Organic Amendments Improved the Productivity and Bio-Fortification of Fine Rice by Improving Physiological Responses and Nutrient Homeostasis under Salinity Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Details
2.2. Measurement of Morphological Traits
2.3. Determination of Relative Water Contents, Electrolyte Leakage, and Photosynthetic Pigments
2.4. Determination of Potential Osmolytes, Oxidative Stress Markers and Antioxidant Activities
2.5. Determination of Yield and Quality Traits
2.6. Statistical Analysis
3. Results
3.1. Organic Amendments Improve Rice Growth under SS
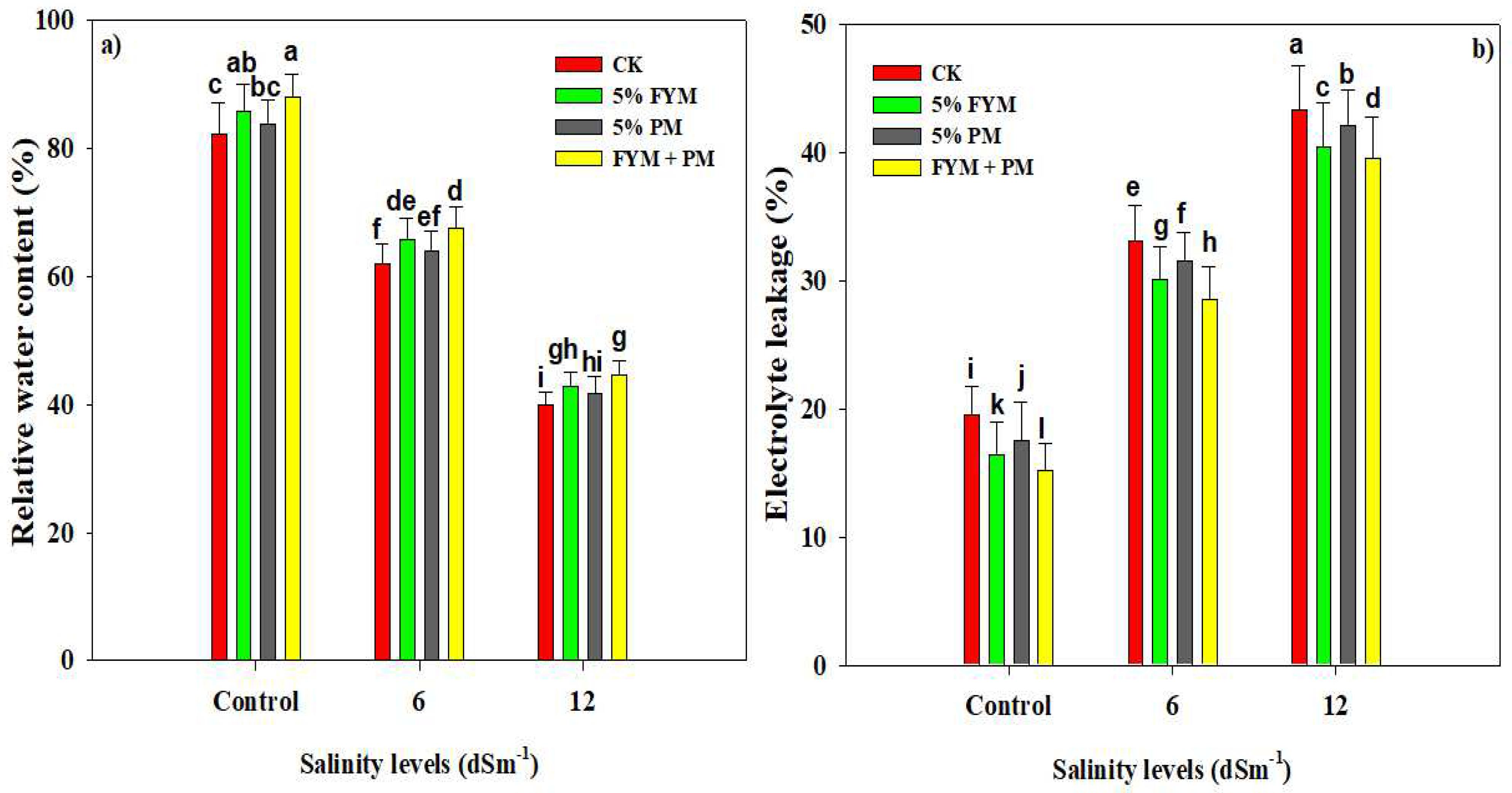
3.2. Organic Amendments Improve Photosynthetic Pigments and RWC and Reduce EL under SS
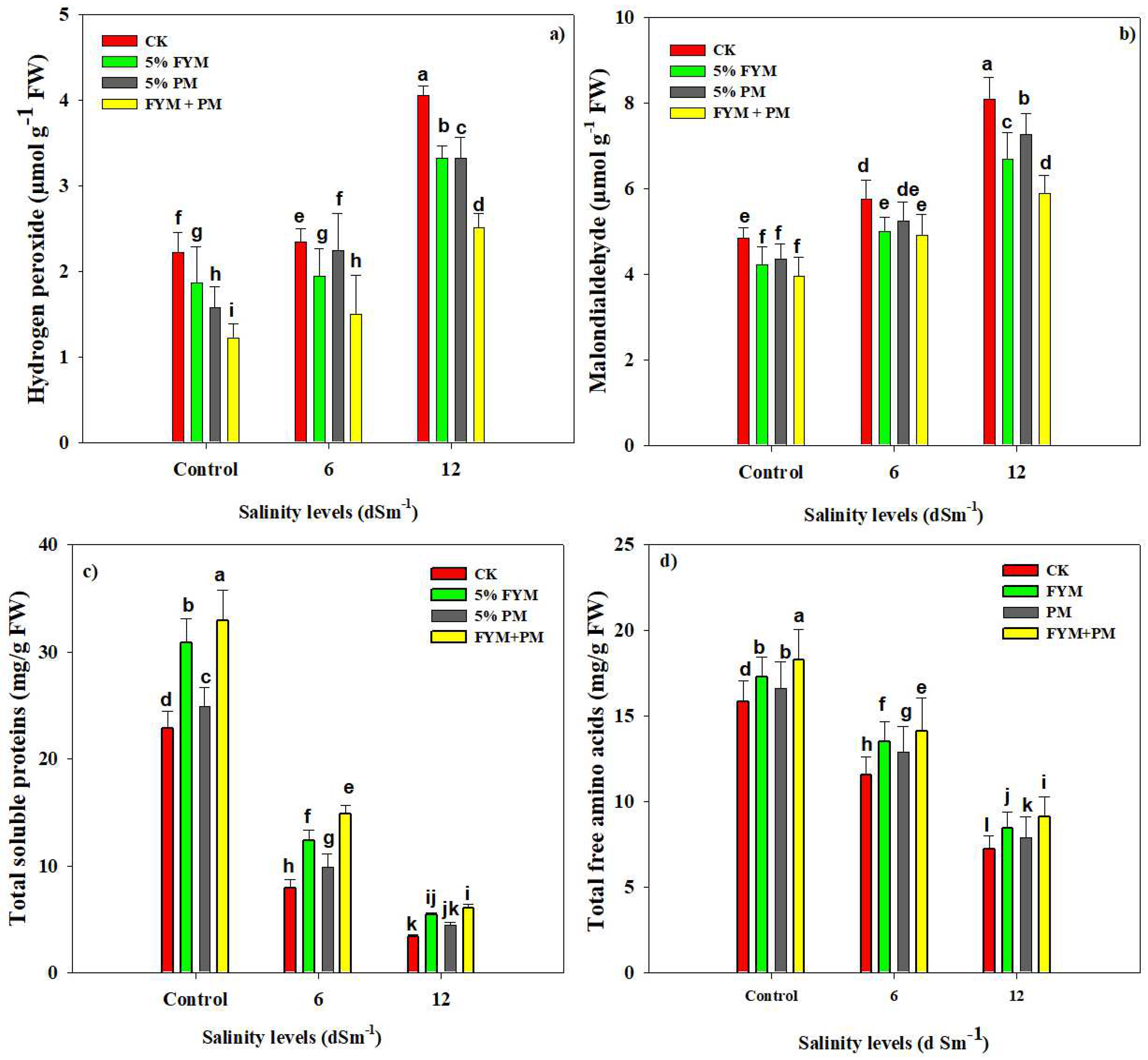
3.3. Organic Amendments Maintain a Lower H2O2 and MDA Accumulation and Increase Accumulation of TSP and FAA in Rice under SS
3.4. Organic Amendments Improve Antioxidant Activities in Rice under SS
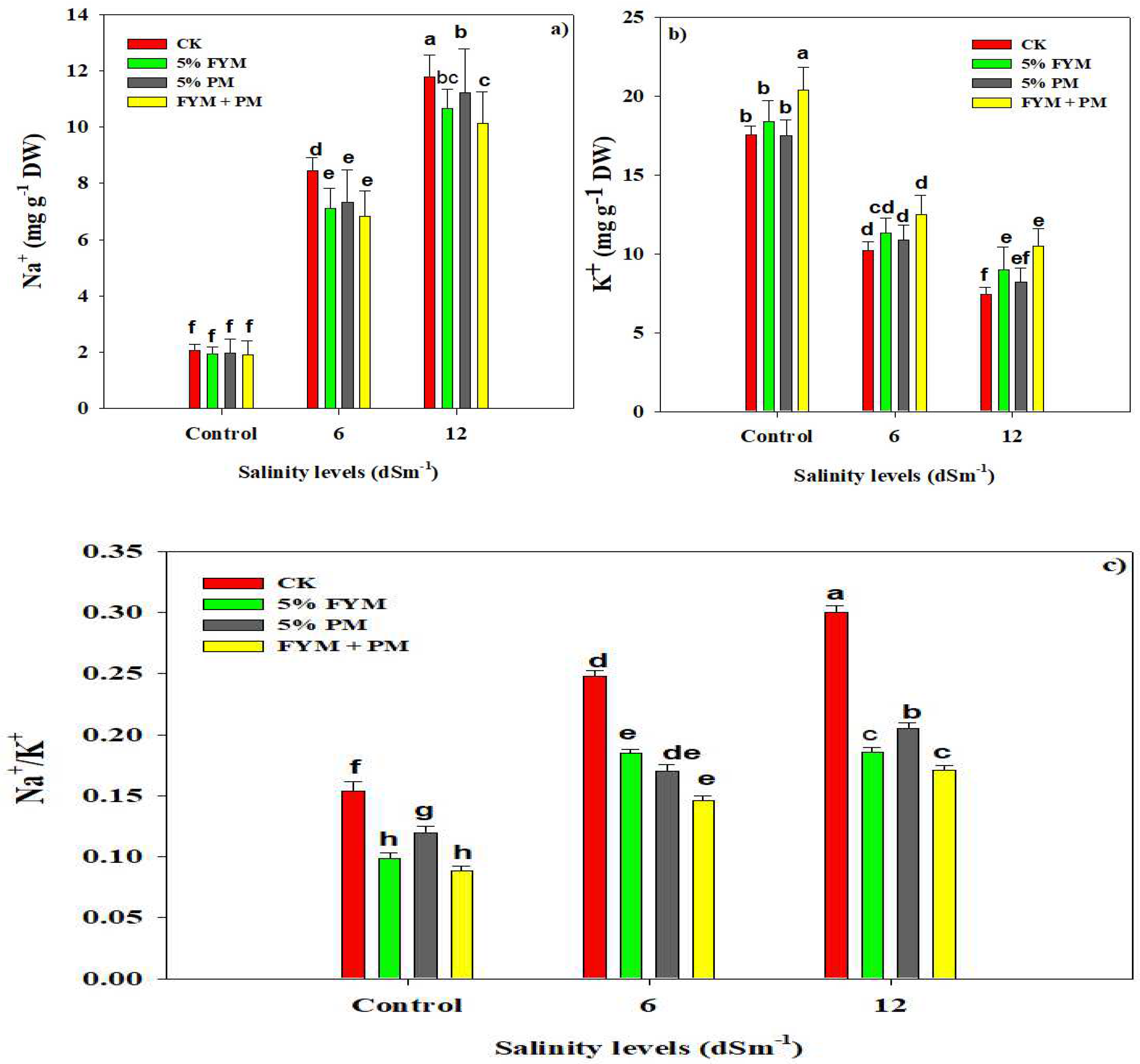
3.5. Organic Amendments Maintain Ionic Homeostasis under SS
3.6. Organic Amendments Increased the Yield of Rice under SS
3.7. Organic Amendments Increase Grain Bio-Fortification under SS
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Al-Zahrani, H.S.; Nahar, K.; Alharby, H.F.; Alsamadany, H.; Hakeem, K.R.; Hasanuzzaman, M. Zinc supplementation enhances glutathione-mediated antioxidant defense and glyoxalase systems to conferring salt tolerance in soybean (Glycine max L.). Agronomy 2022, 12, 1032. [Google Scholar] [CrossRef]
- Otlewska, A.; Migliore, M.; Dybka-Stępień, K.; Manfredini, A.; Struszczyk-Świta, K.; Napoli, R. When salt meddles between plant, soil, and microorganisms. Front. Plant Sci. 2020, 11, 1429. [Google Scholar] [CrossRef] [PubMed]
- Etesami, H.; Maheshwari, D.K. Use of plant growth promoting rhizobacteria (PGPRs) with multiple plant growth promoting traits in stress agriculture: Action mechanisms and future prospects. Ecotoxicol. Environ. Saf. 2018, 156, 225–246. [Google Scholar] [CrossRef] [PubMed]
- Verma, H.; Kumar, D.; Kumar, V.; Kumari, M.; Singh, S.K.; Sharma, V.K. The potential application of endophytes in management of stress from drought and salinity in crop plants. Microorganisms 2021, 9, 1729. [Google Scholar] [CrossRef] [PubMed]
- Yusnawan, E.; Taufiq, A.; Wijanarko, A.; Susilowati, D.N.; Praptana, R.H.; Chandra-Hioe, M.V. Changes in volatile organic compounds from salt-tolerant trichoderma and the biochemical response and growth performance in saline-stressed groundnut. Sustainability 2021, 13, 13226. [Google Scholar] [CrossRef]
- Bouras, H.; Choukr-Allah, R.; Mosseddaq, F.; Bouaziz, A.; Devkota, K.P.; Mouttaqi, A.E. Does phosphorus fertilization increase biomass production and salinity tolerance of blue panicum (Panicum antidotale Retz.) in the Salt-affected soils of arid regions? Agronomy 2022, 12, 791. [Google Scholar] [CrossRef]
- Contreras-Cornejo, H.A.; Macías-Rodríguez, L.; Alfaro-Cuevas, R.; López-Bucio, J. Trichoderma spp. improve growth of Arabidopsis seedlings under salt stress through enhanced root development, osmolite production, and Na+ elimination through root exudates. Mol. Plant-Microbe Interact. 2014, 27, 503–514. [Google Scholar] [CrossRef]
- Székely, Á.; Szalóki, T.; Pauk, J.; Lantos, C.; Ibadzade, M.; Jancsó, M. Salinity tolerance characteristics of marginally located rice varieties in the northernmost rice-growing area in Europe. Agronomy 2022, 12, 652. [Google Scholar] [CrossRef]
- Dustgeer, Z.; Seleiman, M.F.; Imran, K.; Chattha, M.U.; Alhammad, B.A.; Jalal, R.S. Glycine-betaine induced salinity tolerance in maize by regulating the physiological attributes, antioxidant defense system and ionic homeostasis. Not. Bot. Horti Agrobot. Cluj-Napoca 2021, 49, 12248. [Google Scholar] [CrossRef]
