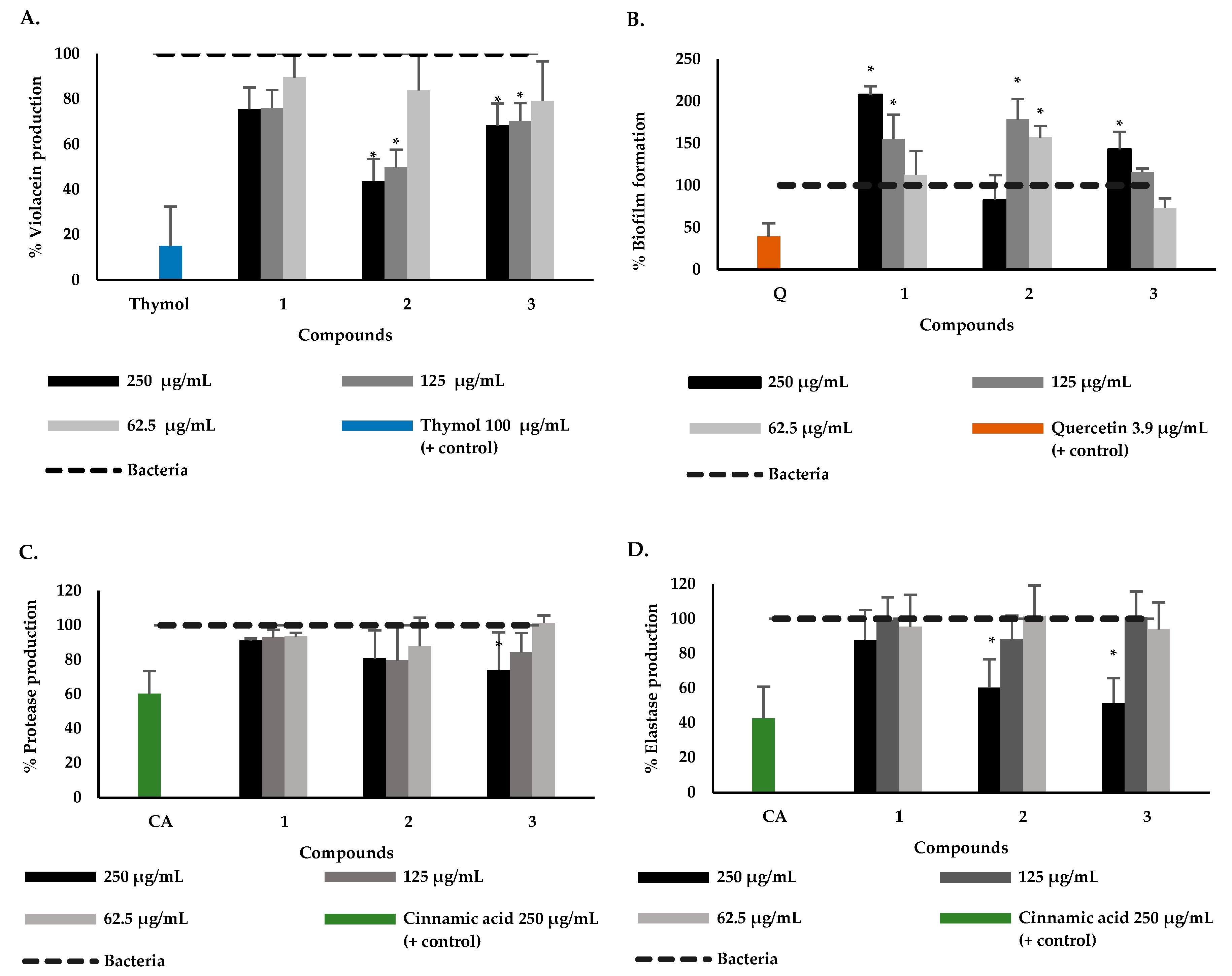
2.1. Phytochemical Study and Anti-QS and Anti-Biofilm Activity
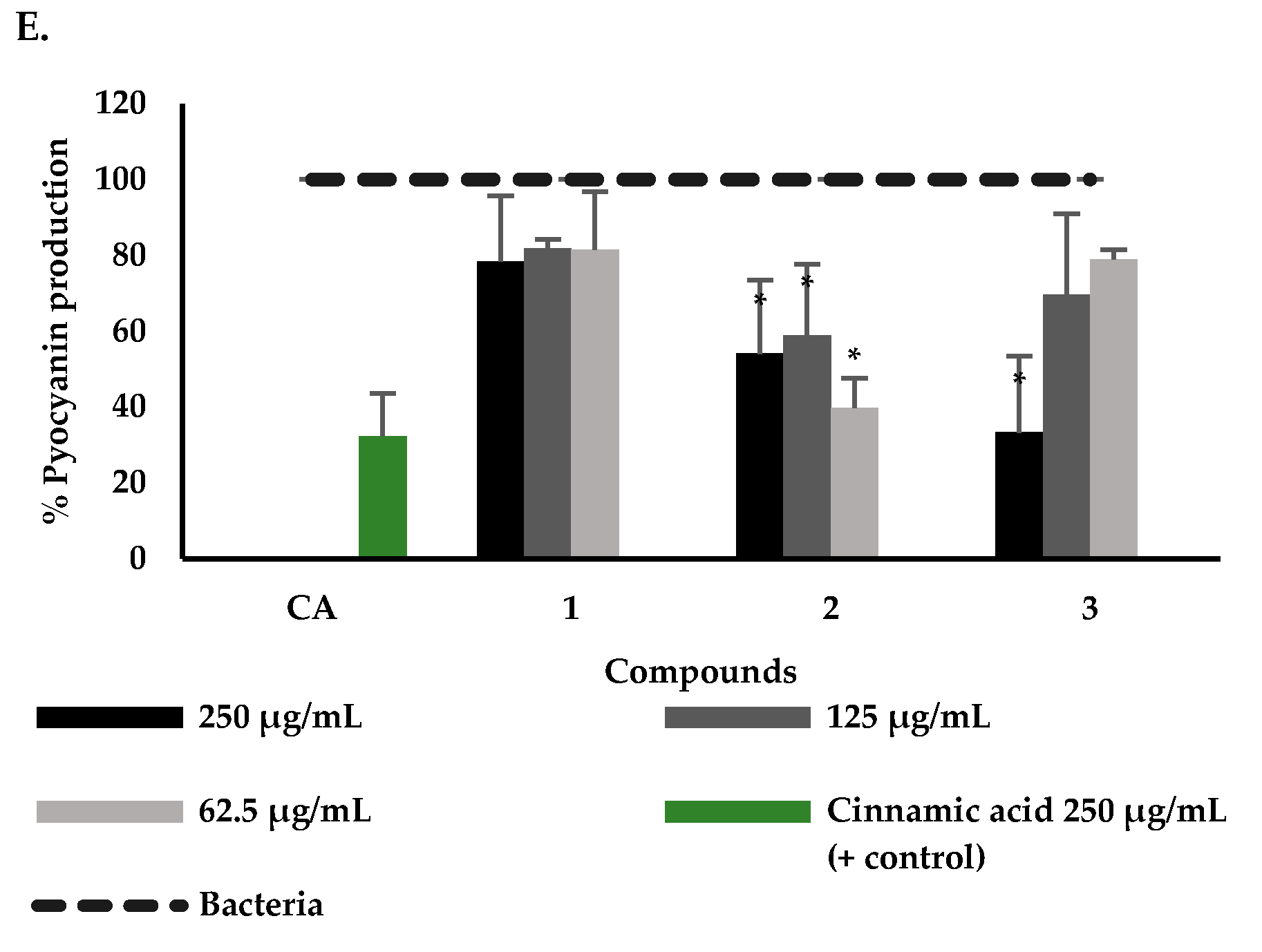
2.2. Effect of P. bogotense Compounds against C. violaceum Quorum Sensing, Biofilm Formation and Production of Virulence Factors in P. aeruginosa
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Plant Material
3.3. Extraction and Isolation of Compounds
3.4. Strains and Bacterial Growth Conditions
3.5. Evaluation of Bacterial Growth on P. aeruginosa
3.6. Biofilm Formation and Quantification Assay in P. aeruginosa
3.7. Quorum Sensing Inhibition Assay: Quantification of Violacein Production from C. violaceum
3.8. Assays on Virulence Factors in P. aeruginosa
3.8.1. Quantification of Pyocyanin Production
3.8.2. Quantification of Elastase Production
3.8.3. Quantification of Protease Production
3.9. Data Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Global Antimicrobial Resistance and Use Surveillance System (GLASS) Report. Available online: https://apps.who.int/iris/bitstream/handle/10665/341666/9789240027336-eng.pdf (accessed on 29 March 2023).
- World Bank. Drug-Resistant Infections: A Threat to Our Economic Future. Available online: www.worldbank.org (accessed on 29 March 2023).
