Relocation of Sr48 to Chromosome 2D Using an Alternative Mapping Population and Development of a Closely Linked Marker Using Diverse Molecular Technologies
Abstract
1. Introduction
2. Results
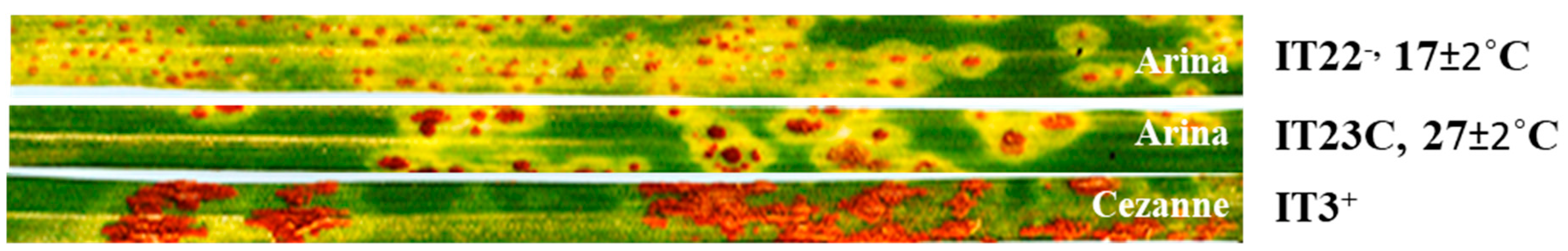
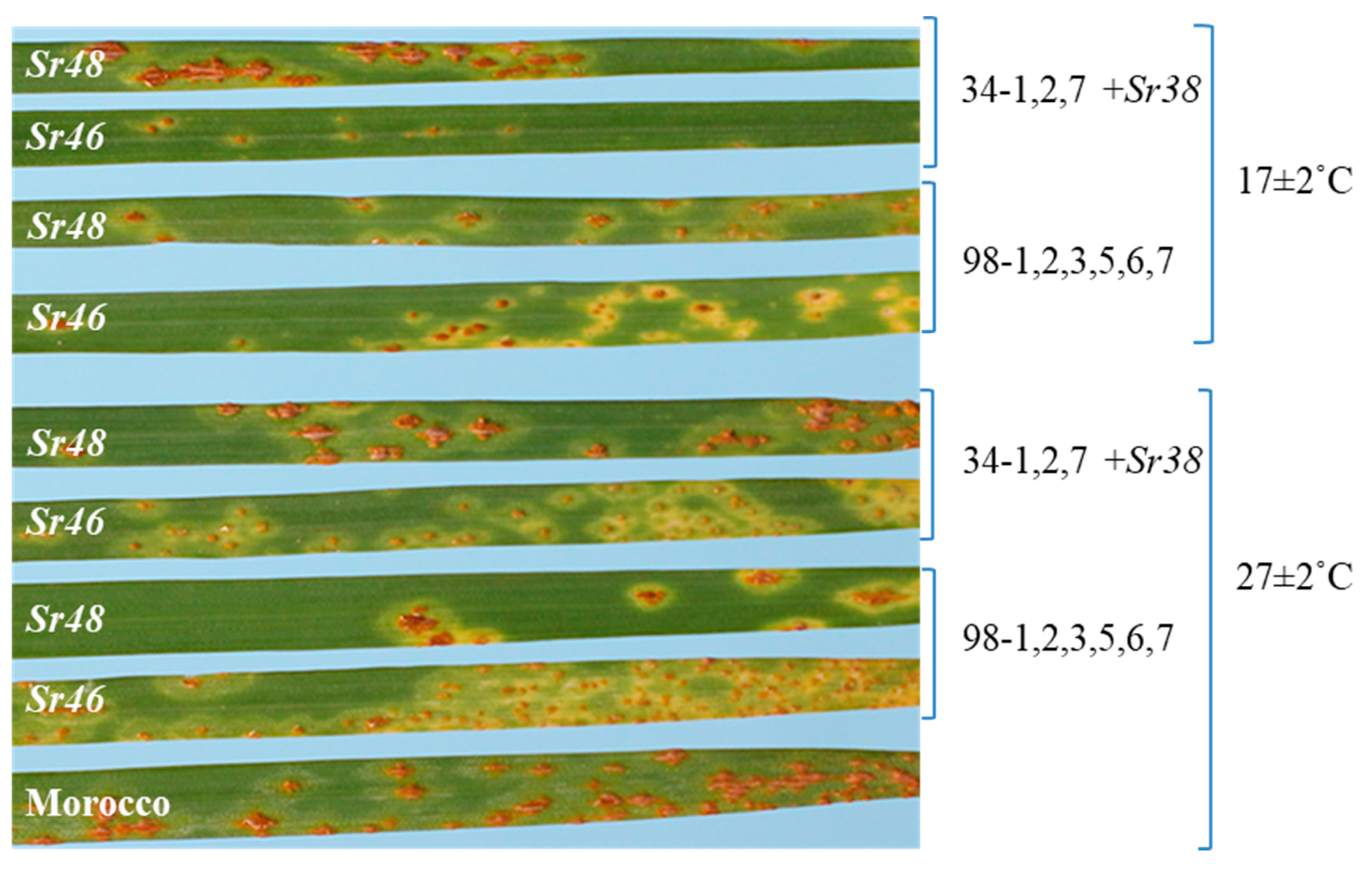
2.1. Rust Response Assessments
2.2. Construction of DArTSeq Linkage Map
2.3. Mapping of Sr48
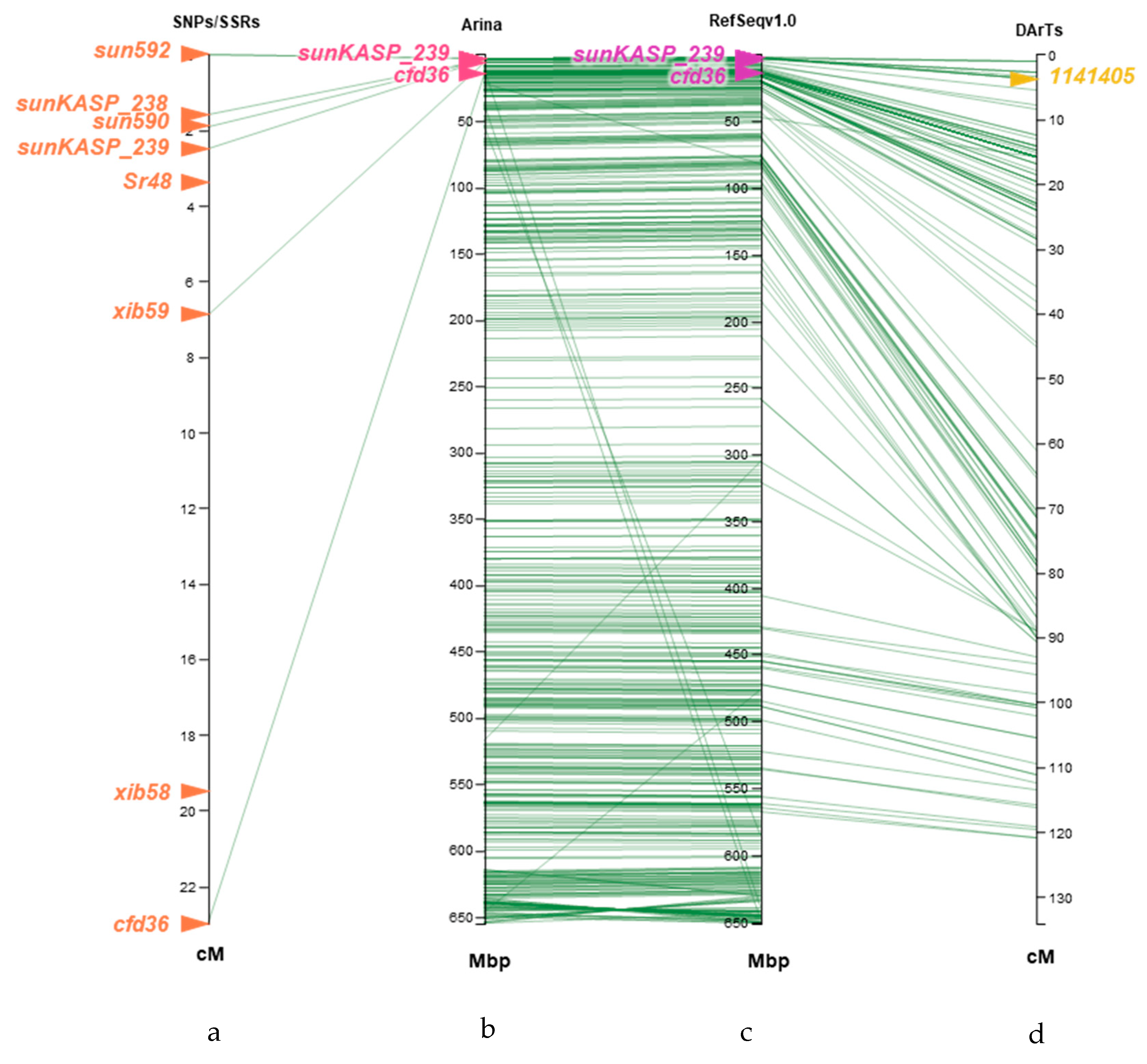
2.4. Comparison of Sr48-Linked Markers Developed with Chromosome Survey Sequences with Arina LrFor (Arina) Pseudomolecule and CS Reference Genome Sequence v1.0
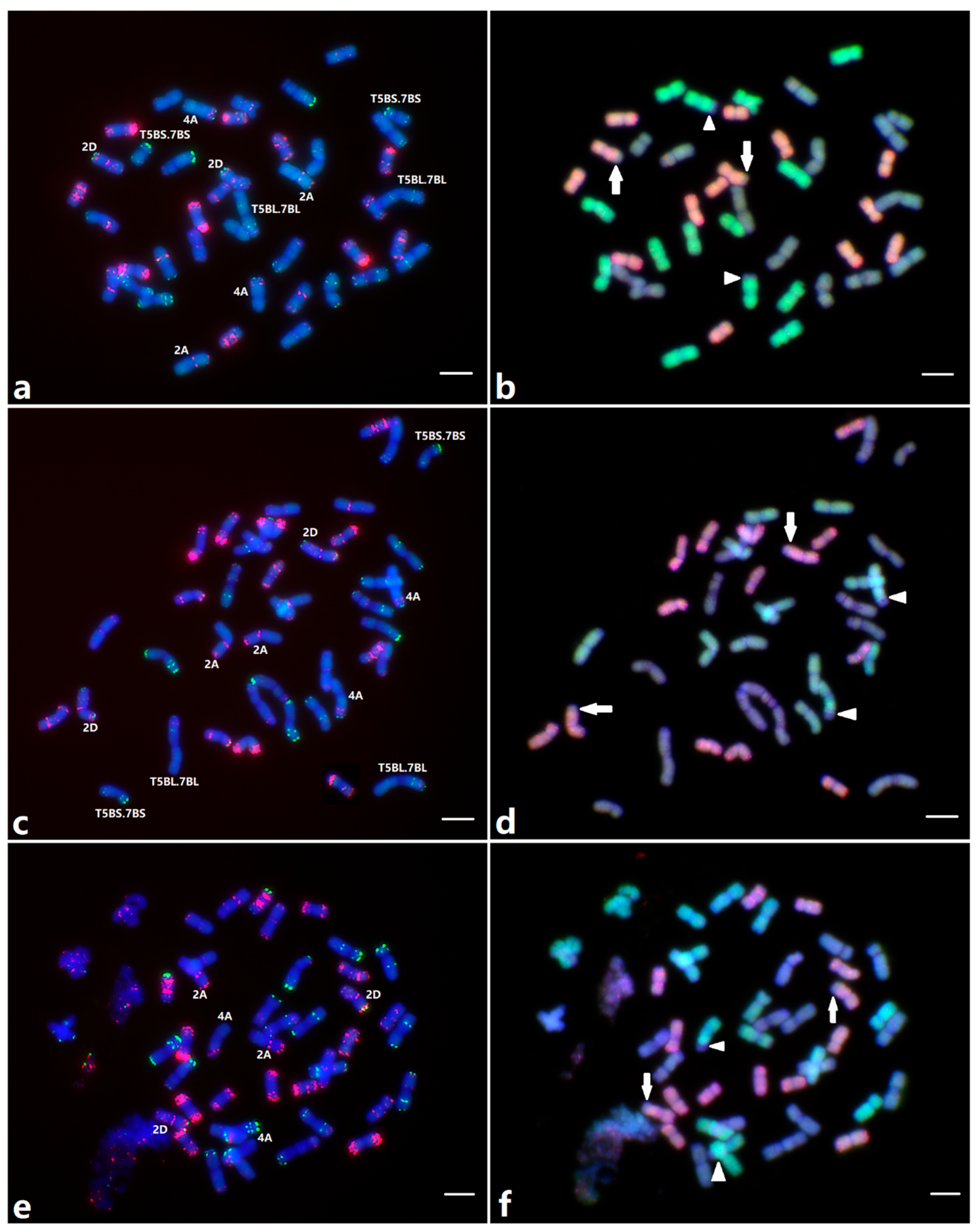
2.5. Molecular Cytogenetic Analysis
2.6. Polymorphism of the Sr48 Linked Marker sunKASP_239
3. Discussion
4. Materials and Methods
4.1. Host Materials
4.2. Greenhouse Screening
4.3. Genotyping of Arina/Cezzane RIL Population
4.4. Development of PCR Based Markers
4.5. Molecular Cytogenetic Analysis
4.6. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shiferaw, B.; Smale, M.; Braun, H.-J.; Duveiller, E.; Reynolds, M.; Muricho, G. Crops that feed the world 10. Past successes and future challenges to the role played by wheat in global food security. Food Secur. 2013, 5, 291–317. [Google Scholar] [CrossRef]
- Atchison, J.; Head, L.; Gates, A. Wheat as food, wheat as industrial substance; comparative geographies of transformation and mobility. Geoforum 2010, 41, 236–246. [Google Scholar] [CrossRef]
- Leonard, K.J.; Szabo, L.J. Stem rust of small grains and grasses caused by Puccinia graminis. Mol. Plant Path. 2005, 6, 99–111. [Google Scholar] [CrossRef] [PubMed]
- Zadoks, J.C. Epidemiology of wheat rust in Europe. FAO Plant Prot. Bull. 1963, 13, 97. [Google Scholar] [CrossRef]
- Singh, S.; Singh, R.P.; Huerta-Espino, J. Stem Rust. In Disease Resistance in Wheat; CABI: Wallingford, UK, 2012; pp. 18–32. [Google Scholar] [CrossRef]
- Pretorius, Z.A.; Singh, R.P.; Wagoire, W.W.; Payne, T.S. Detection of virulence to wheat stem rust resistance gene Sr31 in Puccinia graminis f. sp. tritici in Uganda. Plant Dis. 2000, 84, 203. [Google Scholar] [CrossRef]