- Rahnama, A.; James, R.A.; Poustini, K.; Munns, R. Stomatal conductance as a screen for osmotic stress tolerance in durum wheat growing in saline soil. Funct. Plant Biol. 2010, 37, 255–263. [Google Scholar] [CrossRef]
- Sultan, I.; Khan, I.; Chattha, M.U.; Hassan, M.U.; Barbanti, L.; Calone, R. Improved salinity tolance in early growth stage of maize through salicylic acid foliar application. Ital. J. Agron. 2021, 16, 1810. [Google Scholar]
- Kumar, M.; Tak, Y.; Potkule, J.; Choyal, P.; Tomar, M.; Meena, N.L. Phenolics as plant protective companion against abiotic stress. In Plant Phenolics in Sustainable Agriculture; Springer: Berlin/Heidelberg, Germany, 2020; pp. 277–308. [Google Scholar]
- Seleiman, M.F.; Aslam, M.T.; Alhammad, B.A.; Hassan, M.U.; Maqbool, R.; Chattha, M.U. Salinity stress in wheat: Effects, mechanisms and management strategies. Phyton 2022, 91, 667. [Google Scholar] [CrossRef]
- Zhang, S.; Song, J.; Wang, H.; Feng, G. Effect of salinity on seed germination, ion content and photosynthesis of cotyledons in halophytes or xerophyte growing in Central Asia. J. Plant Ecol. 2010, 3, 259–267. [Google Scholar] [CrossRef]
- Najjar, A.A.; Kuhn, A.J.; Al-Tardeh, S.M.; Kuchendorf, C.M. Microalgae and biochar agro-fertilization of the palestinian rehan barley cultivar under salinity stress. Agronomy 2021, 11, 2309. [Google Scholar] [CrossRef]
- Chahal, S.S.; Choudhary, O.P.; Mavi, M.S. Organic amendments decomposability influences microbial activity in saline soils. Arch. Agron. Soil Sci. 2017, 63, 1875–1888. [Google Scholar] [CrossRef]
- Leogrande, R.; Vitti, C. Use of organic amendments to reclaim saline and sodic soils: A review. Arid Land Res. Manag. 2019, 33, 1–21. [Google Scholar] [CrossRef]
- Wichern, F.; Islam, M.; Hemkemeyer, M.; Watson, C.; Joergensen, R.G. Organic amendments alleviate salinity effects on soil microorganisms and mineralisation processes in aerobic and anaerobic paddy rice soils. Front. Sustain. Food Syst. 2020, 4, 30. [Google Scholar] [CrossRef]
- Khan, I.; Muhammad, A.; Chattha, M.U.; Skalicky, M.; Bilal, C.M.; Ahsin, A.M. Mitigation of salinity induced oxidative damage, growth and yield reduction in fine rice by sugarcane press-mud application. Front. Plant Sci. 2022, 13, 865. [Google Scholar] [CrossRef]
- Li, S.; Liu, R.; Wang, M.; Wang, X.; Shan, H.; Wang, H. Phytoavailability of cadmium to cherry-red radish in soils applied composted chicken or pig manure. Geoderma 2006, 136, 260–271. [Google Scholar] [CrossRef]
- Park, J.; Cho, K.H.; Ligaray, M.; Choi, M.J. Organic matter composition of manure and its potential impact on plant growth. Sustainability 2019, 11, 2346. [Google Scholar] [CrossRef]
- Ahmad, P.; Kumar, A.; Ashraf, M.; Akram, N.A. Salt-induced changes in photosynthetic activity and oxidative defense system of three cultivars of mustard (Brassica juncea L.). Afr. J. Biotechnol. 2012, 11, 2694–2703. [Google Scholar]
- Aini, N.; Dwi Yamika, W.S.; Aini, L.Q.; Kurniawan, A.P. Application of saline tolerant bacteria and soil ameliorants improved growth, yield and nutrient uptake of tomato in saline land. Aust. J. Crop Sci. 2021, 15, 827–834. [Google Scholar] [CrossRef]
- Daur, I.; Tatar, Ö. Effects of gypsum and brassinolide on soil properties, and berseem (Trifolium alexandrinum L.) growth, yield and chemical composition grown on saline soil. Legume Res.-Int. J. 2013, 36, 306–311. [Google Scholar]
- Buttar, G.; Thind, H.; Sekhon, K.; Kaur, A.; Gill, R.; Sidhu, B. Management of saline-sodic water in cotton-wheat cropping system. J. Agric. Sci. Technol. 2017, 19, 465–474. [Google Scholar]
