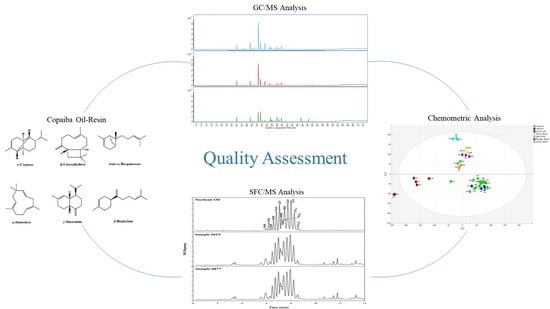
Chemical Characterization and Quality Assessment of Copaiba Oil-Resin Using GC/MS and SFC/MS
Abstract
1. Introduction
2. Results and Discussion
2.1. Characterization of the Chemical Composition of Copaiba Oil-Resin by GC/MS Analysis
2.2. Chemometric Analyses
2.3. Detection of Adulteration and Identification of Adulterants
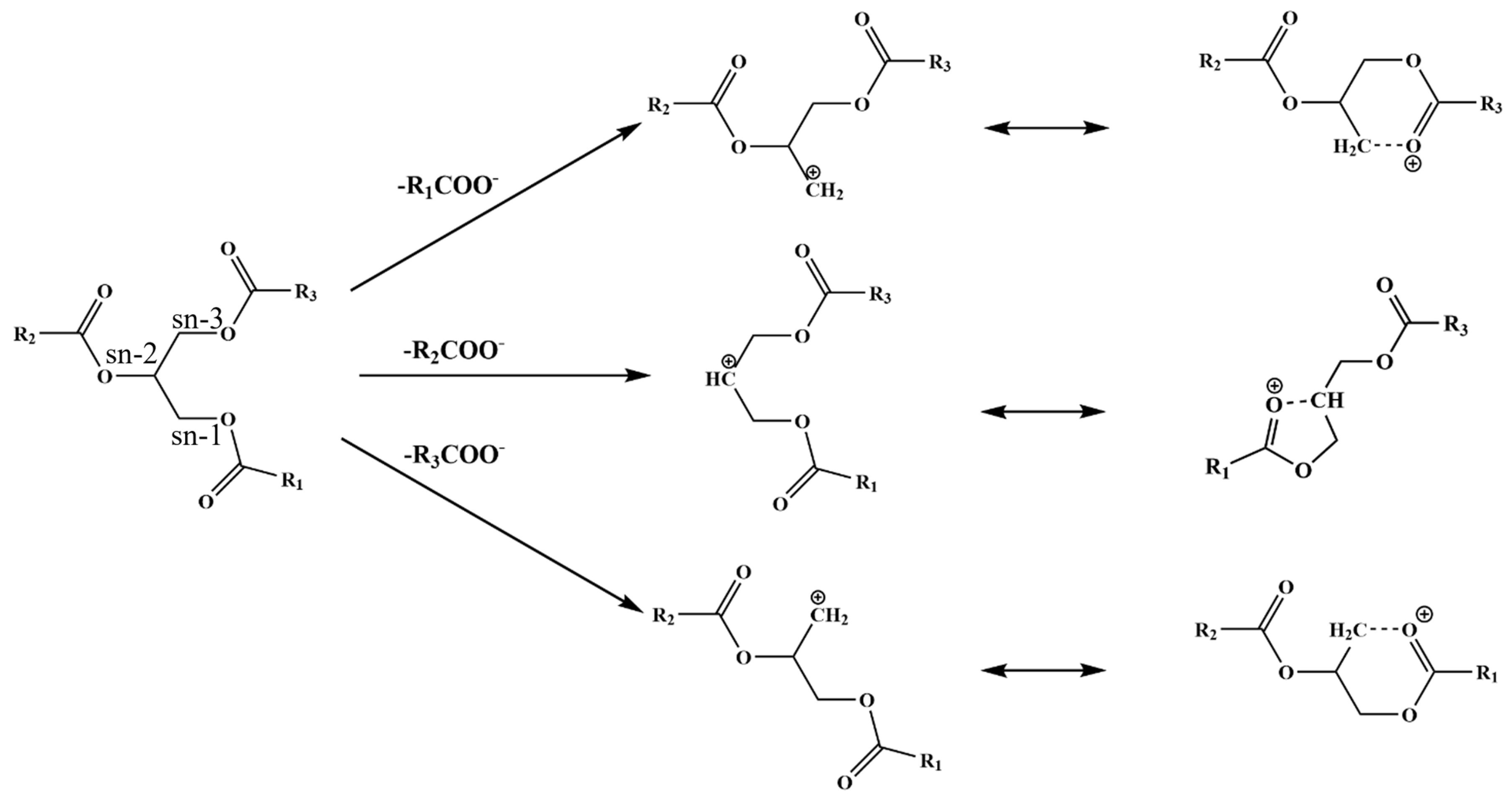
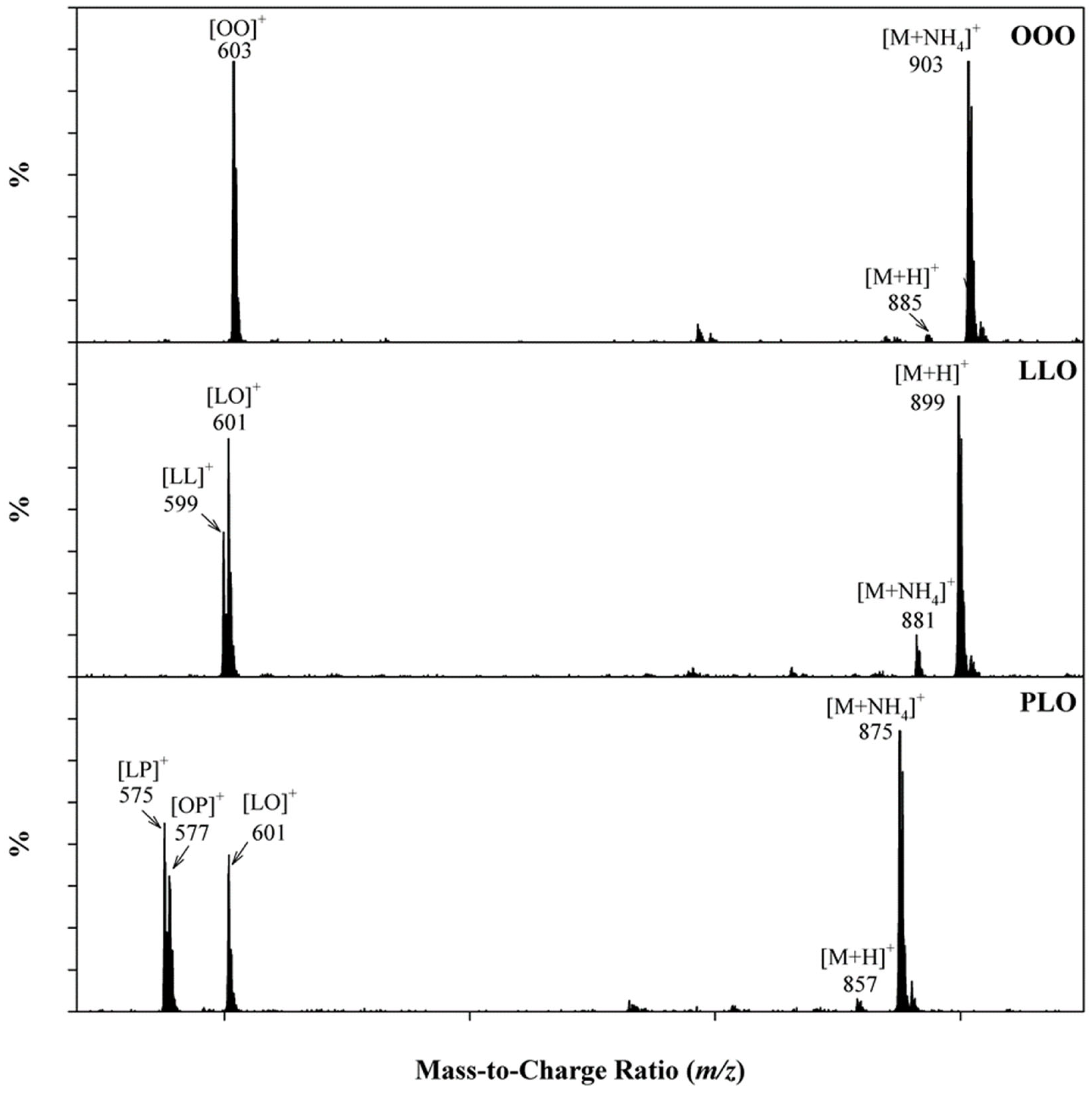
2.3.1. Unambiguous Identification of Triglycerides Using SFC/MS
2.3.2. Detection of Soybean Oil in Commercial Copaiba Oil-Resin Samples
3. Materials and Methods
3.1. Copaiba Oil-Resin Samples
3.2. Chemicals and Reagents
3.3. Sample Preparation
3.4. Gas Chromatography/Mass Spectrometry (GC/MS) Analysis
3.5. Supercritical Fluid Chromatography/Mass Spectrometry (SFC/MS) Analysis
3.6. Statistical Analyses
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Da Trindade, R.; Da Silva, J.K.; Setzer, W.N. Copaifera of the Neotropics: A Review of the Phytochemistry and Pharmacology. Int. J. Mol. Sci. 2018, 19, 1511. [Google Scholar] [CrossRef] [PubMed]
- Arruda, C.; Mejía, J.A.A.; Ribeiro, V.P.; Borges, C.H.G.; Martins, C.H.G.; Veneziani, R.C.S.; Ambrosio, S.R.; Bastos, J.K. Occurrence, chemical composition, biological activities and analytical methods on Copaifera genus—A review. Biomed. Pharmacother. 2019, 109, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Plowden, C. The Ethnobotany of Copaíba (Copaifera) Oleoresin in the Amazon. Econ. Bot. 2004, 58, 729–733. [Google Scholar] [CrossRef]
- dos Santos Menezes, A.C.; Alves, L.D.B.; Goldemberg, D.C.; de Melo, A.C.; Antunes, H.S. Anti-inflammatory and wound healing effect of Copaiba oleoresin on the oral cavity: A systematic review. Heliyon 2022, 8, e08993. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, K.d.S.; Yoshida, M.; Scudeller, V.V. Detection of adulterated copaiba (Copaifera multijuga Hayne) oil-resins by refractive index and thin layer chromatography. Rev. Bras. De Farmacogn. 2009, 19, 57–60. [Google Scholar] [CrossRef]
