Glyoxalase I Assay as a Possible Tool for Evaluation of Biological Activity of Antioxidant-Rich Plant Extracts
Abstract
1. Introduction
2. Results
2.1. Antioxidant Capacity Evaluation of Plant Food Extracts
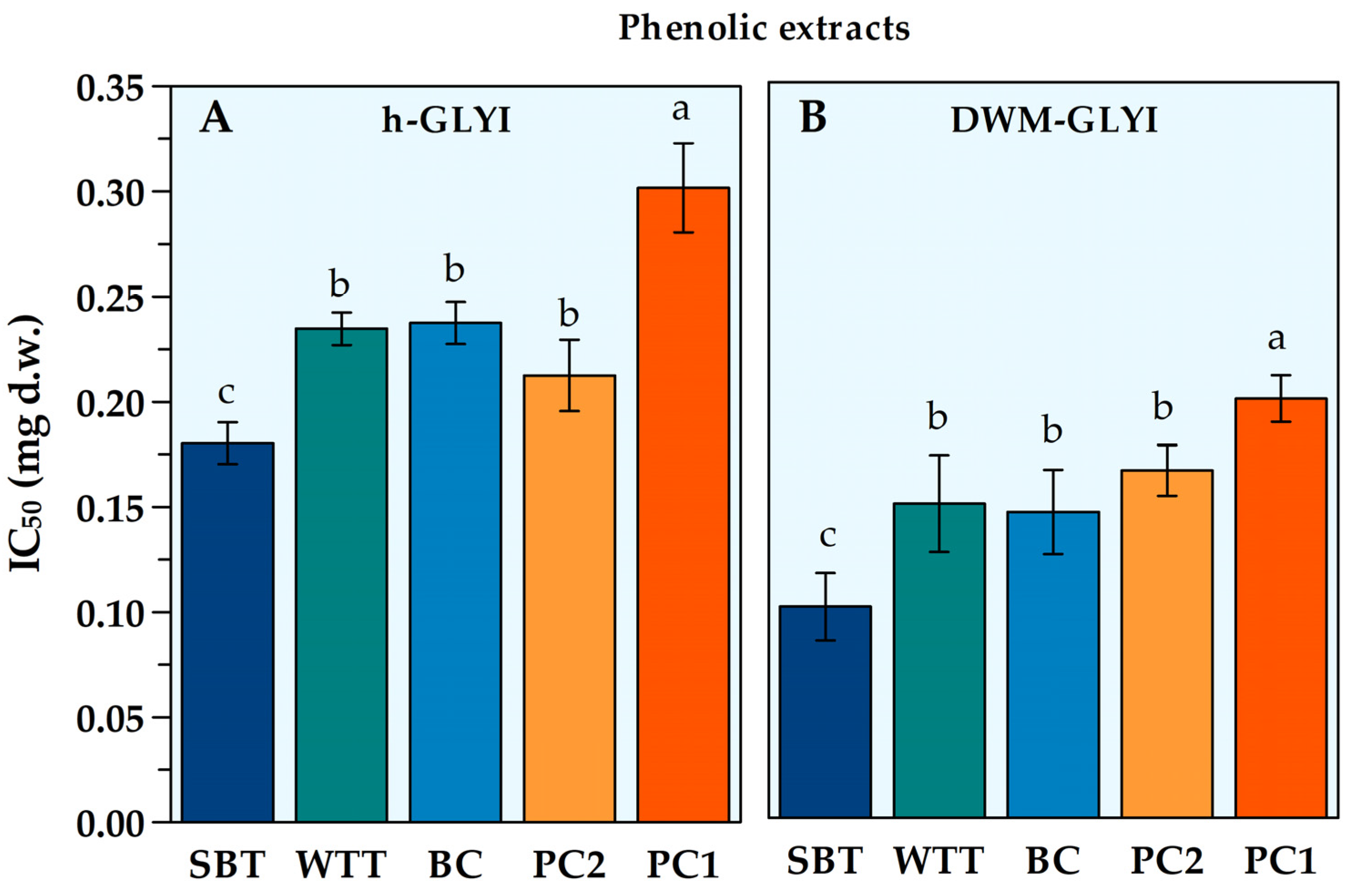
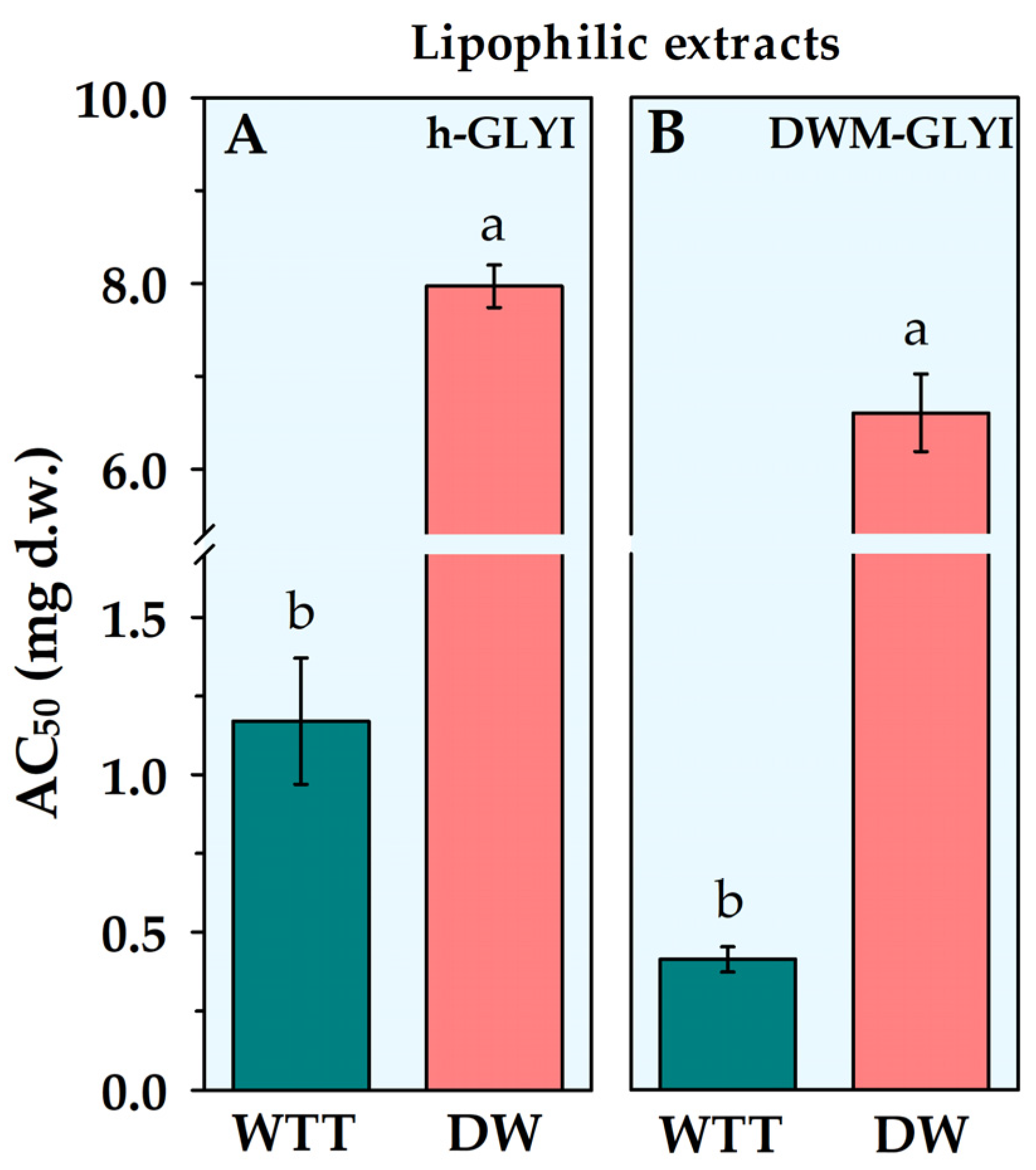
2.2. Evaluation of Effects of Plant Food Extracts on h-GLYI and DWM-GLYI Activities
2.3. Comparison of Performance of DWM-GLYI and h-GLYI Assays
3. Discussion
4. Materials and Methods
4.1. Chemicals and Plant Materials
4.2. Preparation of Plant Food Extracts
4.2.1. Extraction of Lipophilic Compounds from Durum Wheat Whole Flour and Wildtype Tomato Fruits
4.2.2. Extraction of Phenolic Compounds from Durum Wheat Whole Flour, Tomato Fruits and Carrots
4.3. In Vitro Evaluation of Antioxidant Capacity of Plant Food Extracts Using TEAC, ORAC, and LOX-FL Methods
4.3.1. TEAC Method
4.3.2. ORAC Method
4.3.3. LOX-FL Method
4.4. Isolation of Durum Wheat Mitochondria (DWM)
4.5. Glyoxalase I Activity Assay
4.6. Spectrophotometric Determination of Total Carotenoid Content
4.7. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Kasote, D.M.; Katyare, S.S.; Hegde, M.V.; Bae, H. Significance of Antioxidant Potential of Plants and Its Relevance to Therapeutic Applications. Int. J. Biol. Sci. 2015, 11, 982–991. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Zhong, Y. Measurement of Antioxidant Activity. J. Funct. Foods 2015, 18, 757–781. [Google Scholar] [CrossRef]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.D.; Mazur, M.; Telser, J. Free Radicals and Antioxidants in Normal Physiological Functions and Human Disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef] [PubMed]
- Nigro, C.; Leone, A.; Fiory, F.; Prevenzano, I.; Nicolò, A.; Mirra, P.; Beguinot, F.; Miele, C. Dicarbonyl Stress at the Crossroads of Healthy and Unhealthy Aging. Cells 2019, 8, 749. [Google Scholar] [CrossRef]
- Semchyshyn, H.M.; Lushchak, V.I. Interplay Between Oxidative and Carbonyl Stresses: Molecular Mechanisms, Biological Effects and Therapeutic Strategies of Protection. In Oxidative Stress; Lushchak, V., Semchyshyn, H.M., Eds.; IntechOpen: Rijeka, Croatia, 2012. [Google Scholar]
- Mostofa, M.G.; Ghosh, A.; Li, Z.G.; Siddiqui, M.N.; Fujita, M.; Tran, L.S.P. Methylglyoxal—A Signaling Molecule in Plant Abiotic Stress Responses. Free. Radic. Biol. Med. 2018, 122, 96–109. [Google Scholar] [CrossRef]