- Chattha, M.U.; Hassan, M.U.; Barbanti, L.; Chattha, M.B.; Khan, I.; Usman, M. Composted sugarcane by-product press mud cake supports wheat growth and improves soil properties. Int. J. Plant Prod. 2019, 13, 241–249. [Google Scholar] [CrossRef]
- Chattha, M.U.; Hassan, M.U.; Khan, I.; Chattha, M.B.; Mahmood, A.; Nawaz, M. Biofortification of wheat cultivars to combat zinc deficiency. Front. Plant Sci. 2017, 8, 281. [Google Scholar] [CrossRef]
- Kumar, S.; Meena, R.; Jinger, D.; Jatav, H.S.; Banjara, T. Use of pressmud compost for improving crop productivity and soil health. Int. J. Chem. Stud. 2017, 5, 384–389. [Google Scholar]
- Nawaz, M.; Chattha, M.; Chattha, M.; Ahmad, R.; Munir, H.; Usman, M. Assessment of compost as nutrient supplement for spring planted sugarcane (Saccharum officinarum L.). J. Anim. Plant Sci. 2017, 27, 283–293. [Google Scholar]
- Imran, M.; Ashraf, M.; Awan, A.R. Growth, yield and arsenic accumulation by wheat grown in a pressmud amended salt-affected soil irrigated with arsenic contaminated water. Ecotoxicol. Environ. Saf. 2021, 224, 112692. [Google Scholar] [CrossRef]
- Sheoran, P.; Kumar, A.; Singh, A.; Kumar, A.; Parjapat, K.; Sharma, R. Pressmud alleviates soil sodicity stress in a rice–wheat rotation: Effects on soil properties, physiological adaptation and yield-related traits. Land Degrad. Dev. 2021, 32, 2735–2748. [Google Scholar] [CrossRef]
- Muhammad, D.; Khattak, R. Growth and nutrient concentrations of maize in pressmud treated saline-sodic soils. Soil Environ. 2009, 128, 145–155. [Google Scholar]
- Rasheed, A.; Fahad, S.; Aamer, M.; Hassan, M.; Tahir, M.; Wu, Z. Role of genetic factors in regulating cadmium uptake, transport and accumulation mechanisms and quantitative trait loci mapping in rice: A review. Appl. Ecol. Environ. Res. 2020, 18, 4005–4023. [Google Scholar] [CrossRef]
- Rasheed, A.; Gill, R.A.; Hassan, M.U.; Mahmood, A.; Qari, S.; Zaman, Q.U. A critical review: Recent advancements in the use of CRISPR/Cas9 technology to enhance crops and alleviate global food crises. Curr. Issues Mol. Biol. 2021, 43, 1950–1976. [Google Scholar] [CrossRef] [PubMed]
- Rasheed, A.; Hassan, M.U.; Aamer, M.; Batool, M.; Sheng, F.; Ziming, W. A critical review on the improvement of drought stress tolerance in rice (Oryza sativa L.). Not. Bot. Horti Agrobot. Cluj-Napoca 2020, 48, 1756–1788. [Google Scholar] [CrossRef]
- Rasheed, A.; Seleiman, M.F.; Nawaz, M.; Mahmood, A.; Anwar, M.R.; Ayub, M.A. Agronomic and genetic approaches for enhancing tolerance to heat stress in rice: A review. Not. Bot. Horti Agrobot. Cluj-Napoca 2021, 49, 12501. [Google Scholar] [CrossRef]
- Rasheed, A.; Wassan, G.M.; Khanzada, H.; Solangi, A.M.; Han, R.; Li, H. Identification of genomic regions at seedling related traits in response to aluminium toxicity using a new high-density genetic map in rice (Oryza sativa L.). Genet. Resour. Crop Evol. 2021, 68, 1889–1903. [Google Scholar] [CrossRef]
- Yan, G.; Fan, X.; Peng, M.; Yin, C.; Xiao, Z.; Liang, Y. Silicon improves rice salinity resistance by alleviating ionic toxicity and osmotic constraint in an organ-specific pattern. Front. Plant Sci. 2020, 11, 260. [Google Scholar] [CrossRef]
- Hussain, M.; Ahmad, S.; Hussain, S.; Lal, R.; Ul-Allah, S.; Nawaz, A. Rice in saline soils: Physiology, biochemistry, genetics, and management. Adv. Agron. 2018, 148, 231–287. [Google Scholar]
- Hu, Q.; Fu, Y.; Guan, Y.; Lin, C.; Cao, D.; Hu, W.; Sheteiwy, M.; Hu, J. Inhibitory effect of chemical combinations on seed germination and pre-harvest sprouting in hybrid rice. Plant Growth Regul. 2016, 80, 281–289. [Google Scholar] [CrossRef]
- Zhu, J.K. Abiotic stress signaling and responses in plants. Cell 2016, 167, 313–324. [Google Scholar] [CrossRef]
