Preliminary Investigation of Essentially Derived Variety of Tea Tree and Development of SNP Markers
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample and DNA Isolation
2.2. Genotyping by Sequencing (GBS)
2.3. Population Structure Analysis
2.4. Genetic Similarity Analysis
2.5. Core Varieties/Strains Analysis
2.6. SNPs Markers for Rapidly Varieties Identification
3. Result
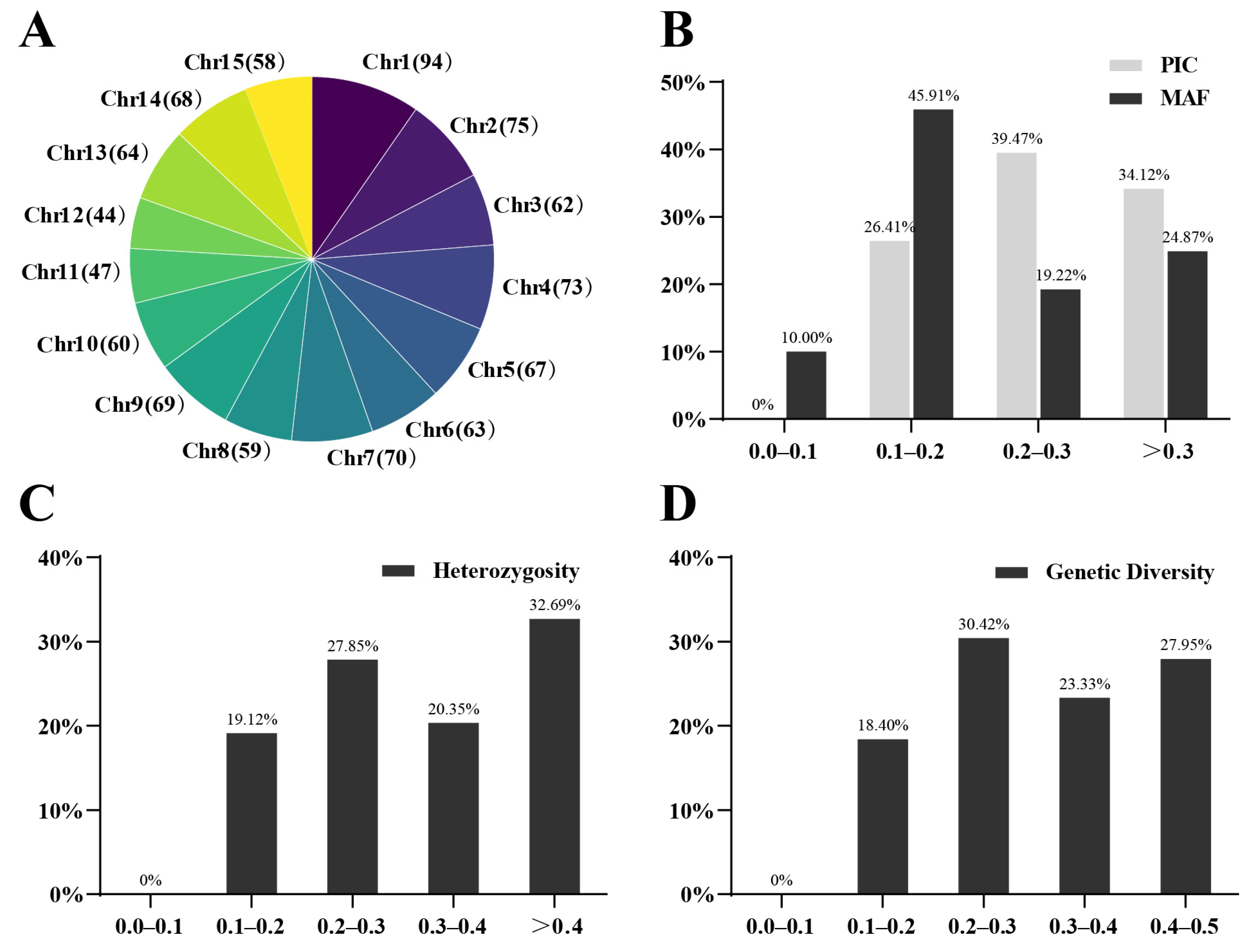
3.1. Genome-Wide Perfect SNPs Discovery of 349 Tea Trees
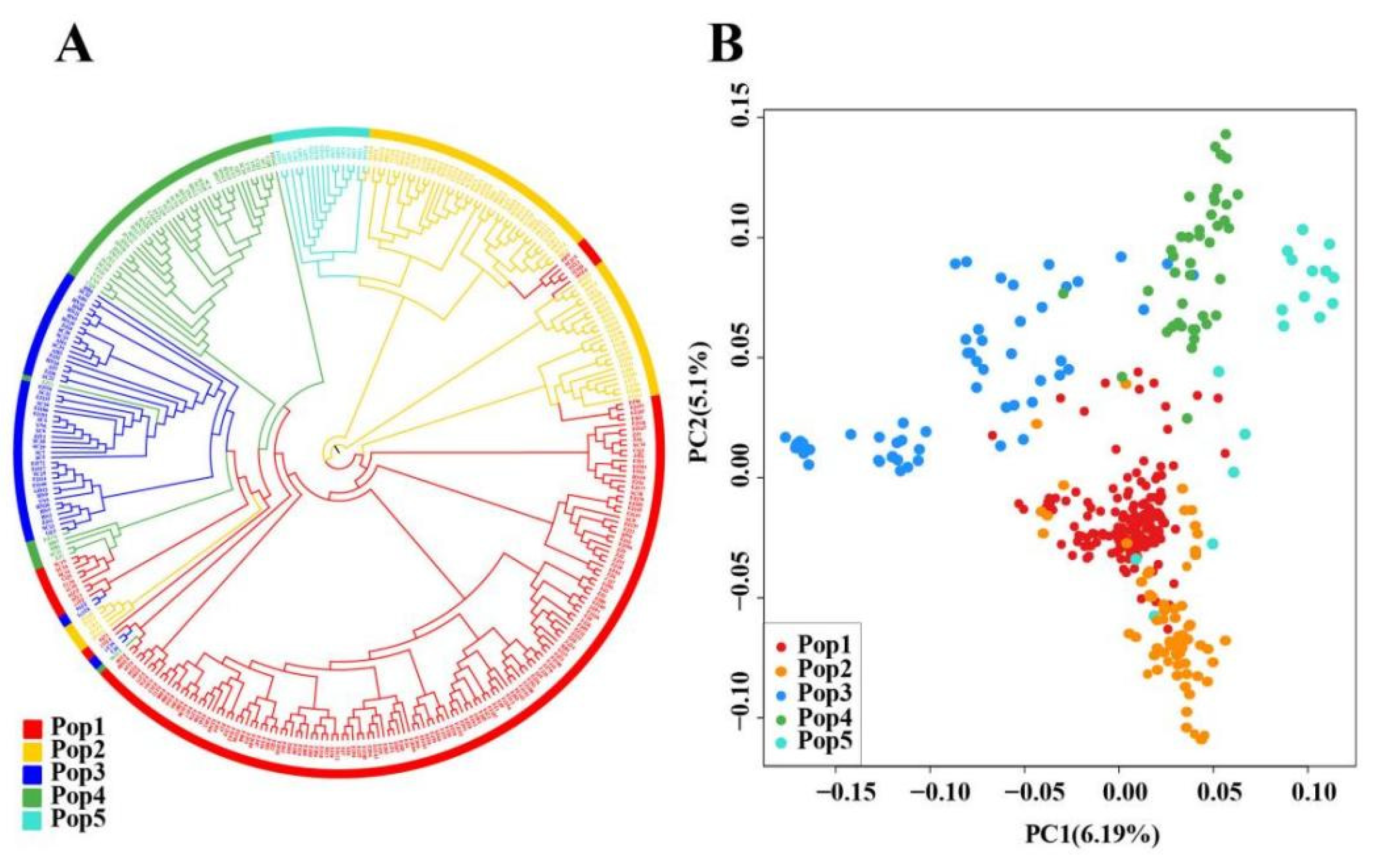
3.2. Population Structure Analysis
3.3. Core SNPs Set Exploration
3.4. GS and EDV Analysis
3.5. Core Varieties Analysis
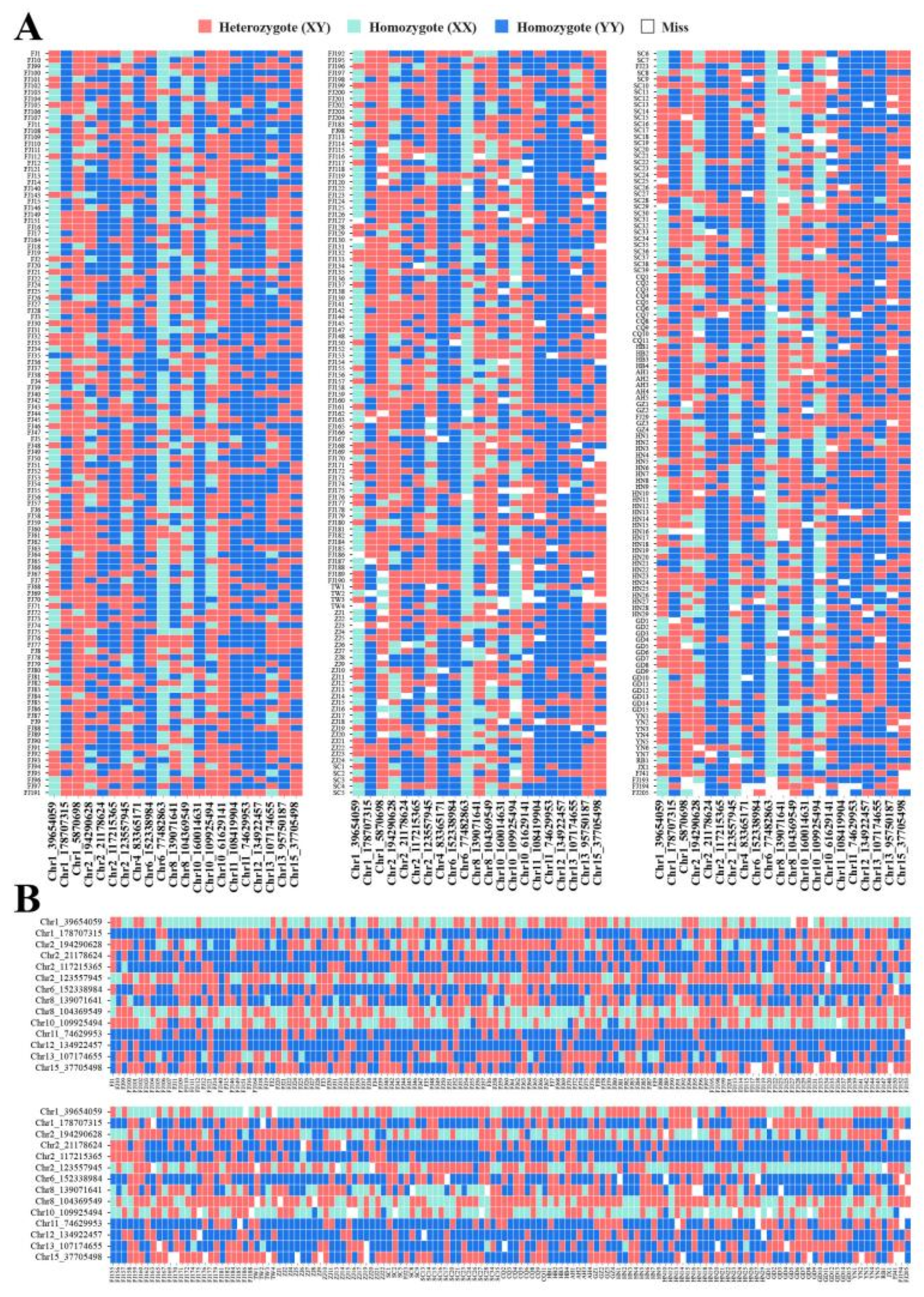
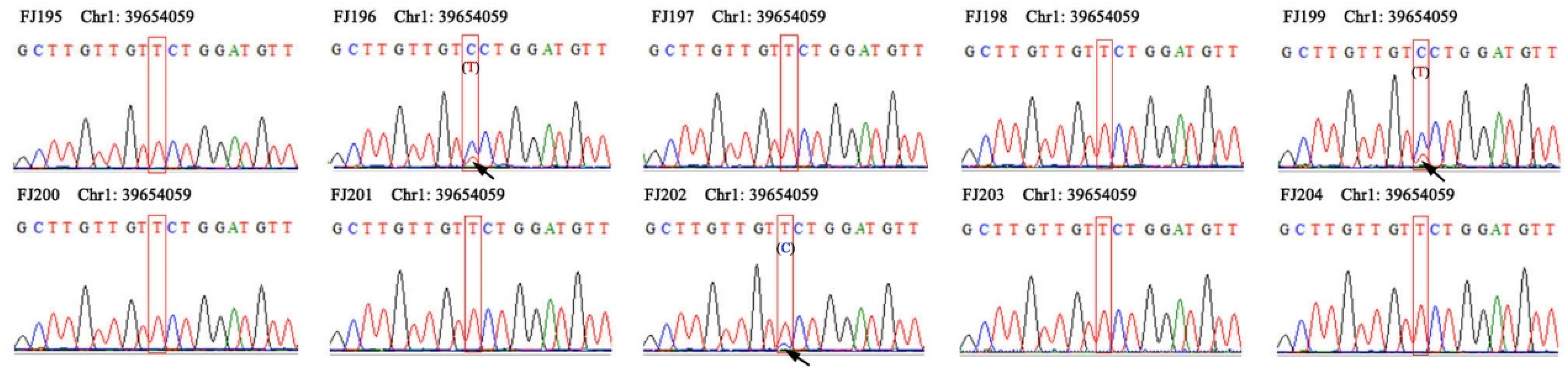
3.6. Screening of SNPs Markers for Rapid Identification
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bostyn, S.J.R. Plant Variety Rights Protection and Essentially Derived Varieties: A Fresh Proposal to Untie the Gordian Knot. GRUR Int. 2020, 69, 785–802. [Google Scholar] [CrossRef]
- Vosman, B. Essentially Derived Varieties in Ornamentals. Acta Hortic. 2009, 836, 161–167. [Google Scholar] [CrossRef]
- Noli, E.; Teriaca, M.S.; Conti, S. Identification of a threshold level to assess essential derivation in durum wheat. Mol. Breed. 2012, 29, 687–698. [Google Scholar] [CrossRef]
- Noli, E.; Teriaca, M.S.; Conti, S. Criteria for the definition of similarity thresholds for identifying essentially derived varieties. Plant Breed. 2013, 132, 525–531. [Google Scholar] [CrossRef]
- Yuan, X.; Li, Z.; Xiong, L.; Song, S.; Zheng, X.; Tang, Z.; Yuan, Z.; Li, L. Effective identification of varieties by nucleotide polymorphisms and its application for essentially derived variety identification in rice. BMC Bioinform. 2022, 23, 30. [Google Scholar] [CrossRef] [PubMed]