- Leandro, L.M.; de Sousa Vargas, F.; Barbosa, P.C.S.; Neves, J.K.O.; Da Silva, J.A.; Veiga-Junior, D.; Florêncio, V. Chemistry and biological activities of terpenoids from copaiba (Copaifera spp.) oleoresins. Molecules 2012, 17, 3866–3889. [Google Scholar] [CrossRef]
- Junior, V.V.; Rosas, E.; Carvalho, M.V.d.; Henriques, M.d.G.M.d.O.; Pinto, A.C. Chemical composition and anti-inflammatory activity of copaiba oils from Copaifera cearensis Huber ex Ducke, Copaifera reticulata Ducke and Copaifera multijuga Hayne—A comparative study. J. Ethnopharmacol. 2007, 112, 248–254. [Google Scholar] [CrossRef]
- Gelmini, F.; Beretta, G.; Anselmi, C.; Centini, M.; Magni, P.; Ruscica, M.; Cavalchini, A.; Facino, R.M. GC–MS profiling of the phytochemical constituents of the oleoresin from Copaifera langsdorffii Desf. and a preliminary in vivo evaluation of its antipsoriatic effect. Int. J. Pharm. 2013, 440, 170–178. [Google Scholar] [CrossRef]
- Zoghbi, M.d.G.B.; Andrade, E.H.; Martins-da-Silva, R.C.; Trigo, J.R. Chemical variation in the volatiles of Copaifera reticulata Ducke (Leguminosae) growing wild in the states of Pará and Amapá, Brazil. J. Essent. Oil Res. 2009, 21, 501–503. [Google Scholar] [CrossRef]
- Xavier-Junior, F.H.; Maciuk, A.; Rochelle do Vale Morais, A.; Alencar, E.d.N.; Garcia, V.L.; Tabosa do Egito, E.S.; Vauthier, C. Development of a gas chromatography method for the analysis of copaiba oil. J. Chromatogr. Sci. 2017, 55, 969–978. [Google Scholar] [CrossRef]
- Souza Barbosa, P.C.; Moreira Wiedemann, L.S.; da Silva Medeiros, R.; de Tarso Barbosa Sampaio, P.; Vieira, G.; Florêncio da Veiga-Junior, V. Phytochemical fingerprints of copaiba oils (Copaifera multijuga Hayne) determined by multivariate analysis. Chem. Biodivers. 2013, 10, 1350–1360. [Google Scholar] [CrossRef]
- Mangabeira da Silva, J.J.; Pena Ribeiro, V.; Lemos, M.; Miller Crotti, A.E.; Rogez, H.; Kenupp Bastos, J. Reliable methods for analyses of volatile compounds of Copaifera oleoresins combining headspace and gas chromatography. Chem. Biodivers. 2020, 17, e1900440. [Google Scholar] [CrossRef]
- Zoghbi, M.d.G.B.; Lameira, O.A.; Oliveira, E.C. Seasonal variation of oleoresin and volatiles from Copaifera martii Hayne growing wild in the state of Pará, Brazil. J. Essent. Oil Res. 2007, 19, 504–506. [Google Scholar] [CrossRef]