- Haque, S.; Ahmad, F.; Dar, S.A.; Jawed, A.; Mandal, R.K.; Wahid, M.; Lohani, M.; Khan, S.; Singh, V.; Akhter, N. Developments in strategies for quorum sensing virulence factor inhibition to combat bacterial drug resistance. Microb. Pathog. 2018, 121, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Li, M.; Yi, G.; Liao, L.; Cheng, Q.; Zhu, J.; Zhang, B.; Wang, Y.; Chen, Y.; Zeng, M. Screening strategies for quorum sensing inhibitors in combating bacterial infections. J. Pharm. Anal. 2022, 12, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Santhakumari, S.; Ravi, A.V. Targeting quorum sensing mechanism: An alternative anti-virulent strategy for the treatment of bacterial infections. S. Afr. J. Bot. 2019, 120, 81–86. [Google Scholar] [CrossRef]
- Saeki, E.K.; Kobayashi, R.K.T.; Nakazato, G. Quorum sensing system: Target to control the spread of bacterial infections. Microb. Pathog. 2020, 142, 104068. [Google Scholar] [CrossRef] [PubMed]
- Vadakkan, K. Molecular mechanism of bacterial quorum sensing and its inhibition by target specific approaches. ACS Symp. Ser. 2020, 1374, 21–234. [Google Scholar] [CrossRef]
- Botelho, J.; Grosso, F.; Peixe, L. Antibiotic resistance in Pseudomonas aeruginosa—mechanisms, epidemiology and evolution. Drug Resist. Updates 2019, 44, 100640. [Google Scholar] [CrossRef]
- Elabbadi, A.; Pont, S.; Verdet, C.; Plésiat, P.; Cretin, F.; Voiriot, G.; Fartoukh, M.; Djibré, M. An unusual community-acquired invasive and multi systemic infection due to ExoU-harboring Pseudomonas aeruginosa strain: Clinical disease and microbiological characteristics. J. Microbiol. Immunol. Infect. 2019, 53, 647–651. [Google Scholar] [CrossRef]
- Høiby, N.; Bjarnsholt, T.; Givskov, M.; Molin, S.; Ciofu, O. Antibiotic resistance of bacterial biofilms. Int. J. Antimicrob. Agents 2010, 35, 322–332. [Google Scholar] [CrossRef]
- Aguilar, F.R.; Aguilar, S.L.; Cubas, D.M.; Coaguila, L.Á.; Fernández, D.A.; Mario, M.M.; Campos, R.; Guevara-Vásquez, G.; Díaz, R.S. Portadores de bacterias multirresistentes de importancia clínica en áreas críticas (UCI-UCIN) de un hospital al norte del Perú. Horiz. Médico 2016, 16, 50–57. [Google Scholar] [CrossRef]
- Karakonstantis, S.; Kritsotakis, E.I.; Gikas, A. Pandrug-resistant gram-negative bacteria: A systematic review of current epidemiology, prognosis and treatment options. J. Antimicrob. Chemother. 2020, 75, 271–282. [Google Scholar] [CrossRef]
- Luo, J.; Dong, B.; Wang, K.; Cai, S.; Liu, T.; Cheng, X.; Lei, D.; Chen, Y.; Li, Y.; Kong, J.; et al. Baicalin inhibits biofilm formation, attenuates the quorum sensing-controlled virulence and enhances Pseudomonas aeruginosa clearance in a mouse peritoneal implant infection model. PLoS ONE 2017, 12, 0176883. [Google Scholar] [CrossRef]
- Silva, A.; Silva, V.; Igrejas, G.; Poeta, P. Chapter 17: Carbapenems and Pseudomonas aeruginosa: Mechanisms and Epidemiology. In Antibiotics and Antimicrobial Resistance Genes in the Environment: Volume 1 in the Advances in Environmental Pollution Research Series; Elsevier: Amsterdam, The Netherlands, 2020; Volume 1, pp. 253–268. ISBN 9780128188828. [Google Scholar]
- Yendo, T.M.; de Pante, C.C.; Miyamoto, D. Pseudomonas aeruginosa as an uncommon agent of infectious panniculitis. An. Bras. Dermatol. 2022, 97, 326–331. [Google Scholar] [CrossRef] [PubMed]
- Sambrano, H.; Castillo, J.C.; Ramos, C.W.; de Mayorga, B.; Chen, O.; Durán, O.; Ciniglio, C.; Aguilar, C.; Cisterna, O.; de Chial, M. Prevalence of antibiotic resistance and virulent factors in nosocomial clinical isolates of Pseudomonas aeruginosa from Panamá. Braz. J. Infect. Dis. 2021, 25, 101038. [Google Scholar] [CrossRef] [PubMed]
- Silby, M.W.; Winstanley, C.; Godfrey, S.A.C.; Levy, S.B.; Jackson, R.W. Pseudomonas genomes: Diverse and adaptable. FEMS Microbiol. Rev. 2011, 35, 652–680. [Google Scholar] [CrossRef] [PubMed]
- Pirnay, J.-P.; Bilocq, F.; Pot, B.; Cornelis, P.; Zizi, M.; Van Eldere, J.; Deschaght, P.; Vaneechoutte, M.; Jennes, S.; Pitt, T.; et al. Pseudomonas aeruginosa population structure revisited. PLoS ONE 2009, 4, e7740. [Google Scholar] [CrossRef] [PubMed]
- WHO. WHO Publishes List of Bacteria for Which New Antibiotics Are Urgently Needed. Available online: https://www.who.int/news/item/27-02-2017-who-publishes-list-of-bacteria-for-which-new-antibiotics-are-urgently-needed (accessed on 29 September 2022).