- Sanderson, J. Plants, People and Practices: The Nature and History of the UPOV Convention. Examining and Identifying Essentially Derived Varieties; Cambridge University Press: London, UK, 2017; pp. 205–230. [Google Scholar] [CrossRef]
- Gao, P.; Ma, H.; Luan, F.; Song, H. DNA Fingerprinting of Chinese Melon Provides Evidentiary Support of Seed Quality Appraisal. PLoS ONE 2012, 7, e52431. [Google Scholar] [CrossRef]
- Zhang, Z.; Feng, X.; Wang, Y.; Xu, W.; Huang, K.; Hu, M.; Zhang, C.; Yuan, H. Advances in research on functional genes of tea plant. Gene 2019, 711, 143940. [Google Scholar] [CrossRef]
- China’s New Seed Law: Molecular Varieties and Macro Developments. Available online: http://news.agropages.com/News/NewsDetail---42896 (accessed on 23 May 2022).
- Seed Law Revised to Strengthen Protection of Seed Industry Intellectual Property Rights. Available online: http://english.moa.gov.cn/datastatistics/202204/t20220408_300834.html (accessed on 9 April 2022).
- Huang, H.; Shi, C.; Liu, Y.; Mao, S.Y.; Gao, L.Z. Thirteen Camellia chloroplast genome sequences determined by high-throughput sequencing: Genome structure and phylogenetic relationships. BMC Evol. Biol. 2014, 14, 151. [Google Scholar] [CrossRef] [PubMed]
- Vosman, B.; Visser, D.; Voort, J.R.V.D.; Smulders, M.J.M.; Eeuwijk, F.V. The establishment of ‘essential derivation’ among rose varieties, using AFLP. Theor. Appl. Genet. 2004, 109, 1718–1725. [Google Scholar] [CrossRef]
- Raina, S.N.; Ahuja, P.S.; Sharma, R.K.; Das, S.C.; Bhardwaj, P.; Negi, R.; Sharma, V.; Singh, S.S.; Sud, R.K.; Kalia, R.K.; et al. Genetic structure and diversity of India hybrid tea. Genet. Resour. Crop Evol. 2012, 59, 1527–1541. [Google Scholar] [CrossRef]
