Characterization of Mineral Composition and Nutritional Value of Acacia Green Pods
Abstract
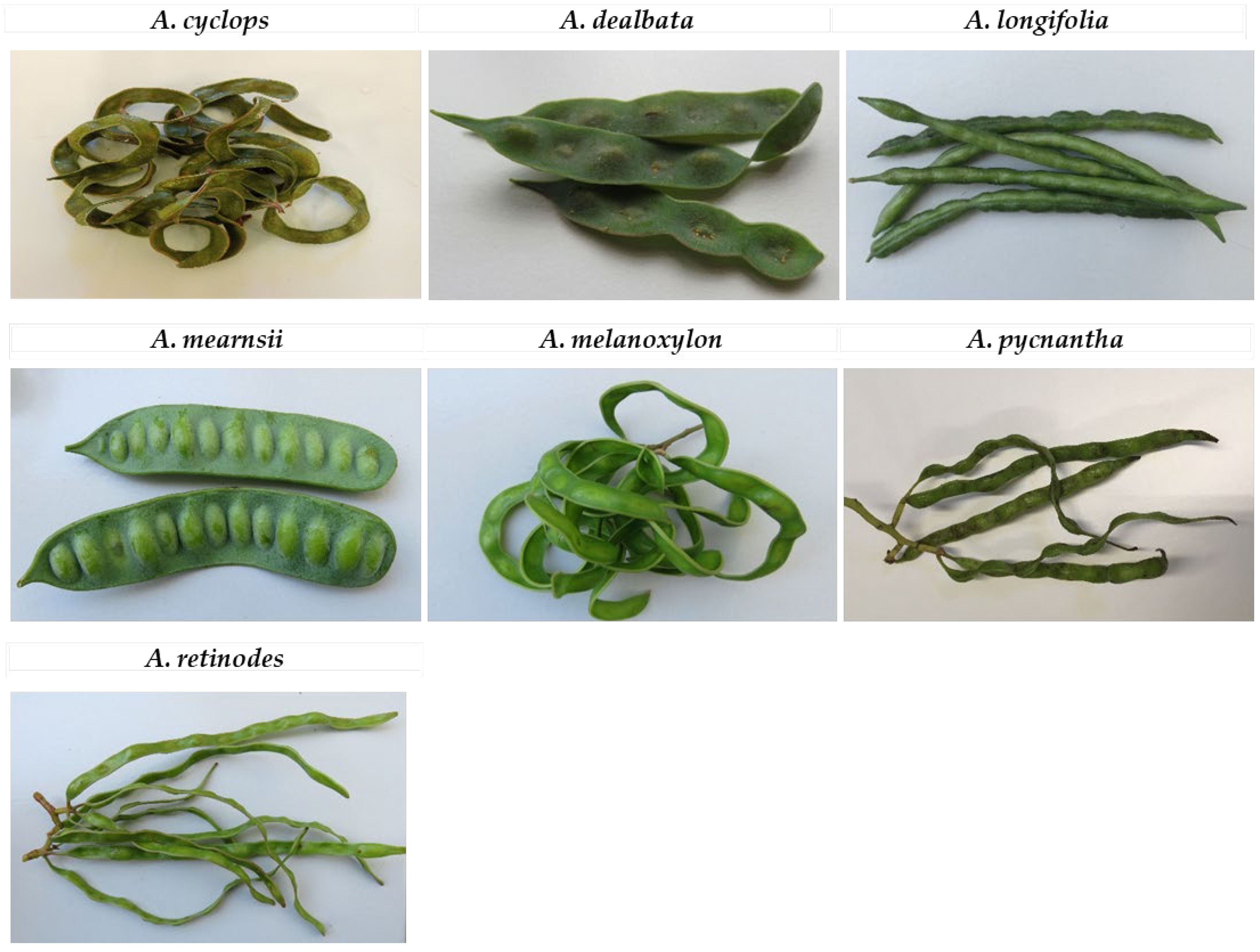
1. Introduction
2. Results and Discussion
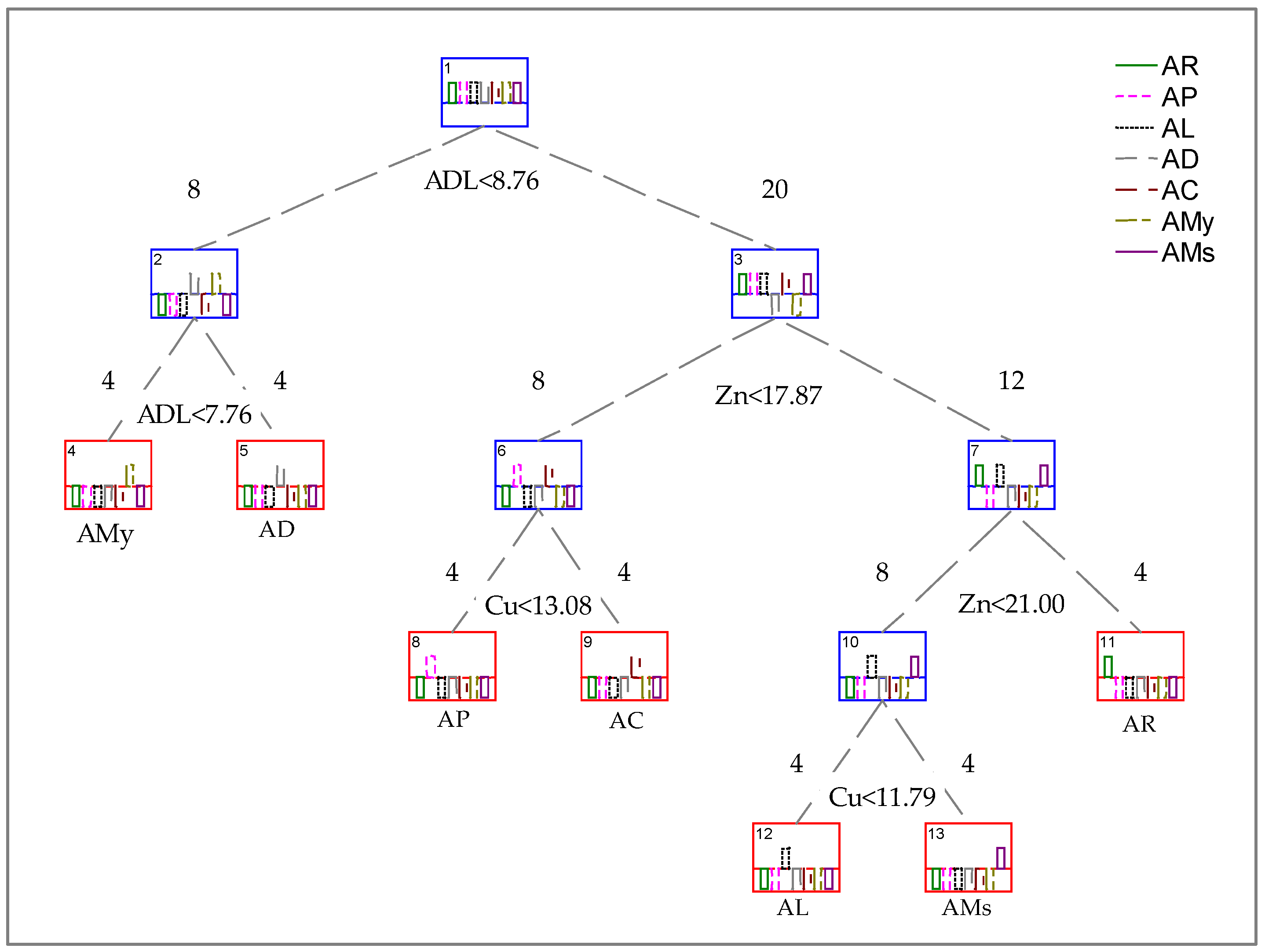
2.1. Nutritional Parameters
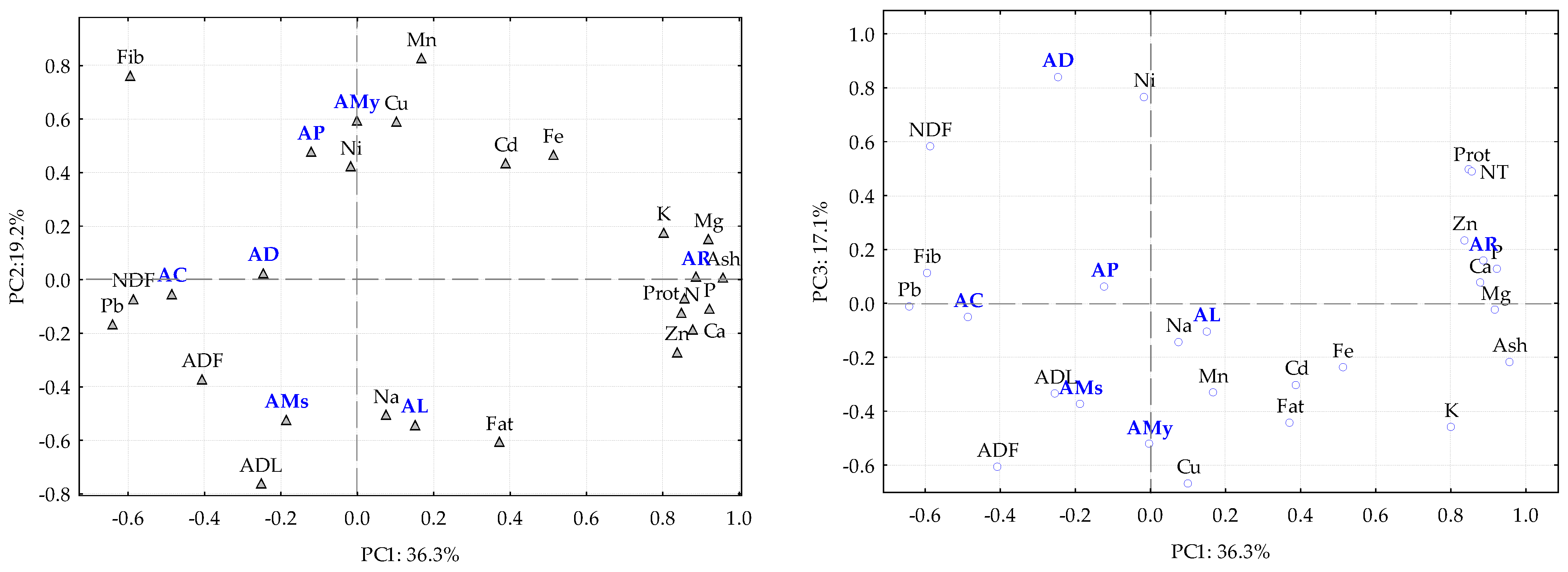
2.2. Elemental Analysis
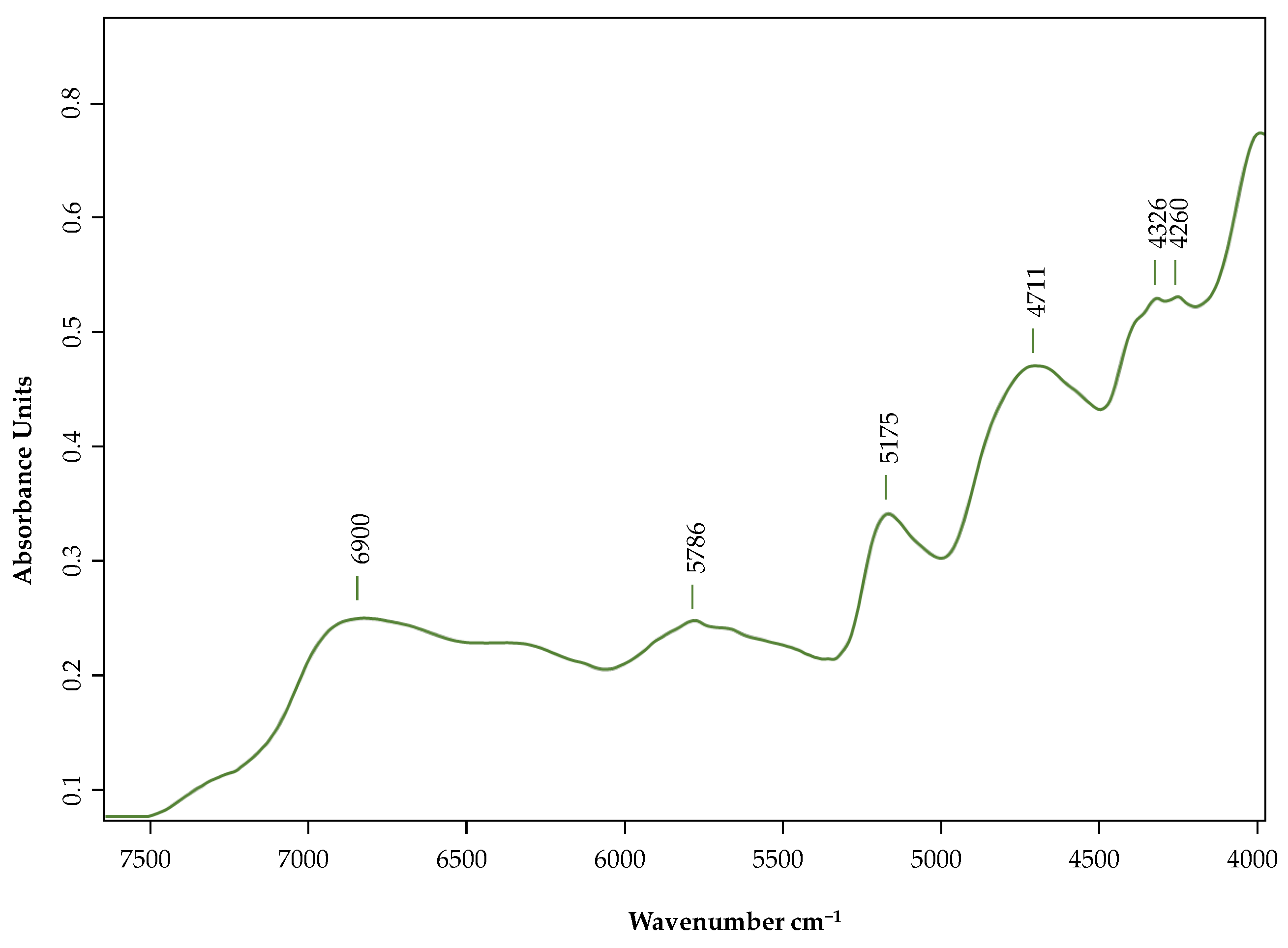
2.3. Spectroscopic Analysis
3. Materials and Methods
3.1. Plant and Soil
3.2. Sample Preparation
3.3. Nutritional Parameters
3.4. Elemental Analysis
3.5. Extraction Conditions
3.6. Spectroscopic Analysis
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Correia, R.; Quintela, J.C.; Duarte, M.P.; Gonçalves, M. Insights for the valorization of biomass from Portuguese invasive Acacia spp. in a biorefinery perspective. Forests 2020, 11, 1342. [Google Scholar] [CrossRef]
- Paula, V.; Pedro, S.I.; Campos, M.G.; Delgado, T.; Estevinho, L.M.; Anjos, O. Special Bioactivities of Phenolics from Acacia dealbata L. with Potential for Dementia, Diabetes and Antimicrobial Treatments. Appl. Sci. 2022, 12, 1022. [Google Scholar] [CrossRef]
- Miller, J.T.; Murphy, D.J.; Brown, G.K.; Richardson, D.M.; González-Orozco, C.E. The evolution and phylogenetic placement of invasive Australian Acacia species. Divers. Distrib. 2011, 17, 848–860. [Google Scholar] [CrossRef]
- Inderjit; Wardle, D.A.; Karban, R.; Callaway, R.M. The ecosystem and evolutionary contexts of allelopathy. Trends Ecol. Evol. 2011, 26, 655–662. [Google Scholar] [CrossRef] [PubMed]
- Chemetova, C.; Ribeiro, H.; Fabião, A.; Gominho, J. Towards sustainable valorisation of Acacia melanoxylon biomass: Characterization of mature and juvenile plant tissues. Environ. Res. 2020, 191, 110090. [Google Scholar] [CrossRef]
- da Costa, R.M.F.; Bosch, M.; Simister, R.; Gomez, L.D.; Canhoto, J.M.; Batista de Carvalho, L.A.E. Valorisation Potential of Invasive Acacia dealbata, A. longifolia and A. melanoxylon from Land Clearings. Molecules 2022, 27, 7006. [Google Scholar] [CrossRef]
- Pedro, S.I.; Rosado, T.; Barroca, C.; Neiva, D.; Alonso-herranz, V.; Gradillas, A.; Garc, A.; Gominho, J.; Gallardo, E. Characterisation of the Phenolic Profile of Acacia retinodes and Acacia mearnsii Flowers’ Extracts. Plants 2022, 11, 1442. [Google Scholar] [CrossRef]