- Gramosa, N.V.; Silveira, E.R. Volatile constituents of Copaifera langsdorffii from the Brazilian northeast. J. Essent. Oil Res. 2005, 17, 130–132. [Google Scholar] [CrossRef]
- do Nascimento, M.E.; Zoghbi, M.d.G.B.; Pinto, J.E.B.P.; Bertolucci, S.K.V. Chemical variability of the volatiles of Copaifera langsdorffii growing wild in the Southeastern part of Brazil. Biochem. Syst. Ecol. 2012, 43, 1–6. [Google Scholar] [CrossRef]
- Cascon, V.; Gilbert, B. Characterization of the chemical composition of oleoresins of Copaifera guianensis Desf., Copaifera duckei Dwyer and Copaifera multijuga Hayne. Phytochemistry 2000, 55, 773–778. [Google Scholar] [CrossRef]
- Tappin, M.R.; Pereira, J.F.; Lima, L.A.; Siani, A.C.; Mazzei, J.L.; Ramos, M.F. Quantitative chemical analysis for the standardization of copaiba oil by high resolution gas chromatograpy. Quim. Nova 2004, 27, 236–240. [Google Scholar] [CrossRef]
- Hanbury, D. On Wood Oil, A Substitute for Copaiba. Am. J. Pharm. (1835–1907) 1856, 4, 159. [Google Scholar]
- Sousa, J.P.B.; Brancalion, A.P.; Souza, A.B.; Turatti, I.C.; Ambrósio, S.R.; Furtado, N.A.; Lopes, N.P.; Bastos, J.K. Validation of a gas chromatographic method to quantify sesquiterpenes in copaiba oils. J. Pharm. Biomed. Anal. 2011, 54, 653–659. [Google Scholar] [CrossRef]
- Wang, M.; Avula, B.; Wang, Y.-H.; Zhao, J.; Avonto, C.; Parcher, J.F.; Raman, V.; Zweigenbaum, J.A.; Wylie, P.L.; Khan, I.A. An integrated approach utilising chemometrics and GC/MS for classification of chamomile flowers, essential oils and commercial products. Food Chem. 2014, 152, 391–398. [Google Scholar] [CrossRef]
- Wang, M.; Zhao, J.; Avula, B.; Wang, Y.-H.; Chittiboyina, A.G.; Parcher, J.F.; Khan, I.A. Quality evaluation of terpinen-4-ol-type Australian tea tree oils and commercial products: An integrated approach using conventional and chiral GC/MS combined with chemometrics. J. Agric. Food Chem. 2015, 63, 2674–2682. [Google Scholar] [CrossRef] [PubMed]