- Singh, P.R.; Hodson, D.P.; Huerta-Espino, J.; Jin, Y.; Bhavani, S.; Njau, P.; Herrera-Foessel, S.; Singh, P.K.; Singh, S.; Govindan, V. The emergence of Ug99 races of the stem rust fungus is a threat to world wheat production. Annu. Rev. Phytopathol. 2011, 49, 465–481. [Google Scholar] [CrossRef]
- Nazari, K.; Mafi, M.; Yahyaoui, A.; Singh, R.P.; Park, R.F. Detection of wheat stem rust race (Puccinia graminis f. sp. tritici), TTKSK (Ug99) in Iran. Plant Dis. 2009, 93, 317. [Google Scholar] [CrossRef]
- Jin, Y.; Szabo, L.J.; Rouse, M.N.; Fetch, T., Jr.; Pretorius, Z.A.; Wanyera, R.; Njau, P. Detection of virulence to resistance gene Sr36 within the TTKS race lineage of Puccinia graminis f. sp. tritici. Plant Dis. 2009, 93, 367–370. [Google Scholar] [CrossRef]
- Jin, Y.; Pretorius, Z.A.; Singh, R.P.; Fetch, T., Jr. Detection of virulence to resistance gene Sr24 within race TTKS of Puccinia graminis f. sp. tritici. Plant Dis. 2008, 92, 923–926. [Google Scholar] [CrossRef]
- Olivera, P.; Newcomb, M.; Szabo, L.J.; Rouse, M.; Johnson, J.; Gale, S.; Luster, D.G.; Hodson, D.; Cox, J.A.; Burgin, L.; et al. Phenotypic and genotypic characterization of race TKTTF of Puccinia graminis f. sp. tritici that caused a wheat stem rust epidemic in Southern Ethiopia in 2013-14. Phytopathology 2015, 105, 917–928. [Google Scholar] [CrossRef]
- Macharia, J.K.; Wanyera, R.; Kilonzo, S. Evaluation of fungicides for controlling stem rust race Ug99 on bread wheat. J. Agric. Sci. Tech. 2013, 3, 404. [Google Scholar]
- Murray, G.M.; Brennan, J.P. Estimating disease losses to the Australian wheat industry. Aust. Plant Pathol. 2009, 38, 558–570. [Google Scholar] [CrossRef]
- Watson, I.A.; Butler, F.C. Wheat Rust Control in Australia, National Conferences and Other Initiatives and Developments; University of Sydney: Sydney, Australia, 1984. [Google Scholar]
- Watson, I.A.; Singh, D. The future for rust resistant wheat in Australia. J. Aust. Inst. Agric. Sci. 1952, 18, 190–197. [Google Scholar]