- Ogawa, S.; Yazaki, Y. Tannins from Acacia mearnsii De Wild. Bark: Tannin determination and biological activities. Molecules 2018, 23, 837. [Google Scholar] [CrossRef]
- Correia, R.; Duarte, M.P.; Maurício, E.M.; Brinco, J.; Quintela, J.C.; da Silva, M.G.; Gonçalves, M. Chemical and Functional Characterization of Extracts from Leaves and Twigs of Acacia dealbata. Processes 2022, 10, 2429. [Google Scholar] [CrossRef]
- Zubair, M.; Azeem, M.; Mumtaz, R.; Younas, M.; Adrees, M.; Zubair, E.; Khalid, A.; Hafeez, F.; Rizwan, M.; Ali, S. Green synthesis and characterization of silver nanoparticles from Acacia nilotica and their anticancer, antidiabetic and antioxidant efficacy. Environ. Pollut. 2022, 304, 119249. [Google Scholar] [CrossRef]
- Elansary, H.O.; Szopa, A.; Kubica, P.; Ekiert, H.; Al-Mana, F.A.; Al-Yafrsi, M.A. Antioxidant and biological activities of Acacia saligna and lawsonia inermis natural populations. Plants 2020, 9, 908. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; He, L.; Zhang, Y.; You, C.; Li, X.; Jiang, P.; Wang, F. Separation of flavonoids with significant biological activity from Acacia mearnsii leaves. RSC Adv. 2023, 13, 9119–9127. [Google Scholar] [CrossRef] [PubMed]
- Madjid, O.A.; Sanni, A.; Lagnika, L. Chemical Diversity and Pharmacological Properties of Genus Acacia. Asian J. Appl. Sci. 2020, 13, 40–59. [Google Scholar] [CrossRef]
- Subhan, N.; Burrows, G.E.; Kerr, P.G.; Obied, H.K. Phytochemistry, Ethnomedicine, and Pharmacology of Acacia, 1st ed.; Elsevier B.V.: Amsterdam, The Netherlands, 2018; Volume 57, ISBN 9780444640574. [Google Scholar]
- Jelassi, A.; El Ayeb-Zakhama, A.; Nejma, A.B.; Chaari, A.; Harzallah-Skhiri, F.; Jannet, H. Ben Phytochemical composition and allelopathic potential of three Tunisian Acacia species. Ind. Crops Prod. 2016, 83, 339–345. [Google Scholar] [CrossRef]
- Aguilera, N.; Becerra, J.; Villaseñor-Parada, C.; Lorenzo, P.; González, L.; Hernández, V. Effects and identification of chemical compounds released from the invasive Acacia dealbata Link. Chem. Ecol. 2015, 31, 479–493. [Google Scholar] [CrossRef]
- Delgadillo Puga, C.; Cuchillo Hilario, M.; Espinosa Mendoza, J.G.; Medina Campos, O.; Molina Jijón, E.; Díaz Martínez, M.; Álvarez Izazaga, M.A.; Ledesma Solano, J.Á.; Pedraza Chaverri, J. Antioxidant activity and protection against oxidative-induced damage of Acacia shaffneri and Acacia farnesiana pods extracts: In vitro and in vivo assays. BMC Complement. Altern. Med. 2015, 15, 435. [Google Scholar] [CrossRef]