- Sripinyowanich, S.; Klomsakul, P.; Boonburapong, B.; Bangyeekhun, T.; Asami, T.; Gu, H.; Buaboocha, T.; Chadchawan, S. Exogenous ABA induces salt tolerance in indica rice (Oryza sativa L.): The role of OsP5CS1 and OsP5CR gene expression during salt stress. Environ. Exp. Bot. 2013, 86, 94–105. [Google Scholar] [CrossRef]
- Chou, T.S.; Chao, Y.Y.; Kao, C.H. Involvement of hydrogen peroxide in heat shock-and cadmium-induced expression of ascorbate peroxidase and glutathione reductase in leaves of rice seedlings. J. Plant Physiol. 2012, 169, 478–486. [Google Scholar] [CrossRef] [PubMed]
- Arnon, D.I. Copper enzymes in isolated chloroplasts. Polyphe-noloxidase in Beta vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Hamilton, P.B.; Van-Slyke, D.D. Amino acid determination with ninhydrin. J. Biol. Chem. 1943, 150, 231–250. [Google Scholar] [CrossRef]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants: Protective role of exogenous polyamines. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar]
- Zhang, X. The measurement and mechanism of lipid peroxidation and SOD, POD and CAT activities in biological system. In Research Methodology of Crop Physiology; Agriculture Press: Beijing, China, 1992; pp. 208–211. [Google Scholar]
- Mukherjee, S.; Choudhuri, M. Implications of water stress-induced changes in the levels of endogenous ascorbic acid and hydrogen peroxide in Vigna seedlings. Physiol. Plant 1983, 58, 166–170. [Google Scholar] [CrossRef]
- Steel, R.G.; Torrie, J.H. Principles and Procedures of Statistics: A Biometrical Approach; McGraw-Hill: New York, NY, USA, 1980. [Google Scholar]
- Sarker, U.; Oba, S. Salinity stress enhances color parameters, bioactive leaf pigments, vitamins, polyphenols, flavonoids and antioxidant activity in selected Amaranthus leafy vegetables. J. Sci. Food Agric. 2019, 99, 2275–2284. [Google Scholar] [CrossRef]
- Sarker, U.; Oba, S. The response of salinity stress-induced A. tricolor to growth, anatomy, physiology, non-enzymatic and enzymatic antioxidants. Front. Plant Sci. 2020, 11, 233–262. [Google Scholar] [CrossRef]
- Kosová, K.; Vítámvás, P.; Prášil, I.T.; Renaut, J. Plant proteome changes under abiotic stress—Contribution of proteomics studies to understanding plant stress response. J. Proteom. 2011, 74, 1301–1322. [Google Scholar] [CrossRef] [PubMed]
- Urbaniak, M.; Wyrwicka, A.; Tołoczko, W.; Serwecińska, L.; Zieliński, M. The effect of sewage sludge application on soil properties and willow (Salix sp.) cultivation. Sci. Total Environ. 2017, 586, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Dineshkumar, R.; Subramanian, J.; Arumugam, A.; Rasheeq, A.A.; Sampathkumar, P. Exploring the microalgae biofertilizer effect on onion cultivation by field experiment. Waste Biomass Valorization 2020, 11, 77–87. [Google Scholar] [CrossRef]
- Liu, Z.; Rong, Q.; Zhou, W.; Liang, G.J. Effects of inorganic and organic amendment on soil chemical properties, enzyme activities, microbial community and soil quality in yellow clayey soil. PLoS ONE 2017, 12, e0172767. [Google Scholar] [CrossRef] [PubMed]
- Fatima, A.; Hussain, S.; Hussain, S.; Ali, B.; Ashraf, U.; Zulfiqar, U. Differential morphophysiological, biochemical, and molecular responses of maize hybrids to salinity and alkalinity stresses. Agronomy 2021, 11, 1150. [Google Scholar] [CrossRef]
- Rao, P.S.; Kumar, C.G.; Reddy, B.V. Sweet sorghum: From theory to practice. In Characterization of Improved Sweet Sorghum Cultivars; Springer: Berlin/Heidelberg, Germany, 2013; pp. 1–15. [Google Scholar]
- Chen, T.; Shabala, S.; Niu, Y.; Chen, Z.H.; Shabala, L.; Meinke, H.; Venkataraman, G.; Pareek, A.; Xu, J.; Zhou, M. Molecular mechanisms of salinity tolerance in rice. Crop J. 2021, 9, 506–520. [Google Scholar] [CrossRef]