- Bariana, H.S. Breeding for disease resistance. In Encyclopedia of Applied Plant Sciences; Thomas, B., Murphy, D.J., Murray, B.G., Eds.; Academic Press: London, UK, 2003; pp. 244–253. [Google Scholar]
- Bariana, H.S.; Brown, G.N.; Bansal, U.K.; Miah, H.; Standen, G.E.; Lu, M. Breeding triple rust resistant wheat cultivars for Australia using conventional and marker-assisted selection technologies. Aust. J. Agri. Res. 2007, 58, 576–587. [Google Scholar] [CrossRef]
- Pathan, A.K.; Park, R.F. Evaluation of seedling and adult plant resistance to stem rust in European wheat cultivars. Euphytica 2007, 155, 87–105. [Google Scholar] [CrossRef]
- Bansal, U.K.; Hayden, M.J.; Keller, B.; Welling, C.R.; Park, R.F.; Bariana, H.S. Relationship between wheat rust resistance genes Yr1 and Sr48 and a microsatellite marker. Plant Pathol. 2009, 58, 1039–1043. [Google Scholar] [CrossRef]
- Salina, E.A.; Leonova, I.N.; Efremova, T.T.; Röder, M.S. Wheat genome structure, translocations during the course of polyploidization. Funct. Integr. Genome 2005, 6, 71–80. [Google Scholar] [CrossRef]
- Hao, M.; Luo, J.; Zhang, L.; Yuan, Z.; Zheng, Y.; Zhang, H.; Liu, D. In situ hybridization analysis indicates that 4AL–5AL–7BS translocation preceded subspecies differentiation of Triticum turgidum. Genome 2013, 56, 303–305. [Google Scholar] [CrossRef]
- Badaeva, E.D.; Dedkova, O.S.; Gay, G.; Pukhalskyi, V.A.; Zelenin, A.V.; Bernard, S.; Bernard, M. Chromosomal rearrangements in wheat: Their types and distribution. Genome 2007, 50, 907–926. [Google Scholar] [CrossRef]
- Kislev, M.E. Stem rust of wheat 3300 years old found in Israel. Science 1982, 216, 993–994. [Google Scholar] [CrossRef]
- Chaves, M.S.; Martinelli, J.A.; Wesp-Guterres, C.; Graichen, F.A.S.; Brammer, S.P.; Scagliusi, S.M.; da Silva, P.R.; Wietholter, P.; Torres, G.A.M.; Lau, E.Y.; et al. The importance for food security of maintaining rust resistance in wheat. Food Secur. 2013, 5, 157–176. [Google Scholar] [CrossRef]