- Abdallah, E.M. Antibacterial Efficacy of Acacia nilotica (L.) Pods Growing in Sudan against Some Bacterial Pathogens. Int. J. Curr. Res. Biosci. Plant Biol. 2016, 3, 6–11. [Google Scholar] [CrossRef]
- Auwal, M.S.; Shuaibu, A.; Ibrahim, A.; Mustapha, M. Antibacterial properties of crude pod extract of Acacia nilotica (Fabaceae). Haryana Vet. 2015, 54, 29–32. [Google Scholar]
- Ahmad, M.; Zaman, F.; Sharif, T.; Zabta, C.M. Antidiabetic and hypolipidemic effects of Aqueous Methanolic extract of Acacia nilotica pods in alloxan-induced diabetic rabbits. Scand. J. Lab. Anim. Sci. 2008, 35, 29–30. [Google Scholar]
- Omara, E.A.; Nada, S.A.; Farrag, A.R.H.; Sharaf, W.M.; El-Toumy, S.A. Therapeutic effect of Acacia nilotica pods extract on streptozotocin induced diabetic nephropathy in rat. Phytomedicine 2012, 19, 1059–1067. [Google Scholar] [CrossRef]
- Gilani, A.H.; Shaheen, F.; Zaman, M.; Janbaz, K.H.; Shah, B.H.; Akhtar, M.S. Studies on antihypertensive and antispasmodic activities of methanol extract of Acacia nilotica pods. Phyther. Res. 1999, 13, 665–669. [Google Scholar] [CrossRef]
- Kankara, S. Acacia nilotica Pods ’ Water Extract Enhances Wound Healing in Sprague-Dawley Rats By Alleviating Oxidative Stress and Suppressing Pro-Inflammatory Cytokines. Niger. J. Sci. Res. 2017, 16, 202–210. [Google Scholar]
- Kneuper, C.L.; Scott, C.B.; Pinchak, W.E. Consumption and dispersion of mesquite seeds by ruminants. J. Range Manag. 2003, 56, 255–259. [Google Scholar] [CrossRef]
- García-Winder, L.R.; Goñi-Cedeño, S.; Olguín-Lara, P.A.; Díaz-Salgado, G.; Arriaga-Jordán, C.M. Huizache (Acacia farnesiana) whole pods (flesh and seeds) as an alternative feed for sheep in Mexico. Trop. Anim. Health Prod. 2009, 41, 1615–1621. [Google Scholar] [CrossRef]
- Barrientos-Ramírez, L.; Vargas-Radillo, J.J.; Rodríguez-Rivas, A.; Ochoa-Ruíz, H.G.; Navarro-Arzate, F.; Zorrilla, J. Evaluation of characteristics of huizache (Acacia farnesiana (L.) Willd.) fruit for potential use in leather tanning or animal feeding. Madera Bosques 2012, 18, 23–35. [Google Scholar]
- Walker, K.P. Fodder potential of leaves and pods of planted Leucaena diversifolia and L. leucocephala species in semi-arid Botswana. Int. Res. J. Agric. Sci. Soil Sci. 2012, 2, 445–450. [Google Scholar]