- Rabbani, N.; Xue, M.; Thornalley, P.J. Dicarbonyls and Glyoxalase in Disease Mechanisms and Clinical Therapeutics. Glycoconj. J. 2016, 33, 513–525. [Google Scholar] [CrossRef]
- Schmitz, J.; Dittmar, I.C.; Brockmann, J.D.; Schmidt, M.; Hüdig, M.; Rossoni, A.W.; Maurino, V.G. Defense against Reactive Carbonyl Species Involves at Least Three Subcellular Compartments Where Individual Components of the System Respond to Cellular Sugar Status. Plant Cell 2017, 29, 3234–3254. [Google Scholar] [CrossRef]
- Lin, J.A.; Wu, C.H.; Lu, C.C.; Hsia, S.M.; Yen, G.C. Glycative Stress from Advanced Glycation End Products (AGEs) and Dicarbonyls: An Emerging Biological Factor in Cancer Onset and Progression. Mol. Nutr. Food Res. 2016, 60, 1850–1864. [Google Scholar] [CrossRef]
- Aragonès, G.; Rowan, S.; Francisco, S.G.; Whitcomb, E.A.; Yang, W.; Perini-Villanueva, G.; Schalkwijk, C.G.; Taylor, A.; Bejarano, E. The Glyoxalase System in Age-Related Diseases: Nutritional Intervention as Anti-Ageing Strategy. Cells 2021, 10, 1852. [Google Scholar] [CrossRef]
- Thornalley, P.J. Glyoxalase I—Structure, Function and a Critical Role in the Enzymatic Defence against Glycation. Biochem. Soc. Trans. 2003, 31, 1343–1348. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Zhou, C.; Huang, M.; Tang, C.; Liu, X.; Yue, Y.; Diao, Q.; Zheng, Z.; Liu, D. Glyoxalase System: A Systematic Review of Its Biological Activity, Related-Diseases, Screening Methods and Small Molecule Regulators. Biomed. Pharmacother. 2020, 131, 110663. [Google Scholar] [CrossRef] [PubMed]
- Jin, T.; Zhao, L.; Wang, H.P.; Huang, M.L.; Yue, Y.; Lu, C.; Zheng, Z.B. Recent Advances in the Discovery and Development of Glyoxalase I Inhibitors. Bioorg. Med. Chem. 2020, 28, 115243. [Google Scholar] [CrossRef] [PubMed]
- Soccio, M.; Marangi, M.; Laus, M.N. Genome-Wide Expression Analysis of Glyoxalase I Genes Under Hyperosmotic Stress and Existence of a Stress-Responsive Mitochondrial Glyoxalase I Activity in Durum Wheat (Triticum durum Desf.). Front. Plant Sci. 2022, 13, 934523. [Google Scholar] [CrossRef] [PubMed]