- Jones, C.J.; Edwards, K.J.; Castaglione, S.; Winfield, M.O.; Sala, F.; van de Wiel, C.C.; Bredemeijer, G.M.; Vosman, B.; Matthes, M.C.; Daly, A.; et al. Reproducibility testing of RAPD, AFLP and SSR markers in plants by a network of European laboratories. Mol. Breed. 2004, 3, 381–390. [Google Scholar] [CrossRef]
- Heckenberger, M.; Bohn, M.; Ziegle, J.S.; Joe, L.K.; Hauser, J.D.; Hutton, M.; Melchinger, A.E. Variation of DNA fingerprints among accessions within maize inbred lines and implications for identification of essentially derived varieties. I. Genetic and technical sources of variation in SSR data. Mol. Breed. 2002, 10, 181–191. [Google Scholar] [CrossRef]
- Heckenberger, M.; Muminovic, J.; Van, J.R.; Peleman, J.; Bohn, M.; Melchinger, A.E. Identification of essentially derived varieties obtained from biparental crosses of homozygous lines. III. AFLP data from maize inbreds and comparison with SSR data. Mol. Breed. 2006, 17, 111–125. [Google Scholar] [CrossRef]
- Kahler, A.L.; Kahler, J.L.; Thompson, S.A.; Ferriss, R.S.; Jones, E.S.; Nelson, B.K.; Mikel, M.A.; Smith, S. North American Study on Essential Derivation in Maize: II. Selection and Evaluation of a Panel of Simple Sequence Repeat Loci. Crop Sci. 2010, 50, 486–503. [Google Scholar] [CrossRef]
- Nelson, B.K.; Kahler, A.L.; Kahler, J.L.; Mikel, M.A.; Thompson, S.A.; Ferriss, R.S.; Smith, S.; Jones, E.S. Evaluation of the numbers of single nucleotide polymorphisms required to measure genetic gain distance in maize (Zea mays L.). Crop Sci. 2011, 51, 1470–1480. [Google Scholar] [CrossRef]
- Rafalski, J.A. Genomic tools for the analysis of genetic diversity. Plant Genet. 2011, 9, 159–162. [Google Scholar] [CrossRef]