- Garcia-Montes De Oca, C.A.; Gonzalez-Ronquillo, M.; Salem, A.Z.M.; Romero-Bernal, J.; Pedraza, J.F.; Estrada, J.G. Chemical composition and in vitro gas production of some legume browse species in subtropical areas of Mexico. Trop. Subtrop. Agroecosyst. 2011, 14, 589–595. [Google Scholar]
- Rubio, E.E.S.; Rodriguez, D.P.; Reyes, L.O.; Buenfil, G.Z. Evaluación del potencial forrajero de árboles y arbustos tropicales para la alimentación de ovinos Tropical trees and shrubs forage potential for sheep feeding. Técnica Pecu. México 2004, 42, 129–144. [Google Scholar]
- Uguru, C.; Lakpini, C.A.M.; Akpa, G.N.; Bawa, G.S. Nutritional Potential of Acacia (Acacia nilotica (L.) Del.) Pods for Growing Red Sokoto Goats. IOSR J. Agric. Vet. Sci. 2014, 7, 43–49. [Google Scholar] [CrossRef]
- Hadi, R.F.; Handayanta, E.; Ngadyastuti, I. Evaluation of in vitro digestibility and pH of Acacia seed pods (Acacia sp.). IOP Conf. Ser. Earth Environ. Sci. 2021, 902, 012003. [Google Scholar] [CrossRef]
- Abdalla, M.S.A.; Babiker, I.A.; Idris, A.M.; Elkalifa, K.F. Potential Nutrient Composition of Acacia seyal Fruits as Fodder for Livestock in the Dry Lands in Sudan. Dev. Anal. Chem. 2014, 1, 25–30. [Google Scholar]
- Zapata-Campos, C.C.; García-Martínez, J.E.; Salinas-Chavira, J.; Ascacio-Valdés, J.A.; Medina-Morales, M.A.; Mellado, M. Chemical composition and nutritional value of leaves and pods of Leucaena leucocephala, Prosopis laevigata and Acacia farnesiana in a xerophilous shrubland. Emirates J. Food Agric. 2020, 32, 723–730. [Google Scholar] [CrossRef]
- McDonald, P.R.A.; Edwards, J.F.D.; Greenhalgh, C.A.; Morgan, L.A. Sinclair Wilkinson, R.G. Animal Nutrition, 7th ed.; Prentice Hall: Hoboken, NJ, USA, 2010. [Google Scholar]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for Dietary Fiber, Neutral Detergent Fiber, and Nonstarch Polysaccharides in Relation to Animal Nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef] [PubMed]
- Goff, J.P. Invited review: Mineral absorption mechanisms, mineral interactions that affect acid—base and antioxidant status, and diet considerations to improve mineral status. J. Dairy Sci. 2018, 101, 2763–2813. [Google Scholar] [CrossRef] [PubMed]
- Raileanu, L.E.; Stoffel, K. Theoretical comparison between the Gini Index and Information Gain criteria. Ann. Math. Artif. Intell. 2004, 41, 77–93. [Google Scholar] [CrossRef]