- Amaning Danquah, C.; Osei-Djarbeng, S.; Appiah, T.; Duah Boakye, Y.; Adu, F. Combating Biofilm and Quorum Sensing: A New Strategy to Fight Infections. In Bacterial Biofilms; IntechOpen: London, UK, 2020. [Google Scholar]
- Mohsenipour, Z.; Hassanshahian, M. The effects of Allium sativum extracts on biofilm formation and activities of six pathogenic bacteria. Jundishapur J. Microbiol. 2015, 8, e18971. [Google Scholar] [CrossRef] [PubMed]
- Qais, F.A.; Ahmad, I. Anti-quorum sensing and biofilm inhibitory effect of some medicinal plants against gram-negative bacterial pathogens: In vitro and in silico investigations. Heliyon 2022, 8, e11113. [Google Scholar] [CrossRef]
- Lu, L.; Hu, W.; Tian, Z.; Yuan, D.; Yi, G.; Zhou, Y.; Cheng, Q.; Zhu, J.; Li, M. Developing natural products as potential anti-biofilm agents. Chin. Med. 2019, 14, 11. [Google Scholar] [CrossRef]
- Abdel Bar, F.M.; Alossaimi, M.A.; Elekhnawy, E.; Alzeer, M.A.A.; Abo Kamer, A.; Moglad, E.; ElNaggar, M.H. Anti-quorum sensing and anti-biofilm activity of Pelargonium × hortorum root extract against Pseudomonas aeruginosa: Combinatorial effect of catechin and gallic acid. Molecules 2022, 27, 7841. [Google Scholar] [CrossRef]
- Ćirić, A.D.; Petrović, J.D.; Glamočlija, J.M.; Smiljković, M.S.; Nikolić, M.M.; Stojković, D.S.; Soković, M.D. Natural products as biofilm formation antagonists and regulators of quorum sensing functions: A comprehensive review update and future trends. S. Afr. J. Bot. 2019, 120, 65–80. [Google Scholar] [CrossRef]
- dos Santos, V.L.P.; Rodrigues, I.C.G.; Berté, R.; Raman, V.; Messias-Reason, I.J.; Budel, J.M. Review of Piper species growing in the Brazilian State of Paraná with emphasize on the vegetative anatomy and biological activities. Bot. Rev. 2021, 87, 23–54. [Google Scholar] [CrossRef]
- Ashokkumar, K.; Murugan, M.; Dhanya, M.K.; Pandian, A.; Warkentin, T.D. Phytochemistry and therapeutic potential of black pepper [Piper nigrum (L.)] essential oil and piperine: A review. Clin. Phytoscience 2021, 7, 52. [Google Scholar] [CrossRef]
- de Tamokou, J.D.; Mbaveng, A.T.; Kuete, V. Chapter 8: Antimicrobial Activities of African Medicinal Spices and Vegetables. In Medicinal Spices and Vegetables from Africa; Academic Press: Cambridge, MA, USA, 2017; pp. 207–237. ISBN 9780128094419. [Google Scholar]
- The Plant List. Available online: http://www.theplantlist.org/ (accessed on 19 October 2022).