- Rousselle, Y.; Jones, E.; Charcosset, A.; Moreau, P.; Robbins, K.R.; Stich, B.; Knaak, C.; Flament, P.; Karaman, Z.; Martinant, J.P.; et al. Study on Essential Derivation in Maize: III. Selection and Evaluation of a Panel of Single Nucleotide Polymorphism Loci for Use in European and North American Germplasm. Crop Sci. 2017, 55, 1170–1180. [Google Scholar] [CrossRef]
- Reyes, V.P.; Kitony, J.K.; Nishiuchi, S.; Makihara, D.; Doi, K. Utilization of Genotyping-by-Sequencing (GBS) for Rice Pre-Breeding and Improvement: A Review. Life 2022, 12, 1752. [Google Scholar] [CrossRef]
- Writing Committee. Tea Plant Varieties in China; Shanghai Science and Technology Press: Shanghai, China, 2001; pp. 15–230. [Google Scholar]
- Yang, Y.; Liang, Y. Tea Plant Clonal Varieties in China. Shanghai Science and Technology Press: Shanghai, China, 2014; pp. 7–268. [Google Scholar]
- Wu, X.; Blair, M.W. Diversity in Grain Amaranths and Relatives Distinguished by Genotyping by Sequencing (GBS). Front. Plant Sci. 2017, 8, 1960. [Google Scholar] [CrossRef] [PubMed]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Li, H. Aligning sequence reads, clone sequences and assembly contigs with BWA-MEM. arXiv 2013, arXiv:1303.3997. [Google Scholar] [CrossRef]
- Xia, E.; Tong, W.; Hou, Y.; An, Y.; Chen, L.; Wu, Q.; Liu, Y.; Yu, J.; Li, F.; Li, R.; et al. The Reference Genome of Tea Plant and Resequencing of 81 Diverse Accessions Provide Insights into Its Genome Evolution and Adaptation. Mol. Plant 2020, 13, 1013–1026. [Google Scholar] [CrossRef]
- Li, H.; Handsaker, B.; Wysoker, A.; Fennell, T.; Ruan, J.; Homer, N. The Sequence Alignment/Map Format and SAMtools. Bioinformatics 2009, 25, 2078–2079. [Google Scholar] [CrossRef]