- Joshi, R.; Sathasivam, R.; Jayapal, P.K.; Patel, A.K.; Van Nguyen, B.; Faqeerzada, M.A.; Park, S.U.; Lee, S.H.; Kim, M.S.; Baek, I.; et al. Comparative Determination of Phenolic Compounds in Arabidopsis thaliana Leaf Powder under Distinct Stress Conditions Using Fourier-Transform Infrared (FT-IR) and Near-Infrared (FT-NIR) Spectroscopy. Plants 2022, 11, 836. [Google Scholar] [CrossRef]
- Türker-Kaya, S.; Huck, C. A Review of Mid-Infrared and Near-Infrared Imaging: Principles, Concepts and Applications in Plant Tissue Analysis. Molecules 2017, 22, 168. [Google Scholar] [CrossRef]
- Xu, L.; Ye, Z.H.; Yan, S.M.; Shi, P.T.; Cui, H.F.; Fu, X.S.; Yu, X.P. Combining local wavelength information and ensemble learning to enhance the specificity of class modeling techniques: Identification of food geographical origins and adulteration. Anal. Chim. Acta 2012, 754, 31–38. [Google Scholar] [CrossRef]
- Hong, X.Z.; Fu, X.S.; Wang, Z.L.; Zhang, L.; Yu, X.P.; Ye, Z.H. Tracing Geographical Origins of Teas Based on FT-NIR Spectroscopy: Introduction of Model Updating and Imbalanced Data Handling Approaches. J. Anal. Methods Chem. 2019, 2019, 1537568. [Google Scholar] [CrossRef]
- Chang, X.; Wei, D.; Su, S.; Guo, S.; Qian, S.; Yan, H.; Zhao, M.; Shang, E.; Qian, D.; Sun, X.; et al. An integrated strategy for rapid discovery and prediction of nucleobases, nucleosides and amino acids as quality markers in different flowering stages of Flos Chrysanthemi using UPLC–MS/MS and FT-NIR coupled with multivariate statistical analysis. Microchem. J. 2020, 153, 104500. [Google Scholar] [CrossRef]
- Beć, K.B.; Grabska, J.; Badzoka, J.; Huck, C.W. Spectra-structure correlations in NIR region of polymers from quantum chemical calculations. The cases of aromatic ring, C=O, C≡N and C-Cl functionalities. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 262, 120085. [Google Scholar] [CrossRef] [PubMed]
- Stuart, B.H. Infrared Spectroscopy: Fundamentals and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2004; ISBN 978-0-470-85428-0. [Google Scholar]
- IUSS Working Group WRB. World Reference Base for Soil Resources 2014; Update 201; IUSS Working Group WRB: Rome, Italy, 2015; ISBN 9789251083697. [Google Scholar]