- Fan, D.; Zhou, C.; Chen, C.; Li, X.; Ma, J.; Hu, Y.; Li, G.; Ruan, J.; Wu, A.; Li, L.; et al. Lignans from the genus Piper L. and their pharmacological activities: An updated review. Fitoterapia 2023, 165, 105403. [Google Scholar] [CrossRef] [PubMed]
- Parmar, V.S.; Jain, S.C.; Bisht, K.S.; Jain, R.; Taneja, P.; Jha, A.; Tyagi, O.D.; Prasad, A.K.; Wengel, J.; Olsen, C.E.; et al. Phytochemistry of the genus Piper. Phytochemistry 1997, 46, 597–673. [Google Scholar] [CrossRef]
- Chakraborty, D.; Shah, B. Antimicrobial, antioxidative and antihemolytic activity of Piper betel leaf extracts. Int. J. Pharm. Pharm. Sci. 2011, 3, 192–199. [Google Scholar]
- Ganesh, P.; Suresh Kumar, R.; Saranraj, P. Phytochemical analysis and antibacterial activity of Pepper (Piper nigrum L.) against some human pathogens. Sch. Res. Libr. Cent. Eur. J. Exp. Biol. 2014, 3, 36–41. [Google Scholar]
- Trivedi, M.N.; Khemani, A.; Vachhani, U.D.; Shah, C.P.; Santani, D.D. Pharmacognostic, phytochemical analysis and antimicrobial activity of two Piper species. Pharm. Glob. 2011, 7, 1–4. [Google Scholar]
- Vallejo, A.; Feitosa, A.; Gourlart, A.; Pires, L.; Mosquera, O. Tamizaje de acción antimicrobiana de 34 extractos vegetales contra bacilos gramnegativos. Salud. Soc. Uptc. 2014, 1, 34–39. [Google Scholar]
- Prabhu, A.; Chembili, V.; Kandal, T.; Punchappady-Devasya, R. Piper nigrum seeds inhibit biofilm formation in Pseudomonas aeruginosa strains. Res. J. Pharm. Technol. 2017, 10, 3894–3898. [Google Scholar] [CrossRef]
- Datta, S.; Jana, D.; Maity, T.R.; Samanta, A.; Banerjee, R. Piper betle leaf extract affects the quorum sensing and hence virulence of Pseudomonas aeruginosa PAO1. 3 Biotech 2016, 6, 18. [Google Scholar] [CrossRef]
- Zaki, A.A. Assessment of anti-quorum sensing activity for some ornamental and medicinal plants native to Egypt. Sci. Pharm. 2013, 81, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Martín-Rodríguez, A.J.; Ticona, J.C.; Jiménez, I.A.; Flores, N.; Fernández, J.J.; Bazzocchi, I.L. Flavonoids from Piper delineatum modulate quorum-sensing-regulated phenotypes in Vibrio harveyi. Phytochemistry 2015, 117, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Martínez, J.; Buitemea-Cantúa, G.V.; Gutierrez-Villagomez, J.M.; García-González, J.P.; Ramírez-Chávez, E.; Molina-Torres, J. Bioautography and GC-MS based identification of piperine and trichostachine as the active quorum quenching compounds in black pepper. Heliyon 2020, 6, e03137. [Google Scholar] [CrossRef]
- Tan, L.Y.; Yin, W.F.; Chan, K.G. Piper nigrum, Piper betle and Gnetum gnemon-Natural food sources with anti-quorum sensing properties. Sensors 2013, 13, 3975–3985. [Google Scholar] [CrossRef]
- Srinivasan, R.; Santhakumari, S.; Ravi, A.V. In vitro antibiofilm efficacy of Piper betle against quorum sensing mediated biofilm formation of luminescent Vibrio harveyi. Microb. Pathog. 2017, 110, 232–239. [Google Scholar] [CrossRef]
- Olivero, J.T.V.; Pájaro, N.P.C.; Stashenko, E. Antiquorum sensing activity of essential oils isolated from different species of the genus Piper. Vitae 2011, 18, 77–82. [Google Scholar] [CrossRef]
- Amrutha, B.; Sundar, K.; Shetty, P.H. Spice oil nanoemulsions: Potential natural inhibitors against pathogenic E. coli and Salmonella spp. from fresh fruits and vegetables. LWT Food Sci. Technol. 2017, 79, 152–159. [Google Scholar] [CrossRef]
- Sen, S.; Rengaian, G. A Review on the Ecology, Evolution and Conservation of Piper (Piperaceae) in India: Future Directions and Opportunities. Bot. Rev. 2021, 88, 333–358. [Google Scholar] [CrossRef]
- Muñoz, D.; Calvache, D.; Yela, J. Especies forestales con potencial agroforestal para las zonas altas en el departamento de Nariño. Rev. De Cienc. Agrícolas 2013, 30, 38–53. [Google Scholar]
- de Bogota, S.A. de Cordoncillo: Beneficioso Para Los Procesos de Restauración Ecológica y Para La Salud—Historial de Noticias—Secretaría Distrital de Ambiente. Available online: https://ambientebogota.gov.co/es/historial-de-noticias/-/asset_publisher/VqEYxdh9mhVF/content/cordoncillo-beneficioso-para-los-procesos-de-restauracion-ecologica-y-para-la-salud?redirect=https%3A%2F%2Fambientebogota.gov.co%2Fes%2Fhistorial-de-noticias%3Fp (accessed on 10 April 2022).
- Secretaria de Ambiente de Bogotá Propiedades de La Planta Cordoncillo Que Habita En Bogotá|Bogota.Gov.Co. Available online: https://bogota.gov.co/mi-ciudad/ambiente/propiedades-de-la-planta-cordoncillo-que-habita-en-bogota (accessed on 29 March 2023).