- DePristo, M.A.; Banks, E.; Poplin, R.; Garimella, K.V.; Maguire, J.R.; Hartl, C.; Philippakis, A.A.; del Angel, G.; Rivas, M.A.; Hanna, M.; et al. A framework for variation discovery and genotyping using next-generation DNA sequencing data. Nat. Genet. 2011, 43, 491–498. [Google Scholar] [CrossRef]
- Danecek, P.; Auton, A.; Abecasis, G.; Albers, C.A.; Banks, E.; DePristo, M.A.; Handsaker, R.E.; Lunter, G.; Marth, G.T.; Sherry, S.T.; et al. The variant call format and VCFtools. Bioinformatics 2011, 27, 2156–2158. [Google Scholar] [CrossRef]
- Liu, K.J.; Muse, S.V. PowerMarker: An integrated analysis environment for genetic marker analysis. Bioinformatics 2005, 21, 2128–2129. [Google Scholar] [CrossRef] [PubMed]
- Alexander, D.H.; Novembre, J.; Lange, K. Fast model-based estimation of ancestry in unrelated individuals. Genome Res. 2009, 19, 1655–1664. [Google Scholar] [CrossRef]
- Purcell, S.; Neale, B.; Todd-Brown, K.; Thomas, L.; Ferreira, M.A.; Bender, D.; Maller, J.; Sklar, P.; de Bakker, P.I.; Daly, M.J.; et al. PLINK: A tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. 2007, 81, 559–575. [Google Scholar] [CrossRef] [PubMed]
- Letunic, I.; Bork, P. Interactive tree of life (iTOL) v3: An online tool for the display and annotation of phylogenetic and other trees. Nucleic Acids Res. 2016, 44, W242–W245. [Google Scholar] [CrossRef] [PubMed]
- Rohlf, F.J. NTSYS-pc: Numerical Taxonomy and Multivariate Analysis System Version 2.1; Applied Biostatistics Inc.: New York, NY, USA, 1997. [Google Scholar]
- Reif, J.C.; Melchinger, A.E.; Frisch, M. Genetical and mathematical properties of similarity and dissimilarity coefficients applied in plant breeding and seed bank management. Crop Sci. 2005, 45, 1–7. [Google Scholar] [CrossRef]
- ISF Guidelines for the Handling of a Dispute on Essential Derivation of Maize Lines. Available online: http://worldseed.org. (accessed on 24 January 2023).