- McIntosh, R.A.; Wellings, C.R.; Park, R.F. Wheat rusts: An atlas of resistance genes. In CSIRO Publications; East Melbourne: Victoria, Australia, 1995. [Google Scholar]
- Mago, R.; Chen, C.; Xia, X.; Whan, X.; Forrest, K.; Basnet, B.R.; Perera, G.; Chandramohan, S.; Randhawa, M.; Hayden, M.; et al. Adult plant stem rust resistance in durum wheat Glossy Huguenot: Mapping to marker development and validation. Theor. Appl. Genet. 2022, 135, 1541–1550. [Google Scholar] [CrossRef] [PubMed]
- Bariana, H.S.; McIntosh, R.A. Cytogenetic studies in wheat XV. Location of rust resistance genes in VPM1 and their genetic linkage with other resistance genes in chromosome 2A. Genome 1993, 36, 476–482. [Google Scholar] [CrossRef] [PubMed]
- Green, G.J.; Knott, D.R.; Watson, I.A.; Pugsley, A.T. Seedling reactions to stem rust of lines of Marquis wheat with substituted genes for rust resistance. Can. J. Plant Sci. 1960, 40, 524–538. [Google Scholar] [CrossRef]
- Tsilo, T.J.; Chao, S.; Jin, Y.; Anderson, J.A. Identification and validation of SSR markers linked to the stem rust resistance gene Sr6 on the short arm of chromosome 2D in wheat. Theor. Appl. Genet. 2009, 118, 515–524. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Rouse, M.N.; Zhang, W.; Jin, Y.; Akhunov, E.; Wei, Y.; Dubcovsky, J. Fine mapping and characterization of Sr21., a temperature-sensitive diploid wheat resistance gene effective against the Puccinia graminis f. sp. tritici Ug99 race group. Theor. Appl. Genet. 2015, 128, 645–656. [Google Scholar] [CrossRef]
- Qi, L.L.; Pumphrey, M.O.; Friebe, B.; Qian, C.; Bowden, R.L.; Rouse, M.N.; Jin, Y.; Gill, B.S. A novel Robertsonian event leads to transfer of a stem rust resistance gene (Sr52). effective against race Ug99 from Daspyrum villosum into wheat. Theor. Appl. Genet. 2011, 123, 159–167. [Google Scholar] [CrossRef]
- Arora, S.; Steuernagel, B.; Gaurav, K.; Chandramohan, S.; Long, Y.; Matny, O.; Johnson, R.; Enk, J.; Periyannan, S.; Singh, N.; et al. Resistance gene cloning from a wild crop relative by sequence capture and association genetics. Nat. Biotechnol. 2019, 37, 139–143. [Google Scholar] [CrossRef]