- Merchak, N.; Rizk, T.; Silvestre, V.; Remaud, G.S.; Bejjani, J.; Akoka, S. Olive oil characterization and classification by 13C NMR with a polarization transfer technique: A comparison with gas chromatography and 1H NMR. Food Chem. 2018, 245, 717–723. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Yu, P.; Chittiboyina, A.G.; Chen, D.; Zhao, J.; Avula, B.; Wang, Y.-H.; Khan, I.A. Characterization, quantification and quality assessment of avocado (Persea americana Mill.) oils. Molecules 2020, 25, 1453. [Google Scholar] [CrossRef] [PubMed]
- Wei, N.; Wang, M.; Adams, S.J.; Yu, P.; Avula, B.; Wang, Y.-H.; Pan, K.; Wang, Y.; Khan, I.A. Comparative study and quality evaluation regarding morphology characters, volatile constituents, and triglycerides in seeds of five species used in traditional Chinese medicine. J. Pharm. Biomed. Anal. 2021, 194, 113801. [Google Scholar] [CrossRef]
- Andrikopoulos, N.K. Triglyceride species compositions of common edible vegetable oils and methods used for their identification and quantification. Food Rev. Int. 2002, 18, 71–102. [Google Scholar] [CrossRef]
- Řezanka, T.; Kolouchová, I.; Nedbalová, L.; Sigler, K. Enantiomeric separation of triacylglycerols containing very long chain fatty acids. J. Chromatogr. A 2018, 1557, 9–19. [Google Scholar] [CrossRef]
- Sirbu, D.; Corno, M.; Ullrich, M.S.; Kuhnert, N. Characterization of triacylglycerols in unfermented cocoa beans by HPLC-ESI mass spectrometry. Food Chem. 2018, 254, 232–240. [Google Scholar] [CrossRef]
- Indelicato, S.; Bongiorno, D.; Pitonzo, R.; Di Stefano, V.; Calabrese, V.; Indelicato, S.; Avellone, G. Triacylglycerols in edible oils: Determination, characterization, quantitation, chemometric approach and evaluation of adulterations. J. Chromatogr. A 2017, 1515, 1–16. [Google Scholar] [CrossRef]
- Yoshida, H.; Tomiyama, Y.; Yoshida, N.; Mizushina, Y. Characteristics of lipid components, fatty acid distributions and triacylglycerol molecular species of adzuki beans (Vigna angularis). Food Chem. 2009, 115, 1424–1429. [Google Scholar] [CrossRef]
- Holčapek, M.; Lísa, M.; Jandera, P.; Kabátová, N. Quantitation of triacylglycerols in plant oils using HPLC with APCI-MS, evaporative light-scattering, and UV detection. J. Sep. Sci. 2005, 28, 1315–1333. [Google Scholar] [CrossRef]