- Wang, J.C.; Hu, J.; Xu, H.M.; Zhang, S. A strategy on constructing core collections by least distance stepwise sampling. Theor. Appl. Genet. 2007, 115, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhang, J.; Han, R.; Zhang, F.; Mao, A.; Luo, J.; Dong, B.; Liu, H.; Tang, H.; Zhang, J.; et al. Target SSR-Seq: A Novel SSR Genotyping Technology Associate With Perfect SSRs in Genetic Analysis of Cucumber Varieties. Front. Plant Sci. 2019, 10, 531. [Google Scholar] [CrossRef]
- Liu, W.; Qian, Z.; Zhang, J.; Yang, J.; Wu, M.; Barchi, L.; Zhao, H.; Sun, H.; Cui, Y.; Wen, C. Impact of fruit shape selection on genetic structure and diversity uncovered from genome-wide perfect SNPs genotyping in eggplant. Mol. Breed. 2019, 39, 140. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, Y.; Qiu, H.; Guo, Y.; Wan, H.; Zhang, X.; Scossa, F.; Alseekh, S.; Zhang, Q.; Wang, P.; et al. Genome assembly of wild tea tree DASZ reveals pedigree and selection history of tea varieties. Nat. Commun. 2020, 11, 3719. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.; Jones, E.; Nelson, B.; Phillips, D.; Wineland, R. Genomics of Plant Genetic Resources: Genomic Approaches and Intellectual Property Protection for Variety Release: A Perspective from the Private Sector; Springer: Dordrecht, The Netherlands, 2014; pp. 27–47. [Google Scholar] [CrossRef]
- Chen, L.; Yang, Y.; Yu, F. Genetic diversity, relationship and molecular discrimination of elite tea germplasms [Camellia sinensis (L.), O. Kuntze] revealed by RAPD markers. Mol. Plant Breed. 2004, 2, 385–390. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, B.; Zhang, J.; Zhang, J.N.; Li, J.S. Enhancement of plant variety protection and regulation using molecular marker technology. Acta Agron. Sin. 2022, 48, 1853–1870. [Google Scholar]
- Debener, T.; Janakiram, T.; Mattiesch, L. Sports and seedlings of rose varieties analyzed with molecular markers. Plant Breed. 2010, 119, 71–74. [Google Scholar] [CrossRef]
- Law, J.; Eeuwijk, F.A. Statistical aspects of essential derivation, with illustrations based on lettuce and barley. Euphytica 2003, 137, 129–137. [Google Scholar] [CrossRef]
- Yu, X.; Xiao, J.; Chen, S.; Yu, Y.; Ma, J.; Lin, Y.; Li, R.; Lin, J.; Fu, Z.; Zhou, Q.; et al. Metabolite signatures of diverse Camellia sinensis tea populations. Nat. Commun. 2020, 11, 5586. [Google Scholar] [CrossRef]
- Paneto, G.G.; de Paula Careta, F. Designing primers for SNaPshot technique. Methods Mol. Biol. 2015, 1275, 165–172. [Google Scholar] [CrossRef]


| No. | IV | EDV | GS |
|---|---|---|---|
| Sample ID, Name (Grade, Time) | Sample ID, Name (Grade, Time) | ||
| 1 | FJ115, Fudingdahaocha (National, 1984) | FJ166, Fuyun595 (Provincial, 1988) | 0.9744 * |
| 2 | FJ193, Jiulongdabaicha (Provincial, 1998) | 0.9805 * | |
| 3 | SC11, Shuke no.1 (Provincial, 2015) | 0.9805 * | |
| 4 | SC30, Gulinniupicha (Provincial, 1985) | 0.9826 * | |
| 5 | SC6, Mengshan no.9 (Provincial, 1989) | 0.9815 * | |
| 6 | SC7, Mengshan no.11 (Provincial, 1989) | 0.9774 * | |
| 7 | SC12, Tezao213 (Provincial, 2003) | SC10, Chuannonghuangyazao (Provincial, 2009) | 0.9703 * |
| 8 | CQ1, Bayutezao (National, 2014) | 0.9774 * | |
| 9 | SC14, Chuanmu28 (Provincial, 2010) | 0.9774 * | |
