Abstract
Current control methods for invasive alien plants (IAPs) have acceptable short-term outcomes but have proven to be unfeasible or unaffordable in the long-term or for large invaded areas. For these reasons, there is an urgent need to develop sustainable approaches to control or restrict the spread of aggressive IAPs. The use of waste derived from IAP control actions could contribute to motivating the long-term management and preservation of local biodiversity while promoting some economic returns for stakeholders. However, this strategy may raise some concerns that should be carefully addressed before its implementation. In this article, we summarize the most common methods to control IAPs, explaining their viability and limitations. We also compile the potential applications of IAP residues and discuss the risks and opportunities associated with this strategy.
1. Introduction
Scientific studies over the last few decades have highlighted invasive alien plants (IAPs) as one of the major threats to ecosystems [1,2,3], especially those growing in protected areas [4,5]. Plant invasions are mainly influenced by direct (e.g., by transport propagules) or indirect (e.g., by altering land use) human actions that involve moving plants around the world for different purposes [6]. For example, many exotic plant species were planted to provide products and benefits that support livelihoods [7]. Once established in a new region, some exotic plants rapidly expand and become invasive, causing significant losses in biodiversity, ecosystem functioning and services, socio-economic values, and human health in the invaded areas [8]. Invasive alien plant species have strong adaptability, fast reproduction, spreading capabilities, and other traits that contribute to their success in their new area. Climate change leading to environmental constraints can also increase opportunities for the establishment of IAPs, which are better able to acquire limited resources or use resources more efficiently than native plant species [9].
Invasive alien plant species have colonized almost all types of terrestrial ecosystems and aquatic environments worldwide, except for Polar biomes. For example, Acacia spp. in Mediterranean areas [1], Prosopis spp. in arid environments [10], Carpobrotus spp. in coastal areas [11], Robinia pseudoacacia L. and Ailanthus altissima (Mill.) Swingle in mixed forests [12], Oxalis pes-caprae L. in ruderal and agricultural lands [13,14], and Eichhornia crassipes (Mart.) Solms in water bodies and courses [15,16]. Invasive plants can form homogeneous stands that have significant impacts on both the above and belowground compartments of invaded ecosystems [17,18,19]. These impacts include changes in resident plant communities and soil microbes [17,18,20,21], animal populations [22,23], soil properties and nutrient cycling [24,25], fire regimes and water flow [2,23,26], and biotic interactions [27,28]. In addition, IAPs can threaten human health and the socio-economy of the region [29]. However, the economic costs associated with the detrimental impacts of IAPs remain largely unknown. According to the most recent database of invasion costs (InvaCost), the economic costs of the biological invasion reached USD 1.22 trillion in the USA [30] and EUR 116.61 billion in Europe [31] between 1960 and 2020, with a clear dominance of damage costs over management expenditure [32], focusing on eradication and control actions [33]. In the Mediterranean Basin as well as in Europe, Ambrosia artimisiifolia L. is the costliest IAP [31,34]. Moreover, the future overall invasion costs are expected to increase, which emphasizes the urgent need to allocate more resources for managing invasive species.
Here, we aim to (i) compile current methods used for controlling and managing IAPs, enumerating their viability and main weaknesses, (ii) present potential applications of IAP waste, and (iii) discuss the risks and opportunities associated with implementing a strategy for using IAP waste which could help to reduce the costs associated with their control.
2. Current Methods to Control Invasive Plants, Their Viability, and Problems
Some IAPs can have positive effects in several areas, including agriculture, the ornamental horticulture industry, and wood production, but their use can result in harmful effects, representing a conflict of interest in their management [35,36]. Harmful IAPs need to be controlled due to the magnitude of their environmental, economic, social, and aesthetic impacts. In dramatic cases of invasion, when IAPs show high vegetative reproduction and are widely distributed across large areas, eradication is extremely difficult and expensive. Therefore, confining invasive populations and limiting their spread through effective strategies should be prioritized.
Traditional management methods for terrestrial and aquatic IAPs include physical (manual or mechanical) methods such as pulling and digging, debarking, harvesting [37], mowing and tilling [38], prescribed fire [39], soil solarization [40], construction of barriers to limit the spread of aquatic weeds [41], usage of chemical (e.g., herbicide application [37,42,43]), biological control [37,44], or a combination of several methods [45,46]. The feasibility of each method depends on the species, the extent of invasion, the characteristics of the habitat, and the effectiveness of the control methods [47]. A recent Australian study based on the stakeholders’ perspective in the management of IAPs noted that decision-makers are more confident in the use of chemicals than biocontrol and mechanical methods in the control of IAPs and believe that it is more feasible to control succulents and herbs/shrubs than monocots and woody vines [48].
Although the existing control methods often produce relatively good short-term outcomes, they are ineffective in eradicating IAPs in the long-term if not applied periodically [3,49,50]. For example, a recent study by Froeschlin et al. [51] in South Africa, a pioneering country in managing IAPs, noted that after 10 years of adopting a combination of control measures (mulching, herbicides, and the sowing of native species) to restore invaded areas, IAPs were not totally extirpated, indicating the need to improve techniques and implement additional efforts to eliminate them. Also, Duarte et al. [52] stressed the importance of frequent follow-up actions to reduce the abundance of Acacia longifolia (Andrews) Willd. in coastal dunes. Success in containing the dispersion or ultimately eradicating IAPs requires consistent post-surveillance and follow-up actions within an integrated strategy framework, which represents a huge challenge in terms of the available budget and execution timeframe [53]. In the absence of ongoing management, escaped individuals from the management can act as new focal dispersal points [51], leading to potential recolonization of the area and a loss of previous control efforts. However, the long-term management of IAPs is often neglected in part due to unaffordable costs for sustained efforts or a lack of interest after controlling the initial target population. Hence, it is necessary to adopt a more economical and efficient strategy to manage IAPs, for example, by deriving benefits from the management of plant invasion.
Besides economic problems, traditional methods also lead to social and environmental side effects. The use of synthetic herbicides can negatively affect human health, including neurological, reproductive, and respiratory diseases, diabetes, and even cancer [54], and the environment by reaching non-target organisms [55], raising concerns about their use. The repeated use of chemicals may also lead to invasive populations developing resistance to herbicides [56]. On the other hand, the introduction of generalist biological control agents can infect non-target species, causing a decline in their populations [57]. Another drawback common to most traditional control methods and anticipated in the previous paragraphs is the poor cost-effectiveness of the relationship between the invader spreading and the controlled area or the eradication time. As an example, the eradication of Alliaria petiolata (Bieb.) Cavara and Grande from an area of Adirondack Park (United States) was predicted to take 11 years with 100% effective control or more than 50 years with only 90% effective control [58]. In general, the management of IAPs is very expensive [31], with control strategies still prioritizing key landscape points to disrupt invasion connectivity and likely reduce costs without covering the whole area [59]. Even the long-term and well-designed South African program “Working for Water” is far from controlling the entire estimated invaded area [60]. Such limitations with frequent uncertain outcomes make traditional control methods unsafe, unfeasible, or unaffordable for large areas.
Recently, proactive strategies such as avoiding introduction, early detection, and rapid intervention (Regulation (EU) No 1143/2014 of the European Parliament and of the Council of 22 October 2014), prioritization of control actions, and citizen awareness have been proposed as key to prevent the spread of IAPs and reduce their negative impacts and control costs [37,61,62,63]. Involving volunteers is also an important component of monitoring and controlling IAPs [64], helping to reduce costs while increasing public awareness [65]. However, this solution is impractical for the rapid control of infested or heavily invaded areas since it is necessary for there to be continuous engagement and training of the volunteers [66]. Therefore, designing cooperative strategies in biosecurity among affected countries [36] is essential for the development of more cost-effective actions against IAPs. Despite these recent strategies preventing the introduction of new exotics, which are totally necessary and welcome, they are still in their infancy and do not greatly improve the control of IAPs already established. For these reasons, it is crucial to find alternative strategies to control current aggressive IAPs in a more sustainable way.
3. Potential Applications of IAP Waste from Management Actions
The use of waste generated by the removal of aggressive IAPs, with the objective of reducing the large costs associated with the control of these species, is a viable alternative. The use of invasive waste can provide novel value-added products, resulting in not only profits for society but also helping to preserve local biodiversity and stimulate the long-term management of invaded areas [67]. With this approach, it would be possible to partially recover invested funds that would otherwise be lost [42,67,68]. Moreover, it prevents the use of limited resources by introducing underused material into the system, which aligns with the circular bioeconomy rationale (Figure 1) and Sustainable Development Goals 12 (responsible consumption and production) and 15 (life on land) of the 2030 Agenda.
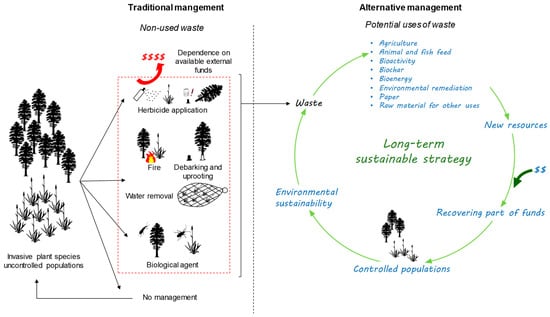
Figure 1.