- Somers, D.J.; Isaac, P.; Edwards, K. A high-density microsatellite consensus map for bread wheat (Triticum aestivum L.). Theor. Appl. Genet. 2004, 109, 1105–1114. [Google Scholar] [CrossRef]
- Tsilo, T.J.; Jin, Y.; Anderson, J.A. Identification of flanking markers for the stem rust resistance gene Sr6 in wheat. Crop Sci. 2010, 50, 1967–1970. [Google Scholar] [CrossRef]
- Stakman, E.C.; Stewart, D.M.; Loegering, W.Q. Identification of physiological races of Puccinia graminis var tritici. In Agricultural Research Service E617; United States Department of Agriculture: Washington, DC, USA, 1962. [Google Scholar]
- Bansal, U.K.; Kazi, A.G.; Singh, B.; Hare, R.A.; Bariana, H.S. Mapping of durable stripe rust resistance in a durum wheat cultivar Wollaroi. Mol. Breed 2014, 33, 51–59. [Google Scholar] [CrossRef]
- Bansal, U.K.; Wicker, T.; Keller, B.; Hayden, M.; Bariana, H.S. Molecular mapping of an adult plant stem rust resistance gene Sr56 in winter wheat cultivar Arina. Theor. Appl. Genet. 2014, 127, 1441–1448. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Zhou, J.; Gong, X.; Zhao, G.; Jia, J.; Qi, X. Identification and validation of a major quantitative trait locus for slow-rusting resistance to stripe rust in wheat. J. Integr. Plant Biol. 2012, 54, 330–344. [Google Scholar] [CrossRef]
- Yu, L.X.; Barbier, H.; Rouse, M.N.; Singh, S.; Singh, R.P.; Bhavani, S.; Huerta-Espino, J.; Sorrells, M.E. A consensus map for Ug99 stem rust resistance loci in wheat. Theor. Appl. Genet. 2014, 127, 1561–1581. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Dundas, I.; Dong, C.; Li, G.; Trethowan, R.; Yang, Z.; Hoxha, S.; Zhang, P. Identification and characterization of a new stripe rust resistance gene Yr83 on rye chromosome 6R in wheat. Theor. Appl. Genet. 2020, 133, 1095–1107. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Friebe, B.; Lukaszewski, A.J.; Gill, B.S. The centromere structure in Robertsonian wheat-rye translocation chromosomes indicates that centric breakage-fusion can occur at different positions within the primary constriction. Chromosoma 2001, 110, 335–344. [Google Scholar] [CrossRef]