| 10 | SC19, Huaqiu no.1 (National, 2014) | 0.9754 * | |
| 11 | AH5, Anhui no.3 (National, 1987) | SC39, Mengshan no.23 (Provincial, 1989) | 0.9897 * |
| 12 | SC33, Mingshanbaihao131(Provincial, 1997) | 0.9887 * | |
| 13 | FJ178, Dayewulong (National, 1985) | FJ126, Jinmudan (National, 2010) | 0.9344 |
| 14 | FJ114, Fuyun no.6 (National, 1987) | SC25, Tianfuhong no.1 (Provincial, 2016) | 0.9805 * |
| 15 | ZJ6, Biyun (National, 1987) | CQ7, Yucha no.2 (Provincial, 2001) | 0.9190 |
| 16 | CQ4, Shuyong no.2 (National, 1987) | CQ10, Shuyong703(National, 1994) | 0.9374 |
| 17 | CQ8, Shuyong no.3 (National, 1994) | SC3, Tianfucha no.28 (National, 2014) | 0.9887 * |
| 18 | FJ113, Zimudan (National, 2010) | SC31, Ziye (Provincial, 2018) | 0.9928 * |
| 19 | ZJ18, Zhenong902 (National, 2002) | ZJ15, Zhenong901 (National, 2020) | 0.9815 * |
| 20 | HN11, Jianbohuang (Provincial, 1987) | HN5, Baihaozao (National, 1994) | 0.9949 * |
| 21 | HN10, Yusun (Provincial, 1997) | HN8, Yulv (National, 2010) | 0.9867 * |
| 22 | ZJ3, Yingshuang (National, 1987) | HN24, Jianghuakucha (Provincial, 1987) | 0.9877 * |
| Pop | Num (All) | Num (Core) | Sample Name (Sample ID) |
|---|---|---|---|
| Pop1 | 159 | 32 | Qizhong-JPC (FJ205); Qidan (FJ192); 1105 (FJ198); Bantianyao (FJ50); Baisuixiang (FJ195); Beidou2 (FJ196); Jiulongqi (FJ201); Jinyaoshi2 (FJ199); Zuishuixian (FJ55); Huangjinya (ZJ5); Biyun (ZJ6); 0206 (FJ19); Dahongmei (FJ104); Bujiantian (FJ45); Laojunmei (FJ88); Zhengyulan (FJ59); Zhengliutiao (FJ100); Zhongcha108 (ZJ1); Yanzhiliu (FJ76); Xiannvsanhua (FJ39); 0207 (FJ20); Yucha no.2 (CQ7); Guilindan (FJ28); Zhenong12 (ZJ19); Liuxiangjianbuzhichun (FJ42); Shiru (FJ112); Xiaoyugui (FJ97); Tieluohan (FJ85); Baojinghuangjincha no.1 (HN6); Xiangtianmei (FJ67); Shanzhizi (FJ83); Luohanqian1 (FJ101) |
| Pop2 | 72 | 14 | 306 (FJ194); Zhengtaiyang2 (FJ202); Ziluolan2 (FJ203); Ruixiang (FJ122); Tieguanyin (FJ185); Chungui (FJ145); Zaoguanyin (FJ175); 0306C (FJ158); Dayewulong (FJ178); Baimaohou (FJ149); 0206-A (FJ134); Benshan (FJ184); Huangdan (FJ120); Rougui (FJ84) |
| Pop3 | 51 | 10 | Fudingdahaocha (FJ115); Fudingdabaicha (FJ116); Mabianlv (SC5); Anhui no.3 (AH5); Mengshan no.23 (SC39); Baihaozao (HN5); yingshuang (ZJ3); Bixiangzao (HN7); Zhengbaihao (FJ31); Zhenong117 (ZJ20) |
| Pop4 | 50 | 10 | Jianhexiangcha (SC18); Zijuan (YN7); Qianmei809 (GZ1); Dayelong (JX1); Qigaixian (FJ176); Xintianwandacha (HN15); Shuyong401 (CQ2); Shuyong no.3 (CQ8); Shuke no.36 (SC23); Bashanzao (SC26) |
| Pop5 | 17 | 4 | Zhilanxiang (GD1); Chengmen (GD6); Laoxianweng (GD3); Wuyedangcong (GD14) |
| Total | 349 | 70 |
| No | Name | SNP | Primer | Tm | Len | Site | PIC | Ho | GD |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Chr1_5870698 | G/T | F: AACACCGAGACGCTTTGGAT | 58 | 385 | 279 | 0.44 | 0.83 | 0.54 |
| R: CCTCGCACCTCACACTTAGG | |||||||||
| 2 | Chr1_39654059 | C/T | F: GTTGGAACCGATGCTAAT | 52 | 426 | 78 | 0.32 | 0.43 | 0.37 |
| R: GCATTCAAAGCCAGAGTAA | |||||||||
| 3 | Chr1_178707315 | C/T | F: TAGAAGTTCCGCAGCACG | 52 | 369 | 157 | 0.28 | 0.36 | 0.32 |
| R: AGGTTCATGTACCACCAT | |||||||||
| 4 | Chr2_21178624 | C/T | F: GCGTTTGTGCAAGGCTGTTA | 58 | 382 | 312 | 0.27 | 0.36 | 0.31 |
| R: AGATGATGCCCTGCTAGCTC | |||||||||