Scheme for the proposed management for the control of highly invasive alien plants.
The waste from IAPs exhibits specific properties (functional, biological, medicinal, etc.) that can be used directly or serve as a basis for new products, as summarized in Table 1. For example, waste biomass, especially that from invasive legumes, but not only them, is primarily a source of nutrients that can be used as fertilizers, horticultural substrates, or soil amendments in agriculture either directly or after a composting process [69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103]. Invasive plants with allelopathic/phytotoxic effects, such as Acacia dealbata Link, Solidago canadensis L., or S. gigantea Aiton, can provide compounds with pesticidal or biostimulant effects appropriate for agricultural purposes [104,105,106,107]. Some IAPs are also a source of bioactive compounds with beneficial antioxidant, antimicrobial, nutraceutical, pharmacological, cosmetic, or therapeutical-related applications (e.g., [16,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132]). Invasive waste can also be used to produce bioenergy, namely bioethanol, biogas, or wood fuel (e.g., [133,134,135,136,137,138,139,140,141,142,143,144,145,146,147,148]), biochar or charcoal for different purposes (e.g., [149,150,151,152,153]), or animal feed (e.g., [154,155,156,157]. Some authors also suggest the use of IAPs for effluent treatments (e.g., [158,159,160,161,162,163,164,165]), paper and packaging materials (e.g., [166,167,168,169,170]), building materials [171], natural fiber composites [172], and bio-adsorbents for textile dyes or others [173,174,175,176,177,178,179,180].

Table 1.
Reported uses for invasive plant species.
4. Remarks on the Risks and Opportunities of Adopting the Use of Waste from IAPs
The use of waste from IAPs to control invasive populations, although not completely new, is advocated by several authors, as mentioned in Table 1 [92,126]. This idea is not broadly accepted among plant invasion ecologists [181], who argue that it can promote the cultivation of IAPs or facilitate their spread due to negligence in the treatment/transport of waste. This concern is valid given that plant invasion is a sensitive issue, and using IAPs poses the risk of incentivizing its growth instead of its control [182]. However, when invasion achieves a dramatic level and traditional control methods prove to be physically or economically insufficient or fail over time, which is associated with the inability to conduct follow-up actions after the end of a management project, the use of IAP waste could be viewed as a sustainable and viable solution to control and restrict the expansion of aggressive IAPs (Figure 1). The control of widespread IAPs, such as Australian acacias (Acacia spp.), water hyacinth (E. crassipes), or pampas grass (Cortaderia selloana (Schult. and Schult.f.) Asch. and Graebn.) [9,20,24,183], which have high vegetative reproduction and/or produce huge quantities of long-lived seeds that make their control difficult even after clearing an invaded area [67], generates large quantities of waste, often left on-site or burned for energy purposes [184]. The abandonment of the plant material may be a fire hazard [2,185], which can also contribute to the death of native plants and increase the risk of re-invasion [2], resulting, in turn, in the loss of control action benefits. In these situations, we suggest that using the waste from control actions is a reasonable approach to manage the overabundance of these IAPs in the long-term. The implementation of this approach may reduce the continuous spread of IAPs and contribute to the partial recovery of management funds, creating conditions for the sustainability of the process (Figure 1). In addition, the conversion of waste biomass from IAPs into new, useful products can promote zero-waste and circular economy approaches [186]. The potential uses of waste derived from IAPs have gained increasing interest in the last years, as summarized in Table 1. The majority of these studies were performed on a laboratory scale and focused on the potential of IAPs for further investigation and application in different areas, but relatively few studies include a cost–benefit analysis of using waste from IAPs. The work of Mudavanhu et al. [187] is one of the studies that discuss the economic implications of using the waste of Acacia cyclops A. Cunn. ex Don fil. for electricity generation in South Africa and concluded that it is a viable and feasible option in comparison with electricity production by diesel generators. On the contrary, Melane et al. [188], also in South Africa, observed that the costs of using biomass of seven non-woody IAPs, namely Arundo donax L., Lantana camara L., Solanum mauritianum Scop., Atriplex nummularia Lindl., Cestrum laevigatum Schlecht, Senna didymobotrya (Fresen.) H. S. Irwin and Barneby, and Chromoleana odorata (L.) R.M.King and H.Rob, were very high when compared to woody IAPs, suggesting that these IAPs are not a profitable resource for the production of electricity. Another study conducted by Valen [189] with the aim of investigating the cost–benefits of Arundo donax as feedstock for the pulp and paper industry in California, indicated an average annual profit of ca. 60% of removal expenses, which is an encouraging indicator of the financial viability of the process. As indicated by Ortega et al. [172], it is important to emphasize that the economic benefits achieved by using IAP biomass should be only to minimize the costs of their management and control and ultimately contribute to the reduction of the area occupied by these species. However, complete economic analyses assessing all aspects of using IAP waste, including management actions, waste transport, waste processing, and the benefit of the final product, are still lacking.
The use of invasive waste should be adopted within a clear regulatory framework to avoid bias in the utilization of this waste [182]. As stated before, the cultivation of IAPs is legally prohibited (Regulation (EU) No 1143/2014 of the European Parliament and of the Council of 22 October 2014), but the use of IAP waste derived from control actions lacks legal policies. To ensure that this strategy is properly applied, it is a prerequisite that a long-term management plan for IAPs is applied. In addition, it is necessary to select a control method (please see Section 2) that minimizes the risk of re-invasion and implement a monitoring plan for the controlled area after management actions. A set of recommendations for control, transport, and waste disposal after the control takes place should also be considered to avoid the dispersion of new propagules and the re-establishment of the invader. For example, control actions should take place when plants have no flowers or fruits/seeds to prevent seed dispersal. The location of disposal sites should be in areas with minimal possibility of IAP establishment, and the area should be monitored to prevent their spread. For IAPs that reproduce by seeds (e.g., Acacia spp. and Cortaderia selloana), the plant material should be left on site to dry. For IAPs that present viable (root or stem) fragments (e.g., Carpobrotus edulis and Eichhornia crassipes), the plant material should be left on site with the roots upward to prevent them from contacting the soil. In both situations, transport should occur when all plant material is completely dry, and the operation should be monitored for the presence of any type of viable structures.
In summary, the use of waste from IAPs is a strategy that feeds on and completes traditional control actions to facilitate the management of aggressive IAPs. However, this strategy should only be conducted in specific and dramatic invasion cases, when traditional control methods have failed over time, and should be conducted under strict regulations. This strategy should be accompanied by a complete economic analysis covering the entire IAP management and use of the waste process.
Author Contributions
Conceptualization, P.L. and M.C.M.; literature search, P.L. and M.C.M.; writing—review and editing, P.L. and M.C.M. All authors have read and agreed to the published version of the manuscript.