- Chen, G.; Zheng, D.; Feng, N.; Zhou, H.; Mu, D.; Zhao, L.; Shen, X.; Rao, G.; Meng, F.; Huang, A. Physiological mechanisms of ABA-induced salinity tolerance in leaves and roots of rice. Sci. Rep. 2022, 12, 1–26. [Google Scholar] [CrossRef]
- Naveed, M.; Aslam, M.K.; Ahmad, Z.; Abbas, T.; Al-Huqail, A.A.; Siddiqui, M.H. Growth Responses, physiological alterations and alleviation of salinity stress in sunflower (Helianthus annuus L.) amended with gypsum and composted cow dung. Sustainability 2021, 13, 6792. [Google Scholar] [CrossRef]
- Kusvuran, A.; Bilgici, M.; Kusvuran, S.; Nazli, R.I. The effect of different organic matters on plant growth regulation and nutritional components under salt stress in sweet sorghum [Sorghum bicolor (L.) Moench.]. Maydica 2021, 66, 9. [Google Scholar]
- Sutrisno, S.; Yusnawan, E. Effect of Manure and Inorganic Fertilizers on Vegetative, Generative Characteristics, Nutrient, and Secondary Metabolite Contents of Mungbean. Biosaintifika J. Biol. Biol. 2018, 10, 56–65. [Google Scholar] [CrossRef]
- Litardo, R.C.M.; Bendezú, S.J.G.; Zenteno, M.D.C.; Pérez-Almeida, I.B.; Parismoreno, L.L.; García, E.D.L. Effect of mineral and organic amendments on rice growth and yield in saline soils. J. Saudi Soc. Agric. Sci. 2022, 21, 29–37. [Google Scholar]
- Soni, P.G.; Yadav, R.; Kumar, A.; Kumar, R.; Datt, C.; Paul, K. Sorghum fodder production and its nutrient composition under different residual sodium carbonate levels in irrigation water. Indian J. Anim. Nutr. 2016, 33, 345–349. [Google Scholar] [CrossRef]
- Hu, Q.; Lin, C.; Guan, Y.; Sheteiwy, M.S.; Hu, W.; Hu, J. Inhibitory effect of eugenol on seed germination and pre-harvest sprouting of hybrid rice (Oryza sativa L.). Sci. Rep. 2017, 7, 5295. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Shabala, S.; Zhang, J.; Ma, G.; Chen, D.; Shabala, L.; Zeng, F.; Chen, Z.H.; Zhou, M.; Venkataraman, G.; et al. Melatonin improves rice salinity stress tolerance by NADPH oxidase-dependent control of the plasma membrane K+ transporters and K+ homeostasis. Plant Cell Environ. 2020, 43, 2591–2605. [Google Scholar] [CrossRef] [PubMed]
- Horie, T.; Costa, A.; Kim, T.H.; Han, M.J.; Horie, R.; Leung, H.Y.; Miyao, A.; Hirochika, H.; An, G.; Schroeder, J.I. Rice OsHKT2; 1 transporter mediates large Na+ influx component into K+-starved roots for growth. EMBO J. 2007, 26, 3003–3014. [Google Scholar] [CrossRef]
- Ashraf, M.; Athar, H.; Harris, P.; Kwon, T. Some prospective strategies for improving crop salt tolerance. Adv. Agron. 2008, 97, 45–110. [Google Scholar]
- Zhanwu, G.; Jiayu, H.; Chunsheng, M.; Jixiang, L.; Xiaoyu, L.; Lidong, L. Effects of saline and alkaline stresses on growth and physiological changes in oat (Avena sativa L.) seedlings. Not. Bot. Horti Agrobot. Cluj-Napoca 2014, 42, 357–362. [Google Scholar]
- Fahad, S.; Bano, A. Effect of salicylic acid on physiological and biochemical characterization of maize grown in saline area. Pak. J. Bot. 2012, 44, 1433–1438. [Google Scholar]
- El-Esawi, M.A.; Alaraidh, I.A.; Alsahli, A.A.; Alzahrani, S.M.; Ali, H.M.; Alayafi, A.A. Serratia liquefaciens KM4 improves salt stress tolerance in maize by regulating redox potential, ion homeostasis, leaf gas exchange and stress-related gene expression. Int. J. Mol. Sci. 2018, 19, 3310. [Google Scholar] [CrossRef]
- Heidari, M.; Jamshidi, P. Effects of salinity and potassium application on antioxidant enzyme activities and physiological parameters in pearl millet. Agric. Sci. China 2011, 10, 228–237. [Google Scholar] [CrossRef]
- Chen, D.; Ma, X.; Li, C.; Zhang, W.; Xia, G.; Wang, M. A wheat aminocyclopropane-1-carboxylate oxidase gene, TaACO1, negatively regulates salinity stress in Arabidopsis thaliana. Plant Cell Rep. 2014, 33, 1815–1827. [Google Scholar] [CrossRef]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Ann. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef] [PubMed]