- Taylor, J.; Butler, D. R package ASMap, efficient genetic linkage map construction and diagnosis. J. Statist. Soft. 2017, 79, 1. [Google Scholar] [CrossRef]
- Kosambi, D.D. The estimation of map distances from recombination values. Ann. Eugen. 1943, 12, 172. [Google Scholar] [CrossRef]
- Voorrip, R.E. MapChart, software for the graphical presentation of linkage maps and QTLs. J. Hered. 2002, 93, 77–78. [Google Scholar] [CrossRef]

| Dartseq Marker | CSS | Position in Physical Map (bp) | STS/SSR (Repeat) | Primer | Forward a | Reverse | |
|---|---|---|---|---|---|---|---|
| Start Site | Stop Site | ||||||
| 1109505 | 2DS_6421726 | 2,944,768 | 2,944,836 | STS | sun582 | AGTACAGGTCGTGGCTGCTT | ATGTCGCCAAGTTCGCATAG |
| STS | sun583 | GGCAAAATGGCAGGATATTG | TCCATCCATGTTCCTGATGA | ||||
| 2258266 | 2DS_4785040 | 2,944,771 | 2,944,703 | STS | sun584 | AAGGCTGACCTTTGACCTGA | CCGACCTCATACCGGTACAT |
| 1141405 | 2DS_5324961 | 2,341,675 | 2,341,622 | STS | sun846 | ATTACTGCGCCTCCTTCTGA | CGACCATTAGCTGACTGCAA |
| STS | sun847 | GCGGCTTGACATCTCATCTT | CACGTATTCGCAGCAAAAGA | ||||
| STS | sun848 | GTTTTGGGTACCGAGCAAAA | TGCCTCGTTGATTGAAATTG | ||||
| STS | sun849 | CCGTTGTCTCCTCTGAAAGC | GCGTTGTAGGTGCTTTGCTT | ||||
| SSR (GCG)3 | sun585 | GTGAATCGTACAAGTGGAATG | ACTCTGCATCGTAAGTCACC | ||||
| SSR (CCG)3 | sun586 | TTAAGGCTAAAACAAATCAGG | CGATACAAAGGCAGATGG | ||||
| SSR (AGA)3 | sun587 | AAGTAGCATTTCTGGAACACA | AATACTTGCACATCTCCTTTG | ||||
| SSR (AGAGA)5 | sun588 | GCTTGCTTCCAGTTCCAG | CTCAAGCAAGCAAATAGTCAT | ||||
| SSR (TCGCCG)6 | sun589 | CCGTCCCACAGGTGTCTC | TCTGAACCAAACACATCCTAC | ||||
| SSR (TCCT)4 | sun590 | TACTCTAGTGACCAACCAACA | GGCCATTACATGGTACTAGTTT | ||||
| SSR (AGCT)4 | sun591 | TTATTCCATTTCAATCTGAGC | CCTCACTAATAATTGAAACACG | ||||
| SSR (GTGTGC)6 | sun592 | TCTGGTGATTTTTGTTAGGAA | AACTCTGTGCATGCTAGTTTC | ||||
| SSR (CTGGAA)6 | sun593 | TCCTCCATCCTCTCTTCC | TTTATGGGTACGTGCTGTATT | ||||