Funding
This work was carried out at the R&D Unit Center for Functional Ecology—Science for People and the Planet (CFE), with reference UIDB/04004/2020, financed by FCT/MCTES through national funds (PIDDAC). This work was also supported by National Funds from FCT—Portuguese Foundation for Science and Technology, under the project UIDB/04033/2020. P.L. and M.C.M. were supported by the Portuguese FCT (grant SFRH/BPD/88504/2012; contract IT057-18-7248 and grant SFRH/BPD/114746/2016, respectively.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gaertner, M.; Den Breeyen, A.; Hui, C.; Richardson, D.M. Impacts of alien plant invasions on species richness in Mediterranean-type ecosystems: A meta-analysis. Prog. Phys. Geogr. 2009, 33, 319–338. [Google Scholar] [CrossRef]
- Le Maître, D.C.; Gaertner, M.; Marchante, E.; Ens, E.-J.; Holmes, P.M.; Pauchard, A.; O’Farrell, P.J.; Rogers, A.M.; Blanchard, R.; Blignaut, J.; et al. Impacts of invasive Australian acacias: Implications for management and restoration. Divers. Distrib. 2011, 17, 1015–1029. [Google Scholar] [CrossRef]
- Simberloff, D. How common are invasion-induced ecosystem impacts? Biol. Invasions 2011, 13, 1255–1268. [Google Scholar] [CrossRef]
- Foxcroft, L.C.; Pyšek, P.; Richardson, D.M.; Genovesi, P.; MacFadyen, S. Plant invasion science in protected areas: Progress and priorities. Biol. Invasions 2017, 19, 1353–1378. [Google Scholar] [CrossRef]
- Shackleton, R.T.; Foxcroft, L.C.; Pyšek, P.; Wood, L.E.; Richardson, D.M. Assessing biological invasions in protected areas after 30 years: Revisiting nature reserves targeted by the 1980s SCOPE programme. Biol. Conserv. 2020, 243, 108424. [Google Scholar] [CrossRef]
- Mack, R.N.; Lonsdale, W.M. Humans as global plant dispersers: Getting more than we bargained for: Current introductions of species for aesthetic purposes present the largest single challenge for predicting which plant immigrants will become future pests. BioScience 2001, 51, 95–102. [Google Scholar] [CrossRef]
- Brundu, G.; Richardson, D.M. Planted forests and invasive alien trees in Europe: A code for managing existing and future plantings to mitigate the risk of negative impacts from invasions. NeoBiota 2016, 30, 5–47. [Google Scholar] [CrossRef]
- Hulme, P.E.; Pyšek, P.; Jarošík, V.; Pergl, J.; Schaffner, U.; Vilà, M. Bias and error in understanding plant invasion impacts. Trends Ecol. Evol. 2013, 28, 212–218. [Google Scholar] [CrossRef]
- Milanović, M.; Knapp, S.; Pyšek, P.; Kühn, I. Trait–environment relationships of plant species at different stages of the introduction process. NeoBiota 2020, 58, 55–74. [Google Scholar] [CrossRef]
- Dzikiti, S.; Ntshidi, Z.; Le Maitre, D.C.; Bugan, R.D.; Mazvimavi, D.; Schachtschneider, K.; Jovanovic, N.Z.; Pienaar, H.H. Assessing water use by Prosopis invasions and Vachellia karroo trees: Implications for groundwater recovery following alien plant removal in an arid catchment in South Africa. For. Ecol. Manag. 2017, 398, 153–163. [Google Scholar] [CrossRef]
- Mugnai, M.; Benesperi, R.; Viciani, D.; Ferretti, G.; Giunti, M.; Giannini, F.; Lazzaro, L. Impacts of the invasive alien Carpobrotus spp. on coastal habitats on a Mediterranean island (Giglio Island, Central Italy). Plants 2022, 11, 2802. [Google Scholar] [CrossRef] [PubMed]
- Radtke, A.; Ambraß, S.; Zerbe, S.; Tonon, G.; Fontana, V.; Ammer, C. Traditional coppice forest management drives the invasion of Ailanthus altissima and Robinia pseudoacacia into deciduous forests. For. Ecol. Manag. 2013, 291, 308–317. [Google Scholar] [CrossRef]
- Lorenzo, P.; González, L.; Ferrero, V. Effect of plant origin and phenological stage on the allelopathic activity of the invasive species Oxalis pes-caprae. Am. J. Bot. 2021, 108, 971–979. [Google Scholar] [CrossRef] [PubMed]
- Volakakis, N.; Kabourakis, E.; Rempelos, L.; Kiritsakis, A.; Leifert, C. Effect of different cover crops, mass-trapping systems and environmental factors on invertebrate activity in table olive orchards—Results from field experiments in Crete, Greece. Agronomy 2022, 12, 2576. [Google Scholar] [CrossRef]
- Pintor-Ibarra, L.F.; Rivera-Prado, J.J.; Ngangyo-Heya, M.; Rutiaga-Quiñones, J.G. Evaluation of the chemical components of Eichhornia crassipes as an alternative raw material for pulp and paper. BioResources 2018, 13, 2800–2813. [Google Scholar] [CrossRef]
- Bakrim, W.B.; Ezzariai, A.; Karouach, F.; Sobeh, M.; Kibret, M.; Hafidi, M.; Kouisni, L.; Yasri, A. Eichhornia crassipes (Mart.) Solms: A comprehensive review of its chemical composition, traditional use, and value-added products. Front. Pharmacol. 2022, 13, 842511. [Google Scholar] [CrossRef]
- Lorenzo, P.; Pazos-Malvido, E.; Rubido-Bará, M.; Reigosa, M.J.; González, L. Invasion by the leguminous tree Acacia dealbata (Mimosaceae) reduces the native understorey plant species in different communities. Aust. J. Bot. 2012, 60, 669–675. [Google Scholar] [CrossRef]
- Lorenzo, P.; Pereira, C.S.; Rodríguez-Echeverría, S. Differential impact on soil microbes of allelopathic compounds released by the invasive Acacia dealbata Link. Soil Biol. Biochem. 2013, 57, 156–163. [Google Scholar] [CrossRef]
- Rodríguez-Echeverría, S.; Afonso, C.; Correia, M.; Lorenzo, P.; Roiloa, S.R. The effect of soil legacy on competition and invasion by Acacia dealbata Link. Plant Ecol. 2013, 214, 1139–1146. [Google Scholar] [CrossRef]
- Lazzaro, L.; Giuliani, C.; Fabiani, A.; Agnelli, A.E.; Pastorelli, R.; Lagomarsino, A.; Benesperi, R.; Calamassi, R.; Foggi, B. Soil and plant changing after invasion: The case of Acacia dealbata in a Mediterranean ecosystem. Sci. Total Environ. 2014, 497, 491–498. [Google Scholar] [CrossRef]
- Keet, J.H.; Ellis, A.G.; Hui, C.; Nóvoa, A.; Le Roux, J.J. Impacts of invasive Australian acacias on soil bacterial community composition, microbial enzymatic activities, and nutrient availability in Fynbos Soils. Microb. Ecol. 2021, 82, 704–721. [Google Scholar] [CrossRef] [PubMed]
- Yapi, T.S.; O’Farrell, P.J.; Dziba, L.E.; Esler, K.J. Alien tree invasion into a South African montane grassland ecosystem: Impact of Acacia species on rangeland condition and livestock carrying capacity. Int. J. Biodiv. Sci. Ecosys. Serv. Manag. 2018, 14, 105–116. [Google Scholar] [CrossRef]
- van Wilgen, B.W.; Zengeya, T.A.; Richardson, D.M. A review of the impacts of biological invasions in South Africa. Biol. Invasions 2022, 24, 27–50. [Google Scholar] [CrossRef]
- Marchante, E.; Kjøller, A.; Struwe, S.; Freitas, H. Short-and long-term impacts of Acacia longifolia invasion on the belowground processes of a Mediterranean coastal dune ecosystem. Appl. Soil Ecol. 2008, 40, 210–217. [Google Scholar] [CrossRef]
- Raghurama, M.; Sankaran, M. Invasive nitrogen-fixing plants increase nitrogen availability and cycling rates in a montane tropical grassland. Plant Ecol. 2022, 223, 13–26. [Google Scholar] [CrossRef]
- Le Maitre, D.C.; Blignaut, J.N.; Clulow, A.; Dzikiti, S.; Everson, C.S.; Grgens, A.H.M.; Gush, M.B. Impacts of plant invasions on terrestrial water flows in South Africa. In Biological Invasions in South Africa; van Wilgen, B.W., Measey, J., Richardson, D.M., Wilson, J.R., Zengeya, T.A., Eds.; Springer: Cham, Germany, 2020; Volume 14, pp. 431–457. [Google Scholar]
- Fletcher, R.A.; Brooks, R.K.; Lakoba, V.T.; Sharma, G.; Heminger, A.R.; Dickinson, C.C.; Barney, J.N. Invasive plants negatively impact native, but not exotic, animals. Glob. Chang. Biol. 2019, 25, 3694–3705. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, J.; Cordero-Rivera, A.; González, L. Impacts of the invasive plant Carpobrotus edulis on herbivore communities on the Iberian Peninsula. Biol. Invasions 2021, 23, 1425–1441. [Google Scholar] [CrossRef]
- Rai, P.K.; Singh, J.S. Invasive alien plant species: Their impact on environment, ecosystem services and human health. Ecol. Indic. 2020, 111, 106020. [Google Scholar]