- Holčapek, M.; Jandera, P.; Zderadička, P.; Hrubá, L. Characterization of triacylglycerol and diacylglycerol composition of plant oils using high-performance liquid chromatography–atmospheric pressure chemical ionization mass spectrometry. J. Chromatogr. A 2003, 1010, 195–215. [Google Scholar] [CrossRef]
- Agapito, F.; Nunes, P.M.; Costa Cabral, B.J.; Borges dos Santos, R.M.; Martinho Simoes, J.A. Energetic differences between the five-and six-membered ring hydrocarbons: Strain energies in the parent and radical molecules. J. Org. Chem. 2008, 73, 6213–6223. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by gas Chromatography/Mass Spectrometry; Allured Publishing Corporation: Carol Stream, IL, USA, 2007; Volume 456. [Google Scholar]


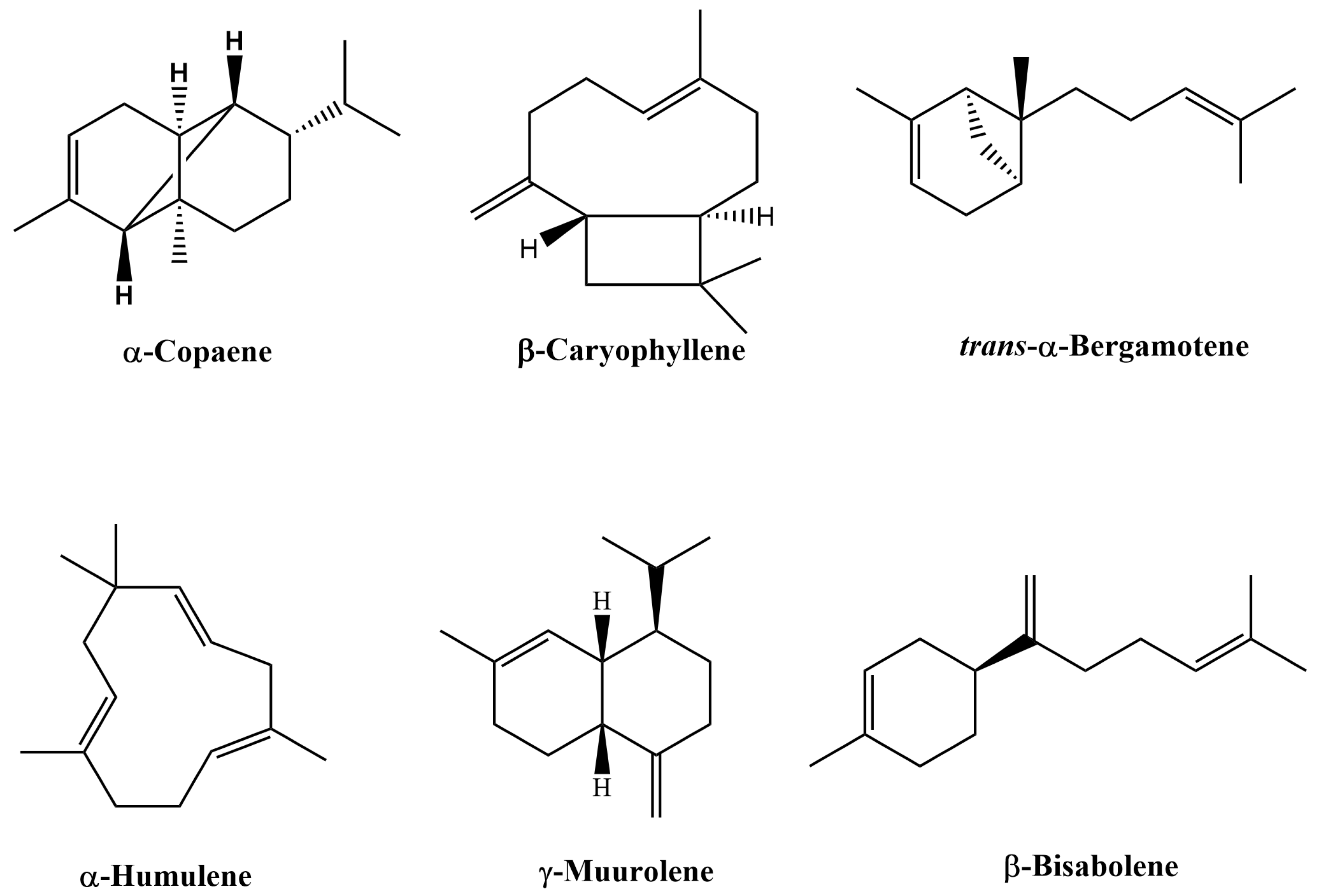



| No. | Compound * | RRICal | RRILit | Authentic Sample | Unknown Sample | Unknown Sample with Known Location | Commercial Sample |
|---|---|---|---|---|---|---|---|
| 1 | δ-EIemene | 1342 | 1338 | 0.84–1.92 (1.15) | 0.00–2.25 (1.00) | 0.05–0.96 (0.35) | 0.00–0.07 (0.04) |
| 2 | α-Cubebene | 1355 | 1351 | 0.65–1.45 (0.83) | 0.00–1.91 (0.73) | 0.20–0.59 (0.35) | 0.00–4.65 (0.78) |
| 3 | α-Ylangene | 1379 | 1375 | 0.13–0.25 (0.18) | 0.09–0.63 (0.27) | 0.04–0.16 (0.08) | 0.00–0.18 (0.08) |
| 4 | α-Copaene | 1384 | 1376 | 7.33–11.25 (9.28) | 3.66–11.3 (8.66) | 3.55–5.96 (5.01) | 0.00–32.93 (5.95) |
| 5 | 7-epi-Sesquithujene | 1395 | 1391 | 0.12–0.21 (0.15) | 0.03–1.95 (0.28) | 0.07–0.26 (0.20) | 0.00–0.16 (0.06) |
| 6 | β-Elemene | 1399 | 1390 | 1.43–1.94 (1.81) | 0.00–2.23 (1.35) | 1.10–1.64 (1.30) | 0.00–6.20 (1.42) |
| 7 | Cyperene | 1409 | 1398 | 0.64–0.80 (0.75) | 0.16–0.73 (0.59) | 0.25–0.49 (0.39) | 0.00–0.02 (0.00) |
| 8 | α-Cedrene | 1419 | 1411 | 0.15–0.20 (0.19) | 0.00–0.21 (0.16) | 0.00–0.32 (0.15) | 0.00–0.03 (0.01) |
| 9 | β-Caryophyllene | 1427 | 1419 | 38.58–45.77 (43.71) | 35.49–67.71 (43.11) | 11.61–56.87 (36.34) | 0.00–43.40 (20.87) |
| 10 | cis-β-Copaene | 1433 | 1432 | 0.22–0.31 (0.24) | 0.12–0.94 (0.34) | 0.04–0.15 (0.09) | 0.00–0.05 (0.03) |
| 11 | γ-Elemene | 1435 | 1436 | 1.46–1.86 (1.76) | 0.00–2.18 (1.42) | 0.00–0.96 (0.21) | 0.00 |
