Meiotic Behaviors of Allotetraploid Citrus Drive the Interspecific Recombination Landscape, the Genetic Structures, and Traits Inheritance in Tetrazyg Progenies Aiming to Select New Rootstocks
Abstract
1. Introduction
2. Results
2.1. DSNP Mining
2.2. Genetic Linkage Maps of Tetraploid Swingle Citrumelo and Volkamer Lemon
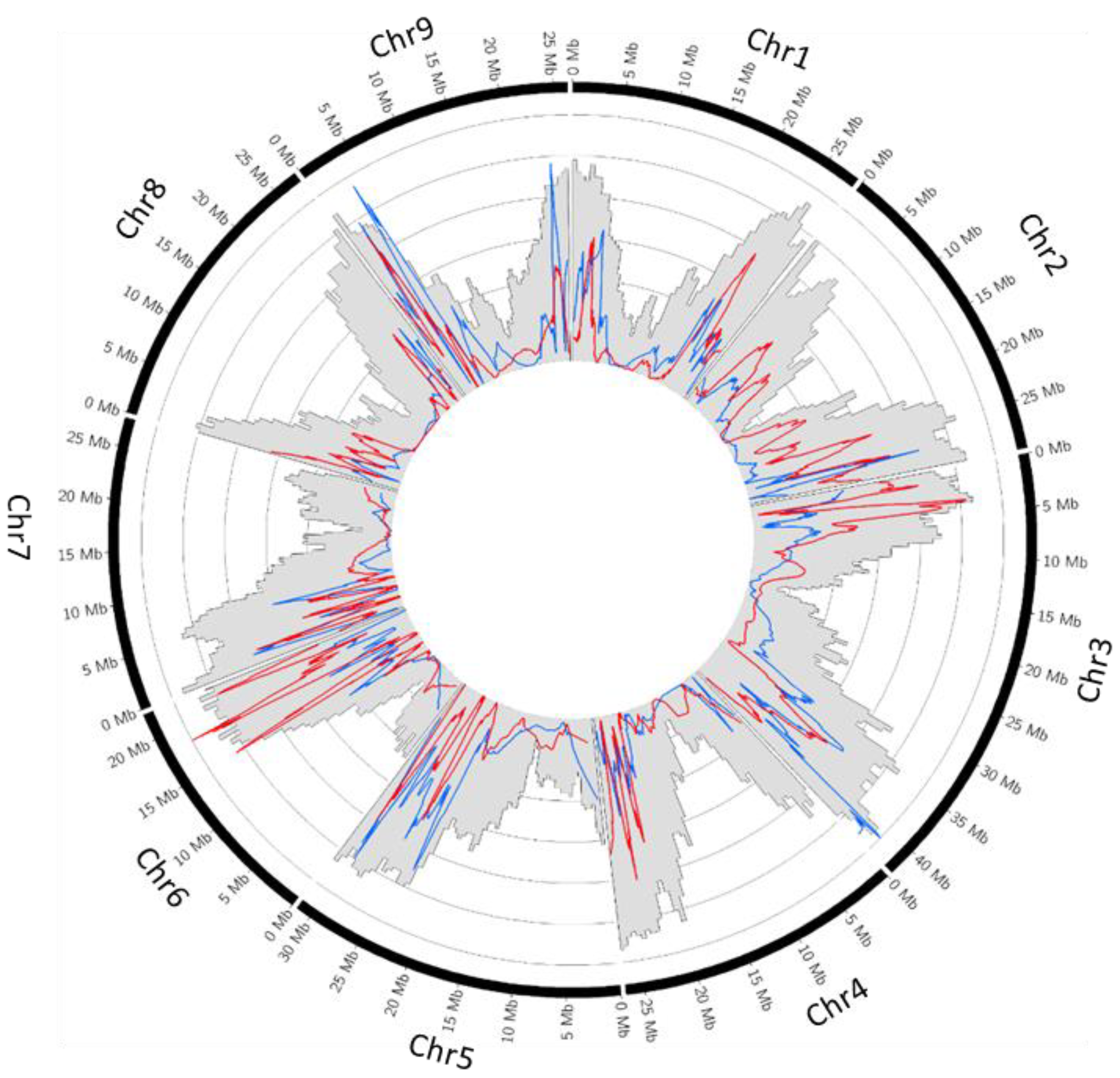
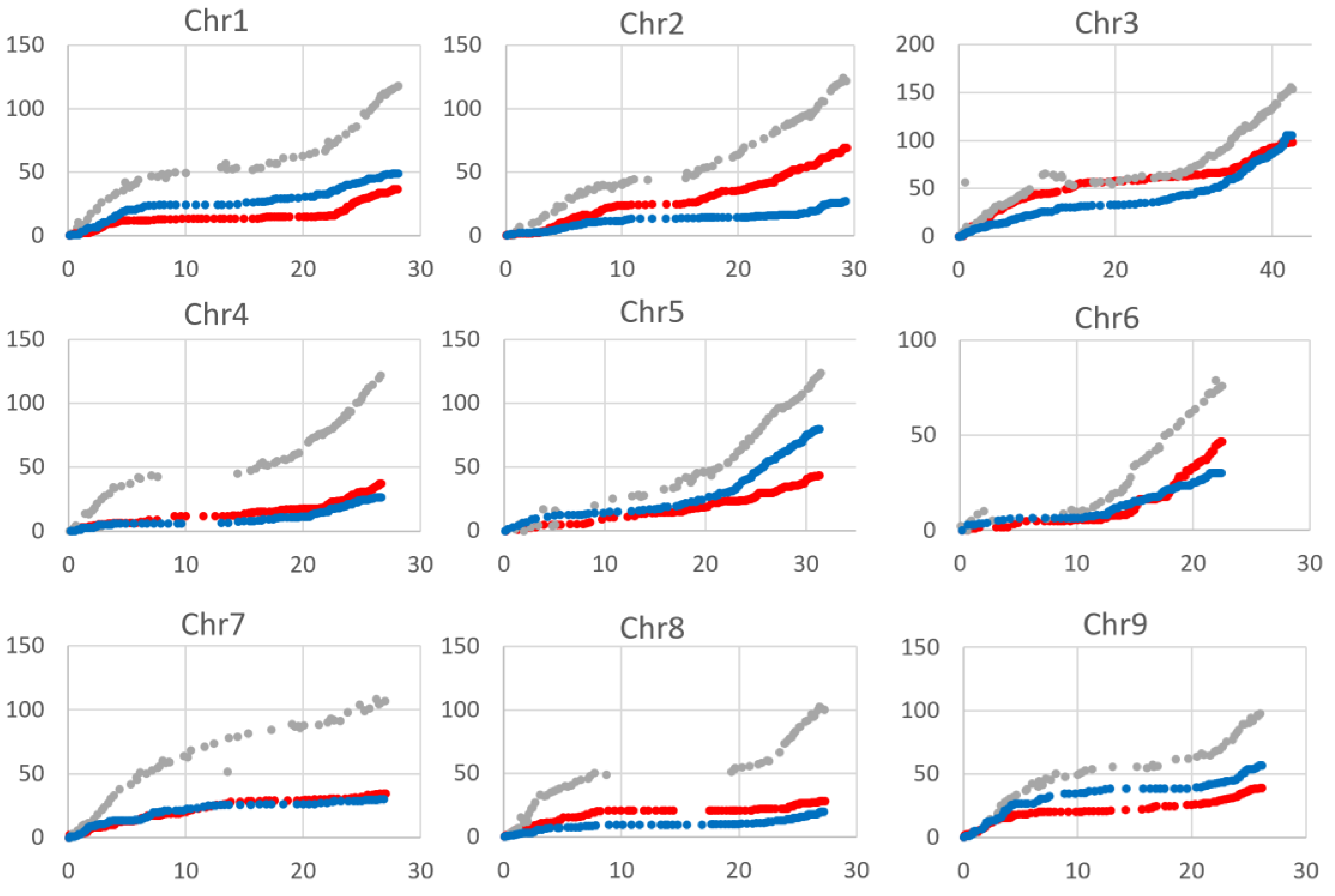
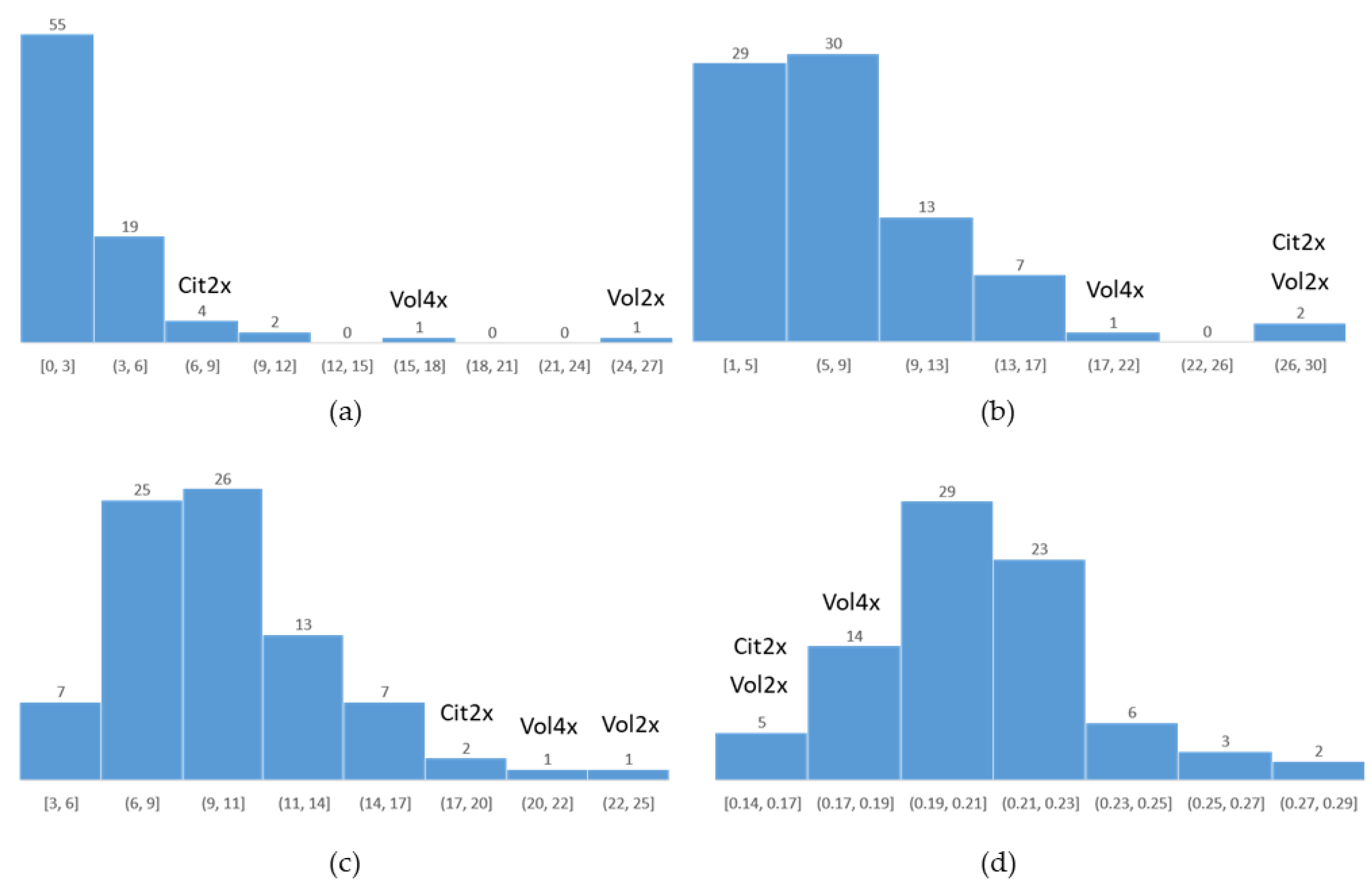
2.3. Recombination Rate
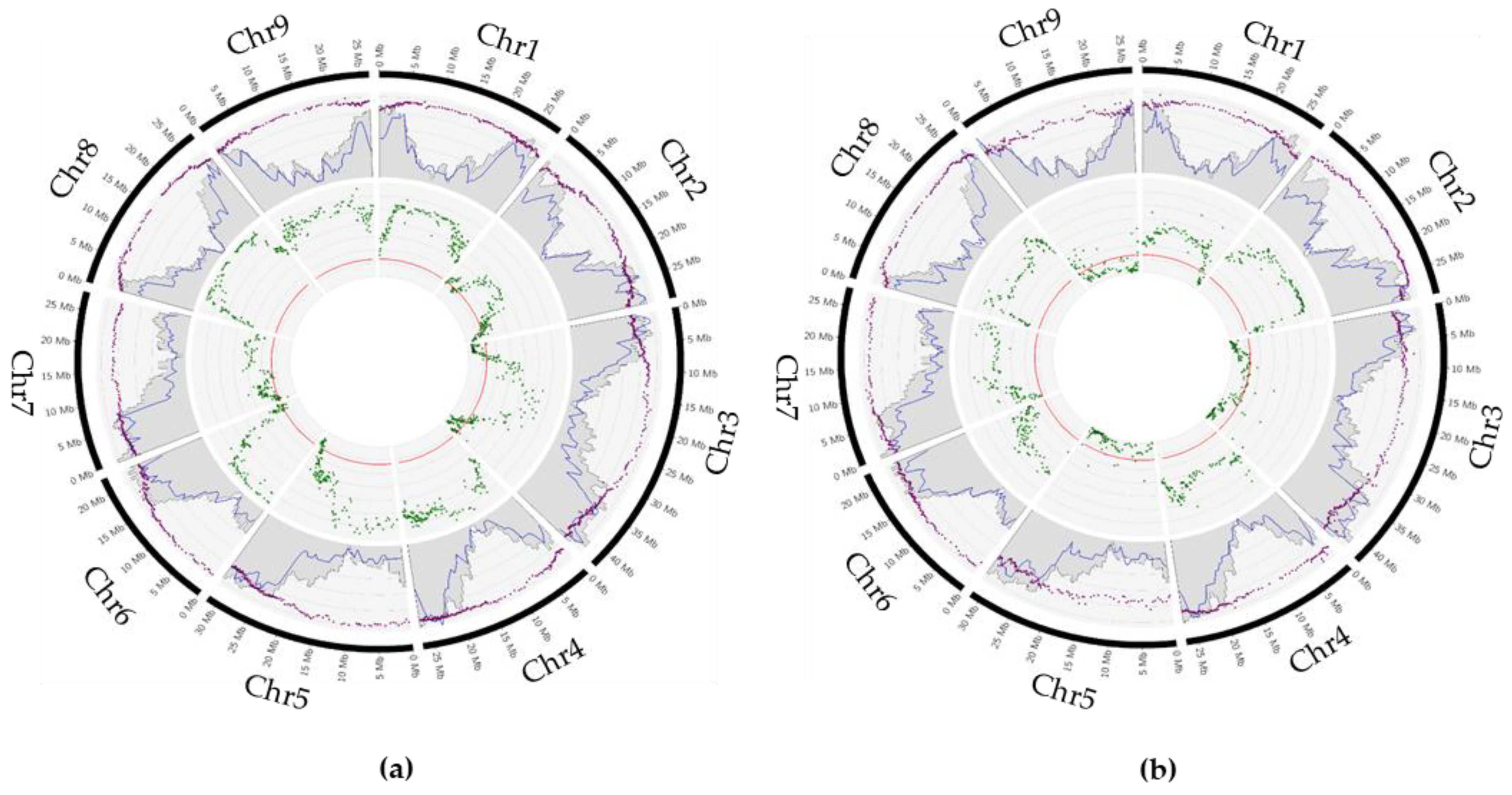
2.4. Parental Heterozygosity Restitution and Meiotic Inheritance Analysis
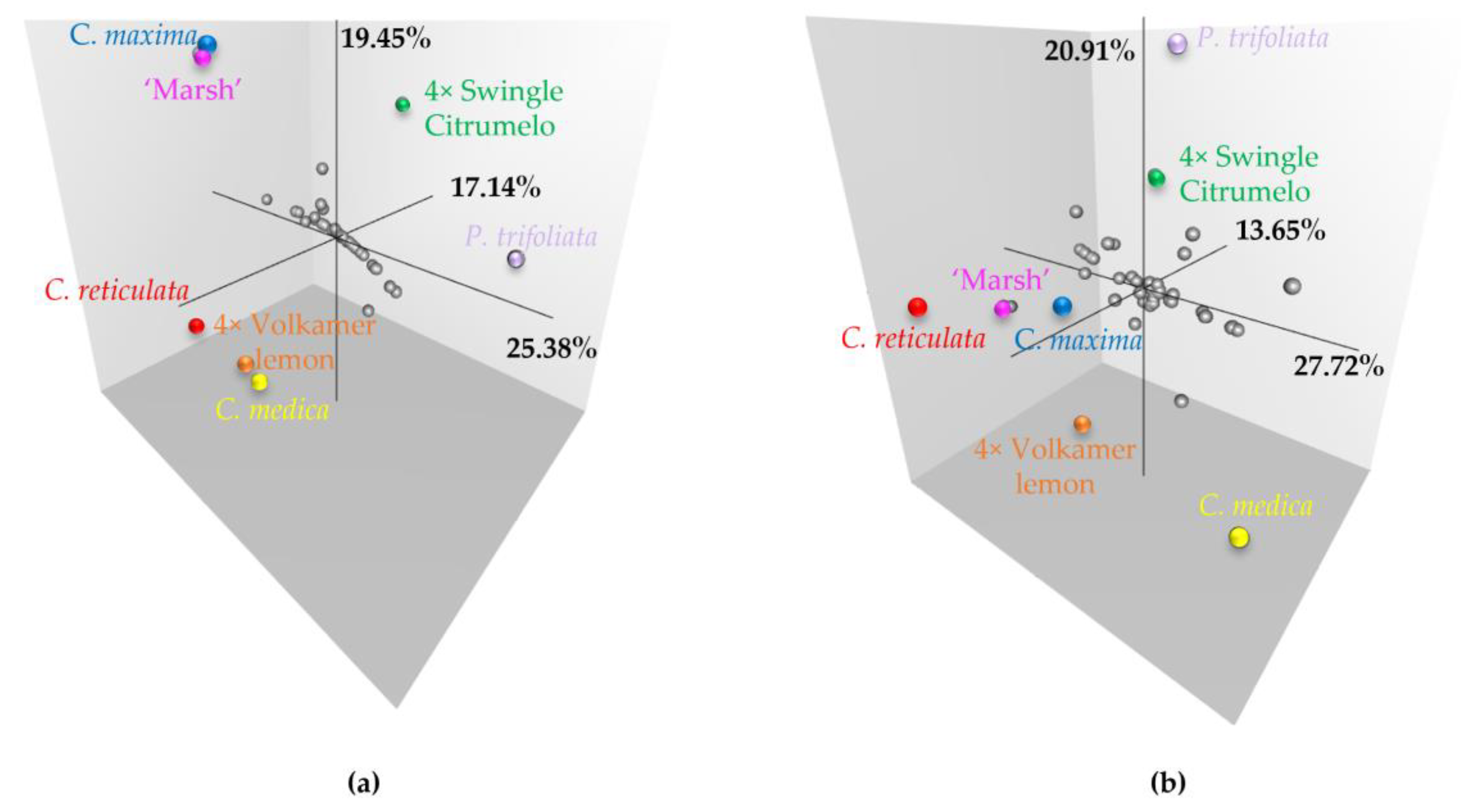
2.5. Impact of Preferential Pairing on Tetraploid Hybrid Diversity Structure
2.6. Inheritance in the Tetraploid Hybrids of Candidate Genes for Pest and Disease Resistance
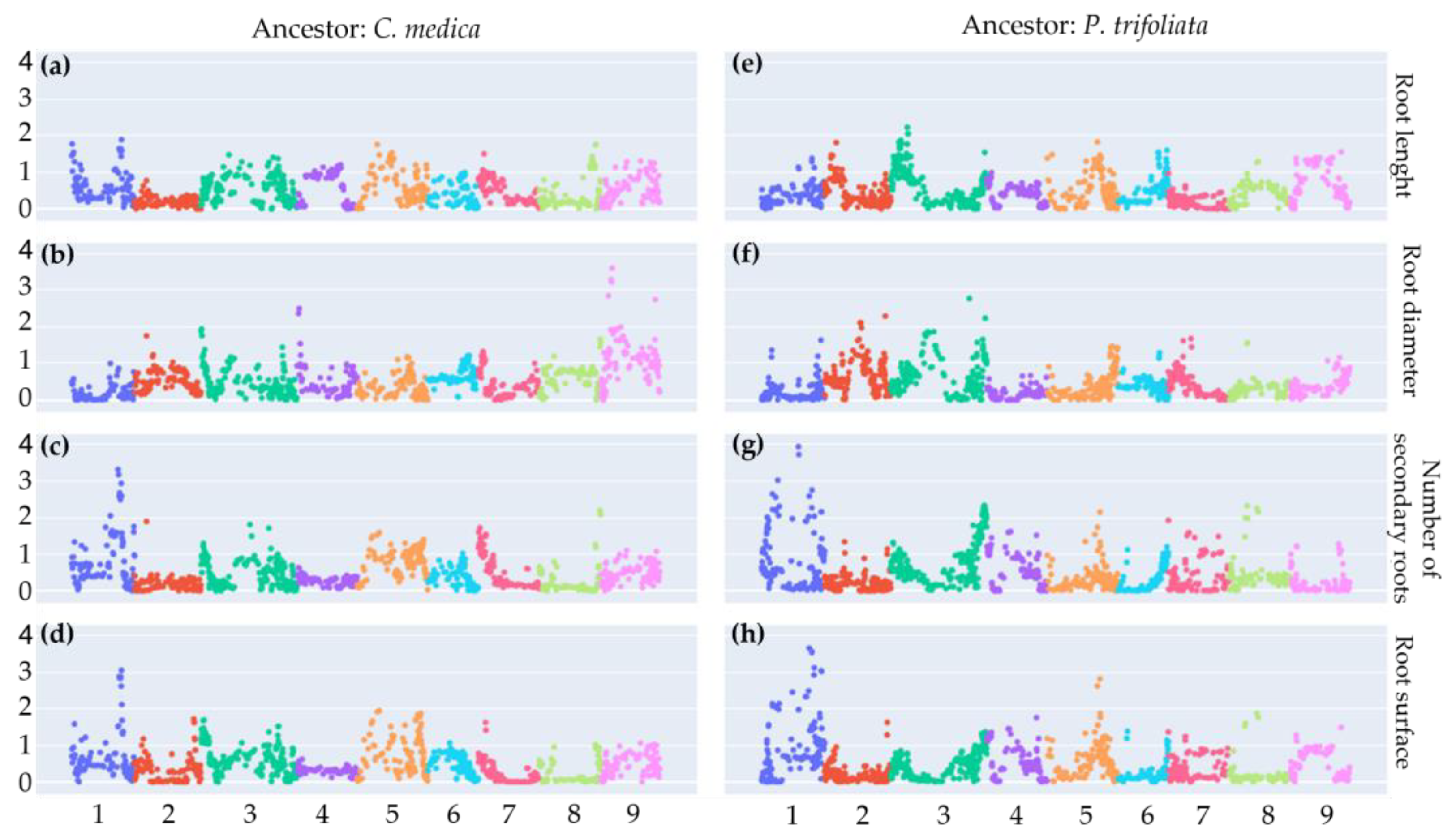
2.7. Root Phenotyping and Genomic Association
3. Discussion
3.1. GBS Coupled with TraceAncestor Was Efficient for Estimating Haplotype Ancestral Doses All along the Genome of the Tetraploid Hybrids
3.2. The Tetraploid Volkamer Lemon and Swingle Citrumelo Parents Display Intermediate Inheritance with a Disomic Tendency and Variability between Parents and Chromosomes for Preferential Chromosome Pairing
3.3. Apparent Interspecific and Intergeneric Recombination Rates Are Limited by Preferential Pairing and Are Very Low in Centromeric-Pericentromeric Areas
3.4. Preferential Chromosome Pairing in Tetraploid Rootstock Resulting from Chromosome Doubling of Interspecific Hybrids Is Unfavorable for QTL Analysis
3.5. Implication for Rootstock Breeding Programs
4. Materials and Methods
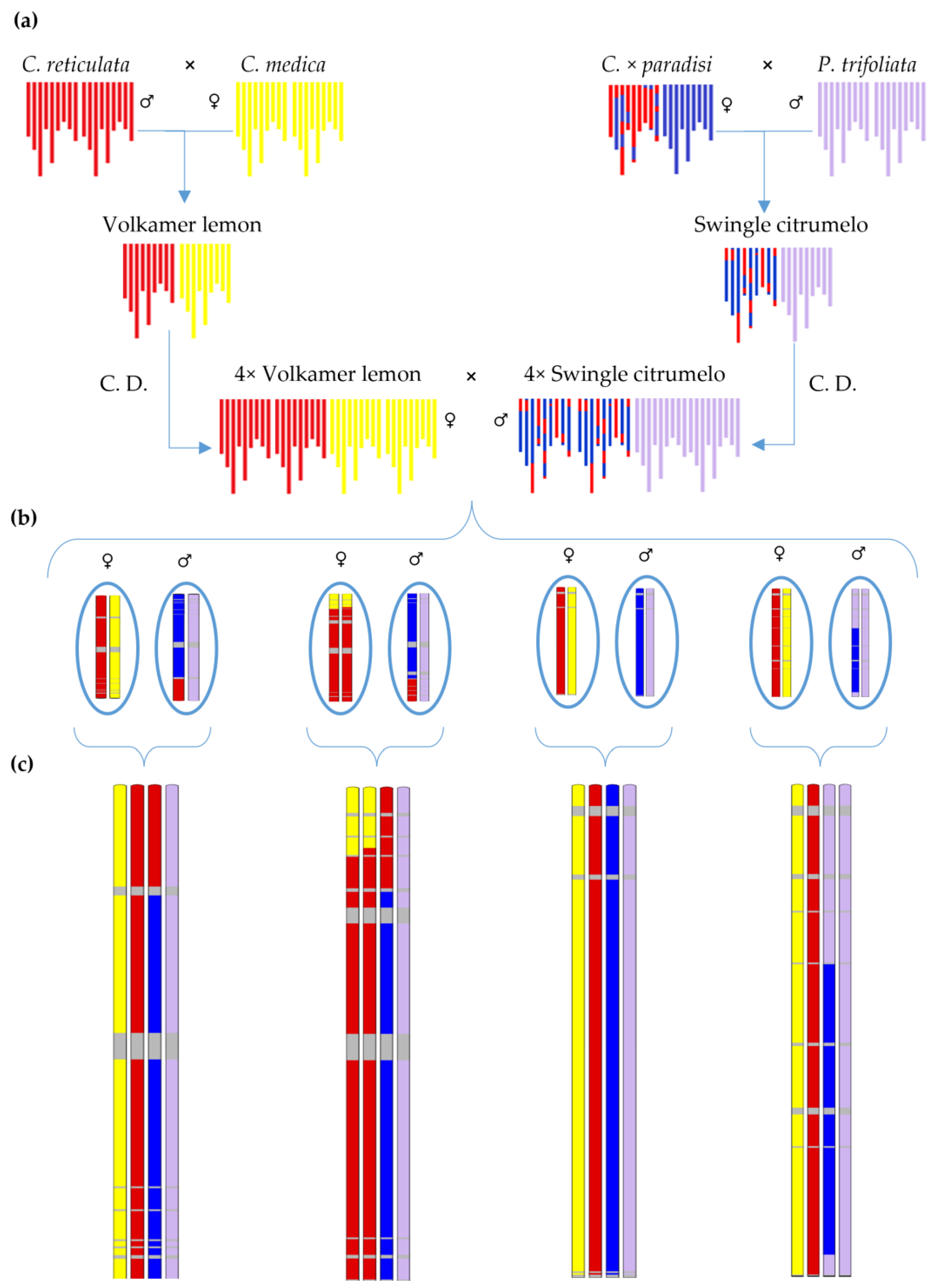
4.1. Plant Material
4.2. DNA Extraction and Genotyping by Sequencing (GBS)
4.3. SNP Calling
4.4. DSNPs Identification
4.5. Matrix Preparation
4.6. Mapping Analysis
4.7. Recombination Rate
4.8. Parental Heterozygosity Restitution (PHR)
4.9. Estimation of Preferential Pairing (PP)