- Fischer, G.F.; Nachtergaele, S.; van V. Prieler, H.T.; Verelst, L.; Wiberg, D. Harmonized World Soil Database v1.2; FAO Soils Portal; Food and Agriculture Organization of the United Nations: Rome, Italy, 2019. [Google Scholar]
- Association of Official Analytical Chemists. Official Methods of Analysis, 17th ed.; Association of Official Analytical Chemists: Gaithersburg, MD, USA, 2000. [Google Scholar]
| Species | DM (% in DM) | Prot (% in DM) | Fat (% in DM) | Fib (% in DM) | NDF (% in DM) | ADF (% in DM) | ADL (% in DM) | Ash (% in DM) |
|---|---|---|---|---|---|---|---|---|
| AMy | 91.41 ± 0.02 | 13.68 ± 0.22 b | 1.48 ± 0.01 c | 19.51 ± 0.15 bc | 44.41 ± 0.15 c | 35.17 ± 0.37 c | 18.82 ± 0.91 e | 3.24 ± 0.08 c |
| AD | 96.79 ± 0.09 | 17.68 ± 0.35 d | 1.09 ± 0.00 b | 22.11 ± 0.29 d | 51.17 ± 0.67 d | 25.74 ± 0.18 a | 8.28 ± 0.59 a | 2.25 ± 0.01 a |
| AC | 96.22 ± 0.09 | 11.82 ± 0.06 a | 1.10 ± 0.02 b | 21.16 ± 0.12 c | 50.15 ± 0.05 d | 32.88 ± 1.01 c | 15.88 ± 0.44 d | 2.62 ± 0.03 b |
| AR | 91.03 ± 0.10 | 21.66 ± 0.27 e | 1.37 ± 0.00 c | 17.87 ± 0.28 ab | 41.72 ± 0.41 ab | 26.92 ± 0.83 a | 10.82 ± 0.39 bc | 5.89 ± 0.02 e |
| AMs | 96.20 ± 0.02 | 14.11 ± 0.17 bc | 1.42 ± 0.05 c | 22.59 ± 0.04 d | 40.85 ± 0.43 ab | 29.78 ± 0.35 b | 7.25 ± 0.58 a | 3.69 ± 0.00 d |
| AP | 93.21 ± 0.02 | 14.96 ± 0.16 c | 0.87 ± 0.00 a | 22.87 ± 0.16 d | 41.67 ± 0.08 ab | 25.83 ± 0.40 a | 8.87 ± 0.04 ab | 3.33 ± 0.02 c |
| AL | 93.80 ± 0.08 | 16.95 ± 0.08 d | 1.82 ± 0.10 d | 17.16 ± 0.91 a | 39.44 ± 0.25 a | 27.24 ± 0.40 ab | 12.69 ± 0.22 c | 3.84 ± 0.05 d |
| Species | NT (%) | P (mg/kg) | Ca (mg/kg) | K (mg/kg) | Na (mg/kg) | Mg (mg/kg) |
|---|---|---|---|---|---|---|
| AMy | 2.18 ± 0.03 b | 0.09 ± 0.00 bc | 2803.61 ± 87.31 a | 18,845.42 ± 398.00 e | 748.86 ± 17.19 ab | 1454.36 ± 67.37 c |
| AD | 2.83 ± 0.06 e | 0.09 ± 0.00 bc | 3082.52 ± 50.33 a | 10,957.26 ± 1528.57 a | 593.86 ± 50.90 a | 1038.80 ± 36.61 a |
| AC | 1.89 ± 0.01 a | 0.06 ± 0.00 a | 3240.21 ± 82.72 ab | 13,154.26 ± 602.65 b | 1415.35 ± 35.22 c | 1288.24 ± 1.99 b |
| AR | 3.46 ± 0.04 f | 0.14 ± 0.00 e | 10,069.31 ± 582.88 e | 23,196.54 ± 1132.75 f | 715.44 ± 34.33 ab | 2532.32 ± 102.94 d |
| AMs | 2.26 ± 0.03 b | 0.10 ± 0.00 d | 4833.96 ± 33.99 d | 17,569.05 ± 422.92 de | 986.52 ± 40.38 b | 1061.40 ± 27.99 a |
| AP | 2.40 ± 0.03 c | 0.09 ± 0.00 b | 3656.00 ± 181.65 bc | 16,231.81 ± 555.47 cd | 1756.47 ± 56.71 c | 1500.28 ± 50.29 c |