- Fantle-Lepczyk, J.E.; Haubrock, P.J.; Kramer, A.M.; Cuthbert, R.N.; Turbelin, A.J.; Crystal-Ornelas, R.; Diagne, C.; Courchamp, F. Economic costs of biological invasions in the United States. Sci. Total Environ. 2022, 806, 151318. [Google Scholar] [CrossRef]
- Haubrock, P.J.; Turbelin, A.J.; Cuthbert, R.N.; Nóvoa, A.; Taylor, N.G.; Angulo, E.; Ballesteros-Mejia, L.; Bodey, T.W.; Capinha, C.; Diagne, C.; et al. Economic costs of invasive alien species across Europe. NeoBiota 2021, 67, 153–190. [Google Scholar] [CrossRef]
- Cuthbert, R.N.; Diagne, C.; Hudgins, E.J.; Turbelin, A.; Ahmed, D.A.; Albert, C.; Bodey, T.W.; Briski, E.; Essl, F.; Haubrock, P.J.; et al. Biological invasion costs reveal insufficient proactive management worldwide. Sci. Total Environ. 2022, 819, 153404. [Google Scholar] [CrossRef] [PubMed]
- Moodley, D.; Angulo, E.; Cuthbert, R.N.; Leung, B.; Turbelin, A.J.; Novoa, A.; Kourantidou, M.; Heringer, G.; Haubrock, P.J.; Renault, D.; et al. Surprisingly high economic costs of biological invasions in protected areas. Biol. Invasions 2022, 24, 1995–2016. [Google Scholar] [CrossRef]
- Kourantidou, M.; Cuthbert, R.N.; Haubrock, P.J.; Novoa, A.; Taylor, N.G.; Leroy, B.; Capinha, C.; Renault, D.; Angulo, E.; Diagne, C.; et al. Economic costs of invasive alien species in the Mediterranean basin. NeoBiota 2021, 67, 427–458. [Google Scholar] [CrossRef]
- Novoa, A.; Dehnen-Schmutz, K.; Fried, J.; Vimercati, G. Does public awareness increase support for invasive species management? Promising evidence across taxa and landscape types. Biol. Invasions 2017, 19, 3691–3705. [Google Scholar] [CrossRef]
- Ricciardi, A.; Iacarella, J.C.; Aldridge, D.C.; Blackburn, T.M.; Carlton, J.T.; Catford, J.A.; Dick, J.T.A.; Hulme, P.; Jeschke, J.M.; Liebhold, A.M.; et al. Four priority areas to advance invasion science in the face of rapid environmental change. Environ. Rev. 2021, 29, 119–141. [Google Scholar] [CrossRef]
- Hussner, A.; Stiers, I.; Verhofstad, M.J.J.M.; Bakker, E.S.; Grutters, B.M.C.; Haury, J.; van Valkenburg, J.L.C.H.; Brundu, G.; Newman, J.; Clayton, J.S.; et al. Management and control methods of invasive alien freshwater aquatic plants: A review. Aquat. Bot. 2017, 136, 112–137. [Google Scholar] [CrossRef]
- Gala-Czekaj, D.; Synowiec, A.; Dąbkowska, T. Self-renewal of invasive goldenrods (Solidago spp.) as a result of different mechanical management of fallow. Agronomy 2021, 11, 1065. [Google Scholar] [CrossRef]
- Gaskin, J.F.; Espeland, E.; Johnson, C.D.; Larson, D.L.; Mangold, J.M.; McGee, R.A.; Milner, C.; Paudel, S.; Pearson, D.E.; Perkins, L.B.; et al. Managing invasive plants on Great Plains grasslands: A discussion of current challenges. Rang. Ecol. Manag. 2021, 78, 235–249. [Google Scholar] [CrossRef]
- Cohen, O.; Bar, P.; Gamliel, A.; Katan, J.; Kurzbaum, E.; Weber, G.; Schubert, I.; Riov, J. Rain-based soil solarization for reducing the persistent seed banks of invasive plants in natural ecosystems–Acacia saligna as a model. Pest Manag. Sci. 2019, 75, 1933–1941. [Google Scholar] [CrossRef]
- Jones, P.; Tummers, J.; Galib, S.; Woodford, D.; Hume, J.; Silva, L.; Braga, R.; Garcia de Leaniz, C.; Vitule, J.; Herder, J.; et al. The use of barriers to limit the spread of aquatic invasive animal species: A global review. Front. Ecol. Evol. 2021, 9, 1–19. [Google Scholar] [CrossRef]
- Souza-Alonso, P.; Lorenzo, P.; Rubido-Bará, M.; González, L. Effectiveness of management strategies in Acacia dealbata Link invasion, native vegetation and soil microbial community responses. For. Ecol. Manag. 2013, 304, 464–472. [Google Scholar] [CrossRef]
- Lazzaro, L.; Tondini, E.; Lombardi, L.; Giunti, M. The eradication of Carpobrotus spp. in the sand-dune ecosystem at Sterpaia (Italy, Tuscany): Indications from a successful experience. Biologia 2020, 75, 199–208. [Google Scholar] [CrossRef]
- Núñez-González, N.; Rodríguez, J.; González, L. Managing the invasive plant Carpobrotus edulis: Is mechanical control or specialized natural enemy more effective? J. Environ. Manag. 2021, 298, 113554. [Google Scholar] [CrossRef]
- Muvengwi, J.; Mbiba, M.; Jimu, L.; Mureva, A.; Dodzo, B. An assessment of the effectiveness of cut and ring barking as a method for control of invasive Acacia mearnsii in Nyanga National Park, Zimbabwe. For. Ecol. Manag. 2018, 427, 1–6. [Google Scholar] [CrossRef]
- Sher, A.A.; El Waer, H.; González, E.; Anderson, R.; Henry, A.L.; Biedron, R.; Yue, P. Native species recovery after reduction of an invasive tree by biological control with and without active removal. Ecol. Eng. 2018, 111, 167–175. [Google Scholar] [CrossRef]
- Verbrugge, L.N.H.; de Hoop, L.; Aukema, R.; Beringen, R.; Creemers, R.C.M.; van Duinen, G.A.; Hollander, H.; de Hullu, E.; Scherpenisse, M.; Spikmans, F.; et al. Lessons learned from rapid environmental risk assessments for prioritization of alien species using expert panels. J. Environ. Manag. 2019, 249, 109405. [Google Scholar] [CrossRef] [PubMed]
- Osunkoya, O.O.; Froese, J.G.; Nicol, S. Management feasibility of established invasive plant species in Queensland, Australia: A stakeholders’ perspective. J. Environ. Manag. 2019, 246, 484–495. [Google Scholar] [CrossRef]
- Delbart, E.; Mahy, G.; Weickmans, B.; Henriet, F.; Crémer, S.; Pieret, N.; Vanderhoeven, S.; Monty, A. Can land managers control Japanese knotweed? Lessons from control tests in Belgium. Environ. Manag. 2012, 50, 1089–1097. [Google Scholar] [CrossRef]
- Frelich, M.; Bzdęga, K. Management of invasive plant species in the valley of the River Ślepiotka in Katowice–The example of the REURIS project. Environ. Socio-Econ. Stud. 2014, 2, 26–37. [Google Scholar] [CrossRef]
- Froeschlin, N.; Privett, S.D.; Richardson, D.M.; Gaertner, M. Fynbos vegetation recovery twelve years after removal of invasive Eucalyptus trees. S. Afr. J. Bot. 2022, 147, 764–773. [Google Scholar] [CrossRef]
- Duarte, L.N.; Marchante, E.; Marchante, H. Managing an invasive tree in coastal dunes: The importance of follow-up treatments to improve the recovery of protected habitats. Front. Environ. Sci. 2023, 11, 1113876. [Google Scholar] [CrossRef]
- Dana, E.D.; García-de-Lomas, J.; Verloove, F.; Vilà, M. Common deficiencies of actions for managing invasive alien species: A decision-support checklist. NeoBiota 2019, 48, 97–112. [Google Scholar] [CrossRef]
- Rani, L.; Thapa, K.; Kanojia, N.; Sharma, N.; Singh, S.; Grewal, A.S.; Srivastav, A.L.; Kaushal, J. An extensive review on the consequences of chemical pesticides on human health and environment. J. Clean. Prod. 2020, 283, 124657. [Google Scholar] [CrossRef]
- Choudri, B.; Charabi, Y.; Ahmed, M. Pesticides and herbicides. Water Environ. Res. 2018, 90, 1663–1678. [Google Scholar] [CrossRef]
- Chauvel, B.; Fried, G.; Follak, S.; Chapman, D.; Kulakova, Y.; Le Bourgeois, T.; Marisavlievic, D.; Monty, A.; Rossi, J.-P.; Starfinger, U.; et al. Monographs on invasive plants in Europe N° 5: Ambrosia trifida L. Bot. Lett. 2021, 168, 167–190. [Google Scholar] [CrossRef]
- Brodeur, J. Host specificity in biological control: Insights from opportunistic pathogens. Evol. Appl. 2012, 5, 470–480. [Google Scholar] [CrossRef]
- Corbin, J.D.; Wolford, M.; Zimmerman, C.L.; Quirion, B. Assessing feasibility in invasive plant management: A retrospective analysis of garlic mustard (Alliaria petiolata) control. Restor. Ecol. 2017, 25, S170–S177. [Google Scholar] [CrossRef]
- Perry, G.L.; Moloney, K.A.; Etherington, T.R. Using network connectivity to prioritise sites for the control of invasive species. J. Appl. Ecol. 2017, 54, 1238–1250. [Google Scholar] [CrossRef]
- Van Wilgen, B.W.; Wannenburgh, A. Co-facilitating invasive species control, water conservation and poverty relief: Achievements and challenges in South Africa’s Working for Water programme. Curr. Opin. Environ. Sustain. 2016, 19, 7–17. [Google Scholar] [CrossRef]