- Blando, F.; Berland, H.; Maiorano, G.; Durante, M.; Mazzucato, A.; Picarella, M.E.; Nicoletti, I.; Gerardi, C.; Mita, G.; Andersen, Ø.M. Nutraceutical Characterization of Anthocyanin-Rich Fruits Produced by “Sun Black” Tomato Line. Front. Nutr. 2019, 6, 133. [Google Scholar] [CrossRef] [PubMed]
- Blando, F.; Marchello, S.; Maiorano, G.; Durante, M.; Signore, A.; Laus, M.N.; Soccio, M.; Mita, G. Bioactive Compounds and Antioxidant Capacity in Anthocyanin-Rich Carrots: A Comparison between the Black Carrot and the Apulian Landrace “Polignano” Carrot. Plants 2021, 10, 564. [Google Scholar] [CrossRef] [PubMed]
- Soccio, M.; Laus, M.N.; Alfarano, M.; Pastore, D. The Soybean Lipoxygenase-Fluorescein Reaction May Be Used to Assess Antioxidant Capacity of Phytochemicals and Serum. Anal. Methods 2016, 8, 4354–4362. [Google Scholar] [CrossRef]
- Soccio, M.; Laus, M.N.; Flagella, Z.; Pastore, D. Assessment of Antioxidant Capacity and Putative Healthy Effects of Natural Plant Products Using Soybean Lipoxygenase-Based Methods. An Overview. Molecules 2018, 23, 3244. [Google Scholar] [CrossRef]
- Ou, B.; Hampsch-Woodill, M.; Prior, R.L. Development and Validation of an Improved Oxygen Radical Absorbance Capacity Assay Using Fluorescein as the Fluorescent Probe. J. Agric. Food Chem. 2001, 49, 4619–4626. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free. Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Laus, M.N.; Tozzi, D.; Soccio, M.; Fratianni, A.; Panfili, G.; Pastore, D. Dissection of Antioxidant Activity of Durum Wheat (Triticum durum Desf.) Grains as Evaluated by the New LOX/RNO Method. J. Cereal Sci. 2012, 56, 214–222. [Google Scholar] [CrossRef]
- Liu, R.H. Whole Grain Phytochemicals and Health. J. Cereal Sci. 2007, 46, 207–219. [Google Scholar] [CrossRef]
- Laus, M.N.; Gagliardi, A.; Soccio, M.; Flagella, Z.; Pastore, D. Antioxidant Activity of Free and Bound Compounds in Quinoa (Chenopodium quinoa Willd.) Seeds in Comparison with Durum Wheat and Emmer. J. Food Sci. 2012, 77, C1150–C1155. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Li, M.; Duan, X.; Abu-Izneid, T.; Rauf, A.; Khan, Z.; Mitra, S.; Emran, T.B.; Aljohani, A.S.M.; Alhumaydhi, F.A.; et al. Phytochemical and Nutritional Profiling of Tomatoes; Impact of Processing on Bioavailability—A Comprehensive Review. Food Rev. Int. 2022; accepted. [Google Scholar] [CrossRef]