- Burhan, A.K.; AL-Taey, D.K. Effect of Potassium humate, humic acid, and compost of rice wastes in the growth and yield of two cultivars of Dill under salt stress conditions. Adv. Nat. Appl. Sci. 2018, 12, 1–6. [Google Scholar]
- Xu, L.; Yan, D.; Ren, X.; Wei, Y.; Zhou, J.; Zhao, H. Vermicompost improves the physiological and biochemical responses of blessed thistle (Silybum marianum Gaertn.) and peppermint (Mentha haplocalyx Briq) to salinity stress. Ind. Crops Prod. 2016, 94, 574–585. [Google Scholar] [CrossRef]
- Chowdhury, S.; Bhusan, D.; Hashem, M.A.; Hoque, M.A. Organic amendments for mitigating soil salinity in rice. Res. Agric. Livest. Fish. 2019, 6, 11–17. [Google Scholar] [CrossRef]
- Mbarki, S.; Skalicky, M.; Talbi, O.; Chakraborty, A.; Hnilicka, F.; Hejnak, V. Performance of Medicago sativa grown in clay soil favored by compost or farmyard manure to mitigate salt stress. Agronomy 2020, 10, 94. [Google Scholar] [CrossRef]
- Mannan, M.A.; Karim, M.A.; Haque, M.M.; Khaliq, Q.A.; Higuchi, H.; Nawata, E. Response of soybean to salinity: II. Growth and yield of some selected genotypes. Trop. Agric. Dev. 2013, 57, 31–40. [Google Scholar]
- Otie, V.; Udo, I.; Shao, Y.; Itam, M.O.; Okamoto, H.; An, P. Salinity effects on morpho-physiological and yield traits of soybean (Glycine max L.) as Mediated by foliar spray with brassinolide. Plants 2021, 10, 541. [Google Scholar] [CrossRef]
- Abbas, A.; Azeem, M.; Naveed, M.; Latif, A.; Bashir, S.; Ali, A. Synergistic use of biochar and acidified manure for improving growth of maize in chromium contaminated soil. Int. J. Phytoremediation 2020, 22, 52–61. [Google Scholar] [CrossRef]
- Al-Ashkar, I.; Alderfasi, A.; El-Hendawy, S.; Al-Suhaibani, N.; El-Kafafi, S.; Seleiman, M.F. Detecting salt tolerance in doubled haploid wheat lines. Agronomy 2019, 9, 211. [Google Scholar] [CrossRef]
- Abbas, G.; Saqib, M.; Rafique, Q.; Rahman, A.; Akhtar, J.; Haq, M. Effect of salinity on grain yield and grain quality of wheat (Triticum aestivum L.). Pak. J. Bot. 2013, 50, 185–189. [Google Scholar]
- Maqsood, T.; Akhtar, J.; Farooq, M.; Haq, M.; Saqib, Z. Biochemical attributes of salt tolerant and salt sensitive maize cultivars to salinity and potassium nutrition. J. Pak J. Agri. Sci. 2008, 45, 1–5. [Google Scholar]
- Hassan, M.U.; Aamer, M.; Nawaz, M.; Rehman, A.; Aslam, T.; Afzal, U. Agronomic Bio-Fortification of Wheat to Combat Zinc Deficiency in Developing Countries. Pak. J. Agric. Res. 2021, 34, 201. [Google Scholar] [CrossRef]
- Hassan, M.U.; Chattha, M.U.; Ullah, A.; Khan, I.; Qadeer, A.; Aamer, M. Agronomic biofortification to improve productivity and grain Zn concentration of bread wheat. Int. J. Agric. Biol. 2019, 21, 615–620. [Google Scholar]
- Mroue, S.; Simeunovic, A.; Robert, H.S. Auxin production as an integrator of environmental cues for developmental growth regulation. J. Exp. Bot. 2018, 69, 201–212. [Google Scholar] [CrossRef]
- Amanullah, I.; Inamullah, X. Dry matter partitioning and harvest index differ in rice genotypes with variable rates of phosphorus and zinc nutrition. Rice Sci. 2016, 23, 78–87. [Google Scholar] [CrossRef]
- Nadeem, F.; Azhar, M.; Anwar-ul-Haq, M.; Sabir, M.; Samreen, T.; Tufail, A. Comparative response of two rice (Oryza sativa L.) cultivars to applied zinc and manganese for mitigation of salt stress. J. Soil Sci. Plant Nutr. 2020, 20, 2059–2072. [Google Scholar] [CrossRef]
- Eissa, M.A. Impact of compost on metals phytostabilization potential of two halophytes species. Int. J. Phytoremediation 2015, 17, 662–668. [Google Scholar] [CrossRef]
- Rekaby, S.A.; Awad, M.Y.; Hegab, S.A.; Eissa, M.A. Effect of some organic amendments on barley plants under saline condition. J. Plant Nutr. 2020, 43, 1840–1851. [Google Scholar] [CrossRef]