| 1375819 | 5B_6422050 | 339,989,574 | 339,989,506 | SSR (AG)6 | sun594 | TTGACTCTCTACCATCCAGAA | AACATATGACTGAAGGACCAA |
| SSR (CGG)4 | sun595 | GTACAGCGAGACATGTGAGAC | GATGTGGAGAACCAAGTCAT | ||||
| STS Marker | KASP Marker | Allele 1 Primer a | Allele 2 Primer b | Common Primer |
|---|---|---|---|---|
| sun849 | sunKASP_238 | GGCTGTCCGCAAATCTTC | GGGCTGTCCGCAAATCTTA | GACAAGTCCGAACATCCACA |
| sunKASP_239 | GGCTATGGCTGATGGAAGAA | GGCTATGGCTGATGGAAGAG | CGACCACAACTCAGCAAAGA | |
| sunKASP_240 | TGGATCCACCGAGAAGGA | GGATCCACCGAGAAGGG | CCCTCCTTCCTTCCTTCCTT | |
| sunKASP_241 | ACATGCACGTCCACAAACA | CACATGCACGTCCACAAACT | CGTTGTGTGCCAGCTAGAAG | |
| sunKASP_242 | TAACTCCAACGGACCCTCAA | TAACTCCAACGGACCCTCAC | GACGATGTGTAGCTCTTGTGG | |
| sunKASP_243 | CTCTCCCTCATTCGCTCAG | CTCTCCCTCATTCGCTCAC | TGTCCGGTGTGCTCCTTATT |
| Cultivars | Marker Allele |
|---|---|
| Arina | A:A |
| Cezanne | G:G |
| Australian cultivars | |
| AGT Katana, Axe, Baxter, Beaufort, Bolac, Calingiri, Carnamah, Catalina, Chara, Chief CL Plus, Cobra, Coolah, Corack, Correll, Crusader, Dart, Derrimut, DS Faraday, EGA Bonnie Rock, EGA Bounty, EGA Burke, EGA Gregory, EGA Wedgetail, EGA Wylie, Elmore CL PLus, Emu Rock, Envoy, Espada, Estoc, Forrest, Fortune, Gauntlet, Gazelle, GBA Sapphire, Giles, Gladius, Grenade CL Plus, Harper, Impala, Impose CL Plus, Janz, Justica CL Plus, King Rock, Kord CL Plus, Kunjin, Lancer, Lang, Lincoln, Livingston, LRPB Arrow, LRPB Flanker, LRPB Kittyhawk, LRPB Reliant, Mace, Mackellar, Magenta, Mansfield, Merinda, Merlin, Ninja, Orion, Phantom, Preston, Scout, Sentinel, SF Adagio, SF Scenario, Shield, Spitfire, SQP Revenue, Strzelecki, Sunco, Sunguard, Sunmax, Suntop, Sunvale, Sunzell, Trojan, Ventura, Waagan, Wallup, Wedin, Westonia, Wyalkatchem, Wylah, Yandanooka, Yitpi, Young | G:G |
| Naparoo | A:A |
| European cultivars | |