- Salehi, B.; Sharifi-Rad, R.; Sharopov, F.; Namiesnik, J.; Roointan, A.; Kamle, M.; Kumar, P.; Martins, N.; Sharifi-Rad, J. Beneficial Effects and Potential Risks of Tomato Consumption for Human Health: An Overview. Nutrition 2019, 62, 201–208. [Google Scholar] [CrossRef]
- Alasalvar, C.; Grigor, J.M.; Zhang, D.; Quantick, P.C.; Shahidi, F. Comparison of Volatiles, Phenolics, Sugars, Antioxidant Vitamins, and Sensory Quality of Different Colored Carrot Varieties. J. Agric. Food Chem. 2001, 49, 1410–1416. [Google Scholar] [CrossRef]
- Gonzali, S.; Mazzucato, A.; Perata, P. Purple as a Tomato: Towards High Anthocyanin Tomatoes. Trends Plant Sci. 2009, 14, 237–241. [Google Scholar] [CrossRef]
- Soccio, M.; Laus, M.N.; Alfarano, M.; Pastore, D. Measuring Activity of Native Plant Sirtuins—The Wheat Mitochondrial Model. Front. Plant Sci. 2018, 9, 961. [Google Scholar] [CrossRef]
- Laus, M.N.; Soccio, M. First Evidence of a Protective Effect of Plant Bioactive Compounds against H2O2-Induced Aconitase Damage in Durum Wheat Mitochondria. Antioxidants 2020, 9, 1256. [Google Scholar] [CrossRef]
- Santel, T.; Pflug, G.; Hemdan, N.Y.A.; Schäfer, A.; Hollenbach, M.; Buchold, M.; Hintersdorf, A.; Linder, I.; Otto, A.; Bigl, M.; et al. Curcumin Inhibits Glyoxalase 1—A Possible Link to Its Anti-Inflammatory and Anti-Tumor Activity. PLoS ONE 2008, 3, e3508. [Google Scholar] [CrossRef]
- Takasawa, R.; Takahashi, S.; Saeki, K.; Sunaga, S.; Yoshimori, A.; Tanuma, S.I. Structure-Activity Relationship of Human GLO I Inhibitory Natural Flavonoids and Their Growth Inhibitory Effects. Bioorg. Med. Chem. 2008, 16, 3969–3975. [Google Scholar] [CrossRef]
- Takasawa, R.; Saeki, K.; Tao, A.; Yoshimori, A.; Uchiro, H.; Fujiwara, M.; Tanuma, S.I. Delphinidin, a Dietary Anthocyanidin in Berry Fruits, Inhibits Human Glyoxalase I. Bioorg. Med. Chem. 2010, 18, 7029–7033. [Google Scholar] [CrossRef] [PubMed]
- Takasawa, R.; Akahane, H.; Tanaka, H.; Shimada, N.; Yamamoto, T.; Uchida-Maruki, H.; Sai, M.; Yoshimori, A.; Tanuma, S.I. Piceatannol, a Natural Trans-Stilbene Compound, Inhibits Human Glyoxalase I. Bioorg. Med. Chem. Lett. 2017, 27, 1169–1174. [Google Scholar] [CrossRef] [PubMed]
- Meeprom, A.; Chan, C.B.; Sompong, W.; Adisakwattana, S. Isoferulic Acid Attenuates Methylglyoxal-Induced Apoptosis in INS-1 Rat Pancreatic β-Cell through Mitochondrial Survival Pathways and Increasing Glyoxalase-1 Activity. Biomed. Pharmacother. 2018, 101, 777–785. [Google Scholar] [CrossRef]