- Novoa, A.; Kaplan, H.; Kumschick, S.; Wilson, J.R.; Richardson, D.M. Soft touch or heavy hand? Legislative approaches for preventing invasions: Insights from cacti in South Africa. Invasive Plant Sci. Manag. 2015, 8, 307–316. [Google Scholar] [CrossRef]
- Epanchin-Niell, R.; Thompson, A.L.; Treakle, T. Public contributions to early detection of new invasive pests. Conserv. Sci. Pract. 2021, 3, e422. [Google Scholar] [CrossRef]
- Price-Jones, V.; Brown, P.M.J.; Adriaens, T.; Tricarico, E.; Farrow, R.A.; Cardoso, A.C.; Gervasini, E.; Groom, Q.; Reyserhove, L.; Schade, S.; et al. Eyes on the aliens: Citizen science contributes to research, policy and management of biological invasions in Europe. NeoBiota 2022, 78, 1–24. [Google Scholar] [CrossRef]
- Encarnação, J.; Teodósio, M.A.; Morais, P. Citizen science and biological invasions: A review. Front. Environ. Sci. 2021, 8, 602980. [Google Scholar] [CrossRef]
- Anđelković, A.A.; Handley, L.L.; Marchante, E.; Adriaens, T.; Brown, P.M.J.; Tricarico, E.; Verbrugge, L.N.H. A review of volunteers’ motivations to monitor and control invasive alien species. NeoBiota 2022, 73, 153–175. [Google Scholar] [CrossRef]
- Jubase, N.; Shackleton, R.T.; Measey, J. Motivations and contributions of volunteer groups in the management of invasive alien plants in South Africa’s Western Cape province. Bothalia Afr. Biodivers. Conserv. 2021, 51, 1–13. [Google Scholar] [CrossRef]
- Ulm, F.; Estorninho, M.; de Jesus, J.G.; de Sousa Prado, M.G.; Cruz, C.; Máguas, C. From a lose–lose to a win–win situation: User-friendly biomass models for Acacia longifolia to aid research, management and valorisation. Plants 2022, 11, 2865. [Google Scholar] [CrossRef] [PubMed]
- Panetta, F.D.; O’Loughlin, L.S.; Gooden, B. Identifying thresholds and ceiling in plant community recovery for optimal management of widespread weeds. NeoBiota 2019, 42, 1–18. [Google Scholar] [CrossRef]
- Brito, L.M.; Mourão, I.; Coutinho, J.; Smith, S.R. Co-composting of invasive Acacia longifolia with pine bark for horticultural use. Environ. Technol. 2015, 36, 1632–1642. [Google Scholar] [CrossRef]
- Brito, L.M.; Reis, M.; Mourão, I.; Coutinho, J. Use of acacia waste compost as an alternative component for horticultural substrates. Commun. Soil Sci. Plant Anal. 2015, 46, 1814–1826. [Google Scholar] [CrossRef]
- Mesa, F.; Torres, J.; Sierra, O.; Escobedo, F.J. Enhanced production of compost from Andean wetland biomass using a bioreactor and photovoltaic system. Biomass Bioenergy 2017, 106, 21–28. [Google Scholar] [CrossRef]
- Mulvaney, M.J.; Wood, C.W.; Balkcom, K.S.; Kemble, J.; Shannon, D.A. No-till with high biomass cover crops and invasive legume mulches increased total soil carbon after three years of collard production. Agroecol. Sustain. Food Syst. 2017, 41, 30–45. [Google Scholar] [CrossRef]
- Cvejić, R.; Klages, S.; Pintar, M.; Resman, L.; Slatnar, A.; Mihelič, R. Invasive plants in support of urban farming: Fermentation-based organic fertilizer from Japanese Knotweed. Agronomy 2021, 11, 1232. [Google Scholar] [CrossRef]
- Liu, H.; Zhao, Q.; Cheng, Y. Composted invasive plant Ageratina adenophora enhanced barley (Hordeum vulgare) growth and soil conditions. PLoS ONE 2022, 17, e0275302. [Google Scholar] [CrossRef]
- Vyankatrao, N.P. Conversion of Parthenium hystorophorus L. weed to compost and vermicompost. Biosci. Discov. 2017, 8, 619–627. [Google Scholar]
- Lorenzo, P.; Álvarez-Iglesias, L.; González, L.; Revilla, P. Assessment of Acacia dealbata as green manure and weed control for maize crop. Renew. Agric. Food Syst. 2022, 37, 322–336. [Google Scholar] [CrossRef]
- Alami, E.; Karimi, M.; Chalavi, V. Investigation of compost and vermicompost of water hyacinth as growing media for Lily (Longiflorum × Asiatic). Int. J. Hortic. Sci. Technol. 2021, 8, 271–280. [Google Scholar]
- Islam, M.N.; Rahman, F.; Papri, S.A.; Faruk, M.O.; Das, A.K.; Adhikary, N.; Debrot, A.O.; Ahsan, M.N. Water hyacinth (Eichhornia crassipes (Mart.) Solms.) as an alternative raw material for the production of bio-compost and handmade paper. J. Environ. Manag. 2021, 294, 113036. [Google Scholar] [CrossRef]
- Gosal, M.; Rayer, D.; Gedoan, S. The effect of water hyacinth (Eichhornia crassipes) organic fertilizer on the vegetative growth of Manado strain yellow maize (Zea mays L.). World J. Adv. Res. Rev. 2022, 15, 450–454. [Google Scholar] [CrossRef]
- Ogutu, P.A. Vermicomposting water hyacinth: Turning Fisherman’s Nightmare into Farmer’s Fortune. Int. J. Res. Innov. Appl. Sci. 2019, IV, 12–14. [Google Scholar]
- Feng, Q.; Wang, B.; Chen, M.; Wu, P.; Lee, X.; Xing, Y. Invasive plants as potential sustainable feedstocks for biochar production and multiple applications: A review. Resour. Conserv. Recycl. 2021, 164, 105204. [Google Scholar] [CrossRef]
- Patwa, D.; Muigai, H.H.; Ravi, K.; Sreedeep, S.; Kalita, P. A novel application of biochar produced from invasive weeds and industrial waste in thermal backfill for crude oil industries. Waste Biomass Valorization 2022, 13, 3025–3042. [Google Scholar] [CrossRef]
- Kleinschroth, F.; Winton, R.S.; Calamita, E.; Niggemann, F.; Botter, M.; Wehrli, B.; Ghazoul, J. Living with floating vegetation invasions. Ambio 2021, 50, 125–137. [Google Scholar] [CrossRef] [PubMed]
- Arutselvy, B.; Rajeswari, G.; Jacob, S. Sequential valorization strategies for dairy wastewater and water hyacinth to produce fuel and fertilizer. J. Food Process Eng. 2021, 44, e13585. [Google Scholar] [CrossRef]
- Harun, I.; Pushiri, H.; Amirul-Aiman, A.J.; Zulkeflee, Z. Invasive water hyacinth: Ecology, impacts and prospects for the rural economy. Plants 2021, 10, 1613. [Google Scholar] [CrossRef]
- Ilo, O.P.; Simatele, M.D.; Nkomo, S.P.L.; Mkhize, N.M.; Prabhu, N.G. The benefits of water hyacinth (Eichhornia crassipes) for Southern Africa: A review. Sustainability 2020, 12, 9222. [Google Scholar] [CrossRef]
- Sladonja, B.; Sušek, M.; Guillermic, J. Review on invasive tree of heaven (Ailanthus altissima (Mill.) Swingle) conflicting values: Assessment of its ecosystem services and potential biological threat. Environ. Manag. 2015, 56, 1009–1034. [Google Scholar] [CrossRef]
- López-Hortas, L.; Rodríguez-González, I.; Díaz-Reinoso, B.; Torres, M.D.; Moure, A.; Domínguez, H. Tools for a multiproduct biorefinery of Acacia dealbata biomass. Ind. Crops Prod. 2021, 169, 113655. [Google Scholar] [CrossRef]
- Souza-Alonso, P.; Puig, C.G.; Pedrol, N.; Freitas, H.; Rodríguez-Echeverría, S.; Lorenzo, P. Exploring the use of residues from the invasive Acacia sp. for weed control. Renew. Agric. Food Syst. 2020, 35, 26–37. [Google Scholar] [CrossRef]
- Brito, L.M.; Mourão, I.; Coutinho, J.; Smith, S. Composting for management and resource recovery of invasive Acacia species. Waste Manag. Res. 2013, 31, 1125–1132. [Google Scholar] [CrossRef]
- Adam, Y.; Sershen, R.S. Maize and pea germination and seedling growth responses to compost generated from biowaste of selected invasive alien plant species. Compost Sci. Util. 2016, 24, 30–41. [Google Scholar] [CrossRef]
- Chemetova, C.; Ribeiro, H.; Fabião, A.; Gominho, J. Towards sustainable valorisation of Acacia melanoxylon biomass: Characterization of mature and juvenile plant tissues. Environ. Res. 2020, 191, 110090. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.H.; Song, W.; Guo, J.Y. Advances in management and utilization of invasive water hyacinth (Eichhornia crassipes) in aquatic ecosystems–a review. Crit. Rev. Biotechnol. 2017, 37, 218–228. [Google Scholar] [CrossRef] [PubMed]
- Kumwimba, M.N.; Dzakpasu, M.; Li, X. Potential of invasive watermilfoil (Myriophyllum spp.) to remediate eutrophic waterbodies with organic and inorganic pollutants. J. Environ. Manag. 2020, 270, 110919. [Google Scholar] [CrossRef] [PubMed]