4.10. Analysis of the Deviation from Expected Gametic Segregation under a Tetrasomic Model
4.11. Inheritance of Candidate Genes in the Hybrid Population
4.12. Root System Architecture Phenotyping
4.13. Quantitative Trait Locus Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Shafqat, W.; Naqvi, S.A.; Maqbool, R.; Haider, M.S.; Jaskani, M.J.; Khan, I.A. Climate Change and Citrus. Citrus 2021, 147. [Google Scholar] [CrossRef]
- Swingle, W.T.; Reece, P.C. The Botany of Citrus and Its Wild Relatives. In The Citrus, Industry; Reuther, W., Webber, H.J., Batchelor, L.D., Eds.; University of California Press: Berkeley, CA, USA, 1967. [Google Scholar]
- Calvez, L.; Dereeper, A.; Mournet, P.; Froelicher, Y.; Bruyère, S.; Morillon, R.; Ollitrault, P. Intermediate Inheritance with Disomic Tendency in Tetraploid Intergeneric Citrus × Poncirus Hybrids Enhances the Efficiency of Citrus Rootstock Breeding. Agronomy 2020, 10, 1961. [Google Scholar] [CrossRef]
- Curk, F.; Ollitrault, F.; Garcia-Lor, A.; Luro, F.; Navarro, L.; Ollitrault, P. Phylogenetic Origin of Limes and Lemons Revealed by Cytoplasmic and Nuclear Markers. Ann. Bot. 2016, 117, 565–583. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, D.; Comte, A.; Curk, F.; Costantino, G.; Luro, F.; Dereeper, A.; Mournet, P.; Froelicher, Y.; Ollitrault, P. Genotyping by Sequencing Can Reveal the Complex Mosaic Genomes in Gene Pools Resulting from Reticulate Evolution: A Case Study in Diploid and Polyploid Citrus. Ann. Bot. 2019, 123, 1231–1251. [Google Scholar] [CrossRef] [PubMed]
- Bowman, K.D.; Joubert, J. Chapter 6—Citrus Rootstocks. In The Genus Citrus; Talon, M., Caruso, M., Gmitter, F.G., Eds.; Woodhead Publishing: Duxford, UK, 2020; pp. 105–127. ISBN 978-0-12-812163-4. [Google Scholar]
- Spiegel-Roy, P.; Goldschmidt, E.E. The Biology of Horticultural Crops. In The Biology of Citrus; Cambridge University Press: Cambridge, UK, 1996; ISBN 978-0-521-33321-4. [Google Scholar]