- Suh, K.S.; Chon, S.; Choi, E.M. Limonene Protects Osteoblasts against Methylglyoxal-Derived Adduct Formation by Regulating Glyoxalase, Oxidative Stress, and Mitochondrial Function. Chem. Biol. Interact. 2017, 278, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Hsu, W.H.; Lee, B.H.; Chang, Y.Y.; Hsu, Y.W.; Pan, T.M. A Novel Natural Nrf2 Activator with PPARγ-Agonist (Monascin) Attenuates the Toxicity of Methylglyoxal and Hyperglycemia. Toxicol. Appl. Pharmacol. 2013, 272, 842–851. [Google Scholar] [CrossRef] [PubMed]
- Truong, C.S.; Seo, E.; Jun, H.S. Psoralea corylifolia L. Seed Extract Attenuates Methylglyoxal-Induced Insulin Resistance by Inhibition of Advanced Glycation End Product Formation. Oxidative Med. Cell. Longev. 2019, 2019, 4310319. [Google Scholar] [CrossRef] [PubMed]
- Cha, S.H.; Hwang, Y.; Heo, S.J.; Jun, H.S. Indole-4-Carboxaldehyde Isolated from Seaweed, Sargassum thunbergii, Attenuates Methylglyoxal-Induced Hepatic Inflammation. Mar. Drugs 2019, 17, 486. [Google Scholar] [CrossRef]
- Yeh, W.J.; Hsia, S.M.; Lee, W.H.; Wu, C.H. Polyphenols with Antiglycation Activity and Mechanisms of Action: A Review of Recent Findings. J. Food Drug Anal. 2017, 25, 84–92. [Google Scholar] [CrossRef]
- Rabbani, N.; Thornalley, P.J. Emerging Glycation-Based Therapeutics—Glyoxalase 1 Inducers and Glyoxalase 1 Inhibitors. Int. J. Mol. Sci. 2022, 23, 2453. [Google Scholar] [CrossRef]
- Pellegrini, N.; Serafini, M.; Colombi, B.; Del Rio, D.; Salvatore, S.; Bianchi, M.; Brighenti, F. Total Antioxidant Capacity of Plant Foods, Beverages and Oils Consumed in Italy Assessed by Three Different in vitro Assays. J. Nutr. 2003, 133, 2812–2819. [Google Scholar] [CrossRef]
- Soccio, M.; Laus, M.N.; Spera, G.P.; Trono, D.; Pompa, M.; Flagella, Z.; Pastore, D. Mitochondrial Proline Oxidation Is Affected by Hyperosmotic Stress in Durum Wheat Seedlings. Ann. Appl. Biol. 2010, 157, 1–11. [Google Scholar] [CrossRef]
- Harris, D. Spectophotometric assay. In Spectophotometry and Spectrofluorimetry: A Practical Approach; Bashford, C.L., Harris, D., Eds.; IRL Press: Oxford, UK, 1987; pp. 59–61. [Google Scholar]