- Quintela-Sabarís, C.; Mendes, L.A.; Domínguez, J. Vermicomposting as a sustainable option for managing biomass of the invasive tree Acacia dealbata Link. Sustainability 2022, 14, 13828. [Google Scholar] [CrossRef]
- Balachandar, R.; Biruntha, M.; Yuvaraj, A.; Thangaraj, R.; Subbaiya, R.; Govarthanan, M.; Kumar, P.; Karmegam, N. Earthworm intervened nutrient recovery and greener production of vermicompost from Ipomoea staphylina–An invasive weed with emerging environmental challenges. Chemosphere 2021, 263, 128080. [Google Scholar] [CrossRef]
- Abbas, A.M.; Novak, S.J.; Fictor, M.; Mostafa, Y.S.; Alamri, S.A.; Alrumman, S.A.; Taher, M.A.; Hashem, M.; Khalaphallah, R. Initial in vitro assessment of the antifungal activity of aqueous extracts from three invasive plant species. Agriculture 2022, 12, 1152. [Google Scholar] [CrossRef]
- Jiao, Y.; Li, Y.; Yuan, L.; Huang, J. Allelopathy of uncomposted and composted invasive aster (Ageratina adenophora) on ryegrass. J. Hazard. Mater. 2021, 402, 123727. [Google Scholar] [CrossRef]
- Liua, H.; Wangc, Y.; Zhaoa, Q. Converting invasive aster (Ageratina adenophora L.) into organic fertilizer source. ScienceAsia 2022, 48, 1–8. [Google Scholar] [CrossRef]
- Li, P.; Chang, Q.; Wang, C.; Cao, J.; Zheng, W. Composting of aerial parts of crofton weed (Eupatorium adenophorum Spreng), the top invasive plant in southwest China. Compost Sci. Util. 2014, 22, 132–137. [Google Scholar] [CrossRef]
- Ulm, F.; Avelar, D.; Hobson, P.; Penha-Lopes, G.; Dias, T.; Máguas, C.; Cruz, C. Sustainable urban agriculture using compost and an open-pollinated maize variety. J. Clean. Prod. 2019, 212, 622–629. [Google Scholar] [CrossRef]
- Devi, C.; Khwairakpam, M. Management of invasive weed Parthenium hysterophorus through vermicomposting using a polyculture of Eisenia fetida and Eudrilus eugeniae. Environ. Sci. Pollut. Res. 2021, 28, 29710–29719. [Google Scholar] [CrossRef] [PubMed]
- Hussain, N.; Abbasi, T.; Abbasi, S.A. Vermicomposting transforms allelopathic parthenium into a benign organic fertilizer. J. Environ. Manag. 2016, 180, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Benelli, G.; Pavela, R.; Cianfaglione, K.; Nagy, D.U.; Canale, A.; Maggi, F. Evaluation of two invasive plant invaders in Europe (Solidago canadensis and Solidago gigantea) as possible sources of botanical insecticides. J. Pest Sci. 2019, 92, 805–821. [Google Scholar] [CrossRef]
- Lorenzo, P.; Reboredo-Durán, J.; Múñoz, L.; González, L.; Freitas, H.; Rodríguez-Echeverría, S. Inconsistency in the detection of phytotoxic effects: A test with Acacia dealbata extracts using two different methods. Phytochem. Lett. 2016, 15, 190–198. [Google Scholar] [CrossRef]
- Lorenzo, P.; Souza-Alonso, P.; Guisande-Collazo, A.; Freitas, H. Influence of Acacia dealbata Link bark extracts on the growth of Allium cepa L. plants under high salinity conditions. J. Sci. Food Agric. 2019, 99, 4072–4081. [Google Scholar] [CrossRef]
- Lorenzo, P.; Reboredo-Durán, J.; Muñoz, L.; Freitas, H.; González, L. Herbicidal properties of the commercial formulation of methyl cinnamate, a natural compound in the invasive silver wattle (Acacia dealbata). Weed Sci. 2020, 68, 69–78. [Google Scholar] [CrossRef]
- Quinty, V.; Colas, C.; Nasreddine, R.; Nehmé, R.; Piot, C.; Draye, M.; Destandau, E.; Da Silva, D.; Chatel, G. Screening and evaluation of dermo-cosmetic activities of the invasive plant species Polygonum cuspidatum. Plants 2022, 12, 83. [Google Scholar] [CrossRef]
- Oliveira, C.S.; Moreira, P.; Resende, J.; Cruz, M.T.; Pereira, C.M.; Silva, A.M.; Santos, S.A.O.; Silvestre, A.J. Characterization and cytotoxicity assessment of the lipophilic fractions of different morphological parts of Acacia dealbata. Int. J. Mol. Sci. 2020, 21, 1814. [Google Scholar] [CrossRef]
- Míguez, C.; Cancela, Á.; Sánchez, Á.; Álvarez, X. Possibilities for exploitation of invasive species, Arundo donax L., as a source of phenol compounds. Waste Biomass Valor. 2022, 13, 4253–4265. [Google Scholar] [CrossRef]
- Okyere, S.K.; Wen, J.; Cui, Y.; Xie, L.; Gao, P.; Wang, J.; Wang, S.; Hu, Y. Toxic mechanisms and pharmacological properties of euptox A, a toxic monomer from A. adenophora. Fitoterapia 2021, 155, 105032. [Google Scholar] [CrossRef]
- Peter, A.; Žlabur, J.Š.; Šurić, J.; Voća, S.; Purgar, D.D.; Pezo, L.; Voća, N. Invasive plant species biomass—Evaluation of functional value. Molecules 2021, 26, 3814. [Google Scholar] [CrossRef] [PubMed]
- Correia, R.; Duarte, M.P.; Maurício, E.M.; Brinco, J.; Quintela, J.C.; da Silva, M.G.; Gonçalves, M. Chemical and functional characterization of extracts from leaves and twigs of Acacia dealbata. Processes 2022, 10, 2429. [Google Scholar] [CrossRef]
- Paula, V.; Pedro, S.I.; Campos, M.G.; Delgado, T.; Estevinho, L.M.; Anjos, O. Special bioactivities of phenolics from Acacia dealbata L. with potential for dementia, diabetes and antimicrobial Treatments. Appl. Sci. 2022, 12, 1022. [Google Scholar] [CrossRef]
- Kaur, A.; Batish, D.R.; Kaur, S.; Chauhan, B.S. An overview of the characteristics and potential of Calotropis procera from botanical, ecological, and economic perspectives. Front. Plant Sci. 2021, 12, 1188. [Google Scholar] [CrossRef]
- Casas, M.P.; López-Hortas, L.; Díaz-Reinoso, B.; Moure, A.; Domínguez, H. Supercritical CO2 extracts from Acacia dealbata flowers. J. Supercrit. Fluids 2021, 173, 105223. [Google Scholar] [CrossRef]
- Ponticelli, M.; Lela, L.; Russo, D.; Faraone, I.; Sinisgalli, C.; Mustapha, M.B.; Esposito, G.; Jannet, H.B.; Costantino, V.; Milella, L. Dittrichia graveolens (L.) Greuter, a rapidly spreading invasive plant: Chemistry and bioactivity. Molecules 2022, 27, 895. [Google Scholar] [CrossRef]
- Arsene, M.M.J.; Viktorovna, P.I.; Mikhaïlovitch, M.K.; Davares, A.K.L.; Parfait, K.; Rehailia, M.; Nikolayevich, S.A.; Stefanovna, G.V.; Sarra, S.; Sulikoevich, K.Z.; et al. In vitro antimicrobial activity, antibioresistance reversal properties, and toxicity screen of ethanolic extracts of Heracleum mantegazzianum Sommier and Levier (giant hogweed), Centaurea jacea L. (brown knapweed), and Chenopodium album L. (Pigweed): Three invasive plants. Open Vet. J. 2022, 12, 584. [Google Scholar]
- Kim, G.J.; Park, S.; Kim, E.; Kwon, H.; Park, H.J.; Nam, J.W.; Roh, S.S.; Choi, H. Antioxidant, pancreatic lipase inhibitory, and tyrosinase inhibitory activities of extracts of the invasive plant Spartina anglica (Cord-Grass). Antioxidants 2021, 10, 242. [Google Scholar] [CrossRef]
- Yildiz, S.; Gurgen, A.; Can, Z.; Tabbouche, S.A.; Kilic, A.O. Some bioactive properties of Acacia dealbata extracts and their potential utilization in wood protection. Drewno 2018, 61, 81–97. [Google Scholar]
- Olayiwola, H.O.; Amiandamhen, S.O.; Meincken, M.; Tyhoda, L. Investigating the suitability of fly ash/metakaolin-based geopolymers reinforced with South African alien invasive wood and sugarcane bagasse residues for use in outdoor conditions. Eur. J. Wood Wood Prod. 2021, 79, 611–627. [Google Scholar] [CrossRef]
- Rodrigues, V.H.; de Melo, M.M.; Portugal, I.; Silva, C.M. Extraction of added-value triterpenoids from Acacia dealbata leaves using supercritical fluid extraction. Processes 2021, 9, 1159. [Google Scholar] [CrossRef]
- Rodrigues, V.H.; de Melo, M.M.; Portugal, I.; Silva, C.M. Lupane-type triterpenoids from Acacia dealbata bark extracted by different methods. Ind. Crops Prod. 2021, 170, 113734. [Google Scholar] [CrossRef]
- Míguez, C.; Cancela, A.; Álvarez, X.; Sánchez, A. The reuse of bio-waste from the invasive species Tradescantia fluminensis as a source of phenolic compounds. J. Clean. Prod. 2022, 336, 130293. [Google Scholar] [CrossRef]
- Borges, A.; José, H.; Homem, V.; Simões, M. Comparison of techniques and solvents on the antimicrobial and antioxidant potential of extracts from Acacia dealbata and Olea europaea. Antibiotics 2020, 9, 48. [Google Scholar] [CrossRef]