| AL | 2.71 ± 0.01 d | 0.09 ± 0.00 c | 4064.88 ± 61.01 c | 14,673.65 ± 180.52 bc | 6975.65 ± 385.21 d | 1531.81 ± 26.91 c |
| (mg/Kg) | AMy | AD | AC | AR | AMs | AP | AL |
|---|---|---|---|---|---|---|---|
| Fe | 45.54 ± 15.13 c | 30.61 ± 1.68 ab | 30.35 ± 1.99 ab | 44.13 ± 4.35 bc | 25.70 ± 0.13 a | 46.50 ± 0.72 c | 46.85 ± 0.88 c |
| Mn | 48.78 ± 2.55 d | 15.04 ± 0.68 a | 13.15 ± 0.34 a | 25.93 ± 1.83 c | 14.73 ± 0.31 a | 50.20 ± 0.91 d | 19.15 ± 0.21 b |
| Cu | 14.62 ± 0.86 e | 10.75 ± 0.10 a | 13.49 ± 0.22 d | 12.99 ± 0.72 cd | 12.13 ± 0.27 bc | 12.73 ± 0.14 cd | 11.55 ± 0.12 ab |
| Zn | 17.50 ± 2.42 ab | 19.30 ± 0.32 b | 15.84 ± 0.31 a | 23.13 ± 2.60 c | 18.67 ± 0.54 ab | 17.00 ± 0.64 ab | 20.24 ± 0.60 bc |
| Pb | 3.80 ± 0.12 ab | 4.63 ± 1.05 b | 4.12 ± 0.18 ab | 1.94 ± 2.30 a | 4.93 ± 0.55 b | 4.83 ± 0.85 b | 4.70 ± 0.12 b |
| Ni | 5.95 ± 0.10 bc | 8.03 ± 0.66 e | 7.20 ± 0.08 de | 7.02 ± 1.02 cde | 4.56 ± 0.12 a | 6.73 ± 0.07 bcd | 5.52 ± 0.62 ab |
| Cd | 0.25 ± 0.03 c | 0.10 ± 0.02 ab | 0.08 ± 0.01 ab | 0.19 ± 0.08 bc | 0.11 ± 0.05 ab | 0.04 ± 0.04 a | 0.04 ± 0.04 a |
| Node | Left Branch | Right Branch | n in Class | Predicted Class | Split Constant | Split Variable | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AR | AP | AL | AD | AC | AMy | AMs | |||||||
| Nutritional content | 1 | 2 | 3 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | AR | −1.0875 | Fat |
| 2 | 4 | 5 | 0 | 4 | 0 | 4 | 0 | 0 | 0 | AP | −22.5317 | Fib | |
| 3 | 6 | 7 | 4 | 0 | 4 | 0 | 4 | 4 | 4 | AR | −11.8166 | ADL | |
| 4 | 0 | 0 | 0 | 4 | 0 | 0 | 0 | AD | |||||
| 5 | 0 | 4 | 0 | 0 | 0 | 0 | 0 | AP | |||||
| 6 | 8 | 9 | 4 | 0 | 0 | 0 | 0 | 4 | 0 | AR | −1.3780 | Fat | |
| 7 | 10 | 11 | 0 | 0 | 4 | 0 | 4 | 0 | 4 | AC | −3.5536 | Ash | |
| 8 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | AR | |||||
| 9 | 0 | 0 | 0 | 0 | 0 | 4 | 0 | AMy | |||||
| 10 | 12 | 13 | 0 | 0 | 0 | 0 | 4 | 0 | 4 | AC | −1.2900 | Fat | |
| 11 | 0 | 0 | 4 | 0 | 0 | 0 | 0 | AL | |||||
| 12 | 0 | 0 | 0 | 0 | 4 | 0 | 0 | AC | |||||
| 13 | 0 | 0 | 0 | 0 | 0 | 0 | 4 | AMs | |||||
| Mineral content | 1 | 2 | 3 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | AR | −11.8 | Cu |
| 2 | 4 | 5 | 0 | 0 | 4 | 4 | 0 | 0 | 0 | AD | −13,292.1 | K | |
| 3 | 6 | 7 | 4 | 4 | 0 | 0 | 4 | 4 | 4 | AR | −16,947.3 | K | |