- Ling, P.; Duncan, L.W.; Deng, Z.; Dunn, D.; Hu, X.; Huang, S.; Gmitter Jr, F.G. Inheritance of Citrus Nematode Resistance and Its Linkage with Molecular Markers. Theor. Appl. Genet. 2000, 100, 1010–1017. [Google Scholar] [CrossRef]
- Gmitter, F.G.; Xiao, S.Y.; Huang, S.; Hu, X.L.; Garnsey, S.M.; Deng, Z. A Localized Linkage Map of the Citrus Tristeza Virus Resistance Gene Region. TAG Theor. Appl. Genet. Theor. Angew. Genet. 1996, 92, 688–695. [Google Scholar] [CrossRef]
- Li, T.; Wu, Q.-S.; Kuča, K.; Rahman, M.M. Responses of Four Citrus Plants to Phytophthora-Induced Root Rot. Sains Malays. 2018, 47, 1693–1700. [Google Scholar] [CrossRef]
- Zhang, C.; Lang, P.; Dane, F.; Ebel, R.C.; Singh, N.K.; Locy, R.D.; Dozier, W.A. Cold Acclimation Induced Genes of Trifoliate Orange (Poncirus Trifoliata). Plant Cell Rep. 2005, 23, 764–769. [Google Scholar] [CrossRef]
- Folimonova, S.Y.; Robertson, C.J.; Garnsey, S.M.; Gowda, S.; Dawson, W.O. Examination of the Responses of Different Genotypes of Citrus to Huanglongbing (Citrus Greening) under Different Conditions. Phytopathology 2009, 99, 1346–1354. [Google Scholar] [CrossRef]
- Alves, M.N.; Lopes, S.A.; Raiol-Junior, L.L.; Wulff, N.A.; Girardi, E.A.; Ollitrault, P.; Pena, L. Resistance to “Candidatus Liberibacter Asiaticus,” the Huanglongbing Associated Bacterium, in Sexually and/or Graft-Compatible Citrus Relatives. Front. Plant Sci. 2021, 11, 617664. [Google Scholar] [CrossRef]
- Soratto, T.A.T.; Curtolo, M.; Marengo, S.; Dezotti, A.L.; Lima, R.P.M.; Gazaffi, R.; Machado, M.A.; Cristofani-Yaly, M. QTL and EQTL Mapping Associated with Host Response to Candidatus Liberibacter Asiaticus in Citrandarins. Trop. Plant Pathol. 2020, 45, 626–645. [Google Scholar] [CrossRef]
- Albrecht, U.; McCollum, G.; Bowman, K.D. Influence of Rootstock Variety on Huanglongbing Disease Development in Field-Grown Sweet Orange (Citrus Sinensis [L.] Osbeck) Trees. Sci. Hortic. 2012, 138, 210–220. [Google Scholar] [CrossRef]