| 12 | trans-α-Bergamotene | 1437 | 1434 | 7.55–8.31 (7.78) | 3.46–8.94 (6.87) | 2.29–14.24 (9.23) | 0.00–6.09 (2.81) |
| 13 | Aromandendrene | 1443 | 1441 | 0.37–0.44 (0.40) | 0.12–0.67 (0.43) | 0.13–0.54 (0.32) | 0.00–0.08 (0.03) |
| 14 | trans-β-Farnesene | 1454 | 1456 | 0.30–0.43 (0.34) | 0.00–0.46 (0.22) | 0.04–0.60 (0.36) | 0.00–0.20 (0.10) |
| 15 | α-Humulene | 1458 | 1454 | 6.26–7.56 (7.10) | 6.07–10.57 (7.67) | 1.91–10.37 (6.50) | 0.00–7.84 (3.57) |
| 16 | allo-Aromadendrene | 1465 | 1460 | 0.46–0.50 (0.48) | 0.20–0.71 (0.48) | 0.00–0.46 (0.35) | 0.00–2.95 (0.49) |
| 17 | Cadina-1(6),4-diene | 1477 | 1463 | 0.50–0.65 (0.57) | 0.18–1.10 (0.53) | 0.18–0.47 (0.29) | 0.00–0.19 (0.05) |
| 18 | γ-Muurolene | 1480 | 1479 | 2.97–3.46 (3.08) | 1.06–4.06 (3.25) | 1.80–4.01 (2.85) | 0.00–0.63 (0.24) |
| 19 | α-Amorphene | 1484 | 1484 | 0.25–0.34 (0.29) | 0.00–0.47 (0.09) | 0.00–0.32 (0.08) | 0.00–0.10 (0.02) |
| 20 | Germacrene D | 1486 | 1481 | 1.34–2.73 (1.84) | 0.00–2.46 (0.99) | 0.00–0.72 (0.14) | 0.00–1.96 (0.33) |
| 21 | 9-epi-β-Caryophyllene | 1488 | 1466 | 0.54–0.69 (0.59) | 0.00–0.76 (0.50) | 0.00–2.74 (0.77) | 0.00–0.69 (0.27) |
| 22 | β-Eudesmene | 1492 | 1490 | 0.47–0.65 (0.57) | 0.26–0.97 (0.47) | 0.41–2.36 (1.29) | 0.00–11.04 (2.42) |
| 23 | Viridiflorene | 1499 | 1496 | 1.49–1.64 (1.54) | 0.00–2.01 (1.02) | 0.00–0.56 (0.09) | 0.00–0.19 (0.03) |
| 24 | α-Selinene | 1501 | 1498 | 0.52–0.61 (0.57) | 0.31–1.02 (0.54) | 0.41–2.15 (1.19) | 0.00–6.19 (1.39) |
| 25 | Bicyclogermacrene | 1504 | 1500 | 0.10–0.22 (0.14) | 0.00–0.22 (0.05) | 0.00–0.06 (0.01) | 0.00–0.43 (0.16) |
| 26 | α-Muurolene | 1506 | 1500 | 0.98–1.40 (1.11) | 0.00–2.54 (1.29) | 0.00–1.90 (0.87) | 0.00–0.44 (0.16) |
| 27 | β-Bisabolene | 1513 | 1505 | 2.78–3.78 (3.13) | 1.59–5.38 (3.16) | 0.63–10.11(5.48) | 0.00–9.31 (4.12) |
| 28 | β-Curcumene | 1516 | 1515 | 0.10–0.18 (0.15) | 0.08–0.27 (0.16) | 0.00–0.91 (0.33) | 0.00–0.51 (0.20) |
| 29 | γ-Cadinene | 1521 | 1513 | 0.95–1.57 (1.27) | 0.66–2.09 (1.54) | 1.44–4.88 (2.49) | 0.00–7.24 (3.64) |
| 30 | δ-Cadinene | 1534 | 1523 | 4.41–5.65 (4.99) | 0.17–6.91 (4.76) | 3.99–7.28 (5.74) | 0.00–8.10 (2.02) |