| 4 | 0 | 0 | 0 | 4 | 0 | 0 | 0 | AD | |||||
| 5 | 0 | 0 | 4 | 0 | 0 | 0 | 0 | AL | |||||
| 6 | 8 | 9 | 0 | 4 | 0 | 0 | 4 | 0 | 0 | AP | −14,709.7 | K | |
| 7 | 10 | 11 | 4 | 0 | 0 | 0 | 0 | 4 | 4 | AR | −20.2 | Zn | |
| 8 | 0 | 0 | 0 | 0 | 4 | 0 | 0 | AC | |||||
| 9 | 0 | 4 | 0 | 0 | 0 | 0 | 0 | AP | |||||
| 10 | 12 | 13 | 0 | 0 | 0 | 0 | 0 | 4 | 4 | AMy | −18,216.0 | K | |
| 11 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | AR | |||||
| 12 | 0 | 0 | 0 | 0 | 0 | 0 | 4 | AMs | |||||
| 13 | 0 | 0 | 0 | 0 | 0 | 4 | 0 | AMy | |||||
| Specie | Origin | Collect Date | Longitude | Latitude | Altitude (m) | Soil Classification |
|---|---|---|---|---|---|---|
| AMy | Vimeiro, Alcobaça | 08/05/2021 | 39.4904 | −9.0367 | 163 | Cambisol |
| AD | Praia de Paredes da Vitoria, Alcobaça | 03/04/2021 | 39.7025 | −9.0483 | 24 | Podzois |
| AC | Tapada da Ajuda, Lisboa | 01/04/2021 | 38.7121 | −9.1908 | 86 | Vertisol |
| AR | Lagoa, Pinhal de Leiria | 01/04/2021 | 39.7478 | −8.9533 | 57 | Podzois |
| AMs | Vimeiro, Alcobaça | 08/05/2021 | 39.4904 | −9.0367 | 163 | Cambisol |
| AP | Silvares, Fundão | 08/05/2021 | 40.1507 | −7.6444 | 477 | Regossolos |
| AL | Tercena, Queluz | 08/05/2021 | 38.7425 | −9.2822 | 90 | Vertisol |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pedro, S.I.; Antunes, C.A.L.; Horta, C.; Pitacas, I.; Gonçalves, J.; Gominho, J.; Gallardo, E.; Anjos, O. Characterization of Mineral Composition and Nutritional Value of Acacia Green Pods. Plants 2023, 12, 1853. https://doi.org/10.3390/plants12091853
Pedro SI, Antunes CAL, Horta C, Pitacas I, Gonçalves J, Gominho J, Gallardo E, Anjos O. Characterization of Mineral Composition and Nutritional Value of Acacia Green Pods. Plants. 2023; 12(9):1853. https://doi.org/10.3390/plants12091853
Chicago/Turabian StylePedro, Soraia I., Carlos A. L. Antunes, Carmo Horta, Inês Pitacas, Joana Gonçalves, Jorge Gominho, Eugenia Gallardo, and Ofélia Anjos. 2023. "Characterization of Mineral Composition and Nutritional Value of Acacia Green Pods" Plants 12, no. 9: 1853. https://doi.org/10.3390/plants12091853
APA StylePedro, S. I., Antunes, C. A. L., Horta, C., Pitacas, I., Gonçalves, J., Gominho, J., Gallardo, E., & Anjos, O. (2023). Characterization of Mineral Composition and Nutritional Value of Acacia Green Pods. Plants, 12(9), 1853. https://doi.org/10.3390/plants12091853