- Stover, E.; McCollum, G. Incidence and Severity of Huanglongbing and Candidatus Liberibacter Asiaticus Titer among Field-Infected Citrus Cultivars. HortScience 2011, 46, 1344–1348. [Google Scholar] [CrossRef]
- George, J.; Lapointe, S.L. Host-Plant Resistance Associated with Poncirus Trifoliata Influence Oviposition, Development and Adult Emergence of Diaphorina Citri (Hemiptera: Liviidae). Pest Manag. Sci. 2019, 75, 279–285. [Google Scholar] [CrossRef]
- Kamiri, M.; Stift, M.; Costantino, G.; Dambier, D.; Kabbage, T.; Ollitrault, P.; Froelicher, Y. Preferential Homologous Chromosome Pairing in a Tetraploid Intergeneric Somatic Hybrid (Citrus Reticulata + Poncirus Trifoliata) Revealed by Molecular Marker Inheritance. Front. Plant Sci. 2018, 9, 1557. [Google Scholar] [CrossRef]
- Ben Yahmed, J.; Costantino, G.; Amiel, P.; Talon, M.; Ollitrault, P.; Morillon, R.; Luro, F. Diversity in the Trifoliate Orange Taxon Reveals Two Main Genetic Groups Marked by Specific Morphological Traits and Water Deficit Tolerance Properties. J. Agric. Sci. 2016, 154, 495–514. [Google Scholar] [CrossRef]
- Khalid, M.F.; Hussain, S.; Anjum, M.A.; Ahmad, S.; Ali, M.A.; Ejaz, S.; Morillon, R. Better Salinity Tolerance in Tetraploid vs Diploid Volkamer Lemon Seedlings Is Associated with Robust Antioxidant and Osmotic Adjustment Mechanisms. J. Plant Physiol. 2020, 244, 153071. [Google Scholar] [CrossRef]
- Khalid, M.F.; Morillon, R.; Anjum, M.A.; Ejaz, S.; Rao, M.J.; Ahmad, S.; Hussain, S. Volkamer Lemon Tetraploid Rootstock Transmits the Salt Tolerance When Grafted with Diploid Kinnow Mandarin by Strong Antioxidant Defense Mechanism and Efficient Osmotic Adjustment. J. Plant Growth Regul. 2022, 41, 1125–1137. [Google Scholar] [CrossRef]
- Mouhaya, W.; Allario, T.; Costantino, G.; Andres, F.; Talon, M.; Luro, F.; Ollitrault, P.; Morillon, R. Citrus Tetraploid Rootstocks Are More Tolerant to Salt Stress than Diploid. Available online: https://agritrop.cirad.fr/547588/ (accessed on 13 September 2021).