| 31 | trans-Cadina-1,4-diene | 1541 | 1534 | 0.11–0.19 (0.14) | 0.07–0.36 (0.18) | 0.09–0.19 (0.15) | 0.00–0.31 (0.06) |
| 32 | α-Cadinene | 1546 | 1538 | 0.32–0.42 (0.35) | 0.16–0.59 (0.37) | 0.00–0.69 (0.24) | 0.00–0.68 (0.12) |
| 33 | trans-γ-Bisabolene | 1550 | 1531 | 0.20–0.33 (0.24) | 0.10–0.49 (0.25) | 0.00–1.73 (0.72) | 0.00–0.43 (0.15) |
| 34 | Selina-3,7(11)-diene | 1551 | 1564 | 0.00–0.17 (0.11) | 0.00–0.35 (0.13) | 0.29–1.11 (0.53) | 0.00–0.10 (0.02) |
| 35 | Caryophyllenyl alcohol | 1578 | 1572 | 0.14–0.19 (0.15) | 0.08–0.58 (0.21) | 0.12–0.81 (0.39) | 0.00–0.11 (0.05) |
| 36 | Caryophyllene oxide | 1590 | 1583 | 0.18–0.23 (0.21) | 0.00–3.53 (0.67) | 0.00–0.37 (0.23) | 0.00–1.45 (0.64) |
| 37 | Gleenol | 1619 | 1587 | 0.13–0.22 (0.17) | 0.00–0.35 (0.19) | 0.00–0.34 (0.22) | 0.00–0.11 (0.02) |
| 38 | Junenol | 1627 | 1619 | 0.10–0.31 (0.19) | 0.10–0.99 (0.35) | 0.37–0.74 (0.50) | 0.00–1.11 (0.22) |
| 39 | τ-MuuroloI | 1652 | 1642 | 0.06–0.16 (0.09) | 0.00–0.73 (0.19) | 0.11–0.79 (0.43) | 0.00–0.40 (0.08) |
| 40 | δ-Cadinol | 1657 | 1646 | 0.04–0.12 (0.07) | 0.00–0.66 (0.17) | 0.12–0.73 (0.50) | 0.00–0.07 (0.02) |
| 41 | α-Cadinol | 1686 | 1654 | 0.13–0.23 (0.17) | 0.07–0.25 (0.18) | 0.11–0.21 (0.15) | 0.00–0.03 (0.01) |
| 42 | Eudesm-7(11)-en-4-ol | 1691 | 1700 | 0.14–0.25 (0.21) | 0.07–0.60 (0.22) | 0.00–0.70 (0.43) | 0.00–0.06 (0.03) |
| 43 | 16-Kaurene | 2035 | 2043 | 0.00 | 0.00–0.22 (0.01) | 0.00–0.40 (0.16) | 0.00–0.15 (0.03) |
| 44 | Manool | 2038 | 2057 | 0.00 | 0.00–0.33 (0.03) | 0.00–0.11 (0.04) | 0.00 |
| 45 | Kolavelool | 2043 | - | 0.00 | 0.00–1.34 (0.14) | 0.00–0.11 (0.03) | 0.00 |
| 46 | Kolavenol | 2323 | 2297 | 0.00 | 0.00–1.07 (0.09) | 0.00–0.34 (0.10) | 0.00–0.18 (0.03) |
| 47 | Methyl kolavenate | 2395 | - | 0.00 | 0.00–0.52 (0.03) | 0.00–0.10 (0.03) | 0.00–3.68 (1.30) |
| NCNPR Code | Information on Label | Country of Origin |
|---|---|---|
| Authentic Samples | ||
| 613 | Raw Copaiba | Brazil |
| 614 | Raw Copaiba | Brazil |
| 615 | Raw Copaiba | Brazil |
| 616 | Raw Copaiba | Brazil |
| 860 | Raw Copaiba | Brazil |
| Unknown Samples | ||
| 327 | Copaifera officinalis | N/A |
| 617 | Raw VC Copaiba | Brazil |
| 618 | Copaiba | Brazil |