- Tene, V.; Malagón, O.; Finzi, P.V.; Vidari, G.; Armijos, C.; Zaragoza, T. An ethnobotanical survey of medicinal plants used in Loja and Zamora-Chinchipe, Ecuador. J. Ethnopharmacol. 2007, 111, 63–81. [Google Scholar] [CrossRef]
- Trelease, W.; Yuncker, G.T. The Piperaceae of Northern South America; University of Illinois Press: Champaign, IL, USA, 1950; Volume 1. [Google Scholar]
- López-Ogalla, J.; García-Palomero, E.; Sánchez-Quesada, J.; Rubio, L.; Delgado, E.; García, P.; Medina, M.; Castro, A.; Muñoz, P. Bioactive prenylated phenyl derivatives derived from marine natural products: Novel scaffolds for the design of BACE inhibitors. Medchemcomm 2014, 5, 474–488. [Google Scholar] [CrossRef]
- Peña, L.A.; Avella, E.; Puentes de Díaz, A.M. Benzoquinona e hidroquinona preniladas y otros constituyentes aislados de Piper bogotense c. dc. Rev. Colomb. De Química 2000, 29, 25–37. [Google Scholar]
- Tangarife-Castaño, V.; Correa-Royero, J.B.; Roa-Linares, V.C.; Pino-Benitez, N.; Betancur-Galvis, L.A.; Durán, D.C.; Stashenko, E.E.; Mesa-Arango, A.C. Anti-dermatophyte, anti-fusarium and cytotoxic activity of essential oils and plant extracts of Piper genus. J. Essent. Oil Res. 2014, 26, 221–227. [Google Scholar] [CrossRef]
- Castañeda, M.L.; Martínez Químico, J.R.; Stanshenko, E.E. Estudio de la composición química y la actividad biológica de los aceites esenciales de diez plantas aromáticas colombianas. Sci. Tech. 2007, XIII, 165–166. [Google Scholar] [CrossRef]
- Durant-Archibold, A.A.; Santana, A.I.; Gupta, M.P. Ethnomedical Uses and Pharmacological Activities of Most Prevalent Species of Genus Piper in Panama: A Review. J. Ethnopharmacol. 2018, 217, 63–82. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, D.R.; Sandoval-Hernandez, A.G.; Delgado, W.A.; Arboleda, G.H.; Cuca, L.E. In vitro anticancer screening of colombian plants from Piper genus (Piperaceae). J. Pharmacogn. Phytother. 2018, 10, 174–181. [Google Scholar] [CrossRef]
- Calderón, Á.I.; Vázquez, Y.; Solís, P.N.; Caballero-George, C.; Zacchino, S.; Gimenez, A.; Pinzón, R.; Cáceres, A.; Tamayo, G.; Correa, M. Screening of latin american plants for cytotoxic activity. Pharm. Biol. 2006, 44, 130–140. [Google Scholar] [CrossRef]
- Da Costa, J.G.; Campos, A.R.; Brito, S.A.; Pereira, C.K.B.; Souza, E.O.; Rodrigues, F.F.G. Biological screening of araripe basin medicinal plants using Artemia salina Leach and pathogenic bacteria. Pharmacogn. Mag. 2010, 6, 331. [Google Scholar] [CrossRef]
- Maxwell, A.; Rampersad, D. Prenylated 4-hydroxybenzoic acid derivatives from Piper marginatum. J. Nat. Prod. 1988, 51, 370–373. [Google Scholar] [CrossRef]
- De Oliveira Chaves, M.C.; De Oliveira Santos, B.V. Constituents from Piper marginatum fruits. Fitoterapia 2002, 73, 547–549. [Google Scholar] [CrossRef]
- Ampofo, S.A.; Roussis, V.; Wiemer, D.F. New prenylated phenolics from Piper auritum. Phytochemistry 1987, 26, 2367–2370. [Google Scholar] [CrossRef]
- Sáez Vega, A.; Rojano, B.; Blair, S.; Segura, C.; Figadere, B.; Seon, B.; Grellier, P.; Sáez, J. Antimalarials and antioxidants compounds from Piper tricuspe (Piperaceae). Pharmacologyonline 2008, 1, 1–8. [Google Scholar]
- López, S.N.; Lopes, A.A.; Batista, J.M.; Flausino, O.; da Bolzani, V.S.; Kato, M.J.; Furlan, M. Geranylation of benzoic acid derivatives by enzymatic extracts from Piper crassinervium (Piperaceae). Bioresour. Technol. 2010, 101, 4251–4260. [Google Scholar] [CrossRef] [PubMed]