- Saleh, B.; Allario, T.; Dambier, D.; Ollitrault, P.; Morillon, R. Tetraploid Citrus Rootstocks Are More Tolerant to Salt Stress than Diploid. Comptes. Rendus Biol. 2008, 331, 703–710. [Google Scholar] [CrossRef]
- Oustric, J.; Morillon, R.; Luro, F.; Herbette, S.; Lourkisti, R.; Giannettini, J.; Berti, L.; Santini, J. Tetraploid Carrizo citrange rootstock (Citrus sinensis Osb. × Poncirus trifoliata L. Raf.) enhances natural chilling stress tolerance of common clementine (Citrus clementina Hort. ex Tan). J. Plant Physiol. 2017, 214, 108–115. [Google Scholar] [CrossRef]
- Oustric, J.; Quilichini, Y.; Morillon, R.; Herbette, S.; Luro, F.; Giannettini, J.; Berti, L.; Santini, J. Tetraploid Citrus Seedlings Subjected to Long-Term Nutrient Deficiency Are Less Affected at the Ultrastructural, Physiological and Biochemical Levels than Diploid Ones. Plant Physiol. Biochem. 2019, 135, 372–384. [Google Scholar] [CrossRef] [PubMed]
- Oustric, J.; Herbette, S.; Morillon, R.; Giannettini, J.; Berti, L.; Santini, J. Influence of Rootstock Genotype and Ploidy Level on Common Clementine (Citrus clementina Hort. Ex Tan) Tolerance to Nutrient Deficiency. Front. Plant Sci. 2021, 12, 403. [Google Scholar] [CrossRef] [PubMed]
- Allario, T.; Brumos, J.; Colmenero-Flores, J.M.; Iglesias, D.J.; Pina, J.A.; Navarro, L.; Talon, M.; Ollitrault, P.; Morillon, R. Tetraploid Rangpur Lime Rootstock Increases Drought Tolerance via Enhanced Constitutive Root Abscisic Acid Production. Plant Cell Environ. 2013, 36, 856–868. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, T.M.; Yahmed, J.B.; Dutra, J.; Maserti, B.E.; Talon, M.; Navarro, L.; Ollitraut, P.; da S. Gesteira, A.; Morillon, R. Better Tolerance to Water Deficit in Doubled Diploid ‘Carrizo Citrange’ Compared to Diploid Seedlings Is Associated with More Limited Water Consumption. Acta Physiol. Plant. 2017, 39, 204. [Google Scholar] [CrossRef]
- Wei, T.; Wang, Y.; Xie, Z.; Guo, D.; Chen, C.; Fan, Q.; Deng, X.; Liu, J. Enhanced ROS Scavenging and Sugar Accumulation Contribute to Drought Tolerance of Naturally Occurring Autotetraploids in Poncirus Trifoliata. Plant Biotechnol. J. 2019, 17, 1394–1407. [Google Scholar] [CrossRef]
- Sivager, G.; Calvez, L.; Bruyere, S.; Boisne-Noc, R.; Brat, P.; Gros, O.; Ollitrault, P.; Morillon, R. Specific Physiological and Anatomical Traits Associated With Polyploidy and Better Detoxification Processes Contribute to Improved Huanglongbing Tolerance of the Persian Lime Compared With the Mexican Lime. Front. Plant Sci. 2021, 12, 1343. [Google Scholar] [CrossRef]
- Sivager, G.; Calvez, L.; Bruyere, S.; Boisne-Noc, R.; Hufnagel, B.; Cebrian-Torrejon, G.; Doménech-Carbó, A.; Gros, O.; Ollitrault, P.; Morillon, R. Better Tolerance to Huanglongbing Is Conferred by Tetraploid Swingle Citrumelo Rootstock and Is Influenced by the Ploidy of the Scion. Front. Plant Sci. 2022, 13, 14. [Google Scholar] [CrossRef]
- Ollitrault, P.; Germanà, M.A.; Froelicher, Y.; Cuenca, J.; Aleza, P.; Morillon, R.; Grosser, J.W.; Guo, W. Ploidy Manipulation for Citrus Breeding, Genetics, and Genomics. In The Citrus Genome; Gentile, A., La Malfa, S., Deng, Z., Eds.; Compendium of Plant Genomes; Springer International Publishing: Cham, The Switzerland, 2020; pp. 75–105. ISBN 978-3-030-15308-3. [Google Scholar]
- Rouiss, H.; Cuenca, J.; Navarro, L.; Ollitrault, P.; Aleza, P. Tetraploid Citrus Progenies Arising from FDR and SDR Unreduced Pollen in 4x X 2x Hybridizations. Tree Genet. Genomes 2017, 13, 10. [Google Scholar] [CrossRef]
- Cuenca, J.; Froelicher, Y.; Aleza, P.; Juárez, J.; Navarro, L.; Ollitrault, P. Multilocus Half-Tetrad Analysis and Centromere Mapping in Citrus: Evidence of SDR Mechanism for 2n Megagametophyte Production and Partial Chiasma Interference in Mandarin Cv ‘Fortune’. Heredity 2011, 107, 462–470. [Google Scholar] [CrossRef]
- Cuenca, J.; Aleza, P.; Juárez, J.; García-Lor, A.; Froelicher, Y.; Navarro, L.; Ollitrault, P. Maximum-Likelihood Method Identifies Meiotic Restitution Mechanism from Heterozygosity Transmission of Centromeric Loci: Application in Citrus. Sci. Rep. 2015, 5, 9897. [Google Scholar] [CrossRef]
- Aleza, P.; Juárez, J.; Cuenca, J.; Ollitrault, P.; Navarro, L. Recovery of Citrus Triploid Hybrids by Embryo Rescue and Flow Cytometry from 2x x 2x Sexual Hybridisation and Its Application to Extensive Breeding Programs. Plant Cell Rep. 2010, 29, 1023–1034. [Google Scholar] [CrossRef]
- Aleza, P.; Froelicher, Y.; Schwarz, S.; Agustí, M.; Hernández, M.; Juárez, J.; Luro, F.; Morillon, R.; Navarro, L.; Ollitrault, P. Tetraploidization Events by Chromosome Doubling of Nucellar Cells Are Frequent in Apomictic Citrus and Are Dependent on Genotype and Environment. Ann. Bot. 2011, 108, 37–50. [Google Scholar] [CrossRef]
- Cameron, J.W.; Frost, H.B. Genetics, Breeding and Nucellar Embryony. Citrus Ind. 1968, I, 325–370. [Google Scholar]
- Barrett, H.C. Colchicine-Induced Polyploidy in Citrus. Bot. Gaz. 1974, 135, 29–41. [Google Scholar] [CrossRef]
- Aleza, P.; Juárez, J.; Ollitrault, P.; Navarro, L. Production of Tetraploid Plants of Non Apomictic Citrus Genotypes. Plant Cell Rep. 2009, 28, 1837–1846. [Google Scholar] [CrossRef]
- Grosser, J.W.; Kainth, D.; Dutt, M. Production of Colchicine-Induced Autotetraploids in Pummelo (Citrus grandis Osbeck) through Indirect Organogenesis. HortScience 2014, 49, 944–948. [Google Scholar] [CrossRef]
- Dambier, D.; Barantin, P.; Boulard, G.; Costantino, G.; Mournet, P.; Perdereau, A.; Morillon, R.; Ollitrault, P. Genomic Instability in Somatic Hybridization between Poncirus and Citrus Species Aiming to Create New Rootstocks. Agriculture 2022, 12, 134. [Google Scholar] [CrossRef]
- Dambier, D.; Benyahia, H.; Pensabene-Bellavia, G.; Aka Kaçar, Y.; Froelicher, Y.; Belfalah, Z.; Lhou, B.; Handaji, N.; Printz, B.; Morillon, R.; et al. Somatic Hybridization for Citrus Rootstock Breeding: An Effective Tool to Solve Some Important Issues of the Mediterranean Citrus Industry. Plant Cell Rep. 2011, 30, 883–900. [Google Scholar] [CrossRef]
- Grosser, J.W.; Ollitrault, P.; Olivares-Fuster, O. Somatic Hybridization in Citrus: An Effective Tool to Facilitate Variety Improvement. Vitr. Cell. Dev. Biol.—Plant 2000, 36, 434–449. [Google Scholar] [CrossRef]
- Grosser, J.W.; Chandler, J.L. Somatic Hybridization of High Yield, Cold-Hardy and Disease Resistant Parents for Citrus Rootstock Improvement. J. Hortic. Sci. Biotechnol. 2000, 75, 641–644. [Google Scholar] [CrossRef]
- Ollitrault, P.; Vanel, F.; Froelicher, Y.; Dambier, D. Creation of Triploid Citrus Hybrids by Electrofusion of Haploid and Diploid Protoplasts. Available online: https://agritrop.cirad.fr/477415/ (accessed on 13 September 2021).