| 5 | Chr2_117215365 | C/T | F: ACGAAAGGCCGTTTCTGGAT | 58 | 395 | 234 | 0.23 | 0.28 | 0.26 |
| R: ACTAGTTTGCCACCCCATCG | |||||||||
| 6 | Chr2_123557945 | G/T | F: TCCCTCATCCCTCCTCAAGG | 60 | 297 | 59 | 0.38 | 0.65 | 0.47 |
| R: CGACACAAATGGAGTCCGA | |||||||||
| 7 | Chr2_194290628 | A/C | F: CCCATAGGACCGGACATCA | 60 | 404 | 169 | 0.39 | 0.5 | 0.49 |
| R: AGCTTGGTTAGGGTCTTCGC | |||||||||
| 8 | Chr4_83365171 | A/G | F: GGCTTAGTTAATGGTGAT | 50 | 443 | 327 | 0.23 | 0.24 | 0.26 |
| R: GGAAGGTATGGGTTGTAT | |||||||||
| 9 | Chr6_77482863 | A/G | F: TTTCCTCGTTTTGGTTAG | 50 | 350 | 197 | 0.17 | 0.12 | 0.18 |
| R: ATATTTCGGCAAGGTTTA | |||||||||
| 10 | Chr6_152338984 | C/T | F: AGCGTTGAAGCAGCATTTGG | 60 | 510 | 286 | 0.26 | 0.31 | 0.29 |
| R: TTGTCCCAGTTGCAACAGGT | |||||||||
| 11 | Chr8_104369549 | A/G | F: GCAAGCTCTATGTGCCTTGC | 58 | 490 | 140 | 0.36 | 0.59 | 0.44 |
| R: GCCTTGTTGTGAAGCGAAG | |||||||||
| 12 | Chr8_139071641 | C/T | F: AGCTCCGATATCCCTTGGGT | 60 | 511 | 142 | 0.39 | 0.44 | 0.5 |
| R: GAGTTAAGGACCCTGTGCC | |||||||||
| 13 | Chr10_61629141 | C/T | F: GTCGGGTCATCATCCGGATC | 60 | 528 | 242 | 0.43 | 0.72 | 0.52 |
| R: GCAAGTGGCTTTCAGTCAGC | |||||||||
| 14 | Chr10_109925494 | A/G | F: AGTGAGCTGGCACAAGTGTT | 60 | 315 | 216 | 0.4 | 0.44 | 0.47 |
| R: AGCCCACTTTAGCACCATCC | |||||||||
| 15 | Chr10_160014631 | C/G | F: TCCTGTTGAGTTGGGTAG | 50 | 445 | 141 | 0.39 | 0.32 | 0.46 |
| R: ATCGTCCTTGGAATACTT | |||||||||
| 16 | Chr11_74629953 | C/T | F: GCGAGCAATGTTTCCACGTT | 60 | 396 | 339 | 0.21 | 0.23 | 0.23 |
| R: CGCACAAGCCTATTGCCTTG | |||||||||
| 17 | Chr11_108419904 | C/T | F: CCAACTTGTTAGCCCCAAGG | 60 | 464 | 192 | 0.27 | 0.24 | 0.29 |
| R: CTGTGGCAGGTCGACATCTT | |||||||||
| 18 | Chr12_134922457 | A/G | F: AGCAGGAGCAGACACCTTT | 58 | 513 | 216 | 0.19 | 0.22 | 0.21 |
| R: GAATTGGCACATGCTGCTCC | |||||||||
| 19 | Chr13_95750187 | C/T | F: CTACCACCCCTAAGAGGCCT | 58 | 452 | 80 | 0.4 | 0.63 | 0.49 |
| R: TTCTGCATCGCCTCGATACC | |||||||||
| 20 | Chr13_107174655 | A/G | F: ATTTGAAGAATGCCGGGGC | 58 | 323 | 140 | 0.35 | 0.38 | 0.42 |
| R: CCTCGCATCTCCTTTTCGGT | |||||||||
| 21 | Chr15_37705498 | A/C | F: TGGGTATGGCTGCAAGATGG | 60 | 537 | 131 | 0.41 | 0.55 | 0.48 |
| R: CCCAAACAAACACCCCCAT | |||||||||
| Average value | 0.32 | 0.42 | 0.38 | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, L.; Li, X.; Liu, F.; Zhao, J.; Zhang, Y.; Zheng, W.; Fan, L. Preliminary Investigation of Essentially Derived Variety of Tea Tree and Development of SNP Markers. Plants 2023, 12, 1643. https://doi.org/10.3390/plants12081643
Li L, Li X, Liu F, Zhao J, Zhang Y, Zheng W, Fan L. Preliminary Investigation of Essentially Derived Variety of Tea Tree and Development of SNP Markers. Plants. 2023; 12(8):1643. https://doi.org/10.3390/plants12081643
Chicago/Turabian StyleLi, Li, Xiangru Li, Fei Liu, Jialin Zhao, Yan Zhang, Weiming Zheng, and Li Fan. 2023. "Preliminary Investigation of Essentially Derived Variety of Tea Tree and Development of SNP Markers" Plants 12, no. 8: 1643. https://doi.org/10.3390/plants12081643
APA StyleLi, L., Li, X., Liu, F., Zhao, J., Zhang, Y., Zheng, W., & Fan, L. (2023). Preliminary Investigation of Essentially Derived Variety of Tea Tree and Development of SNP Markers. Plants, 12(8), 1643. https://doi.org/10.3390/plants12081643