| Salinity Stress | OA | PH (cm) | RL (cm) | SFW (g) | RFW (g) | SDW (g) | RDW (g) | LPP |
|---|---|---|---|---|---|---|---|---|
| Control | Control | 76.91d ± 0.21 | 7.60c ± 0.21 | 23.75c ± 0.25 | 4.08d ± 1.20 | 13.92bc ± 0.52 | 2.25de ± 0.40 | 28.58d ± 0.34 |
| 5% FYM | 82.17b ± 0.22 | 9.33ab ± 0.70 | 24.58a ± 0.16 | 5.29b ± 0.44 | 15.00b ± 0.76 | 4.00b ± 0.36 | 32.67b ± 0.82 | |
| 5% PM | 79.17c ± 0.09 | 8.75b ± 0.25 | 24.00b ± 0.59 | 4.71c ± 0.56 | 14.00c ± 0.24 | 3.00c ± 0.14 | 30.42c ± 0.57 | |
| FYM + PM | 85.19a ± 0.41 | 10.07c ± 0.38 | 25.33a ± 0.24 | 5.95a ± 0.62 | 16.92a ± 0.88 | 4.83a ± 0.29 | 35.33a ± 0.65 | |
| 6 dS/m | Control | 64.00h ± 0.36 | 5.35g ± 0.06 | 13.16de ± 0.22 | 2.62gh ± 0.50 | 9.50ef ± 0.65 | 1.42f ± 0.08 | 19.75g ± 0.33 |
| 5% FYM | 69.16f ± 0.32 | 6.63de ± 0.26 | 17.25cd ± 0.29 | 3.25ef ± 0.37 | 11.00de ± 0.49 | 2.45cd ± 0.14 | 22.50g ± 0.22 | |
| 5% PM | 66.25g ± 0.25 | 5.96ef ± 0.45 | 15.08de ± 1.05 | 2.96fg ± 0.36 | 10.41e ± 0.46 | 2.12de ± 0.12 | 21.00g ± 0.72 | |
| FYM + PM | 72.00e ± 0.47 | 7.35cd ± 0.14 | 19.00c ± 0.76 | 3.70de ± 0.40 | 12.58cd ± 0.32 | 2.75cd ± 0.16 | 24.00e ± 0.59 | |
| 12 dS/m | Control | 55.50j ± 0.62 | 3.54i ± 0.19 | 6.16h ± 0.35 | 1.62j ± 0.35 | 4.25h ± 0.69 | 1.31f ± 0.04 | 10.50j ± 0.29 |
| 5% FYM | 58.91i ± 0.42 | 4.25hi ± 0.19 | 7.92fg ± 0.28 | 1.99ij ± 0.61 | 8.42g ± 0.25 | 1.32f ± 0.23 | 13.16i ± 0.35 | |
| 5% PM | 57.58ij ± 0.71 | 3.79i ± 0.16 | 7.08gh ± 0.58 | 1.87ij ± 0.22 | 6.75g ± 0.55 | 1.26f ± 0.11 | 11.87i ± 0.19 | |
| FYM + PM | 62.08h ± 0.55 | 4.79gh ± 0.42 | 8.75ef ± 0.38 | 2.26hi ± 0.26 | 8.50f ± 0.56 | 1.62ef ± 0.21 | 14.50h ± 0.22 |
| Salinity Stress | OA | Chlorophyll a (mg/g FW) | Chlorophyll b (mg/g FW) | Carotenoids (mg/g FW) |
|---|---|---|---|---|
| Control | Control | 0.54c ± 0.0011 | 0.33b ± 0.0063 | 3.70d ± 0.072 |
| 5% FYM | 0.57b ± 0.0018 | 0.35a ± 0.0027 | 4.11b ± 0.028 | |
| 5% PM | 0.55c ± 0.0014 | 0.34b ± 0.0030 | 3.86c ± 0.086 | |
| FYM + PM | 0.59a ± 0.0030 | 0.36a ± 0.0054 | 4.34a ± 0.074 | |
| 6 dS/m | Control | 0.44d ± 0.0032 | 0.27cd ± 0.0067 | 2.48g ± 0.055 |
| 5% FYM | 0.46d ± 0.0018 | 0.28c ± 0.0054 | 2.86f ± 0.072 | |
| 5% PM | 0.45d ± 0.0016 | 0.28c ± 0.0039 | 2.73f ± 0.086 | |
| FYM + PM | 0.47d ± 0.0011 | 0.30c ± 0.0049 | 3.06e ± 0.033 | |
| 12 dS/m | Control | 0.38f ± 0.0027 | 0.21f ± 0.0066 | 1.18k ± 0.074 |
| 5% FYM | 0.39f ± 0.0014 | 0.23e ± 0.0047 | 1.61i ± 0.049 | |
| 5% PM | 0.38f ± 0.0024 | 0.22e ± 0.0029 | 1.35j ± 0.037 | |
| FYM + PM | 0.41e ± 0.0012 | 0.23e ± 0.0064 | 1.94h ± 0.034 |
| Salinity Stress | OA | APX (U/mg Protein) | CAT (U/mg Protein) | POD) (U/µg Protein) | Ascorbic Acid (mg/g FW) |
|---|---|---|---|---|---|
| Control | Control | 6.62g ± 0.40 | 1.83l ± 0.30 | 0.13j ± 0.0052 | 15.14l ± 0.47 |
| 5% FYM | 6.90g ± 0.67 | 2.24k ± 0.12 | 0.18h ± 0.0006 | 17.74j ± 0.20 | |
| 5% PM | 7.13g ± 0.21 | 2.74j ± 0.087 | 0.15i ± 0.0026 | 16.41k ± 0.27 | |
| FYM + PM | 7.40g ± 0.16 | 3.21i ± 0.082 | 0.20g ± 0.0034 | 19.07i ± 0.14 | |
| 6 dS/m | Control | 19.96f ± 0.22 | 3.68h ± 0.14 | 0.25f ± 0.0010 | 20.34h ± 0.19 |
| 5% FYM | 21.31f ± 0.37 | 4.15g ± 0.012 | 0.30d ± 0.0030 | 22.93f ± 0.11 | |
| 5% PM | 21.87f ± 1.57 | 4.62f ± 0.16 | 0.27e ± 0.0020 | 21.54g ± 0.23 | |
| FYM + PM | 26.83e ± 0.75 | 5.09e ± 0.21 | 0.38b ± 0.0015 | 24.20e ± 0.24 | |
| 12 dS/m | Control | 20.44d ± 0.51 | 5.55d ± 0.22 | 0.27e ± 0.0023 | 25.59d ± 0.40 |
| 5% FYM | 26.84c ± 0.48 | 6.02c ± 0.15 | 0.33c ± 0.0026 | 28.10b ± 0.40 | |
| 5% PM | 32.71b ± 0.98 | 6.49b ± 0.13 | 0.25f ± 0.0012 | 26.86c ± 0.54 | |
| FYM + PM | 38.74a ± 1.32 | 6.96a ± 0.15 | 0.45a ± 0.0008 | 29.40a ± 0.34 |