- Ollitrault, P.; Guo WenWu, G.W.; Grosser, J.W. Somatic Hybridization. In Citrus Genetics, Breeding and Biotechnology; Khan, I.A., Ed.; CABI: Wallingford, UK, 2007; pp. 235–260. ISBN 978-0-85199-019-4. [Google Scholar]
- Guo, W.W.; Wu, R.C.; Cheng, Y.J.; Deng, X.X. Production and Molecular Characterization of Citrus Intergeneric Somatic Hybrids between Red Tangerine and Citrange. Plant Breed. 2007, 126, 72–76. [Google Scholar] [CrossRef]
- Grosser, J.W.; Gmitter, F.G. Protoplast Fusion for Production of Tetraploids and Triploids: Applications for Scion and Rootstock Breeding in Citrus. Plant Cell Tissue Organ Cult. (PCTOC) 2011, 104, 343–357. [Google Scholar] [CrossRef]
- Grosser, J.W.; Graham, J.H.; McCoy, C.W.; Hoyte, A.; Rubio, H.M.; Bright, D.B.; Chandler, J.L. Development of “Tetrazyg” Rootstocks Tolerant of the Diaprepes/Phytophthora Complex under Greenhouse Conditions. Proc. Fla. State Hortic. Soc. 2003, 116, 263–267. [Google Scholar]
- Grosser, J.W.; Barthe, G.A.; Castle, B.; Gmitter, F.G.J.; Lee, O. The Development of Improved Tetraploid Citrus Rootstocks to Facilitate Advanced Production Systems and Sustainable Citriculture in Florida. Acta Hortic. 2015, 1065, 319–327. [Google Scholar] [CrossRef]
- Grosser, J.W.; Graham, J.H.; Hoyte, A.; Rubio, H.M.; Bright, D.B.; Gmitter, J.; Chen, C.X.; Gmitter, F.G. Continued Development of Rootstocks Tolerant of the Phytophthora-Diaprepes Complex via Greenhouse Screening. Proc. Fla. State Hortic. Soc. 2007, 120, 103–109. [Google Scholar]
- Stebbins, G.L. Types of Polyploids: Their Classification and Significance. In Advances in Genetics; Demerec, M., Ed.; Academic Press: Cambridge, MA, USA, 1947; Volume 1, pp. 403–429. [Google Scholar]
- Chen, Z.J. Genetic and Epigenetic Mechanisms for Gene Expression and Phenotypic Variation in Plant Polyploids. Annu. Rev. Plant Biol. 2007, 58, 377–406. [Google Scholar] [CrossRef]
- Stift, M.; Berenos, C.; Kuperus, P.; van Tienderen, P.H. Segregation Models for Disomic, Tetrasomic and Intermediate Inheritance in Tetraploids: A General Procedure Applied to Rorippa (Yellow Cress) Microsatellite Data. Genetics 2008, 179, 2113–2123. [Google Scholar] [CrossRef]
- Aleza, P.; Cuenca, J.; Juárez, J.; Navarro, L.; Ollitrault, P. Inheritance in Doubled-Diploid Clementine and Comparative Study with SDR Unreduced Gametes of Diploid Clementine. Plant Cell Rep. 2016, 35, 1573–1586. [Google Scholar] [CrossRef]
- Rouiss, H.; Bakry, F.; Froelicher, Y.; Navarro, L.; Aleza, P.; Ollitrault, P. Origin of C. latifolia and C. aurantiifolia Triploid Limes: The Preferential Disomic Inheritance of Doubled-Diploid ‘Mexican’ Lime Is Consistent with an Interploid Hybridization Hypothesis. Ann. Bot. 2018, 121, 571–585. [Google Scholar] [CrossRef]
- Peng, Z.; Bredeson, J.V.; Wu, G.A.; Shu, S.; Rawat, N.; Du, D.; Parajuli, S.; Yu, Q.; You, Q.; Rokhsar, D.S.; et al. A Chromosome-Scale Reference Genome of Trifoliate Orange (Poncirus Trifoliata) Provides Insights into Disease Resistance, Cold Tolerance and Genome Evolution in Citrus. Plant J. 2020, 104, 1215–1232. [Google Scholar] [CrossRef]
- Huang, M.; Roose, M.L.; Yu, Q.; Du, D.; Yu, Y.; Zhang, Y.; Deng, Z.; Stover, E.; Gmitter, F.G.J. Construction of High-Density Genetic Maps and Detection of QTLs Associated With Huanglongbing Tolerance in Citrus. Front. Plant Sci. 2018, 9, 1694. [Google Scholar] [CrossRef]
- Aleza, P.; Cuenca, J.; Hernández, M.; Juárez, J.; Navarro, L.; Ollitrault, P. Genetic Mapping of Centromeres in the Nine Citrus clementina Chromosomes Using Half-Tetrad Analysis and Recombination Patterns in Unreduced and Haploid Gametes. BMC Plant Biol. 2015, 15, 80. [Google Scholar] [CrossRef]
- Storey, J.D. A Direct Approach to False Discovery Rates. J. R. Stat. Soc. Ser. B Stat. Methodol. 2002, 64, 479–498. [Google Scholar] [CrossRef]
- De Jong, W.S.; De Jong, D.M.; Bodis, M. A Fluorogenic 5’ Nuclease (TaqMan) Assay to Assess Dosage of a Marker Tightly Linked to Red Skin Color in Autotetraploid Potato. TAG Theor. Appl. Genet. Theor. Angew. Genet. 2003, 107, 1384–1390. [Google Scholar] [CrossRef]
- Esselink, G.D.; Nybom, H.; Vosman, B. Assignment of Allelic Configuration in Polyploids Using the MAC-PR (Microsatellite DNA Allele Counting-Peak Ratios) Method. TAG Theor. Appl. Genet. Theor. Angew. Genet. 2004, 109, 402–408. [Google Scholar] [CrossRef]
- Ferrante, S.P.; Lucretti, S.; Reale, S.; De Patrizio, A.; Abbate, L.; Tusa, N.; Scarano, M.-T. Assessment of the Origin of New Citrus Tetraploid Hybrids (2n = 4x) by Means of SSR Markers and PCR Based Dosage Effects. Euphytica 2010, 173, 223–233. [Google Scholar] [CrossRef]
- Cuenca, J.; Aleza, P.; Navarro, L.; Ollitrault, P. Assignment of SNP Allelic Configuration in Polyploids Using Competitive Allele-Specific PCR: Application to Citrus Triploid Progeny. Ann. Bot. 2013, 111, 731–742. [Google Scholar] [CrossRef]
- Rouiss, H.; Cuenca, J.; Navarro, L.; Ollitrault, P.; Aleza, P. Unreduced Megagametophyte Production in Lemon Occurs via Three Meiotic Mechanisms, Predominantly Second-Division Restitution. Front. Plant Sci. 2017, 8, 1211. [Google Scholar] [CrossRef]
- Ahmed, D.; Curk, F.; Evrard, J.C.; Froelicher, Y.; Ollitrault, P. Preferential Disomic Segregation and C. micrantha/C. medica Interspecific Recombination in Tetraploid ‘Giant Key’ Lime; Outlook for Triploid Lime Breeding. Front. Plant Sci. 2020, 11, 939. [Google Scholar] [CrossRef]
- Garavello, M.; Cuenca, J.; Garcia-Lor, A.; Ortega, N.; Navarro, L.; Ollitrault, P.; Aleza, P. Male and Female Inheritance Patterns in Tetraploid ‘Moncada’ Mandarin. Plant Cell Rep. 2020, 39, 335–349. [Google Scholar] [CrossRef]
- Oueslati, A.; Salhi-Hannachi, A.; Luro, F.; Vignes, H.; Mournet, P.; Ollitrault, P. Genotyping by Sequencing Reveals the Interspecific C. maxima/C. reticulata Admixture along the Genomes of Modern Citrus Varieties of Mandarins, Tangors, Tangelos, Orangelos and Grapefruits. PLoS ONE 2017, 12, e0185618. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.A.; Terol, J.; Ibanez, V.; López-García, A.; Pérez-Román, E.; Borredá, C.; Domingo, C.; Tadeo, F.R.; Carbonell-Caballero, J.; Alonso, R.; et al. Genomics of the Origin and Evolution of Citrus. Nature 2018, 554, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Xie, K.-D.; Xia, Q.-M.; Wang, X.-P.; Liang, W.-J.; Wu, X.-M.; Grosser, J.W.; Guo, W.-W. Cytogenetic and SSR-Marker Evidence of Mixed Disomic, Tetrasomic, and Intermediate Inheritance in a Citrus Allotetraploid Somatic Hybrid between ‘Nova’ Tangelo and ‘HB’ Pummelo. Tree Genet. Genomes 2015, 11, 112. [Google Scholar] [CrossRef]
- Jeridi, M.; Perrier, X.; Rodier-Goud, M.; Ferchichi, A.; D’Hont, A.; Bakry, F. Cytogenetic Evidence of Mixed Disomic and Polysomic Inheritance in an Allotetraploid (AABB) Musa Genotype. Ann. Bot. 2012, 110, 1593–1606. [Google Scholar] [CrossRef]
- Chen, C.; Bowman, K.D.; Choi, Y.A.; Dang, P.M.; Rao, M.N.; Huang, S.; Soneji, J.R.; McCollum, T.G.; Gmitter, F.G. EST-SSR Genetic Maps for Citrus sinensis and Poncirus trifoliata. Tree Genet. Genomes 2008, 4, 1–10. [Google Scholar] [CrossRef]
- Curtolo, M.; Soratto, T.A.T.; Gazaffi, R.; Takita, M.A.; Machado, M.A.; Cristofani-Yaly, M. High-Density Linkage Maps for Citrus sunki and Poncirus trifoliata Using DArTseq Markers. Tree Genet. Genomes 2017, 14, 5. [Google Scholar] [CrossRef]