- Neiva, D.M.; Luís, A.; Gominho, J.; Domingues, F.; Duarte, A.P.; Pereira, H. Bark residues valorization potential regarding antioxidant and antimicrobial extracts. Wood Sci. Technol. 2020, 54, 559–585. [Google Scholar] [CrossRef]
- Marinas, I.C.; Oprea, E.; Geana, E.I.; Tutunaru, O.; Pircalabioru, G.G.; Zgura, I.; Chifiriuc, M.C. Valorization of Gleditsia triacanthos invasive plant cellulose microfibers and phenolic compounds for obtaining multi-functional wound dressings with antimicrobial and antioxidant properties. Int. J. Mol. Sci. 2020, 22, 33. [Google Scholar] [CrossRef]
- Yáñez, R.; Gómez, B.; Martínez, M.; Gullón, B.; Alonso, J.L. Valorization of an invasive woody species, Acacia dealbata, by means of Ionic liquid pretreatment and enzymatic hydrolysis. J. Chem. Technol. Biotechnol. 2014, 89, 1337–1343. [Google Scholar] [CrossRef]
- Iyer, A.; Bestwick, C.S.; Duncan, S.H.; Russell, W.R. Invasive plants are a valuable alternate protein source and can contribute to meeting climate change targets. Front. Sustain. Food Syst. 2021, 5, 575056. [Google Scholar] [CrossRef]
- Iyer, A.; Guerrier, L.; Leveque, S.; Bestwick, C.S.; Duncan, S.H.; Russell, W.R. High throughput method development and optimised production of leaf protein concentrates with potential to support the agri-industry. J. Food Meas. Characterizat. 2022, 16, 49–65. [Google Scholar] [CrossRef]
- Sowndhararajan, K.; Santhanam, R.; Hong, S.; Jhoo, J.W.; Kim, S. Suppressive effects of acetone extract from the stem bark of three Acacia species on nitric oxide production in lipopolysaccharide-stimulated RAW 264.7 macrophage cells. Asian Pac. J. Trop. Biomed. 2016, 6, 658–664. [Google Scholar] [CrossRef]
- Martínez-Espinosa, J.C.; Ramírez-Morales, M.A.; Carrera-Cerritos, R. Silver nanoparticles synthesized using Eichhornia crassipes extract from Yuriria lagoon, and the perspective for application as antimicrobial agent. Crystals 2022, 12, 814. [Google Scholar] [CrossRef]
- Jayaweera, M.W.; Dilhani, J.A.; Kularatne, R.K.; Wijeyekoon, S.L. Biogas production from water hyacinth (Eichhornia crassipes (Mart.) Solms) grown under different nitrogen concentrations. J. Environ. Sci. Health Part A 2007, 42, 925–932. [Google Scholar] [CrossRef] [PubMed]
- Van Meerbeek, K.; Appels, L.; Dewil, R.; Calmeyn, A.; Lemmens, P.; Muys, B.; Hermy, M. Biomass of invasive plant species as a potential feedstock for bioenergy production. Biofuels Bioprod. Biorefining 2015, 9, 273–282. [Google Scholar] [CrossRef]
- Ferreira, S.; Gil, N.; Queiroz, J.A.; Duarte, A.P.; Domingues, F.C. An evaluation of the potential of Acacia dealbata as raw material for bioethanol production. Bioresour. Technol. 2011, 102, 4766–4773. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Weng, C.; Huang, H.; Achal, V.; Wang, D. Optimization of bioethanol production using whole plant of water hyacinth as substrate in simultaneous saccharification and fermentation process. Front. Microbiol. 2016, 6, 1411. [Google Scholar] [CrossRef]
- Carlini, M.; Castellucci, S.; Mennun, A. Water hyacinth biomass: Chemical and thermal pre-treatment for energetic utilization in anaerobic digestion process. Energy Procedia 2018, 148, 431–438. [Google Scholar] [CrossRef]
- Alves, J.L.F.; da Silva, J.C.G.; da Silva Filho, V.F.; Alves, R.F.; de Araujo Galdino, W.V.; De Sena, R.F. Kinetics and thermodynamics parameters evaluation of pyrolysis of invasive aquatic macrophytes to determine their bioenergy potentials. Biomass Bioenergy 2019, 121, 28–40. [Google Scholar] [CrossRef]
- Nunes, L.J.; Raposo, M.A.; Meireles, C.I.; Pinto Gomes, C.J.; Ribeiro, N.M.A. Control of invasive forest species through the creation of a value chain: Acacia dealbata biomass recovery. Environments 2020, 7, 39. [Google Scholar] [CrossRef]
- Van Tran, G.; Unpaprom, Y.; Ramaraj, R. Methane productivity evaluation of an invasive wetland plant, common reed. Biomass Conver. Biorefinery 2020, 10, 689–695. [Google Scholar] [CrossRef]
- da Costa, R.M.; Bosch, M.; Simister, R.; Gomez, L.D.; Canhoto, J.M.; Batista de Carvalho, L.A. Valorisation potential of invasive Acacia dealbata, A. longifolia and A. melanoxylon from land clearings. Molecules 2022, 27, 7006. [Google Scholar] [CrossRef]
- Liao, R.; Gao, B.; Fang, J. Invasive plants as feedstock for biochar and bioenergy production. Bioresour. Technol. 2013, 140, 439–442. [Google Scholar] [CrossRef]
- Albaugh, T.J.; Rubilar, R.A.; Maier, C.A.; Acuna, E.A.; Cook, R.L. Biomass and nutrient mass of Acacia dealbata and Eucalyptus globulus bioenergy plantations. Biomass Bioenergy 2017, 97, 162–171. [Google Scholar] [CrossRef]
- Stafford, W.; Blignaut, J. Reducing landscape restoration costs: Feasibility of generating electricity from invasive alien plant biomass on the Agulhas Plain, South Africa. Ecosyst. Serv. 2017, 27, 224–231. [Google Scholar] [CrossRef]
- Ngorima, A.; Shackleton, C.M. Livelihood benefits and costs from an invasive alien tree (Acacia dealbata) to rural communities in the Eastern Cape, South Africa. J. Environ. Manag. 2019, 229, 158–165. [Google Scholar] [CrossRef]
- Carson, B.D.; Lishawa, S.C.; Tuchman, N.C.; Monks, A.M.; Lawrence, B.A.; Albert, D.A. Harvesting invasive plants to reduce nutrient loads and produce bioenergy: An assessment of Great Lakes coastal wetlands. Ecosphere 2018, 9, e02320. [Google Scholar] [CrossRef]
- Awasthi, M.; Kaur, J.; Rana, S. Bioethanol production through water hyacinth, Eichhornia crassipes via optimization of the pretreatment conditions. Int. J. Emerg. Technol. Adv. Eng. 2013, 3, 42–46. [Google Scholar]
- Ruan, T.; Zeng, R.; Yin, X.Y.; Zhang, S.X.; Yang, Z.H. Water hyacinth (Eichhornia crassipes) biomass as a biofuel feedstock by enzymatic hydrolysis. BioResources 2016, 11, 2372–2380. [Google Scholar] [CrossRef]
- Łapczyńska-Kordon, B.; Ślipek, Z.; Słomka-Polonis, K.; Styks, J.; Hebda, T.; Francik, S. Physicochemical properties of biochar produced from goldenrod plants. Materials 2022, 15, 2615. [Google Scholar] [CrossRef]
- Raj, F.R.M.S.; Boopathi, G.; Kalpana, D.; Jaya, N.V.; Pandurangan, A. Sustainable development through restoration of Prosopis juliflora species into activated carbon as electrode material for supercapacitors. Diam. Relat. Mater. 2022, 121, 108767. [Google Scholar] [CrossRef]
- Yang, L.; Deng, Y.; Shu, Z.; Chen, Q.; Yang, H.; Tan, X. Application of invasive plants as biochar precursors in the field of environment and energy storage. Front. Environ. Sci. 2022, 10, 902915. [Google Scholar] [CrossRef]
- Muñoz, C.; Mendonça, R.; Baeza, J.; Berlin, A.; Saddler, J.; Freer, J. Bioethanol production from bio-organosolv pulps of Pinus radiata and Acacia dealbata. J. Chem. Technol. Biotechnol. Int. Res. Process Environ. Clean Technol. 2007, 82, 767–774. [Google Scholar] [CrossRef]
- Nguyen, X.C.; Nguyen, T.T.H.; Nguyen, T.H.C.; Le, Q.V.; Vo, T.Y.B.; Tran, T.C.P.; La, D.D.; Kumar, G.; Nguyen, V.K.; Chang, S.W.; et al. Sustainable carbonaceous biochar adsorbents derived from agro-wastes and invasive plants for cation dye adsorption from water. Chemosphere 2021, 282, 131009. [Google Scholar] [CrossRef] [PubMed]
- Tham, H.T.; Udén, P. Effect of water hyacinth (Eichhornia crassipes) silage on intake and nutrient digestibility in cattle fed rice straw and cottonseed cake. Asian-Australas. J. Anim. Sci. 2013, 26, 646–653. [Google Scholar] [CrossRef] [PubMed]
- Korkut, A.Y.; Gunes, A.; Kop, A.; Cakar, H.; Akat, O.; Guney, M.A.; Ozkul, B.; Koru, E.; Suzer, C.; Cirik, S.; et al. Preliminary study for utilization of some invasive aquatic plants as raw material for aquaculture feeds. Fresenius Environ. Bull. 2016, 25, 4915–4920. [Google Scholar]
- Moselhy, M.A.; Borba, J.P.; Borba, A.E. Production of high-quality silage from invasive plants plus agro-industrial by-products with or without bacterial inoculation. Biocata. Agric. Biotechnol. 2022, 39, 102251. [Google Scholar] [CrossRef]