| Salinity Stress | OA | TPP | GPP | PP | PL (cm) | GYPP (g) |
|---|---|---|---|---|---|---|
| Control | Control | 5.75c ± 0.16 | 128.00d ± 0.45 | 11.00d ± 0.23 | 21.00cd ± 0.89 | 30.50d ± 0.44 |
| 5% FYM | 8.00b ± 0.24 | 132.00b ± 0.57 | 14.00b ± 0.49 | 23.08ab ± 0.21 | 33.17b ± 0.44 | |
| 5% PM | 6.25c ± 0.08 | 130.00c ± 0.23 | 12.00c ± 0.36 | 22.16bc ± 0.32 | 31.92c ± 0.29 | |
| FYM + PM | 9.50a ± 0.21 | 135.00a ± 0.14 | 16.00a ± 0.36 | 24.25a ± 0.29 | 34.33a ± 0.52 | |
| 6 dS/m | Control | 4.08d ± 0.16 | 115.00g ± 0.56 | 6.00g ± 0.83 | 16.00gh ± 0.015 | 9.33h ± 0.57 |
| 5% FYM | 5.75d ± 0.25 | 120.00e ± 0.49 | 8.58e ± 0.167 | 18.20ef ± 0.42 | 11.50f ± 0.55 | |
| 5% PM | 4.66d ± 0.49 | 118.00f ± 0.36 | 7.16f ± 0.417 | 17.17fg ± 0.29 | 10.42g ± 0.40 | |
| FYM + PM | 6.25c ± 0.41 | 122.00e ± 0.27 | 10.08d ± 0.360 | 19.37de ± 0.55 | 12.50e ± 0.24 | |
| 12 dS/m | Control | 2.25e ± 0.25 | 81.00i ± 0.24 | 3.00i ± 0.315 | 12.00k ± 0.53 | 3.50k ± 0.30 |
| 5% FYM | 4.00d ± 0.33 | 85.00gh ± 0.43 | 4.08h ± 0.518 | 13.58ij ± 0.33 | 4.00j ± 0.29 | |
| 5% PM | 3.00e ± 0.40 | 83.00hi ± 0.24 | 3.50hi ± 0.215 | 12.70jk ± 0.34 | 3.85j ± 0.15 | |
| FYM + PM | 4.50d ± 0.21 | 88.00gh ± 0.27 | 5.16g ± 0.222 | 14.54hi ± 0.41 | 4.50i ± 0.21 |
| Salinity Stress | OA | Protein (%) | Iron (mg/kg DW) | Zinc (mg/kg DW) |
|---|---|---|---|---|
| 0 | Control | 6.65c ± 0.70 | 36.55e ± 1.54 | 21.20de ± 0.14 |
| 5% FYM | 6.93b ± 1.49 | 47.45b ± 1.68 | 26.20b ± 0.027 | |
| 5% PM | 6.60c ± 1.21 | 42.40c ± 1.32 | 23.03c ± 0.074 | |
| FYM + PM | 7.11a ± 0.70 | 54.10a ± 1.17 | 29.70a ± 0.065 | |
| 6 dS/m | Control | 5.82e ± 0.47 | 32.30f ± 1.64 | 19.18f ± 0.074 |
| 5% FYM | 6.16d ± 0.47 | 41.35c ± 1.83 | 23.08c ± 0.066 | |
| 5% PM | 5.99de ± 1.25 | 37.65de ± 0.93 | 21.03de ± 0.099 | |
| FYM + PM | 6.16d ± 2.16 | 45.53b ± 0.92 | 26.23b ± 0.027 | |
| 12 dS/m | Control | 5.27f ± 0.62 | 28.13g ± 0.77 | 16.53g ± 0.071 |
| 5% FYM | 5.81e ± 0.83 | 37.25e ± 1.36 | 22.40cd ± 0.067 | |
| 5% PM | 5.37f ± 0.85 | 33.10f ± 0.84 | 20.38ef ± 0.072 | |
| FYM + PM | 5.95e ± 0.65 | 40.05cd ± 0.65 | 24.95b ± 0.044 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, I.; Mahmood, S.; Chattha, M.U.; Bilal Chattha, M.; Ahmad, S.; Awan, M.I.; Alqahtani, F.M.; Hashem, M.; Alhaithloul, H.A.S.; Qari, S.H.; et al. Organic Amendments Improved the Productivity and Bio-Fortification of Fine Rice by Improving Physiological Responses and Nutrient Homeostasis under Salinity Stress. Plants 2023, 12, 1644. https://doi.org/10.3390/plants12081644
Khan I, Mahmood S, Chattha MU, Bilal Chattha M, Ahmad S, Awan MI, Alqahtani FM, Hashem M, Alhaithloul HAS, Qari SH, et al. Organic Amendments Improved the Productivity and Bio-Fortification of Fine Rice by Improving Physiological Responses and Nutrient Homeostasis under Salinity Stress. Plants. 2023; 12(8):1644. https://doi.org/10.3390/plants12081644
Chicago/Turabian StyleKhan, Imran, Sikandar Mahmood, Muhammad Umer Chattha, Muhammad Bilal Chattha, Shahbaz Ahmad, Masood Iqbal Awan, Fatmah M. Alqahtani, Mohamed Hashem, Haifa Abdulaziz Sakit Alhaithloul, Sameer H. Qari, and et al. 2023. "Organic Amendments Improved the Productivity and Bio-Fortification of Fine Rice by Improving Physiological Responses and Nutrient Homeostasis under Salinity Stress" Plants 12, no. 8: 1644. https://doi.org/10.3390/plants12081644
APA StyleKhan, I., Mahmood, S., Chattha, M. U., Bilal Chattha, M., Ahmad, S., Awan, M. I., Alqahtani, F. M., Hashem, M., Alhaithloul, H. A. S., Qari, S. H., Mahmood, F., & Hassan, M. U. (2023). Organic Amendments Improved the Productivity and Bio-Fortification of Fine Rice by Improving Physiological Responses and Nutrient Homeostasis under Salinity Stress. Plants, 12(8), 1644. https://doi.org/10.3390/plants12081644