- Bernet, G.P.; Fernandez-Ribacoba, J.; Carbonell, E.A.; Asins, M.J. Comparative Genome-Wide Segregation Analysis and Map Construction Using a Reciprocal Cross Design to Facilitate Citrus Germplasm Utilization. Mol. Breed. 2010, 25, 659–673. [Google Scholar] [CrossRef]
- Xu, Y.-Y.; Liu, S.-R.; Gan, Z.-M.; Zeng, R.-F.; Zhang, J.-Z.; Hu, C.-G. High-Density Genetic Map Construction and Identification of QTLs Controlling Leaf Abscission Trait in Poncirus Trifoliata. Int. J. Mol. Sci. 2021, 22, 5723. [Google Scholar] [CrossRef]
- Ollitrault, P.; Ahmed, D.; Costantino, G.; Evrard, J.-C.; Cardi, C.; Mournet, P.; Perdereau, A.; Froelicher, Y. Segregation Distortion for Male Parents in High Density Genetic Maps from Reciprocal Crosses between Two Self-Incompatible Cultivars Confirms a Gametophytic System for Self-Incompatibility in Citrus. Agriculture 2021, 11, 379. [Google Scholar] [CrossRef]
- Ollitrault, P.; Terol, J.; Chen, C.; Federici, C.T.; Lotfy, S.; Hippolyte, I.; Ollitrault, F.; Bérard, A.; Chauveau, A.; Cuenca, J.; et al. A Reference Genetic Map of C. clementina Hort. Ex Tan.; Citrus Evolution Inferences from Comparative Mapping. BMC Genom. 2012, 13, 593. [Google Scholar] [CrossRef]
- Parker, J.S.; Palmer, R.W.; Whitehorn, M.A.F.; Edgar, L.A. Chiasma Frequency Effects of Structural Chromosome Change. Chromosoma 1982, 85, 673–686. [Google Scholar] [CrossRef]
- Chambers, S.R.; Hunter, N.; Louis, E.J.; Borts, R.H. The Mismatch Repair System Reduces Meiotic Homeologous Recombination and Stimulates Recombination-Dependent Chromosome Loss. Mol. Cell. Biol. 1996, 16, 6110–6120. [Google Scholar] [CrossRef]
- Liharska, T.; Wordragen, M.; Kammen, A.; Zabel, P.; Koornneef, M. Tomato Chromosome 6: Effect of Alien Chromosomal Segments on Recombinant Frequencies. Genome 1996, 39, 485–491. [Google Scholar] [CrossRef]
- Chetelat, R.T.; Meglic, V.; Cisneros, P. A Genetic Map of Tomato Based on BC(1) Lycopersicon Esculentum x Solanum Lycopersicoides Reveals Overall Synteny but Suppressed Recombination between These Homeologous Genomes. Genetics 2000, 154, 857–867. [Google Scholar] [CrossRef]
- Opperman, R.; Emmanuel, E.; Levy, A.A. The Effect of Sequence Divergence on Recombination between Direct Repeats in Arabidopsis. Genetics 2004, 168, 2207–2215. [Google Scholar] [CrossRef]
- Li, L.; Jean, M.; Belzile, F. The Impact of Sequence Divergence and DNA Mismatch Repair on Homeologous Recombination in Arabidopsis. Plant J. Cell Mol. Biol. 2006, 45, 908–916. [Google Scholar] [CrossRef]
- Pecinka, A.; Fang, W.; Rehmsmeier, M.; Levy, A.A.; Mittelsten Scheid, O. Polyploidization Increases Meiotic Recombination Frequency in Arabidopsis. BMC Biol. 2011, 9, 1–7. [Google Scholar] [CrossRef]
- Pelé, A.; Falque, M.; Trotoux, G.; Eber, F.; Nègre, S.; Gilet, M.; Huteau, V.; Lodé, M.; Jousseaume, T.; Dechaumet, S.; et al. Amplifying Recombination Genome-Wide and Reshaping Crossover Landscapes in Brassicas. PLoS Genet. 2017, 13, e1006794. [Google Scholar] [CrossRef]
- Elshire, R.J.; Glaubitz, J.C.; Sun, Q.; Poland, J.A.; Kawamoto, K.; Buckler, E.S.; Mitchell, S.E. A Robust, Simple Genotyping-by-Sequencing (GBS) Approach for High Diversity Species. PLoS ONE 2011, 6, e19379. [Google Scholar] [CrossRef]
- Sonah, H.; Bastien, M.; Iquira, E.; Tardivel, A.; Légaré, G.; Boyle, B.; Normandeau, É.; Laroche, J.; Larose, S.; Jean, M.; et al. An Improved Genotyping by Sequencing (GBS) Approach Offering Increased Versatility and Efficiency of SNP Discovery and Genotyping. PLoS ONE 2013, 8, e54603. [Google Scholar] [CrossRef]
- Herten, K.; Hestand, M.S.; Vermeesch, J.R.; Van Houdt, J.K.J. GBSX: A Toolkit for Experimental Design and Demultiplexing Genotyping by Sequencing Experiments. BMC Bioinform. 2015, 16, 73. [Google Scholar] [CrossRef] [PubMed]
- Martin, M. Cutadapt Removes Adapter Sequences from High-Throughput Sequencing Reads. EMBnet. J. 2011, 17, 10–12. [Google Scholar] [CrossRef]
- Garsmeur, O.; Droc, G.; Antonise, R.; Grimwood, J.; Potier, B.; Aitken, K.S.; Jenkins, J.; Martin, G.; Charron, C.; Hervouet, C.; et al. A Mosaic Monoploid Reference Sequence for the Highly Complex Genome of Sugarcane. Nat. Commun. 2018, 9, 2638. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Durbin, R. Fast and Accurate Short Read Alignment with Burrows-Wheeler Transform. Bioinformatics 2009, 25, 1754–1760. [Google Scholar] [CrossRef]
- Dereeper, A.; Homa, F.; Andres, G.; Sempere, G.; Sarah, G.; Hueber, Y.; Dufayard, J.-F.; Ruiz, M. SNiPlay3: A Web-Based Application for Exploration and Large Scale Analyses of Genomic Variations. Nucleic Acids Res. 2015, 43, W295–W300. [Google Scholar] [CrossRef]
- Nei, M. Analysis of Gene Diversity in Subdivided Populations. Proc. Natl. Acad. Sci. USA 1973, 70, 3321–3323. [Google Scholar] [CrossRef]
- Perrier, X.; Jacquemoud-Collet, J.P. DARwin Software. 2006. Available online: http://darwin.cirad.fr/Darwin (accessed on 6 September 2022).
- Adler, D.; Nenadí, O.; Zucchini, W. Rgl: A r-Library for 3d Visualization with Opengl. In Proceedings of the 35th Symposium of the Interface: Computing Science and Statistics, Salt Lake City, UT, USA, 12–15 March 2003; Volume 35, pp. 419–429. [Google Scholar]
- Van Os, H.; Stam, P.; Visser, R.G.F.; van Eck, H.J. SMOOTH: A Statistical Method for Successful Removal of Genotyping Errors from High-Density Genetic Linkage Data. TAG Theor. Appl. Genet. Theor. Angew. Genet. 2005, 112, 187–194. [Google Scholar] [CrossRef]
- Grandke, F.; Ranganathan, S.; van Bers, N.; de Haan, J.R.; Metzler, D. PERGOLA: Fast and Deterministic Linkage Mapping of Polyploids. BMC Bioinform. 2017, 18, 12. [Google Scholar] [CrossRef]
- Krzywinski, M.; Schein, J.; Birol, İ.; Connors, J.; Gascoyne, R.; Horsman, D.; Jones, S.J.; Marra, M.A. Circos: An Information Aesthetic for Comparative Genomics. Genome Res. 2009, 19, 1639–1645. [Google Scholar] [CrossRef]
- Rasche, H.; Hiltemann, S. Galactic Circos: User-Friendly Circos Plots within the Galaxy Platform. GigaScience 2020, 9, giaa065. [Google Scholar] [CrossRef]
- Siberchicot, A.; Bessy, A.; Guéguen, L.; Marais, G.A. MareyMap Online: A User-Friendly Web Application and Database Service for Estimating Recombination Rates Using Physical and Genetic Maps. Genome Biol. Evol. 2017, 9, 2506–2509. [Google Scholar] [CrossRef]
- Kamiri, M.; Stift, M.; Srairi, I.; Costantino, G.; Moussadik, A.E.; Hmyene, A.; Bakry, F.; Ollitrault, P.; Froelicher, Y. Evidence for Non-Disomic Inheritance in a Citrus Interspecific Tetraploid Somatic Hybrid between C. Reticulata and C. Limon Using SSR Markers and Cytogenetic Analysis. Plant Cell Rep. 2011, 30, 1415–1425. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B Methodol. 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Yang, Z.-N.; Ye, X.-R.; Molina, J.; Roose, M.L.; Mirkov, T.E. Sequence Analysis of a 282-Kilobase Region Surrounding the Citrus Tristeza Virus Resistance Gene (Ctv) Locus in Poncirus Trifoliata L. Raf. Plant Physiol. 2003, 131, 482–492. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of Image Analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Bradbury, P.J.; Zhang, Z.; Kroon, D.E.; Casstevens, T.M.; Ramdoss, Y.; Buckler, E.S. TASSEL: Software for Association Mapping of Complex Traits in Diverse Samples. Bioinformatics 2007, 23, 2633–2635. [Google Scholar] [CrossRef]