- Lichtenthaler, H.K. Chlorophylls and Carotenoids: Pigments of Photosynthetic Biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar]
| Plant Food | Extract | AC (μmol Trolox eq./g d.w.) | ||
|---|---|---|---|---|
| LOX-FL | ORAC | TEAC | ||
| DW | Phenolic | 3.10 ± 0.05 g | 24.15 ± 1.50 e | 9.80 ± 0.80 d |
| DW | Lipophilic | 0.52 ± 0.06 h | 1.58 ± 0.17 f | 0.25 ± 0.01 f |
| WTT | Lipophilic | 14.90 ± 0.40 e | 31.10 ± 2.00 d | 3.70 ± 0.03 e |
| WTT | Phenolic | 8.78 ± 0.19 f | 89.62 ± 1.79 c | 3.80 ± 0.06 e |
| SBT | Phenolic | 27.14 ± 0.92 d | 199.9 ± 16.1 a | 10.10 ± 0.71 d |
| BC | Phenolic | 221.2 ± 24.02 a | 199.1 ± 14.4 a | 52.40 ± 0.25 a |
| PC1 | Phenolic | 39.32 ± 0.09 c | 113.1 ± 6.3 b | 19.51 ± 1.20 c |
| PC2 | Phenolic | 81.25 ± 2.39 b | 113.4 ± 4.5 b | 29.13 ± 0.03 b |
| Plant Food | Extract | IC50 or AC50 (mg d.w.) | ||
|---|---|---|---|---|
| h-GLYI | DWM-GLYI | DWM-GLYI/h-GLYI (% Variation) | ||
| DW | Lipophilic | 7.97 ± 0.23 a | 6.60 ± 0.42 a | +17% ** |
| WTT | Lipophilic | 1.17 ± 0.20 a | 0.412 ± 0.040 a | +65% ** |
| WTT | Phenolic | 0.235 ± 0.008 b | 0.150 ± 0.023 b | +36 ** |
| SBT | Phenolic | 0.180 ± 0.010 b | 0.101 ± 0.016 b | +44% ** |
| BC | Phenolic | 0.237 ± 0.010 b | 0.146 ± 0.020 b | +37% ** |
| PC1 | Phenolic | 0.302 ± 0.021 b | 0.200 ± 0.011 b | +34% ** |
| PC2 | Phenolic | 0.213 ± 0.017 b | 0.165 ± 0.012 b | +22% * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laus, M.N.; Blando, F.; Soccio, M. Glyoxalase I Assay as a Possible Tool for Evaluation of Biological Activity of Antioxidant-Rich Plant Extracts. Plants 2023, 12, 1150. https://doi.org/10.3390/plants12051150
Laus MN, Blando F, Soccio M. Glyoxalase I Assay as a Possible Tool for Evaluation of Biological Activity of Antioxidant-Rich Plant Extracts. Plants. 2023; 12(5):1150. https://doi.org/10.3390/plants12051150
Chicago/Turabian StyleLaus, Maura Nicoletta, Federica Blando, and Mario Soccio. 2023. "Glyoxalase I Assay as a Possible Tool for Evaluation of Biological Activity of Antioxidant-Rich Plant Extracts" Plants 12, no. 5: 1150. https://doi.org/10.3390/plants12051150
APA StyleLaus, M. N., Blando, F., & Soccio, M. (2023). Glyoxalase I Assay as a Possible Tool for Evaluation of Biological Activity of Antioxidant-Rich Plant Extracts. Plants, 12(5), 1150. https://doi.org/10.3390/plants12051150