- Malami, I. Prenylated benzoic acid derivatives from Piper species as source of anti-infective agents. Int. J. Pharm. Sci. Res. 2012, 3, 1554–1559. [Google Scholar] [CrossRef]
- Lee, B.; Boucher, H.W. Targeting Antimicrobial-Resistant Bacterial Respiratory Tract Pathogens. Curr Opin Pulm Med. 2015, 21, 293–303. [Google Scholar] [CrossRef] [PubMed]
- Rüegg, T.; Calderón, A.I.; Queiroz, E.F.; Solís, P.N.; Marston, A.; Rivas, F.; Ortega-Barría, E.; Hostettmann, K.; Gupta, M.P. 3-Farnesyl-2-hydroxybenzoic acid is a new anti-Helicobacter pylori compound from Piper multiplinervium. J. Ethnopharmacol. 2006, 103, 461–467. [Google Scholar] [CrossRef]
- Fang, H.Y.; Chokkalingam, U.; Chiou, S.F.; Hwang, T.L.; Chen, S.L.; Wang, W.L.; Sheu, J.H. Bioactive chemical constituents from the brown alga Homoeostrichus formosana. Int. J. Mol. Sci. 2015, 16, 736–746. [Google Scholar] [CrossRef]
- Steindler, L.; Venturi, V. Detection of quorum-sensing N -acyl homoserine lactone signal molecules by bacterial biosensors. FEMS Microbiol. Lett. 2007, 266, 1–9. [Google Scholar] [CrossRef]
- Papenfort, K.; Bassler, B.L. Quorum sensing signal–response systems in Gram-negative bacteria. Nat. Rev. Microbiol. 2016, 14, 576–588. [Google Scholar] [CrossRef]
- Blosser, R.S.; Gray, K.M. Extraction of violacein from Chromobacterium violaceum provides a new quantitative bioassay for N-acyl homoserine lactone autoinducers. J. Microbiol. Methods 2000, 40, 47–55. [Google Scholar] [CrossRef]
- Rai, N.; Rai, R.; Venkatesh, K.V. Quorum Sensing Biosensors. In Quorum Sensing vs Quorum Quenching: A Battle with No End in Sight; Springer India: New Delhi, India, 2015; pp. 173–183. [Google Scholar]
- Lozano Juzgado, R. Nuevas Estrategias En La Lucha Contra La Resistencia a Los Antibióticos: Inhibición Del Quorum Sensing. Bachelor’s Thesis, Universidad Complutense, Madrid, España, 2017. [Google Scholar]
- Ouyang, J.; Sun, F.; Feng, W.; Sun, Y.; Qiu, X.; Xiong, L.; Liu, Y.; Chen, Y. Quercetin is an effective inhibitor of quorum sensing, biofilm formation and virulence factors in Pseudomonas aeruginosa. J. Appl. Microbiol. 2016, 120, 966–974. [Google Scholar] [CrossRef]
- Geng, Y.F.; Yang, C.; Zhang, Y.; Tao, S.N.; Mei, J.; Zhang, X.C.; Sun, Y.J.; Zhao, B.T. An innovative role for luteolin as a natural quorum sensing inhibitor in Pseudomonas aeruginosa. Life Sci. 2021, 274, 119325. [Google Scholar] [CrossRef]
- Plyuta, V.; Zaitseva, J.; Lobakova, E.; Zagoskina, N.; Kuznetsov, A.; Khmel, I. Effect of plant phenolic compounds on biofilm formation by Pseudomonas aeruginosa. APMIS 2013, 121, 1073–1081. [Google Scholar] [CrossRef] [PubMed]
- Ugurlu, A.; Karahasan Yagci, A.; Ulusoy, S.; Aksu, B.; Bosgelmez-Tinaz, G. Phenolic compounds affect production of pyocyanin, swarming motility and biofilm formation of Pseudomonas aeruginosa. Asian Pac. J. Trop. Biomed. 2016, 8, 698–701. [Google Scholar] [CrossRef]
- Thi, M.T.T.; Wibowo, D.; Rehm, B.H.A. Pseudomonas Aeruginosa Biofilms. Int J Mol Sci. 2020, 21, 8671. [Google Scholar] [CrossRef] [PubMed]
- Rajkumari, J.; Borkotoky, S.; Murali, A.; Suchiang, K.; Mohanty, S.K.; Busi, S. Cinnamic acid attenuates quorum sensing associated virulence factors and biofilm formation in Pseudomonas aeruginosa PAO1. Biotechnol. Lett. 2018, 40, 1087–1100. [Google Scholar] [CrossRef] [PubMed]