| Chromosome | C. maxima | C. medica | C. reticulata | P. trifoliata | Total |
|---|---|---|---|---|---|
| C1 | 1101 | 2220 | 1338 | 3028 | 7687 |
| C2 | 1153 | 2654 | 1923 | 3724 | 9454 |
| C3 | 1579 | 3938 | 2420 | 5024 | 12,961 |
| C4 | 977 | 2221 | 1577 | 2962 | 7737 |
| C5 | 1118 | 2193 | 1471 | 2970 | 7752 |
| C6 | 885 | 1924 | 1325 | 2485 | 6619 |
| C7 | 880 | 1975 | 1320 | 2626 | 6801 |
| C8 | 935 | 2018 | 1319 | 2570 | 6842 |
| C9 | 816 | 2087 | 1248 | 2448 | 6599 |
| Total | 9444 | 21,230 | 13,941 | 27,837 | 72,452 |
| % | 13.03 | 29.3 | 19.24 | 38.42 | 100 |
| Swingle Citrumelo | Volkamer Lemon | |||
|---|---|---|---|---|
| LG | Mks/LG | LG Size cM | Mks/LG | LG Size cM |
| 1 | 149 | 36.47 | 106 | 49.16 |
| 2 | 172 | 68.94 | 126 | 27.10 |
| 3 | 236 | 98.46 | 187 | 105.41 |
| 4 | 143 | 37.09 | 108 | 26.40 |
| 5 | 139 | 43.34 | 105 | 79.72 |
| 6 | 116 | 46.59 | 94 | 30.25 |
| 7 | 128 | 34.59 | 96 | 30.09 |
| 8 | 119 | 28.20 | 97 | 20.10 |
| 9 | 116 | 38.97 | 97 | 56.78 |
| Total | 1318 | 432.64 | 1016 | 425 |
| Chromosome | Swingle Citrumelo | Volkamer Lemon | ||
|---|---|---|---|---|
| PHR (Rank) | PP (Rank) | PHR (Rank) | PP (Rank) | |
| Chr1 | 0.889 ± 0.004 (a) | 0.742 ± 0.007 (d) | 0.826 ± 0.008 (a) | 0.583 ± 0.019 (d) |
| Chr2 | 0.802 ± 0.006 (b) | 0.625 ± 0.033 (b) | 0.911 ± 0.008 (b) | 0.81 ± 0 (f) |
| Chr3 | 0.790 ± 0.007 (c) | 0.69 ± 0.054 (c) | 0.719 ± 0.008 (c) | 0.317 ± 0.061 (c) |
| Chr4 | 0.919 ± 0.004 (d) | 0.85 ± 0.043 (e) | 0.872 ± 0.005 (d) | 0.548 ± 0.061(d) |
| Chr5 | 0.849 ± 0.011 (e) | 0.848 ± 0.040 (e) | 0.692 ± 0.008 (e) | 0 ± 0 (a) |
| Chr6 | 0.890 ± 0.012 (adf) | 0.925 (f) | 0.869 ± 0.006 (d) | 0.675 ± 0.012 (e) |
| Chr7 | 0.817 ± 0.003 (g) | 0.502 ± 0.029 (a) | 0.874 ± 0.007 (d) | 0.663 ± 0.036 (e) |
| Chr8 | 0.933 ± 0.006 (h) | 0.923 ± 0.050 (f) | 0.897 ± 0.006 (f) | 0.672 ± 0.057 (e) |
| Chr9 | 0.917± 0.003 (f) | 0.76 ± 0.045 (d) | 0.764 ± 0.008 (g) | 0.25 ± 0.054 (b) |
| Whole genome | 0.859 ± 0.004 | 0.763 ± 0.054 | 0.817 ± 0.005 | 0.502 ± 0.099 |
| (a) Chromosome | N | −log(q-Value) | N < −log(0.05) | % |
|---|---|---|---|---|
| Chr1 | 149 | 4.276 ± 0.149 (c) | 149 | 100% |
| Chr2 | 172 | 1.874 ± 0.129 (ef) | 124 | 72.09% |
| Chr3 | 236 | 1.634 ± 0.171 (f) | 108 | 45.76% |
| Chr4 | 143 | 5.300 ± 0.115 (b) | 143 | 100% |
| Chr5 | 139 | 3.345 ± 0.275 (d) | 122 | 87.77% |
| Chr6 | 116 | 4.368 ± 0.378 (c) | 108 | 93.10 |
| Chr7 | 128 | 2.084 ± 0.102 (e) | 120 | 93.75% |
| Chr8 | 119 | 5.628 0.201 (a) | 119 | 100% |
| Chr9 | 116 | 5.097 ± 0.111 (b) | 116 | 100% |
| Total | 1318 | 3.492 ± 0.104 | 1109 | 84.14% |
| (b) Chromosome | N | −log(q-value) | N > −log(0.05) | % |
| Chr1 | 106 | 2.565 ± 0.178 (d) | 94 | 88.68% |
| Chr2 | 126 | 4.700 ± 0.222 (a) | 126 | 100% |
| Chr3 | 187 | 0.549 ± 0.087 (f) | 18 | 9.63% |
| Chr4 | 108 | 3.738 ± 0.113 (c) | 108 | 100% |
| Chr5 | 105 | 0.407 ± 0.098 (f) | 7 | 6.67% |
| Chr6 | 94 | 3.594 ± 0.153 (c) | 94 | 100% |
| Chr7 | 96 | 3.578 ± 0.210 (c) | 96 | 100% |
| Chr8 | 97 | 4.346 ± 0.190 (b) | 97 | 100% |
| Chr9 | 97 | 0.959 ± 0.157 (e) | 24 | 24.74% |
| Total | 1016 | 2.568 ± 0.113 | 664 | 65.35% |
| Gene ID | Trait | Location on P. trifoliata Reference Genome | Hybrids % with n Dose of Poncirus | |||||
|---|---|---|---|---|---|---|---|---|
| Scaffold | Start | End | 0 | 1 | 2 | N | ||
| Ptrif.0006s1501.1 | Huanglongbing tolerance | Scaffold 6 | 17843557 | 17847864 | 4.21 | 83.16 | 9.47 | 3.16 |
| Ptrif.0009s1449.1 | Scaffold 9 | 16743738 | 16750183 | 5.26 | 90.53 | 1.05 | 3.16 | |
| Ptrif.0009s1451.1 | Scaffold 9 | 16761424 | 16771587 | |||||
| Ptrif.0009s1453.1 | Scaffold 9 | 16803290 | 16817272 | |||||
| Ptrif.0009s1458.2 | Scaffold 9 | 16966396 | 16974146 | |||||
| Ptrif.0009s2550.1 | Scaffold 9 | 16873308 | 16878288 | |||||
| Ptrif.0009s2650.1 | Scaffold 9 | 16878879 | 16881473 | |||||
| Ptrif.0009s1595.1 | Scaffold 9 | 19302619 | 19305258 | 4.21 | 88.42 | 2.11 | 5.26 | |
| Ptrif.0009s1596.1 | Scaffold 9 | 19351433 | 19355794 | |||||
| Ptrif.0009s1599.1 | Scaffold 9 | 19383517 | 19386756 | |||||
| Ptrif.0009s1600.1 | Scaffold 9 | 19400932 | 19403616 | |||||
| Ptrif.0007s1586.1 | Citrus tristeza virus disease resistance | Scaffold 7 | 11780276 | 11787445 | 10.53 | 77.89 | 9.47 | 2.11 |
| Ptrif.0007s1587.1 | Scaffold 7 | 11823916 | 11826636 | |||||
| Ptrif.0007s1590.1 | Scaffold 7 | 11860949 | 11863630 | |||||
| Ptrif.0007s1595.1 | Scaffold 7 | 11908817 | 11912236 | |||||
| Ptrif.0007s1378.1 | Nematode resistance | Scaffold 7 | 9840644 | 9843519 | 11.58 | 78.95 | 7.37 | 2.11 |
| Ptrif.0007s1394.1 | Scaffold 7 | 9974384 | 9977152 | 11.58 | 76.84 | 9.47 | 2.11 | |
| Ptrif.0007s1395.1 | Scaffold 7 | 9985962 | 9988577 | |||||
| Ptrif.0007s1396.1 | Scaffold 7 | 9990366 | 9991216 | |||||
| Ptrif.0007s1398.1 | Scaffold 7 | 10007442 | 10010437 | |||||
| Ptrif.0007s1402.1 | Scaffold 7 | 10043069 | 10045927 | |||||
| Ptrif.0007s1404.1 | Scaffold 7 | 10056890 | 10060283 | |||||
| Ptrif.0007s1406.2 | Scaffold 7 | 10065703 | 10067962 | |||||
| Ptrif.0007s1411.2 | Scaffold 7 | 10097497 | 10100154 | |||||
| Ptrif.0007s1415.1 | Scaffold 7 | 10123503 | 10126433 | |||||
| Ptrif.0007s2703.1 | Scaffold 7 | 10139209 | 10144561 | |||||
| Ptrif.0007s1481.1 | Scaffold 7 | 10687572 | 10691702 | 11.58 | 78.95 | 9.47 | 0 | |
| Ptrif.0007s1484.1 | Scaffold 7 | 10729463 | 10732201 | |||||
| Ptrif.0007s1501.1 | Scaffold 7 | 10812404 | 10852651 | |||||
| Ptrif.0007s1502.1 | Scaffold 7 | 10860634 | 10863348 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calvez, L.; Dereeper, A.; Perdereau, A.; Mournet, P.; Miranda, M.; Bruyère, S.; Hufnagel, B.; Froelicher, Y.; Lemainque, A.; Morillon, R.; et al. Meiotic Behaviors of Allotetraploid Citrus Drive the Interspecific Recombination Landscape, the Genetic Structures, and Traits Inheritance in Tetrazyg Progenies Aiming to Select New Rootstocks. Plants 2023, 12, 1630. https://doi.org/10.3390/plants12081630
Calvez L, Dereeper A, Perdereau A, Mournet P, Miranda M, Bruyère S, Hufnagel B, Froelicher Y, Lemainque A, Morillon R, et al. Meiotic Behaviors of Allotetraploid Citrus Drive the Interspecific Recombination Landscape, the Genetic Structures, and Traits Inheritance in Tetrazyg Progenies Aiming to Select New Rootstocks. Plants. 2023; 12(8):1630. https://doi.org/10.3390/plants12081630
Chicago/Turabian StyleCalvez, Lény, Alexis Dereeper, Aude Perdereau, Pierre Mournet, Maëva Miranda, Saturnin Bruyère, Barbara Hufnagel, Yann Froelicher, Arnaud Lemainque, Raphaël Morillon, and et al. 2023. "Meiotic Behaviors of Allotetraploid Citrus Drive the Interspecific Recombination Landscape, the Genetic Structures, and Traits Inheritance in Tetrazyg Progenies Aiming to Select New Rootstocks" Plants 12, no. 8: 1630. https://doi.org/10.3390/plants12081630
APA StyleCalvez, L., Dereeper, A., Perdereau, A., Mournet, P., Miranda, M., Bruyère, S., Hufnagel, B., Froelicher, Y., Lemainque, A., Morillon, R., & Ollitrault, P. (2023). Meiotic Behaviors of Allotetraploid Citrus Drive the Interspecific Recombination Landscape, the Genetic Structures, and Traits Inheritance in Tetrazyg Progenies Aiming to Select New Rootstocks. Plants, 12(8), 1630. https://doi.org/10.3390/plants12081630