| 621 | Oil of Balsam Copaiba | N/A |
| 622 | Copaiba | N/A |
| 623 | Copaifera officinalis | N/A |
| 624 | Copaiba | Brazil |
| 625 | Copaiba | N/A |
| 626 | Copaiba | N/A |
| 629 | Copaifera | Brazil |
| 630 | Copaiba | N/A |
| 863 | Copaiba balsam | N/A |
| 865 | Copaiba | N/A |
| 871 | Copaiba | N/A |
| 873 | Copaiba | N/A |
| 878 | Raw Copaiba | N/A |
| 880 | Copaifera officinalis | N/A |
| 888 | Copaifera officinalis | Tapaua, Brazil |
| 889 | Copaifera officinalis | Tapaua, Brazil |
| 890 | Copaifera officinalis | Tapaua, Brazil |
| 891 | Copaifera officinalis | Apui, Brazil |
| 892 | Copaifera officinalis | Apui, Brazil |
| 893 | Copaifera officinalis | Apui, Brazil |
| 894 | Copaifera officinalis | Parintins, Brazil |
| 895 | Copaifera officinalis | Parintins, Brazil |
| 896 | Copaifera officinalis | Parintins, Brazil |
| 897 | Copaifera officinalis | Labrea, Brazil |
| 898 | Copaifera officinalis | Labrea, Brazil |
| 899 | Copaifera officinalis | Labrea, Brazil |
| Samples from Commercial Sources | ||
| 619 | Copaiba | Peru |
| 620 | Copaifera | Ecuador |
| 628 | Copaiba | Peru |
| 862 | Copaiba | Peru |
| 874 | Copaifera | Ecuador |
| 877 | Copaiba | Peru |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.; Wang, M.; Zhao, J.; Ali, Z.; Hawwal, M.F.; Khan, I.A. Chemical Characterization and Quality Assessment of Copaiba Oil-Resin Using GC/MS and SFC/MS. Plants 2023, 12, 1619. https://doi.org/10.3390/plants12081619
Lee J, Wang M, Zhao J, Ali Z, Hawwal MF, Khan IA. Chemical Characterization and Quality Assessment of Copaiba Oil-Resin Using GC/MS and SFC/MS. Plants. 2023; 12(8):1619. https://doi.org/10.3390/plants12081619
Chicago/Turabian StyleLee, Joseph, Mei Wang, Jianping Zhao, Zulfiqar Ali, Mohammed F. Hawwal, and Ikhlas A. Khan. 2023. "Chemical Characterization and Quality Assessment of Copaiba Oil-Resin Using GC/MS and SFC/MS" Plants 12, no. 8: 1619. https://doi.org/10.3390/plants12081619
APA StyleLee, J., Wang, M., Zhao, J., Ali, Z., Hawwal, M. F., & Khan, I. A. (2023). Chemical Characterization and Quality Assessment of Copaiba Oil-Resin Using GC/MS and SFC/MS. Plants, 12(8), 1619. https://doi.org/10.3390/plants12081619