- Sethupathy, S.; Ananthi, S.; Selvaraj, A.; Shanmuganathan, B.; Vigneshwari, L.; Balamurugan, K.; Mahalingam, S.; Pandian, S.K. Vanillic acid from Actinidia deliciosa impedes virulence in Serratia marcescens by affecting S-layer, flagellin and fatty acid biosynthesis proteins. Sci. Rep. 2017, 7, 16328. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Zhang, L. The Hierarchy Quorum Sensing Network in Pseudomonas Aeruginosa. Protein Cell. 2015, 6, 26–41. [Google Scholar] [CrossRef]
- Asfour, H. Anti-Quorum Sensing Natural Compounds. J Microsc Ultrastruct. 2018, 6, 1. [Google Scholar] [CrossRef]
- Bouyahya, A.; Dakka, N.; Et-Touys, A.; Abrini, J.; Bakri, Y. Medicinal Plant Products Targeting Quorum Sensing for Combating Bacterial Infections. Asian Pac J Trop Med. 2017, 10, 729–743. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute (CLSI). Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically; Approved Standard—Tenth Edition. CLSI Document M07-A10. Wayne, PA: Clinical and Laboratory Standards Institute; 2015, 11th ed.; CLSI standard M07, Ed.; Clinical and Laboratory Standards Institute: Wayne, NJ, USA, 2018; ISBN 1-56238-836-3. [Google Scholar]
- O’Toole, G.A. Microtiter dish biofilm formation assay. J. Vis. Exp. 2011, 47, e2437. [Google Scholar] [CrossRef]
- O’Toole, G.A.; Kolter, R. Initiation of biofilm formation in Pseudomonas fluorescens WCS365 proceeds via multiple, convergent signalling pathways: A genetic analysis. Mol. Microbiol. 1998, 28, 449–461. [Google Scholar] [CrossRef] [PubMed]
- Luís, Â.; Duarte, A.; Gominho, J.; Domingues, F.; Duarte, A.P. Chemical composition, antioxidant, antibacterial and anti-quorum sensing activities of Eucalyptus globulus and Eucalyptus radiata essential oils. Ind. Crops Prod. 2016, 79, 274–282. [Google Scholar] [CrossRef]
- Aleksic, I.; Ristivojevic, P.; Pavic, A.; Radojević, I.; Čomić, L.R.; Vasiljevic, B.; Opsenica, D.; Milojković-Opsenica, D.; Senerovic, L. Anti-quorum sensing activity, toxicity in zebrafish (Danio rerio) embryos and phytochemical characterization of Trapa natans leaf extracts. J. Ethnopharmacol. 2018, 222, 148–158. [Google Scholar] [CrossRef] [PubMed]
- Filloux, A.; Ramos, J. Pseudomonas Methods and Protocols Methods in Molecular Biology 1149; Filloux, A., Ramos, J.-L., Eds.; Springer: New York, NY, USA, 2014; Volume 1149, ISBN 978-1-4939-0472-3. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sierra-Quitian, A.G.; Hernandez-Moreno, L.V.; Pabon-Baquero, L.C.; Prieto-Rodriguez, J.A.; Patiño-Ladino, O.J. Antiquorum and Antibiofilm Activities of Piper bogotense C. DC. against Pseudomonas aeruginosa and Identification of Bioactive Compounds. Plants 2023, 12, 1901. https://doi.org/10.3390/plants12091901
Sierra-Quitian AG, Hernandez-Moreno LV, Pabon-Baquero LC, Prieto-Rodriguez JA, Patiño-Ladino OJ. Antiquorum and Antibiofilm Activities of Piper bogotense C. DC. against Pseudomonas aeruginosa and Identification of Bioactive Compounds. Plants. 2023; 12(9):1901. https://doi.org/10.3390/plants12091901
Chicago/Turabian StyleSierra-Quitian, Andrés G., Lida V. Hernandez-Moreno, Ludy C. Pabon-Baquero, Juliet A. Prieto-Rodriguez, and Oscar J. Patiño-Ladino. 2023. "Antiquorum and Antibiofilm Activities of Piper bogotense C. DC. against Pseudomonas aeruginosa and Identification of Bioactive Compounds" Plants 12, no. 9: 1901. https://doi.org/10.3390/plants12091901
APA StyleSierra-Quitian, A. G., Hernandez-Moreno, L. V., Pabon-Baquero, L. C., Prieto-Rodriguez, J. A., & Patiño-Ladino, O. J. (2023). Antiquorum and Antibiofilm Activities of Piper bogotense C. DC. against Pseudomonas aeruginosa and Identification of Bioactive Compounds. Plants, 12(9), 1901. https://doi.org/10.3390/plants12091901