| Apu, Atson, Bastian, Batalj, Bjarne, Børsum, Brons, Canon, Dala, Dalarna, Diamant, Diamant ll, Drabant, Dragon, ELS 6404-102-3, Extra Kolben, Fagott, Fram l, Fram ll, Fylgia l, Fylgia ll, Haarajärvi ME0102 Apu, Halland, Horsmanaho ME201 Timantti, J-03, Järvenkylä ME0302 Timantti, JO 3524, JO 8023, Jokikylä ME0505 Apu, Kärn, Kärn ll, Kimmo, Kiuru, Laitiala AP0103, Landvårkveite, Lantvete från Dalarna, Lantvete från Halland, Lavett, Manu, Monola ME1301, Møystad, MS 273-150, Naxos, Nemares, Nora, Norrøna, østby, Polkka, Pompe, Pondus, Prins, Progress, Rang, Reno, Ring, Rival, Rollo, Rubin, Runar, Ruso, Saffran, Safir, Sappo, Sibirian, Skirne, Snøgg II, Snøgg l, Sober, Sopu, Sport, Svenno, Timantti, Timantti Paavo, Tjalve, Touko, Troll, Trym, Ulla, Vinjett, Walter, Zebra | G:G |
| Avle, Blanka, Boru, Dacke, Kadett, Vitus, William, WW 20299 | A:A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nsabiyera, V.; Qureshi, N.; Li, J.; Randhawa, M.; Zhang, P.; Forrest, K.; Bansal, U.; Bariana, H. Relocation of Sr48 to Chromosome 2D Using an Alternative Mapping Population and Development of a Closely Linked Marker Using Diverse Molecular Technologies. Plants 2023, 12, 1601. https://doi.org/10.3390/plants12081601
Nsabiyera V, Qureshi N, Li J, Randhawa M, Zhang P, Forrest K, Bansal U, Bariana H. Relocation of Sr48 to Chromosome 2D Using an Alternative Mapping Population and Development of a Closely Linked Marker Using Diverse Molecular Technologies. Plants. 2023; 12(8):1601. https://doi.org/10.3390/plants12081601
Chicago/Turabian StyleNsabiyera, Vallence, Naeela Qureshi, Jianbo Li, Mandeep Randhawa, Peng Zhang, Kerrie Forrest, Urmil Bansal, and Harbans Bariana. 2023. "Relocation of Sr48 to Chromosome 2D Using an Alternative Mapping Population and Development of a Closely Linked Marker Using Diverse Molecular Technologies" Plants 12, no. 8: 1601. https://doi.org/10.3390/plants12081601
APA StyleNsabiyera, V., Qureshi, N., Li, J., Randhawa, M., Zhang, P., Forrest, K., Bansal, U., & Bariana, H. (2023). Relocation of Sr48 to Chromosome 2D Using an Alternative Mapping Population and Development of a Closely Linked Marker Using Diverse Molecular Technologies. Plants, 12(8), 1601. https://doi.org/10.3390/plants12081601