- Pratiwi, D.Y.; Andhikawati, A. Utilization of water hyacinth (Eichhornia crassipes) as fish feed ingredient. Asian J. Fish. Aquat. Res. 2021, 13, 35–42. [Google Scholar] [CrossRef]
- Teixeira, A.R.; Jorge, N.; Fernandes, J.R.; Lucas, M.S.; Peres, J.A. Textile dye removal by Acacia dealbata link. pollen adsorption combined with UV-A/NTA/Fenton process. Top. Catal. 2022, 65, 1045–1061. [Google Scholar] [CrossRef]
- Carneiro, M.T.; Barros, A.Z.B.; Morais, A.I.S.; Carvalho Melo, A.L.F.; Bezerra, R.D.S.; Osajima, J.A.; Silva-Filho, E.C. Application of water hyacinth biomass (Eichhornia crassipes) as an adsorbent for methylene blue dye from aqueous medium: Kinetic and isothermal study. Polymers 2022, 14, 2732. [Google Scholar] [CrossRef]
- Zhang, L.; Cheng, H.; Pan, D.; Wu, Y.; Ji, R.; Li, W.; Jiang, X.; Han, J. One-pot pyrolysis of a typical invasive plant into nitrogen-doped biochars for efficient sorption of phthalate esters from aqueous solution. Chemosphere 2021, 280, 130712. [Google Scholar] [CrossRef]
- Almeida, R.; Cisneros, F.; Mendes, C.V.; Carvalho, M.G.V.; Rasteiro, M.G.; Gamelas, J.A. Valorisation of invasive plant species in the production of polyelectrolytes. Ind. Crops Prod. 2021, 167, 113476. [Google Scholar] [CrossRef]
- Jorge, N.; Teixeira, A.R.; Lucas, M.S.; Peres, J.A. Agro-industrial wastewater treatment with Acacia dealbata coagulation/flocculation and photo-Fenton-based processes. Recycling 2022, 7, 54. [Google Scholar] [CrossRef]
- Peng, H.; Wang, Y.; Tan, T.L.; Chen, Z. Exploring the phytoremediation potential of water hyacinth by FTIR Spectroscopy and ICP-OES for treatment of heavy metal contaminated water. Int. J. Phytoremediation 2020, 22, 939–951. [Google Scholar] [CrossRef] [PubMed]
- Panneerselvam, B.; Priya, K.S. Phytoremediation potential of water hyacinth in heavy metal removal in chromium and lead contaminated water. Int. J. Environ. Anal. Chem. 2021, 2021, 1–6. [Google Scholar] [CrossRef]
- Saha, P.; Shinde, O.; Sarkar, S. Phytoremediation of industrial mines wastewater using water hyacinth. Int. J. Phytoremediation 2017, 19, 87–96. [Google Scholar] [CrossRef]
- Kavčič, U.; Karlovits, I. The influence of process parameters of screen-printed invasive plant paper electrodes on cyclic voltammetry. Nord. Pulp Paper Res. J. 2020, 35, 299–307. [Google Scholar] [CrossRef]
- Karlovits, I.; Lavrič, G.; Kavčič, U.; Zorić, V. Electrophotography toner adhesion on agro-industrial residue and invasive plant papers. J. Adhes. Sci. Technol. 2021, 35, 2636–2651. [Google Scholar] [CrossRef]
- Karlovits, I.; Kavčič, U. Flexo printability of agro and invasive papers. Cellulose 2022, 29, 4613–4627. [Google Scholar] [CrossRef]
- Starešinič, M.; Boh Podgornik, B.; Javoršek, D.; Leskovšek, M.; Možina, K. Fibers obtained from invasive alien plant species as a base material for paper production. Forests 2021, 12, 527. [Google Scholar] [CrossRef]
- Kapun, T.; Zule, J.; Fabjan, E.; Hočevar, B.; Grilc, M.; Likozar, B. Engineered invasive plant cellulose fibers as resources for papermaking. Eur. J. Wood Wood Prod. 2022, 80, 501–514. [Google Scholar] [CrossRef]
- Ranesi, A.; Faria, P.; Correia, R.; Freire, M.T.; Veiga, R.; Gonçalves, M. Gypsum mortars with Acacia dealbata biomass waste additions: Effect of different fractions and contents. Buildings 2022, 12, 339. [Google Scholar] [CrossRef]
- Ortega, Z.; Romero, F.; Paz, R.; Suárez, L.; Benítez, A.N.; Marrero, M.D. Valorization of invasive plants from Macaronesia as filler materials in the production of natural fiber composites by rotational molding. Polym. 2021, 13, 2220. [Google Scholar] [CrossRef] [PubMed]
- Portela-Grandío, A.; Peleteiro, S.; Yáñez, R.; Romaní, A. Integral valorization of Acacia dealbata wood in organic medium catalyzed by an acidic ionic liquid. Bioresour. Technol. 2021, 342, 126013. [Google Scholar] [CrossRef] [PubMed]
- Rani, B.S.J.; Venkatachalam, S. A neoteric approach for the complete valorization of Typha angustifolia leaf biomass: A drive towards environmental sustainability. J. Environ. Manag. 2022, 318, 115579. [Google Scholar] [CrossRef]
- Neiva, D.M.; Rencoret, J.; Marques, G.; Gutiérrez, A.; Gominho, J.; Pereira, H.; Del Río, J.C. Lignin from tree barks: Chemical structure and valorization. ChemSusChem 2020, 13, 4537–4547. [Google Scholar] [CrossRef]
- Lim, C.J.; Arumugam, M.; Lim, C.K.; Ee, G.C.L. Mercerizing extraction and physicochemical characterizations of lignocellulosic fiber from the leaf waste of Mikania micrantha Kunth ex HBK. J. Nat. Fibers 2018, 17, 726–737. [Google Scholar] [CrossRef]
- Čuk, N.; Šala, M.; Gorjanc, M. Development of antibacterial and UV protective cotton fabrics using plant food waste and alien invasive plant extracts as reducing agents for the in-situ synthesis of silver nanoparticles. Cellulose 2021, 28, 3215–3233. [Google Scholar] [CrossRef]
- Arana-Cuenca, A.; Tovar-Jiménez, X.; Favela-Torres, E.; Perraud-Gaime, I.; González-Becerra, A.E.; Martínez, A.; Moss-Acosta, C.L.; Mercado-Flores, Y.; Téllez-Jurado, A. Use of water hyacinth as a substrate for the production of filamentous fungal hydrolytic enzymes in solid-state fermentation. 3 Biotech 2019, 9, 21. [Google Scholar] [CrossRef]
- Linhares, T.; de Amorim, M.T.P. LCA of textile dyeing with Acacia dealbata tree bark: A case study research. Procedia Eng. 2017, 200, 365–369. [Google Scholar] [CrossRef]
- Lock Toy Ki, Y.; Garcia, A.; Pelissier, F.; Olszewski, T.K.; Babst-Kostecka, A.; Legrand, Y.M.; Grison, C. Mechanochemistry and eco-bases for sustainable Michael addition reactions. Molecules 2022, 27, 3306. [Google Scholar] [CrossRef]
- Shackleton, R.T.; Vimercati, G.; Probert, A.F.; Bacher, S.; Kull, C.A.; Novoa, A. Consensus and controversy in the discipline of invasion science. Conserv. Biol. 2022, 36, e13931. [Google Scholar] [CrossRef]
- Dehnen-Schmutz, K.; Novoa, A. Advances in the management of invasive plants. In Global Plant Invasions; Clements, D.R., Upadhyaya, M.K., Joshi, S., Shrestha, A., Eds.; Springer: Cham, Germany, 2022; pp. 317–330. [Google Scholar]
- Suárez, L.; Díaz, T.E.; Benavente-Ferraces, I.; Plaza, C.; Almeida, M.; Centeno, T.A. Hydrothermal treatment as a complementary tool to control the invasive Pampas grass (Cortaderia selloana). Sci. Total Environ. 2022, 807, 150796. [Google Scholar] [CrossRef] [PubMed]
- Correia, R.; Quintela, J.C.; Duarte, M.P.; Gonçalves, M. Insights for the valorization of biomass from Portuguese invasive acacia spp. in a biorefinery perspective. Forests 2020, 11, 1342. [Google Scholar] [CrossRef]
- Nunes, L.J.R.; Rodrigues, A.M.; Loureiro, L.M.E.F.; Sá, L.C.R.; Matias, J.C.O. Energy recovery from invasive species: Creation of value chains to promote control and eradication. Recycling 2021, 6, 21. [Google Scholar] [CrossRef]
- Vrabič-Brodnjak, U.; Možina, K. Invasive alien plant species for use in paper and packaging materials. Fibers 2022, 10, 94. [Google Scholar] [CrossRef]
- Mudavanhu, S.; Blignaut, J.; Nkambule, N.; Morokong, T.; Vundla, T. A cost-benefit analysis of using Rooikrans as biomass feedstock for electricity generation: A case study of the De Hoop nature reserve, South Africa. S. Afr. J. Econ. Manag. Sci. 2016, 19, 788–813. [Google Scholar] [CrossRef]
- Melane, M.; Ham, C.; Meincken, M. Characteristics of selected non-woody invasive alien plants in South Africa and an evaluation of their potential for electricity generation. J. Energy S. Afr. 2017, 28, 92–98. [Google Scholar] [CrossRef]
- Valen, M.A. Economic opportunities for biomass harvest of invasive giant reed (Arundo donax L.) in Southern California as feedstock for the pulp and paper industry. Master’s Thesis, Harvard Extension School, Cambridge, MA, USA, 2017. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).